Trace Element Contents in Maize following the Application of Organic Materials to Reduce the Potential Adverse Effects of Nitrogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology of the Vegetation Experiment
2.2. Methodology of Laboratory Testing and Statistical Analyses
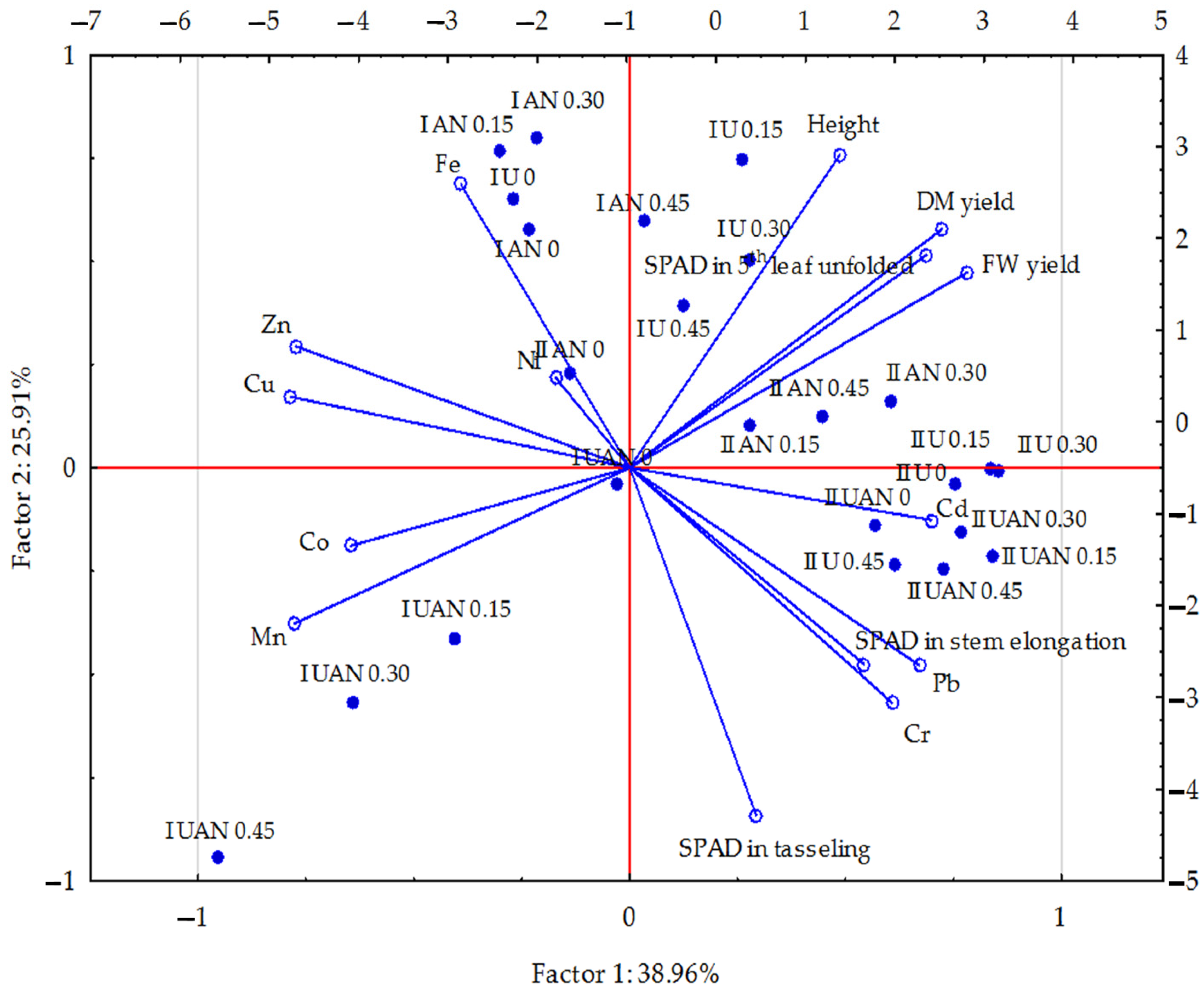
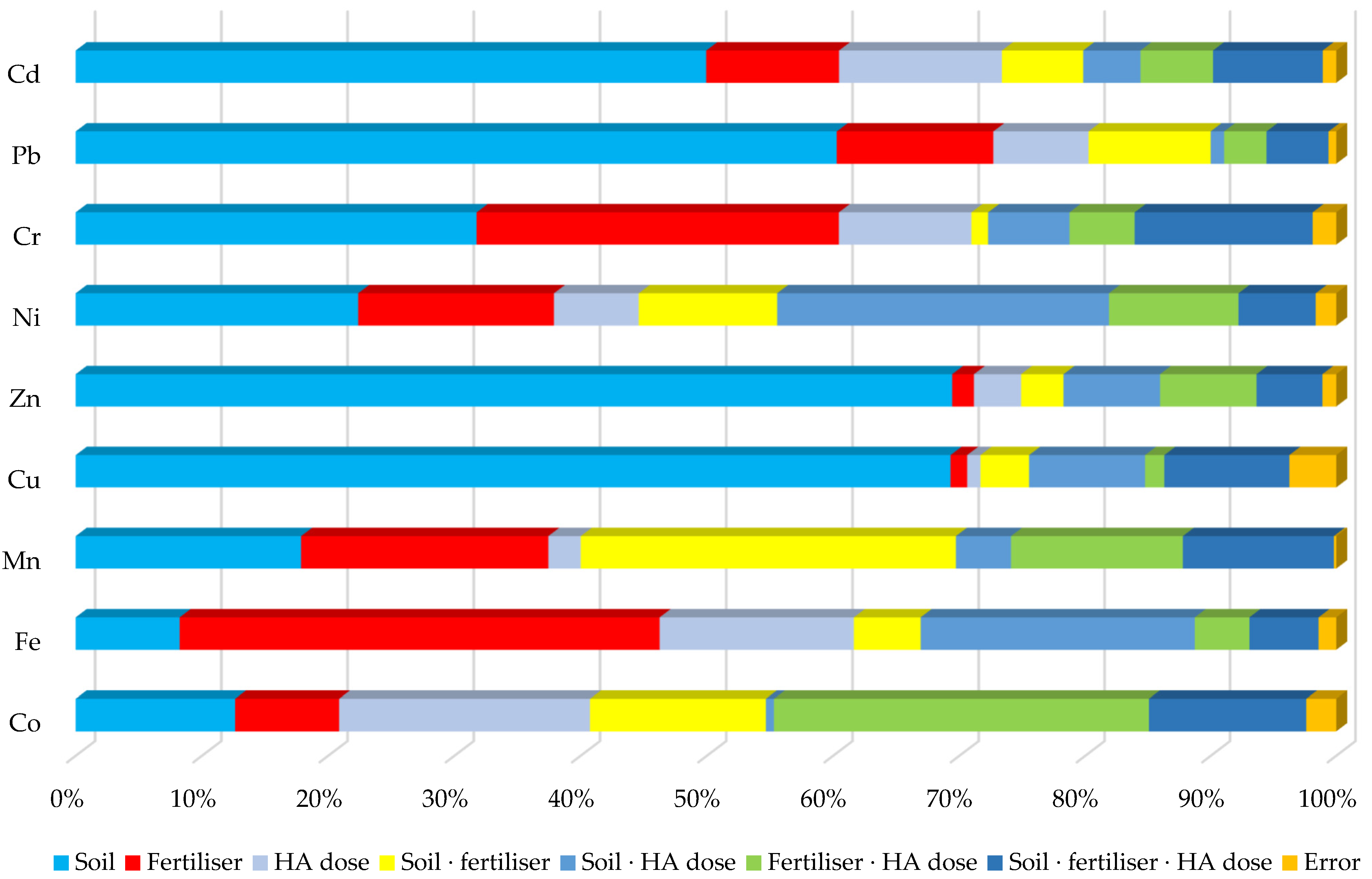
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of differentiated nitrogen fertilization on the enzymatic activity of the soil for sweet potato (Ipomoea batatas L. [Lam.]) cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- Tkaczyk, P.; Bednarek, W.; Dresler, S.; Krzyszczak, J.; Baranowski, P.; Brodowska, M.S. Content of certain macro-and microelements in orchard soils in relation to agronomic categories and reaction of these soils. J. Elem. 2018, 23, 1361–1372. [Google Scholar] [CrossRef]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The effect of application of organic manures and mineral fertilizers on the state of soil organic matter and nutrients in the long-term field experiment. J. Soils Sediments 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Krasilnikov, P.; Taboada, M.A.; Amanullah. Fertilizer use, soil health and agricultural sustainability. Agriculture 2022, 12, 462. [Google Scholar] [CrossRef]
- Singh, B. Are Nitrogen fertilizers deleterious to soil health? Agronomy 2018, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xu, Z.; Zhao, J.; Wang, Y.; Yu, Z. Excessive nitrogen application decreases grain yield and increases nitrogen loss in a wheat–soil system. Acta Agric. Scand. B Soil Plant Sci. 2011, 61, 681–692. [Google Scholar] [CrossRef]
- Liu, C.W.; Sung, Y.; Chen, B.C.; Lai, H.Y. Effects of nitrogen fertilizers on the growth and nitrate content of lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public Health 2014, 11, 4427–4440. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.Y.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- Skorupka, M.; Nosalewicz, A. Ammonia volatilization from fertilizer urea—A new challenge for agriculture and industry in view of growing global demand for food and energy crops. Agriculture 2021, 11, 822. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Xie, J.; Yu, J.; Li, J.; Zhang, J.; Tang, C.; Niu, T.; Patience, B.E. Effect of slow-release fertilizer on soil fertility and growth and quality of wintering Chinese chives (Allium tuberm Rottler ex Spreng.) in greenhouses. Sci. Rep. 2021, 11, 8070. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Zhang, M.; Chen, Q.; Zheng, L.; Li, Y.C.; Sun, L. The combined application of controlled-release urea and fulvic acid improved the soil nutrient supply and maize yield. Arch. Agron. Soil Sci. 2021, 67, 633–646. [Google Scholar] [CrossRef]
- Shrestha, J.; Yadav, D.N.; Amgain, L.P.; Sharma, J.P. Effects of nitrogen and plant density on maize (Zea mays L.) phenology and grain yield. Curr. Agri. Res. 2018, 6. [Google Scholar] [CrossRef]
- Okumura, R.S.; Mariano, D.C.; Zacche, P.V.C. Use of nitrogen fertilizer in corn: A review. Braz. J. Appl. Technol. Agric. Sci. 2011, 4, 226–244. [Google Scholar]
- Asibi, A.E.; Chai, Q.A.; Coulter, J. Mechanisms of nitrogen use in maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, M.K.; Tahir, M.M.; Rahim, N. Effect of N fertilizer source and timing on yield and N use efficiency of rainfed maize (Zea mays L.) in Kashmir-Pakistan. Geoderma 2013, 195, 87–93. [Google Scholar] [CrossRef]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; Van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [Green Version]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trend Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Burakova, A.; Bakšiene, E. Leaching losses of main nutrients by incorporating organic fertilisers into light texture soils Haplic Luvisol. Environ. Eng. Res. 2021, 26, 200190. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecký, M.; Amirahmadi, E.; Moudrý, J., Jr.; Menšík, L. Preliminary findings on cadmium bioaccumulation and photosynthesis in rice (Oryza sativa L.) and maize (Zea mays L.) using biochar made from C3- and C4-originated straw. Plants 2022, 11, 1424. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecký, M.; Amirahmadi, E.; Bucur, D.; Walkiewicz, A. Interaction of biochar with chemical, green and biological nitrogen fertilizers on nitrogen use efficiency indices. Agronomy 2022, 12, 2106. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J. Effects of zeolite on aggregation, nutrient availability, and growth characteristics of corn (Zea mays L.) in cadmium-contaminated soils. Water Air Soil Pollut. 2022, 233, 436. [Google Scholar] [CrossRef]
- Fageria, N.K. Role of Soil Organic Matter in Maintaining Sustainability of Cropping Systems. Commun. Soil Sci. Plant Anal. 2012, 43, 2063–2113. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Debaene, G.; Smreczak, B. Characterization of soil organic matter individual fractions (fulvic acids, humic acids, and humins) by spectroscopic and electrochemical techniques in agricultural soils. Agronomy 2021, 11, 1067. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Maji, D.; Misra, P.; Singh, S.; Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 2017, 110, 97–108. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant Biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Zaborowska, M.; Woźny, G.; Wyszkowska, A.; Kucharski, J. Biostimulation of the activity of microorganisms and soil enzymes through fertilisation with composts. Soil Res. 2018, 56, 737–751. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef] [Green Version]
- Pukalchik, M.; Kydralieva, K.; Yakimenko, O.; Fedoseeva, E.; Terekhova, V. Outlining the potential role of humic products in modifying biological properties of the soil—A review. Front. Environ. Sci. 2019, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef] [PubMed]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.M.; Pitumpe Arachchige, P.S.; Ciampitti, I.A.; Hettiarachchi, G.M.; Maurmann, L.; Trivelin, P.C.O.; Prasad, P.V.V.; Sunoj, S.V.J. Co-addition of humic substances and humic acids with urea enhances foliar nitrogen use efficiency in sugarcane (Saccharum officinarum L.). Heliyon 2020, 6, e05100. [Google Scholar] [CrossRef] [PubMed]
- Savarese, C.; Cozzolino, V.; Verrillo, M.; Vinci, G.; De Martino, A.; Scopa, A.; Piccolo, A. Combination of humic biostimulants with a microbial inoculum improves lettuce productivity, nutrient uptake, and primary and secondary metabolism. Plant Soil 2022, 1–30. [Google Scholar] [CrossRef]
- Scaglia, B.; Nunes, R.R.; Oliveira Rezende, M.O.; Tambone, F.; Adani, F. Investigating organic molecules responsible of auxin-like activity of humic acid fraction extracted from vermicompost. Sci. Total Environ. 2016, 562, 289–295. [Google Scholar] [CrossRef]
- Türkmen, Ö.; Dursun, A.; Turan, M.; Erdinç, Ç. Calcium and humic acid affect seed germination, growth, and nutrient content of tomato (Lycopersicon esculentum L.) seedlings under saline soil conditions. Acta Agric. Scand. Sect. B Plant Soil Sci. 2004, 54, 168–174. [Google Scholar] [CrossRef]
- Vieira, J.H.; Silva, L.K.D.S.; Oliveira, L.C.D.; Carmo, J.B.D.; Rosa, L.M.T.; Botero, W.G. Evaluation of germination of chilli pepper using humic substances and humic acids. J. Environ. Sci. 2018, 12, 33–39. [Google Scholar] [CrossRef]
- Delfine, S.; Tognetti, R.; Ersilio Desiderio, A.A. Effect of foliar application of N and humic acids on growth and yield of durum wheat. Agron. Sustain. Dev. 2005, 25, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.D.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From lab to field: Role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef]
- Galambos, N.; Compant, S.; Moretto, M.; Sicher, C.; Puopolo, G.; Wäckers, F.; Sessitsch, A.; Pertot, I.; Perazzolli, M. Humic acid enhances the growth of tomato promoted by endophytic bacterial strains through the activation of hormone-, growth-, and transcription-related processes. Front. Plant Sci. 2020, 11, 582267. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, F.; Nasiri, Y.; Asadi, M.; Hassanpouraghdam, M.B.; Golestaneh, S.; Pirsarandib, Y. Fertilizer type and humic acid improve the growth responses, nutrient uptake, and essential oil content on Coriandrum sativum L. Sci. Rep. 2022, 12, 7437. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, N.A.; Perminova, I.V. Interactions between humic substances and microorganisms and their implications for nature-like bioremediation technologies. Molecules 2021, 26, 2706. [Google Scholar] [CrossRef] [PubMed]
- Burlakovs, J.; Kļaviņš, M.; Osinska, L.; Purmalis, O. The impact of humic substances as remediation agents to the speciation forms of metals in soil. APCBEE Procedia 2013, 5, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Liu, J.; Huo, L.; Li, Y.C.; Li, X.; Xia, L.; Zhou, Z.; Zhang, M.; Li, B. Humic acids derived from Leonardite to improve enzymatic activities and bioavailability of nutrients in a calcareous soil. Int. J. Agric. Biol. Eng. 2020, 13, 200–205. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Kordala, N. Use of organic materials to limit the potential negative effect of nitrogen on maize in different soils. Materials 2022, 15, 5755. [Google Scholar] [CrossRef]
- Cappelini, L.; Diniz, L.; Fornazari, A.; Alberice, J.; Eugenio, P.; Vieira, E. Application of the humic substances and ammonia in order to minimize losses on nitrogen fertilization. Agric. Sci. 2020, 11, 211–222. [Google Scholar] [CrossRef]
- Rong, Q.; Zhong, K.; Huang, H.; Li, C.; Zhang, C.; Nong, X. Humic acid reduces the available cadmium, copper, lead, and zinc in soil and their uptake by tobacco. Appl. Sci. 2020, 10, 1077. [Google Scholar] [CrossRef] [Green Version]
- Nabulo, G.; Black, C.R.; Young, S.D. Trace metal uptake by tropical vegetables grown on soil amended with urban sewage sludge. Environ. Pollut. 2011, 159, 368–376. [Google Scholar] [CrossRef]
- Shen, M.; Liu, L.; Li, D.W.; Zhou, W.N.; Zhou, Z.P.; Zhang, C.F.; Luo, Y.Y.; Wang, H.B.; Li, H.Y. The effect of endophytic Peyronellaea from heavy metal-contaminated and uncontaminated sites on maize growth, heavy metal absorption and accumulation. Fungal Ecol. 2013, 6, 539–545. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y. Effect of soil pH and organic matter content on heavy metals availability in maize (Zea mays L.) rhizospheric soil of non-ferrous metals smelting area. Environ. Monit. Assess. 2019, 191, 634. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xiong, Y.; Xu, X.; Xu, F.; Hussain, S.; Xiong, H.; Yuan, J. Deep placement of controlled release urea effectively enhanced nitrogen use efficiency and fresh ear yield of sweet corn in fluvo-aquic soil. Sci. Rep. 2019, 9, 20307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; World Soil Resources Report. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources; Reports No. 106; FAO: Rome, Italy, 2015; p. 192. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 18 November 2021).
- Microwave Assisted Acid Digestion of Sediment, Sludges, Soils, and Oils; US-EPA Method 3051A; United States Environmental Protection Agency: Washington, DC, USA, 2007; pp. 1–30. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 24 January 2022).
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analysis and Evaluation of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. [Google Scholar]
- SPAD 502 Plus Chlorophyll Meter. Product Manual; Spectrum Technologies, Inc.: Aurora, IL, USA, 2009; pp. 1–24. Available online: https://www.specmeters.com/assets/1/22/2900P_SPAD_502.pdf (accessed on 30 November 2022).
- PN-R-04032; Soil and Mineral Materials—Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warsaw, Poland, 1998; pp. 1–12.
- Tibco. Statistica Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA, 2021. [Google Scholar]
- Wyszkowski, M.; Brodowska, M.S. Potassium and nitrogen fertilization vs. trace element content of maize (Zea mays L.). Agriculture 2021, 11, 96. [Google Scholar] [CrossRef]
- Zhou, Q. Interaction between heavy metals and nitrogen fertilizers applied to soil-vegetable systems. Bull. Environ. Contam. Toxicol. 2003, 71, 338–344. [Google Scholar] [CrossRef]
- Razanov, S.F.; Tkachuk, O.P.; Razanova, A.M.; Bakhmat, M.I.; Bakhmat, O.M. Intensity of heavy metal accumulation in plants of Silybum marianum L. in conditions of field rotation. Ukr. J. Ecol. 2020, 10, 131–136. [Google Scholar] [CrossRef]
- Wångstrand, H.; Eriksson, J.; Öborn, I. Cadmium concentration in winter wheat as affected by nitrogen fertilization. Eur. J. Agron. 2007, 26, 209–214. [Google Scholar] [CrossRef]
- Rutkowska, B.; Szulc, W.; Sosulski, T.; Stępień, W. Soil micronutrient availability to crops affected by long-term inorganic and organic fertilizer applications. Plant Soil Environ. 2014, 60, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Brodowska, M.S.; Wyszkowski, M.; Bujanowicz-Haraś, B. Mineral Fertilization and Maize Cultivation as Factors Which Determine the Content of Trace Elements in Soil. Agronomy 2022, 12, 286. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S. Content of trace elements in soil fertilized with potassium and nitrogen. Agriculture 2020, 10, 398. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, M.; Marshall, F.M. The role of organic vs. inorganic fertilizers in reducing phytoavailability of heavy metals in a wastewater-irrigated area. Ecol. Eng. 2010, 36, 1733–1740. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; He, P. Changes of heavy metals in soil and wheat grain under long-term environmental impact and fertilization practices in North China. J. Plant Nutr. 2018, 41, 1970–1979. [Google Scholar] [CrossRef]
- Czarnecki, S.; Düring, R.A. Influence of long-term mineral fertilization on metal contents and properties of soil samples taken from different locations in Hesse, Germany. Soil 2015, 1, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Zhang, S.; Tian, Y.; Guo, W.; Wang, J. The influence of humic acids on the accumulation of lead (Pb) and cadmium (Cd) in tobacco leaves grown in different soils. J. Soil Sci. Plant Nutr. 2013, 13, 43–53. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ağar, G.; Dursun, A.; Kul, R.; Alim, Z.; Argin, S. Humic + Fulvic acid mitigated Cd adverse effects on plant growth, physiology and biochemical properties of garden cress. Sci. Rep. 2021, 1, 8040. [Google Scholar] [CrossRef]
- Yang, T.; Hodson, M.E. Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil. Sci. Total Environ. 2019, 647, 290–300. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef]
- Ifansyah, H. Soil pH and solubility of aluminum, iron, and phosphorus in Ultisols: The roles of humic acid. J. Trop. Soils 2013, 18, 203–208. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Cong, L.; Wang, X.; Shi, S. Effect of fulvic acid on the phosphorus availability in acid soil. J. Soil Sci. Plant Nutr. 2013, 13, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowska-Malina, J. Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Appl. Soil Ecol. 2017, 123, 542–545. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; De Martino, A.; Scognamiglio, F.; di Meo, V. Soil washing with solutions of humic substances from manure compost removes heavy metal contaminants as a function of humic molecular composition. Chemosphere 2019, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Chen, B.D.; Zhu, Y.G. Humic acids increase the phyto availability of Cd and Pb to wheat plants cultivated in freshly spiked, contaminated soil. J. Soils Sediments 2006, 6, 236–242. [Google Scholar] [CrossRef]
- Bai, Y.; Gu, C.; Tao, T.; Chen, G.; Shan, Y. Straw incorporation increases solubility and uptake of cadmium by rice plants. Acta Agric. Scand. B Soil Plant Sci. 2013, 63, 193–199. [Google Scholar] [CrossRef]
| HA Dose g kg−1 of Soil | Sand | Loamy Sand | ||||||
|---|---|---|---|---|---|---|---|---|
| Ammonium Nitrate | Urea | UAN | Average | Ammonium Nitrate | Urea | UAN | Average | |
| Cadmium content (mg kg−1 DM) | ||||||||
| 0 | 0.135 g | 0.071 a−c | 0.068 ab | 0.091 A | 0.123 e–g | 0.180 h | 0.175 h | 0.159 D |
| 0.05 | 0.101 de | 0.106 d–f | 0.073 a–c | 0.093 A | 0.174 e–g | 0.239 i | 0.185 h | 0.199 F |
| 0.10 | 0.093 cd | 0.138 g | 0.119 e–g | 0.117 B | 0.128 fg | 0.222 i | 0.176 h | 0.175 E |
| 0.15 | 0.051 a | 0.089 h | 0.093 b–d | 0.078 C | 0.123 e–g | 0.176 h | 0.084 b–d | 0.128 B |
| Average | 0.095 AB | 0.101 B | 0.088 A | 0.095 A | 0.137 C | 0.204 E | 0.155 D | 0.165 B |
| r | −0.972 | 0.389 | 0.674 | −0.141 | −0.240 | −0.120 | −0.766 | −0.511 |
| Lead content (mg kg−1 DM) | ||||||||
| 0 | 0.462 b | 0.610 c | 0.739 d–f | 0.604 A | 0.934 ij | 1.036 kl | 0.841 gh | 0.937 E |
| 0.05 | 0.255 a | 0.677 c–e | 0.754 ef | 0.562 D | 0.770 f−g | 0.893 h–j | 0.883 hi | 0.849 B |
| 0.10 | 0.321 a | 0.461 b | 0.671 cd | 0.484 C | 0.908 h–j | 0.969 jk | 0.688 de | 0.855 B |
| 0.15 | 0.472 b | 0.689 de | 0.715 d–f | 0.625 A | 0.934 i–j | 1.049 l | 1.135 m | 1.039 F |
| Average | 0.378 B | 0.609 C | 0.720 D | 0.569 A | 0.887 A | 0.987 E | 0.887 A | 0.920 B |
| r | 0.116 | 0.026 | −0.552 | −0.026 | 0.227 | 0.207 | 0.478 | 0.454 |
| Chromium content (mg kg−1 DM) | ||||||||
| 0 | 1.132 ab | 0.947 a | 1.757 e–g | 1.279 F | 1.486 cd | 2.355 ij | 2.148 h−j | 1.996 DE |
| 0.05 | 1.330 bc | 1.193 ab | 1.987 gh | 1.503 A | 2.134 h–j | 2.118 hi | 2.369 h–j | 2.207 F |
| 0.10 | 1.204 ab | 2.047 h | 2.384 j | 1.878 BC | 1.925 f–h | 2.005 gh | 2.399 j | 2.110 EF |
| 0.15 | 1.371 bc | 1.659 d–e | 1.563 c–e | 1.531 A | 1.665 d–f | 1.685 d–f | 2.178 h−j | 1.843 B |
| Average | 1.259 A | 1.462 B | 1.923 D | 1.548 A | 1.803 C | 2.041 E | 2.274 F | 2.039 B |
| r | 0.689 | 0.789 | −0.068 | 0.590 | 0.148 | −0.984 | 0.120 | −0.460 |
| HA Dose g kg−1 of Soil | Sand | Loamy Sand | ||||||
|---|---|---|---|---|---|---|---|---|
| Ammonium Nitrate | Urea | UAN | Average | Ammonium Nitrate | Urea | UAN | Average | |
| Nickel content (mg kg−1 DM) | ||||||||
| 0 | 1.258 cd | 2.194 fg | 1.315 cd | 1.589 AB | 0.432 a | 2.160 fg | 2.160 fg | 1.584 AB |
| 0.05 | 1.348 cd | 2.352 hi | 1.414 d | 1.705 BC | 1.526 de | 2.103 fg | 1.830 ef | 1.820 C |
| 0.10 | 3.216 j | 2.716 i | 2.357 hi | 2.763 D | 0.951 bc | 2.037 fg | 1.217 b–d | 1.402 A |
| 0.15 | 3.145 j | 2.717 i | 2.060 fg | 2.641 D | 0.834 ab | 1.554 de | 1.219 b–d | 1.202 E |
| Average | 2.242 E | 2.495 F | 1.787 C | 2.174 B | 0.936 A | 1.964 D | 1.607 B | 1.502 A |
| r | 0.896 | 0.945 | 0.814 | 0.887 | 0.180 | −0.876 | −0.947 | −0.767 |
| Zinc content (mg kg−1 DM) | ||||||||
| 0 | 15.08 h−j | 12.94 gh | 10.07 ef | 12.70 E | 8.93 de | 3.74 ab | 7.58 cd | 6.75 C |
| 0.05 | 22.24 k | 13.36 g−i | 11.74 fg | 15.78 B | 4.22 ab | 3.35 bc | 5.79 bc | 4.45 A |
| 0.10 | 16.89 j | 15.47 ij | 17.13 j | 16.50 B | 3.89 ab | 5.94 fg | 6.03 bc | 5.29 A |
| 0.15 | 10.20 ef | 12.18 fg | 10.26 ef | 10.88 D | 3.89 ab | 8.15 c−e | 4.53 ab | 5.52 A |
| Average | 16.10 D | 13.49 C | 12.30 B | 13.96 B | 5.23 A | 5.30 A | 5.98 A | 5.50 A |
| r | −0.519 | −0.016 | 0.233 | −0.232 | −0.808 | 0.921 | −0.919 | −0.387 |
| Copper content (mg kg−1 DM) | ||||||||
| 0 | 1.771 ab | 2.408 gh | 2.075 d–f | 2.085 D | 2.036 c–e | 1.698 ab | 1.781 a−c | 1.838 C |
| 0.05 | 2.186 e–h | 2.378 gh | 2.126 d–g | 2.230 A | 1.916 b–d | 1.792 a–c | 1.728 ab | 1.812 C |
| 0.10 | 2.370 gh | 2.336 gh | 2.224 e–h | 2.310 A | 1.544 a | 1.660 ab | 1.767 ab | 1.657 B |
| 0.15 | 2.212 e–h | 2.297 f–h | 2.357 gh | 2.289 A | 1.561 a | 1.630 a | 1.561 a | 1.584 B |
| Average | 2.135 B | 2.355 C | 2.196 B | 2.228 B | 1.765 A | 1.695 A | 1.709 A | 1.723 A |
| r | 0.761 | −0.998 | 0.982 | 0.880 | −0.930 | −0.616 | −0.791 | −0.969 |
| HA Dose g kg−1 of Soil | Sand | Loamy Sand | ||||||
|---|---|---|---|---|---|---|---|---|
| Ammonium Nitrate | Urea | UAN | Average | Ammonium Nitrate | Urea | UAN | Average | |
| Manganese content (mg kg−1 DM) | ||||||||
| 0 | 74.14 j | 73.95 j | 80.84 k | 76.31 B | 102.59 m | 44.22 a | 67.50 h | 71.44 A |
| 0.05 | 69.32 hi | 63.95 fg | 84.16 kl | 72.48 AB | 85.08 l | 46.67 a | 53.94 b | 61.90 E |
| 0.10 | 74.05 j | 62.50 ef | 110.63 n | 82.39 F | 57.66 cd | 55.93 bc | 66.51 gh | 60.03 D |
| 0.15 | 59.99 de | 72.01 ij | 101.69 m | 77.90 B | 72.16 | 53.55 b | 45.08 a | 56.93 C |
| Average | 69.38 A | 68.10 A | 94.33 E | 77.27 B | 79.37 D | 50.09 B | 58.26 C | 62.57 A |
| r | −0.732 | −0.164 | 0.809 | 0.462 | −0.802 | 0.867 | −0.658 | −0.937 |
| Iron content (mg kg−1 DM) | ||||||||
| 0 | 39.52 i | 32.59 h | 22.03 e–g | 31.38 D | 35.14 h | 20.39 d–f | 16.98 cd | 24.17 A |
| 0.05 | 35.93 hi | 32.08 h | 20.06 d–f | 29.36 D | 20.39 a | 11.92 ab | 12.98 bc | 15.10 B |
| 0.10 | 35.26 h | 18.47 de | 19.29 d–f | 24.34 A | 23.11 fg | 19.74 d–f | 18.79 de | 20.55 C |
| 0.15 | 22.00 e–g | 16.63 cd | 8.08 a | 15.57 B | 25.32 g | 18.28 de | 23.18 fg | 22.26 AC |
| Average | 33.18 C | 24.94 B | 17.37 A | 25.16 B | 25.99 B | 17.58 A | 17.98 A | 20.52 A |
| r | −0.894 | −0.926 | −0.874 | −0.961 | −0.537 | 0.050 | 0.745 | −0.009 |
| Cobalt content (mg kg−1 DM) | ||||||||
| 0 | 0.487 g | 0.694 h | 0.466 fg | 0.549 F | 0.652 h | 0.249 a–d | 0.320 b–e | 0.407 BC |
| 0.05 | 0.463 fg | 0.378 ef | 0.529 g | 0.457 D | 0.552 g | 0.223 ab | 0.337 de | 0.371 AB |
| 0.10 | 0.296 a–e | 0.226 ab | 0.510 g | 0.344 A | 0.236 a–c | 0.205 a | 0.331 c–e | 0.257 E |
| 0.15 | 0.252 a–d | 0.464 fg | 0.549 g | 0.422 CD | 0.243 a–d | 0.266 a–d | 0.469 fg | 0.326 A |
| Average | 0.375 A | 0.441 B | 0.514 D | 0.443 B | 0.421 B | 0.236 C | 0.364 A | 0.340 A |
| r | −0.955 | −0.556 | 0.838 | −0.751 | −0.934 | 0.157 | 0.811 | −0.714 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowski, M.; Brodowska, M.S.; Kordala, N. Trace Element Contents in Maize following the Application of Organic Materials to Reduce the Potential Adverse Effects of Nitrogen. Materials 2023, 16, 215. https://doi.org/10.3390/ma16010215
Wyszkowski M, Brodowska MS, Kordala N. Trace Element Contents in Maize following the Application of Organic Materials to Reduce the Potential Adverse Effects of Nitrogen. Materials. 2023; 16(1):215. https://doi.org/10.3390/ma16010215
Chicago/Turabian StyleWyszkowski, Mirosław, Marzena S. Brodowska, and Natalia Kordala. 2023. "Trace Element Contents in Maize following the Application of Organic Materials to Reduce the Potential Adverse Effects of Nitrogen" Materials 16, no. 1: 215. https://doi.org/10.3390/ma16010215
APA StyleWyszkowski, M., Brodowska, M. S., & Kordala, N. (2023). Trace Element Contents in Maize following the Application of Organic Materials to Reduce the Potential Adverse Effects of Nitrogen. Materials, 16(1), 215. https://doi.org/10.3390/ma16010215






