Preparation of Hierarchical Porous Silicon Carbide Monoliths via Ambient Pressure Drying Sol–Gel Process Followed by High-Temperature Pyrolysis
Abstract
1. Introduction
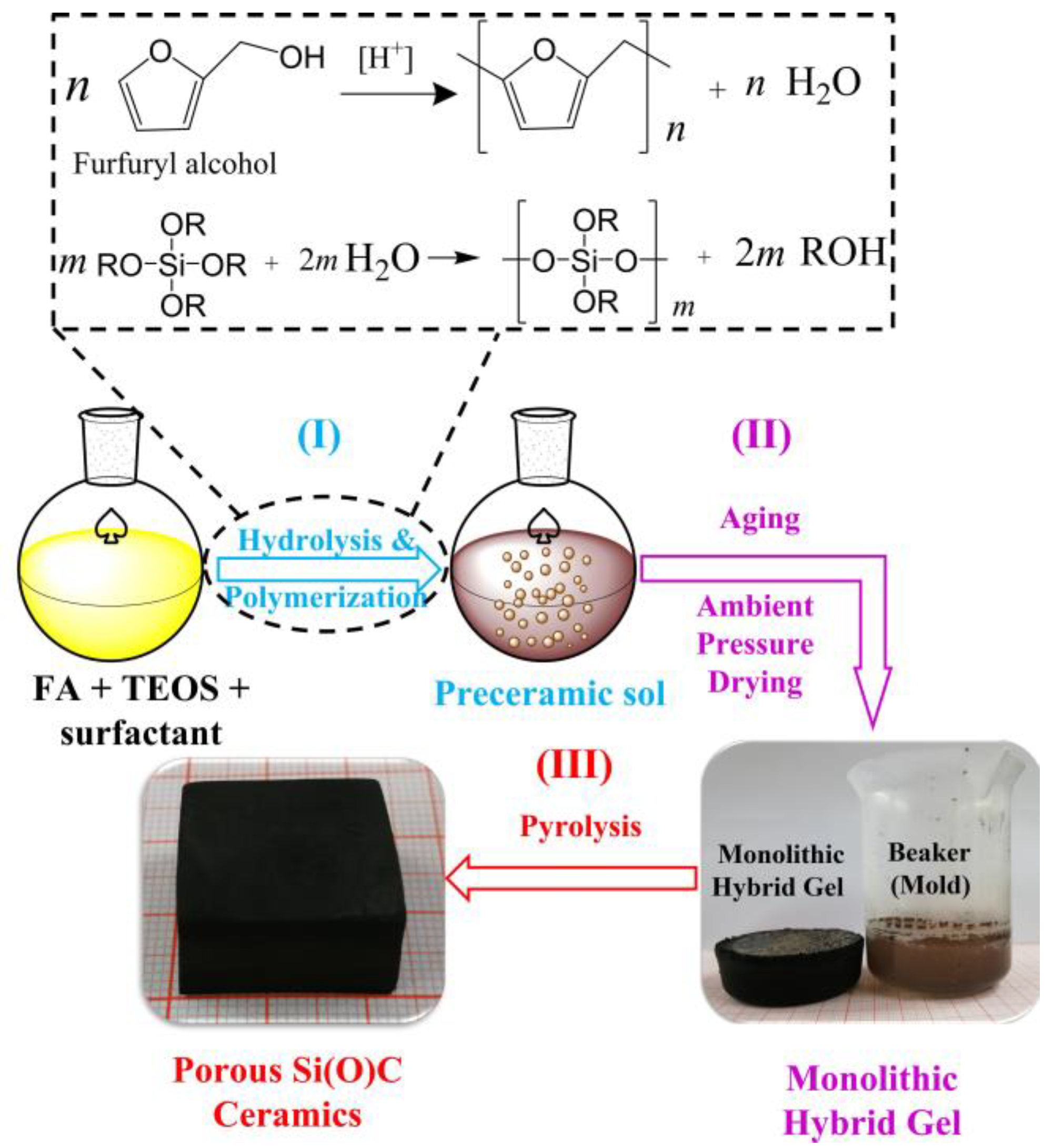
2. Materials and Methods
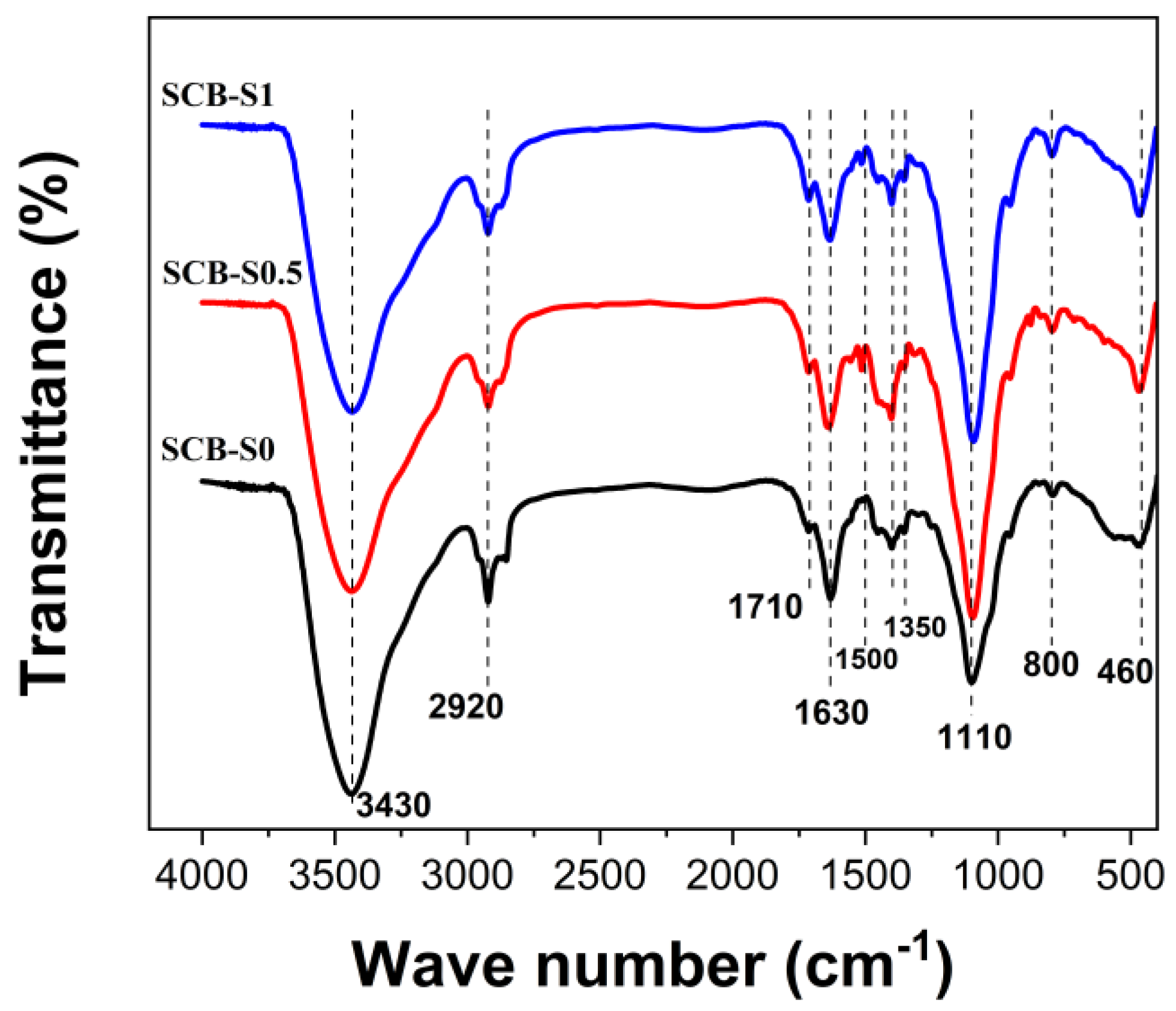
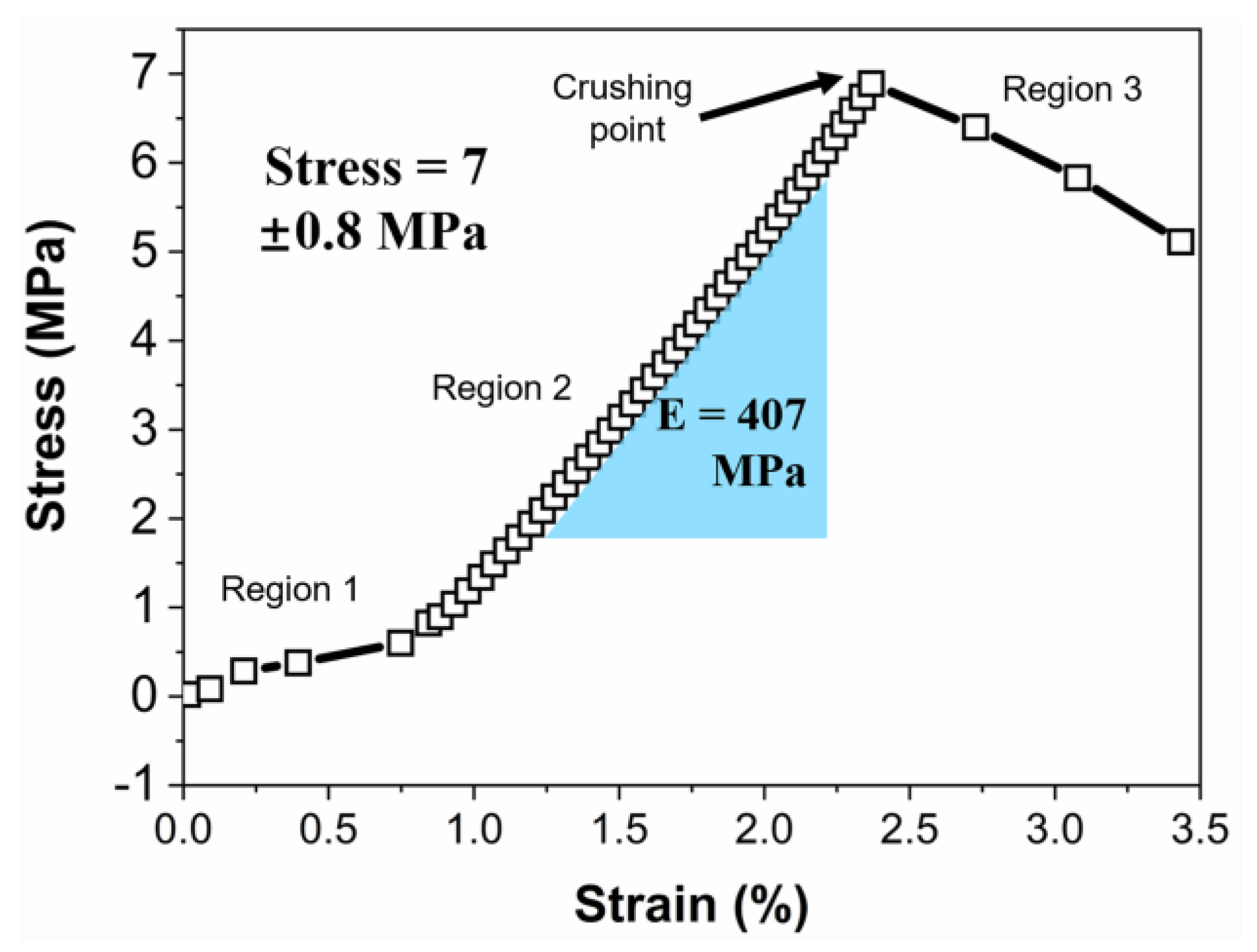
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feinle, A.; Elsaesser, M.S.; Husing, N. Sol-gel synthesis of monolithic materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3377–3399. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu, C.; Zeydanli, D.; Colombo, P. Porous polymer derived ceramics. Mater. Sci. Eng. R Rep. 2016, 106, 1–30. [Google Scholar] [CrossRef]
- Franks, G.V.; Tallon, C.; Studart, A.R.; Sesso, M.L.; Leo, S. Colloidal processing: Enabling complex shaped ceramics with unique multiscale structures. J. Am. Ceram Soc. 2017, 100, 458–490. [Google Scholar] [CrossRef]
- Antonietti, M.; Fechler, N.; Fellinger, T.-P. Carbon aerogels and monoliths: Control of porosity and nanoarchitecture via sol–gel routes. Chem. Mater. 2014, 26, 196–210. [Google Scholar] [CrossRef]
- Li, F.; Huang, X.; Liu, J.-X.; Zhang, G.-J. Sol-gel derived porous ultra-high temperature ceramics. J. Adv. Ceram 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.-G.; Huang, X.; Liu, J.-X.; Bao, W.; Zhang, G.-J.; Wang, H. Preparation of ZrC/SiC porous self-supporting monoliths via sol-gel process using polyethylene glycol as phase separation inducer. J. Eur. Ceram Soc. 2018, 38, 4806–4813. [Google Scholar] [CrossRef]
- Li, F.; Huang, X. Preparation of highly porous ZrB2/ZrC/SiC composite monoliths using liquid precursors via direct drying process. J. Eur. Ceram Soc. 2018, 38, 1103–1111. [Google Scholar] [CrossRef]
- Moreno, R. Better ceramics through colloid chemistry. J. Eur. Ceram Soc. 2020, 40, 559–587. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Jia, H.; Chen, G.; Li, S.; Chen, K.; Wang, C.-A.; Qiao, L. Facile fabrication of cordierite-based porous ceramics with magnetic properties. J. Adv. Ceram 2022, 11, 1583–1595. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Hong, Z.; Wang, S.; Gao, X.; Gu, X. Preparation of hollow fiber membranes from mullite particles with aid of sintering additives. J. Adv. Ceram 2021, 10, 78–87. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Li, D.; Zeng, L.; Gao, S. Formation of hierarchical Si3N4 foams by protein-based gelcasting and chemical vapor infiltration. J. Adv. Ceram 2021, 10, 187–193. [Google Scholar] [CrossRef]
- Eom, J.-H.; Kim, Y.-W.; Raju, S. Processing and properties of macroporous silicon carbide ceramics: A review. J. Asian Ceram Soc. 2013, 1, 220–242. [Google Scholar] [CrossRef]
- Song, L.; Fan, B.; Chen, Y.; Gao, Q.; Li, Z.; Wang, H.; Zhang, X.; Li, G.; Li, H.; Zhang, R. Ultralight and hyperelastic SiC nanofiber aerogel spring for personal thermal energy regulation. J. Adv. Ceram 2022, 11, 1235–1248. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kuntz, J.D.; Satcher, J.J.H.; Baumann, T.F. Synthesis and characterization of monolithic, high surface area SiO2/C and SiC/C composites. J. Mater. Chem. 2010, 20, 4840–4844. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Nakanishi, K.; Hanada, T. Fabrication of macroporous silicon carbide ceramics by intramolecular carbothermal reduction of phenyl-bridged polysilsesquioxane. J. Mater. Chem. 2009, 19, 7716–7720. [Google Scholar] [CrossRef]
- Durif, C.; Wynn, M.; Balestrat, M.; Franchin, G.; Kim, Y.-W.; Leriche, A.; Miele, P.; Colombo, P.; Bernard, S. Open-celled silicon carbide foams with high porosity from boron-modified polycarbosilanes. J. Eur. Ceram Soc. 2019, 39, 5114–5122. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Fukushima, M.; Colombo, P. Silicon carbide-based foams from direct blowing of polycarbosilane. J. Eur. Ceram Soc. 2012, 32, 503–510. [Google Scholar] [CrossRef]
- Yu, Z.L.; Yang, N.; Apostolopoulou-Kalkavoura, V.; Qin, B.; Ma, Z.Y.; Xing, W.Y.; Qiao, C.; Bergstrom, L.; Antonietti, M.; Yu, S.H. Fire-Retardant and Thermally Insulating Phenolic-Silica Aerogels. Angew. Chem. Int. Ed. Engl. 2018, 57, 4538–4542. [Google Scholar] [CrossRef]
- Jin, G.-Q.; Guo, X.-Y. Synthesis and characterization of mesoporous silicon carbide. Micropor. Mesopor. Mat. 2003, 60, 207–212. [Google Scholar] [CrossRef]
- Zera, E.; Campostrini, R.; Aravind, P.R.; Blum, Y.; Sorarù, G.D. Novel SiC/C Aerogels Through Pyrolysis of Polycarbosilane Precursors. Adv. Eng. Mater. 2014, 16, 814–819. [Google Scholar] [CrossRef]
- Wang, H.; Yao, J. Use of poly(furfuryl alcohol) in the fabrication of nanostructured carbons and nanocomposites. Ind. Eng. Chem. Res. 2006, 45, 6393–6404. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. (Eds.) Chapter 8-Drying. In Sol-Gel Science; Academic Press: San Diego, CA, USA, 1990; pp. 452–513. [Google Scholar]
- Li, F.; Liu, J.-X.; Huang, X.; Bao, W.; Zhang, G.-J.; Wang, H. Carbothermal conversion of self-supporting organic/inorganic interpenetrating networks to porous metal boride monoliths. J. Am. Ceram. Soc. 2019, 102, 5746–5762. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Li, Z.-Q.; Wu, K.-L.; Lu, Y.-J.; Xu, Y.-F.; Song, X.-J. Poly(furfuryl alcohol) nanospheres: A facile synthesis approach based on confinement effect of polymer and a template for synthesis of metal oxide hollow nanospheres. B Mater. Sci. 2015, 38, 1859–1865. [Google Scholar] [CrossRef][Green Version]
- Zanchetta, E.; Cattaldo, M.; Franchin, G.; Schwentenwein, M.; Homa, J.; Brusatin, G.; Colombo, P. Stereolithography of SiOC Ceramic Microcomponents. Adv. Mater. 2016, 28, 370–376. [Google Scholar] [CrossRef]
- Qiu, H.-Y.; Guo, W.-M.; Zou, J.; Zhang, G.-J. ZrB2 powders prepared by boro/carbothermal reduction of ZrO2: The effects of carbon source and reaction atmosphere. Powder Technol. 2012, 217, 462–466. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Cheng, Y.-B. Morphology control of mesoporous silica-carbon nanocomposites via phase separation of poly(furfuryl alcohol) and silica in the sol–gel synthesis. J. Sol-Gel Sci. Techn. 2017, 82, 664–674. [Google Scholar] [CrossRef]
- Brezny, R.; Green, D.J. Mechanical behavior of cellular ceramics. In Materials Science and Technology; Wiley-VCH: Weinheim, MA, USA, 2006; pp. 467–516. [Google Scholar]

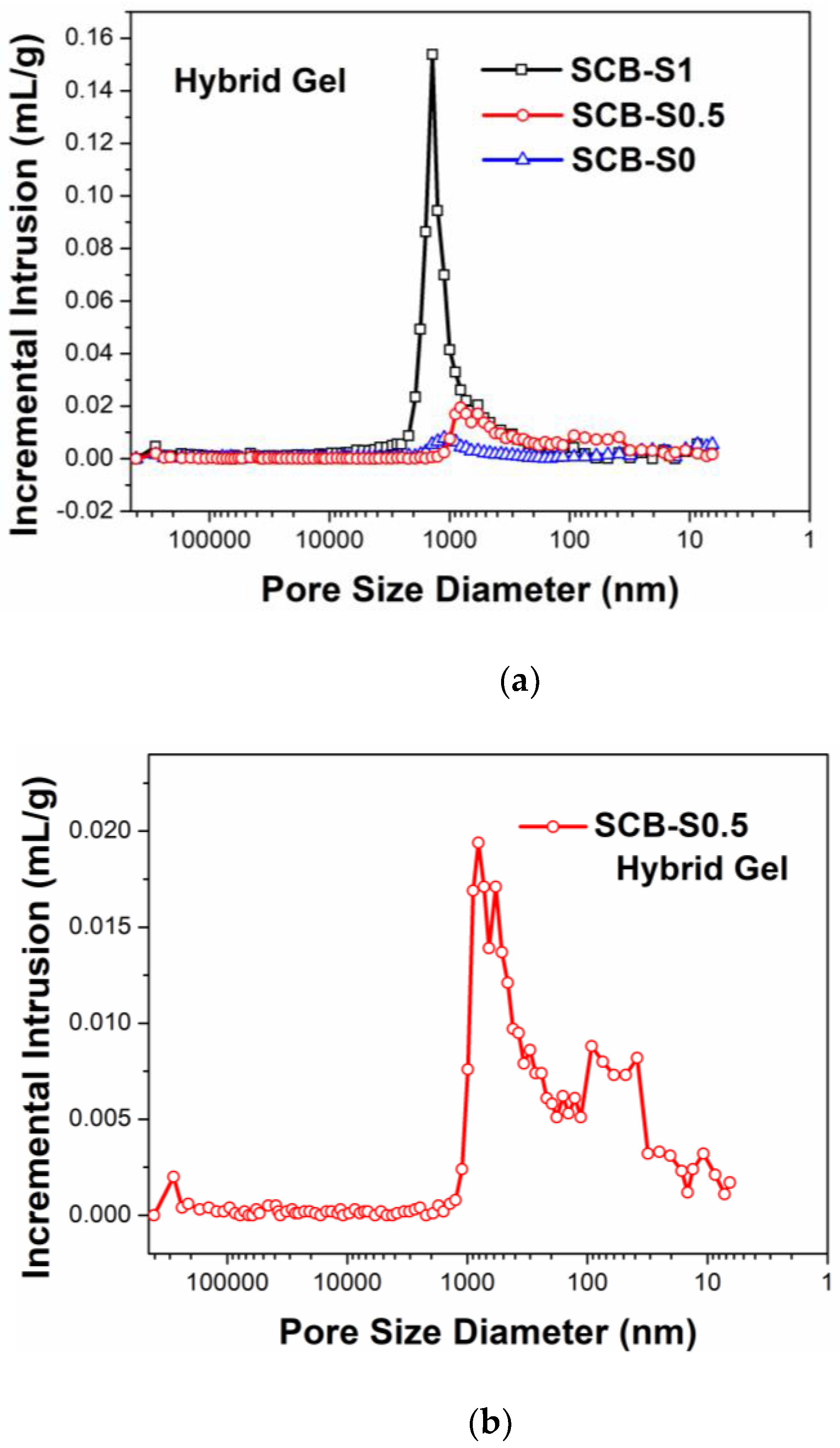
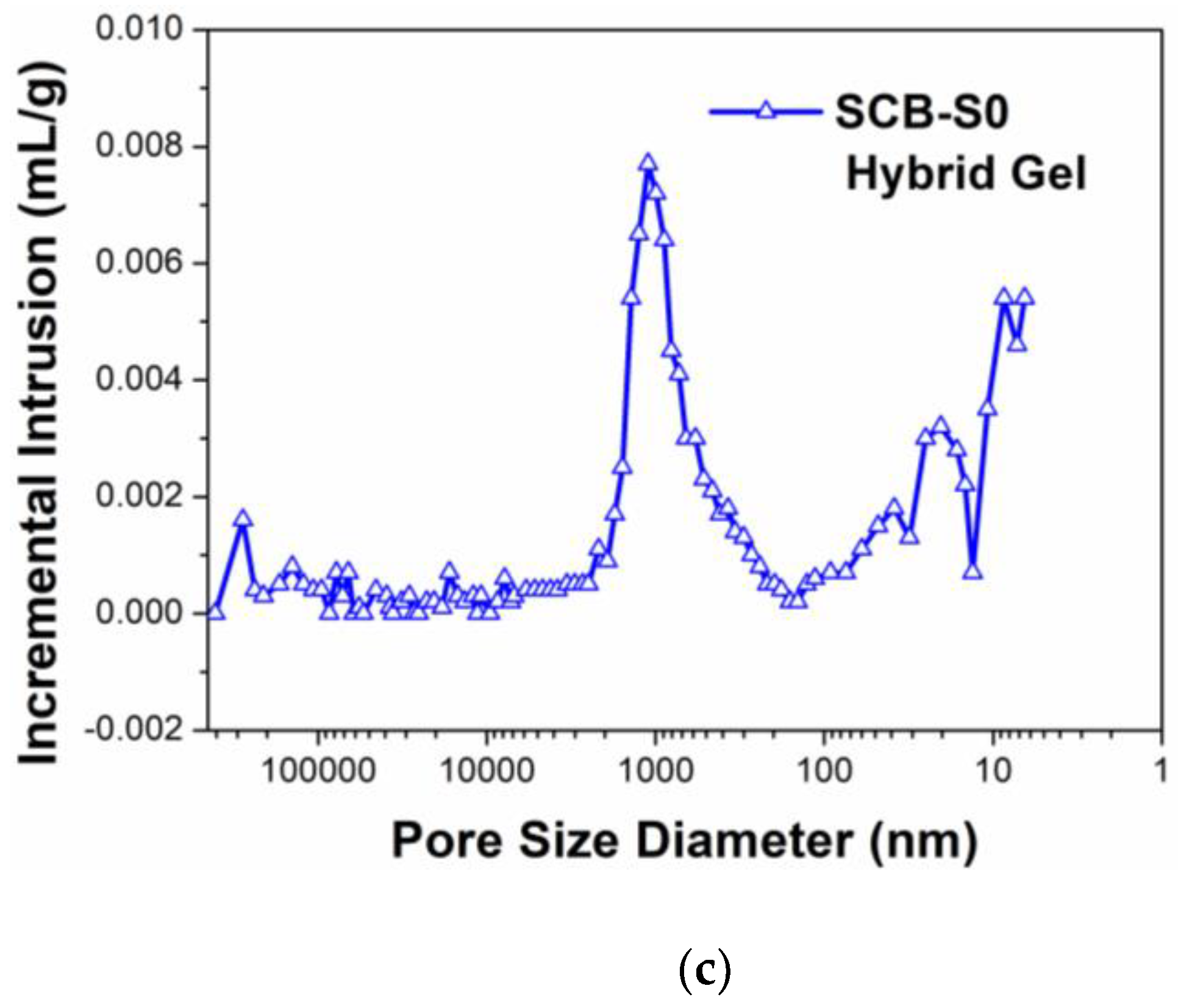
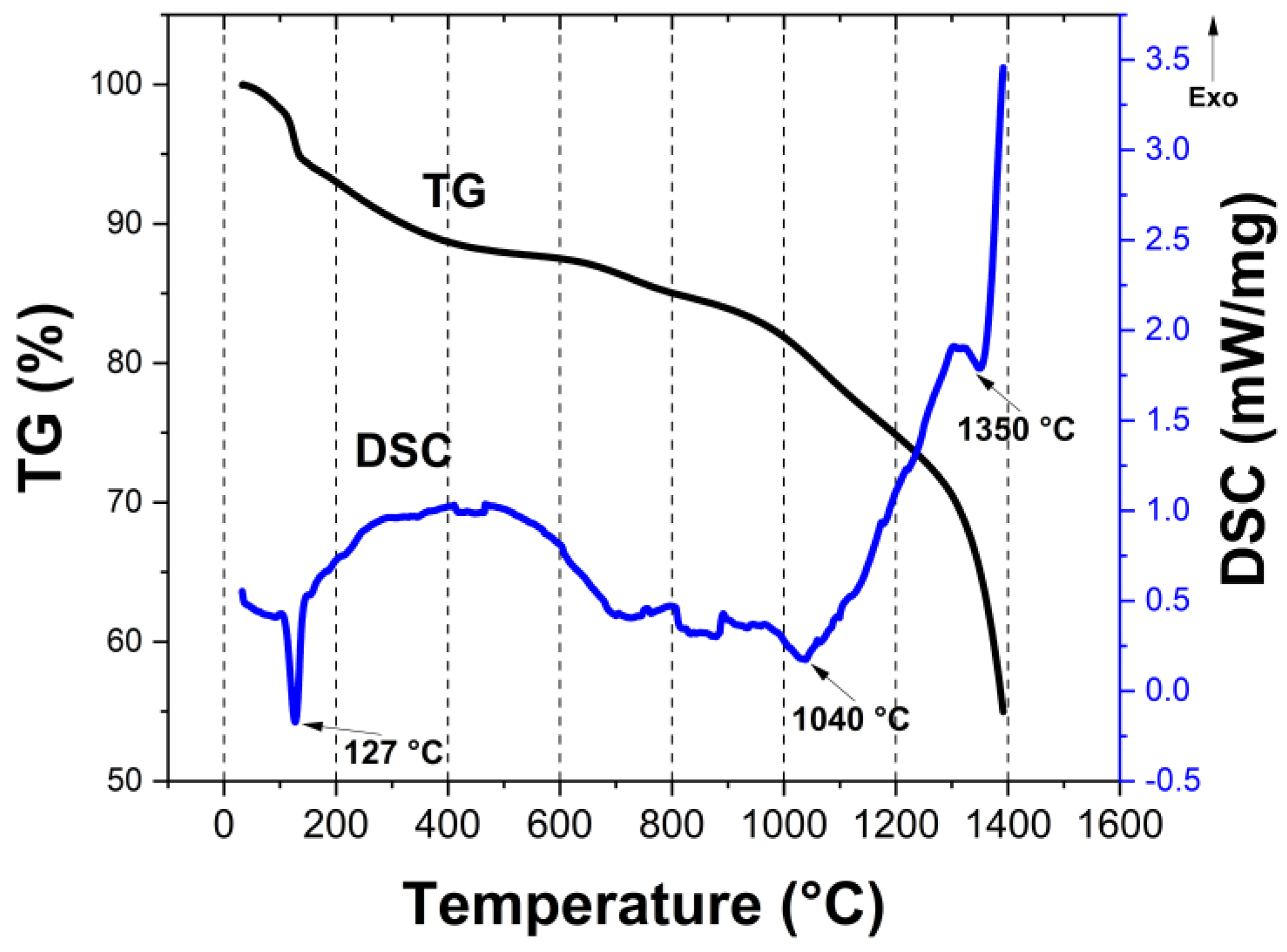
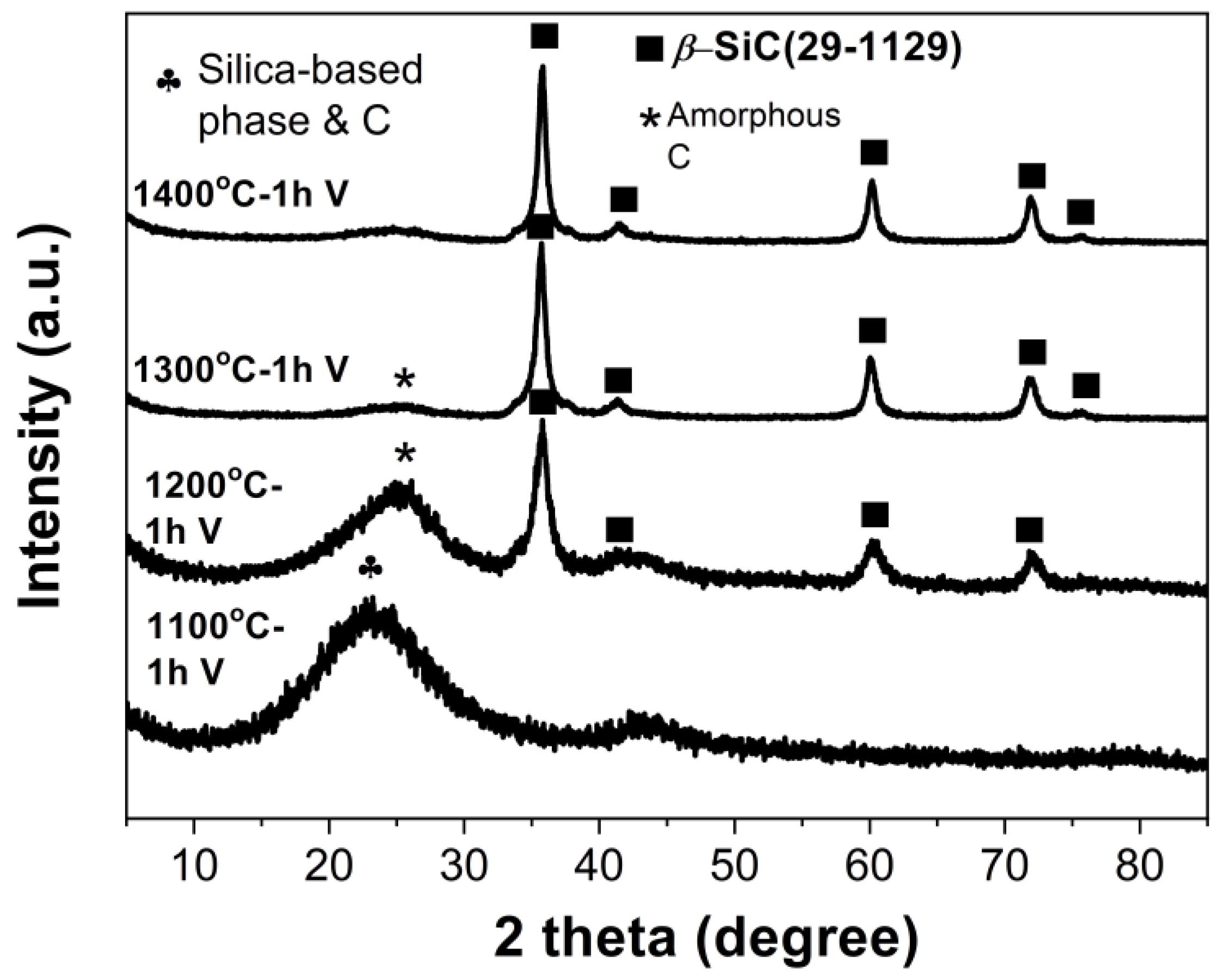

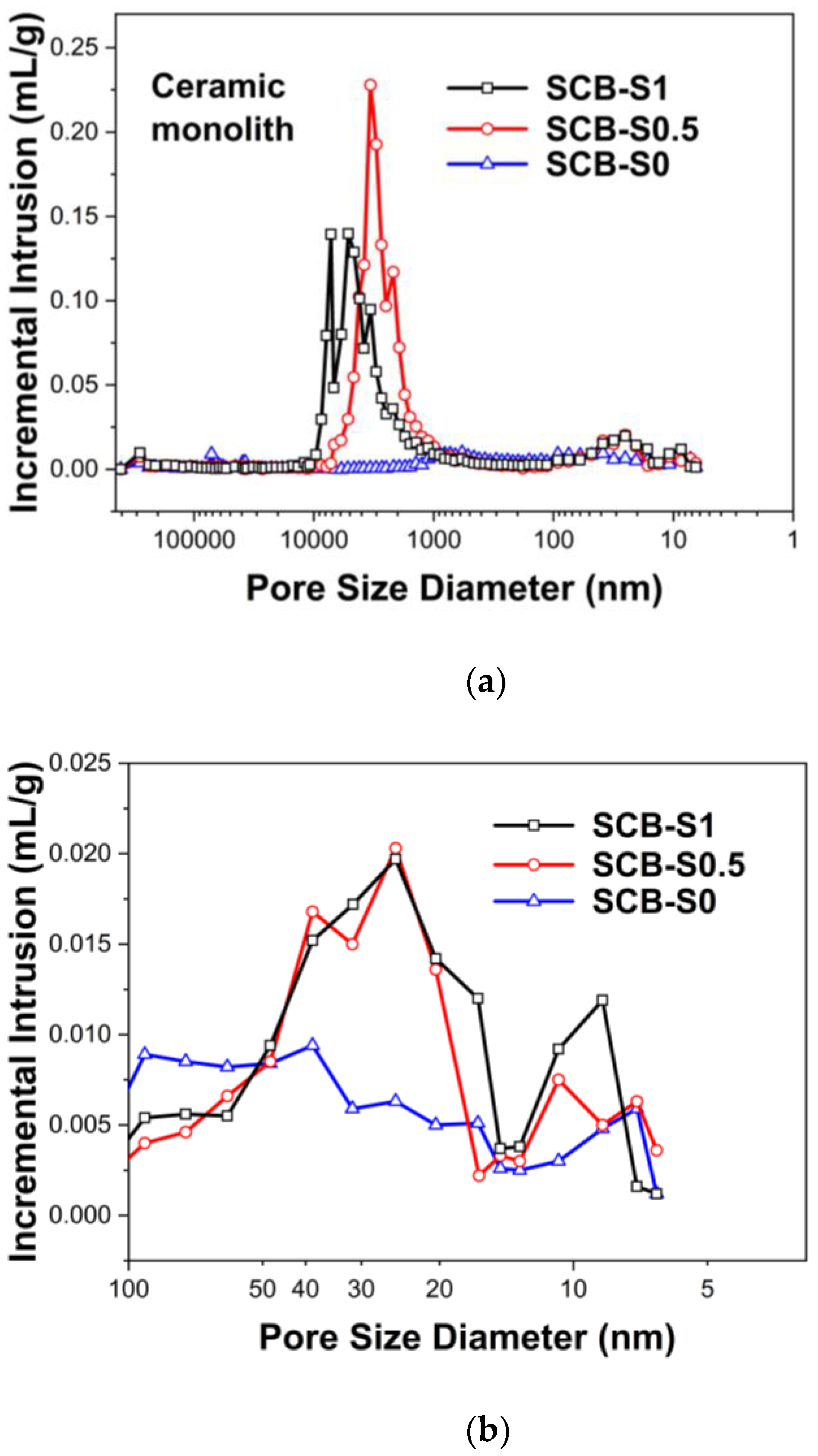
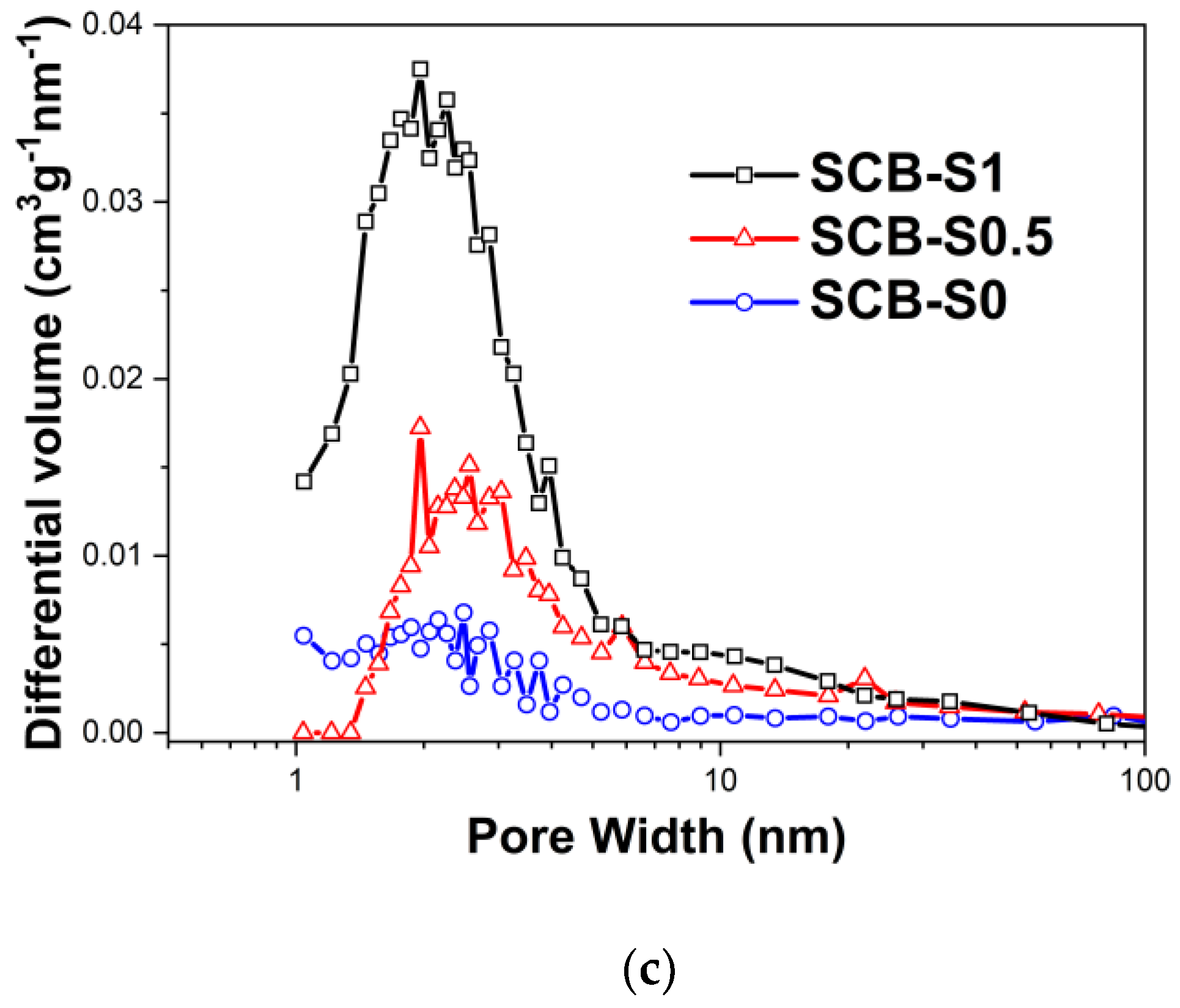
| Ceramic Monoliths | Macropore Size 1 (µm) | Mesopore Size 2 (nm) | Bulk Density (g/cm3) | Porosity (%) | Specific Surface Area (m2/g) | |
|---|---|---|---|---|---|---|
| 1 | 2 | |||||
| SCB-S0 | 0.39 | 2.5 | 1.25 | 34.6 | 15.9 | 30.5 |
| SCB-S0.5 | 3.06 | 2.0 | 0.72 | 61.5 | 24.6 | 65.0 |
| SCB-S1 | 4.50 | 2.0 | 0.42 | 71.3 | 26.5 | 145.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhou, L.; Liu, J.-X.; Zhang, G.-J. Preparation of Hierarchical Porous Silicon Carbide Monoliths via Ambient Pressure Drying Sol–Gel Process Followed by High-Temperature Pyrolysis. Materials 2023, 16, 220. https://doi.org/10.3390/ma16010220
Li F, Zhou L, Liu J-X, Zhang G-J. Preparation of Hierarchical Porous Silicon Carbide Monoliths via Ambient Pressure Drying Sol–Gel Process Followed by High-Temperature Pyrolysis. Materials. 2023; 16(1):220. https://doi.org/10.3390/ma16010220
Chicago/Turabian StyleLi, Fei, Lin Zhou, Ji-Xuan Liu, and Guo-Jun Zhang. 2023. "Preparation of Hierarchical Porous Silicon Carbide Monoliths via Ambient Pressure Drying Sol–Gel Process Followed by High-Temperature Pyrolysis" Materials 16, no. 1: 220. https://doi.org/10.3390/ma16010220
APA StyleLi, F., Zhou, L., Liu, J.-X., & Zhang, G.-J. (2023). Preparation of Hierarchical Porous Silicon Carbide Monoliths via Ambient Pressure Drying Sol–Gel Process Followed by High-Temperature Pyrolysis. Materials, 16(1), 220. https://doi.org/10.3390/ma16010220






