Recovery of Biophenols from Olive Vegetation Waters by Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Carbon Nanotubes
2.1.2. Olive Vegetation Waters (OVWs) and Their Pre-Treatment
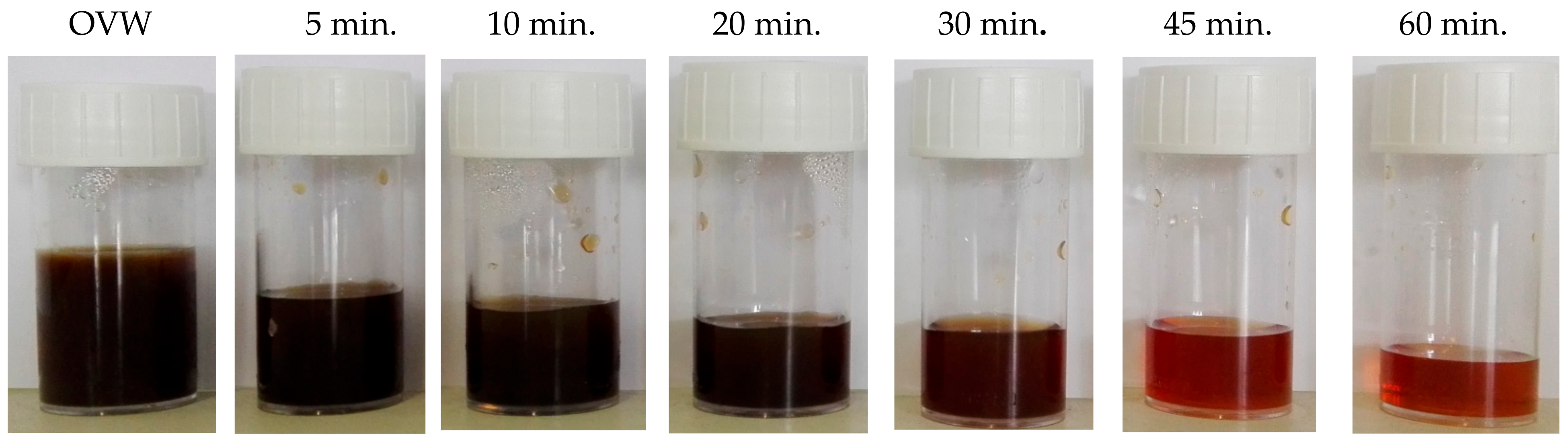
2.2. Adsorption Tests
After Adsorption Tests
2.3. Characterization
3. Results and Discussion
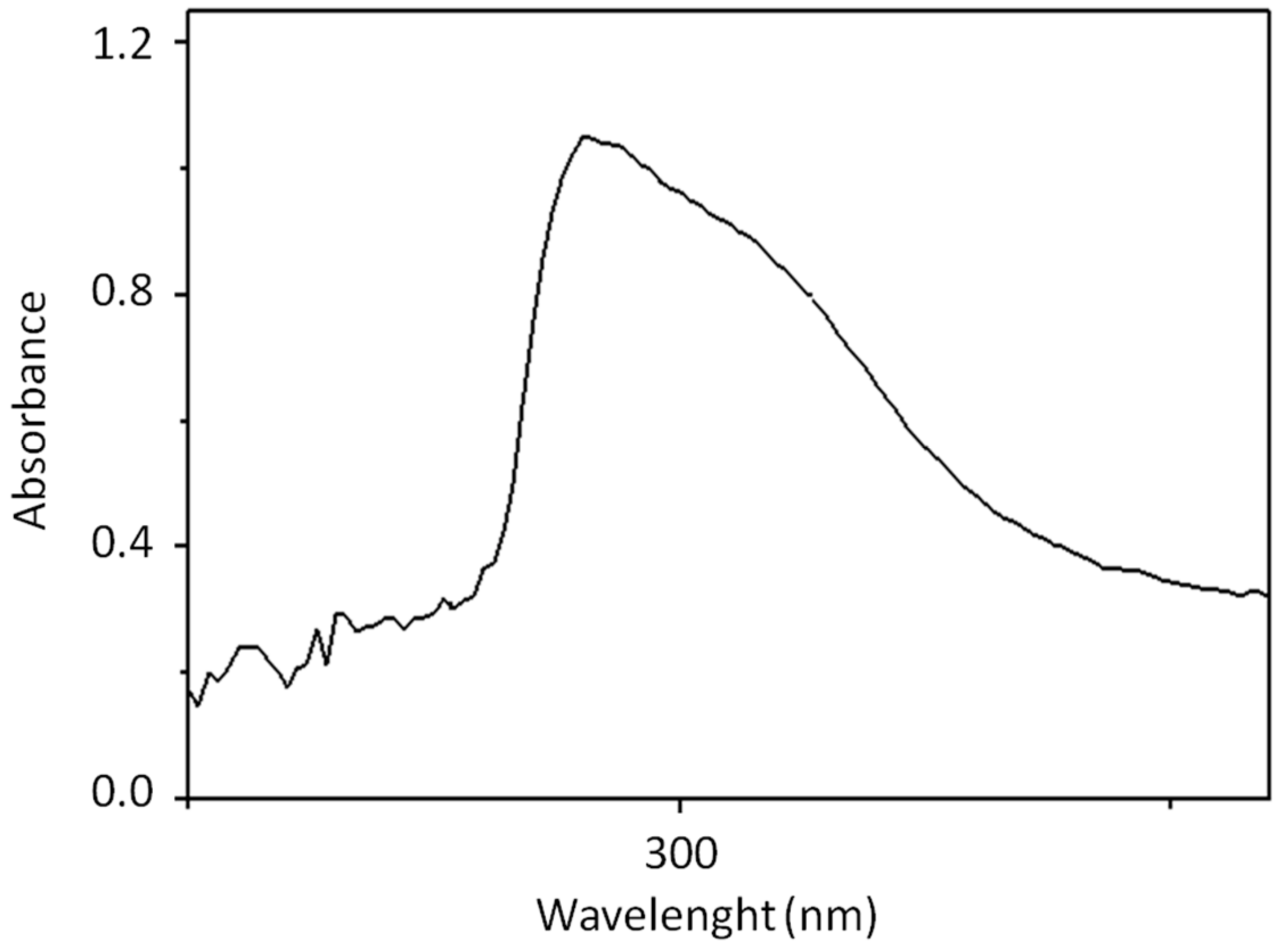
3.1. OVWs Characterization
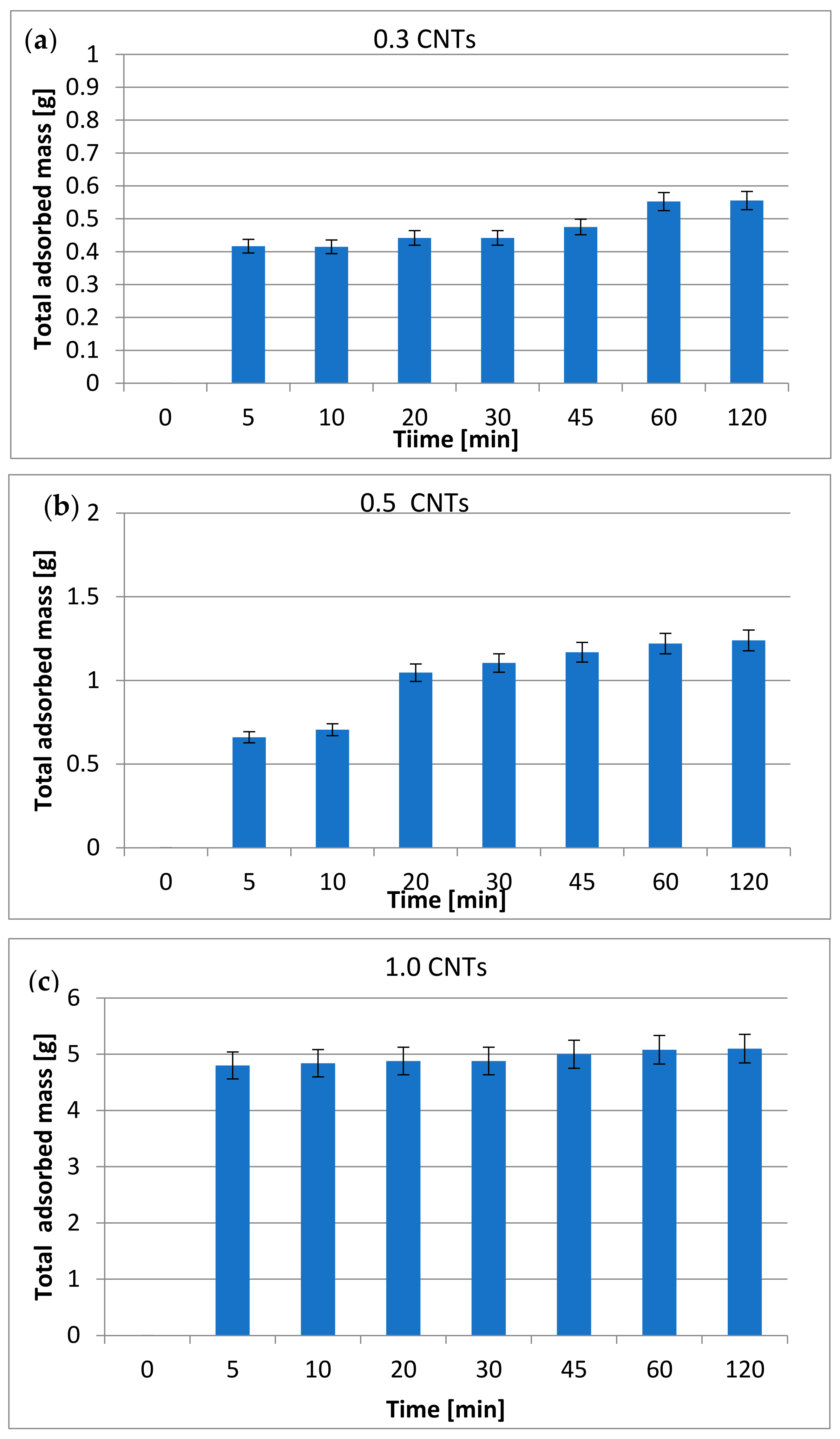
3.2. Total Mass Adsorbed after the Tests
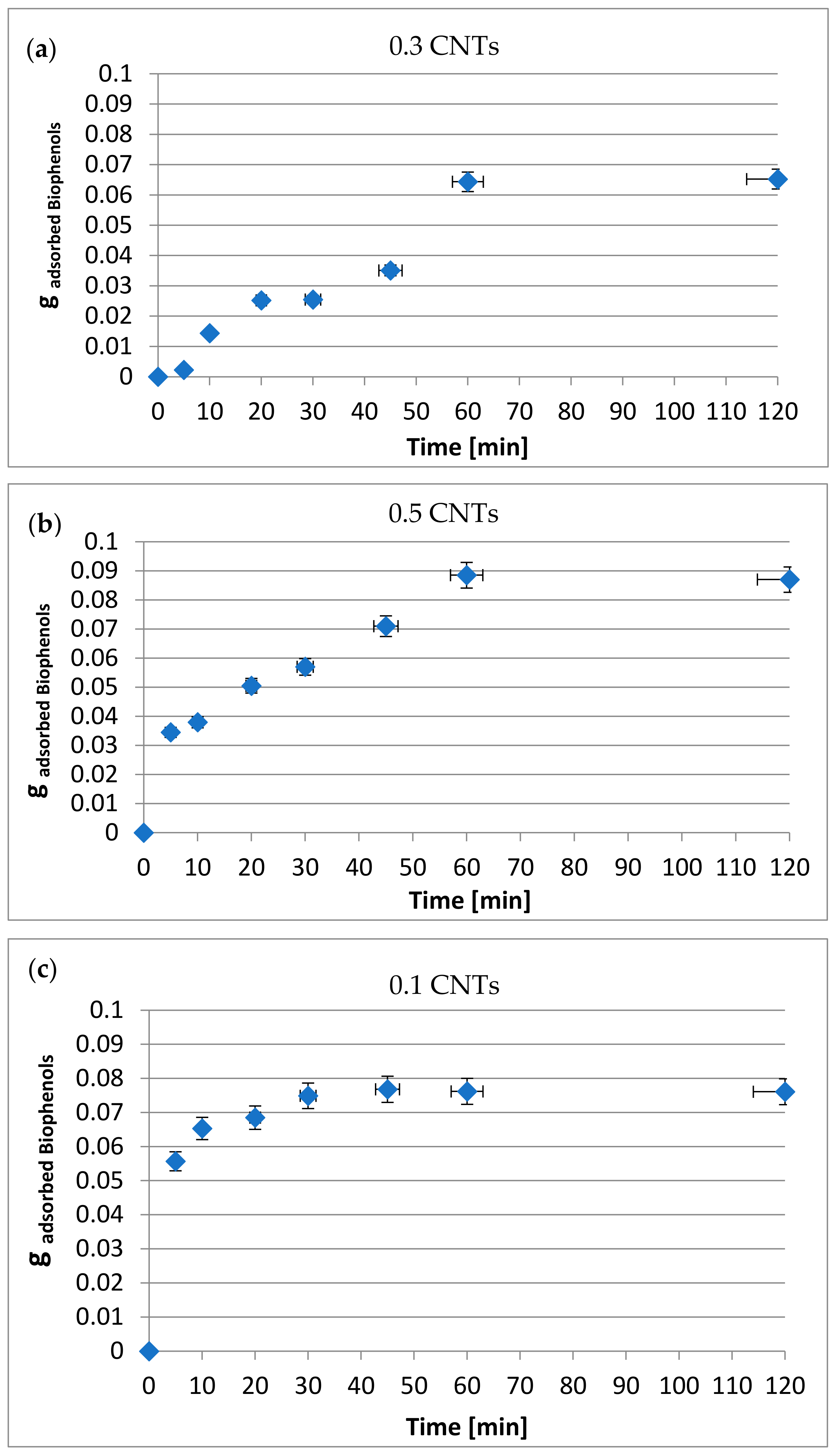
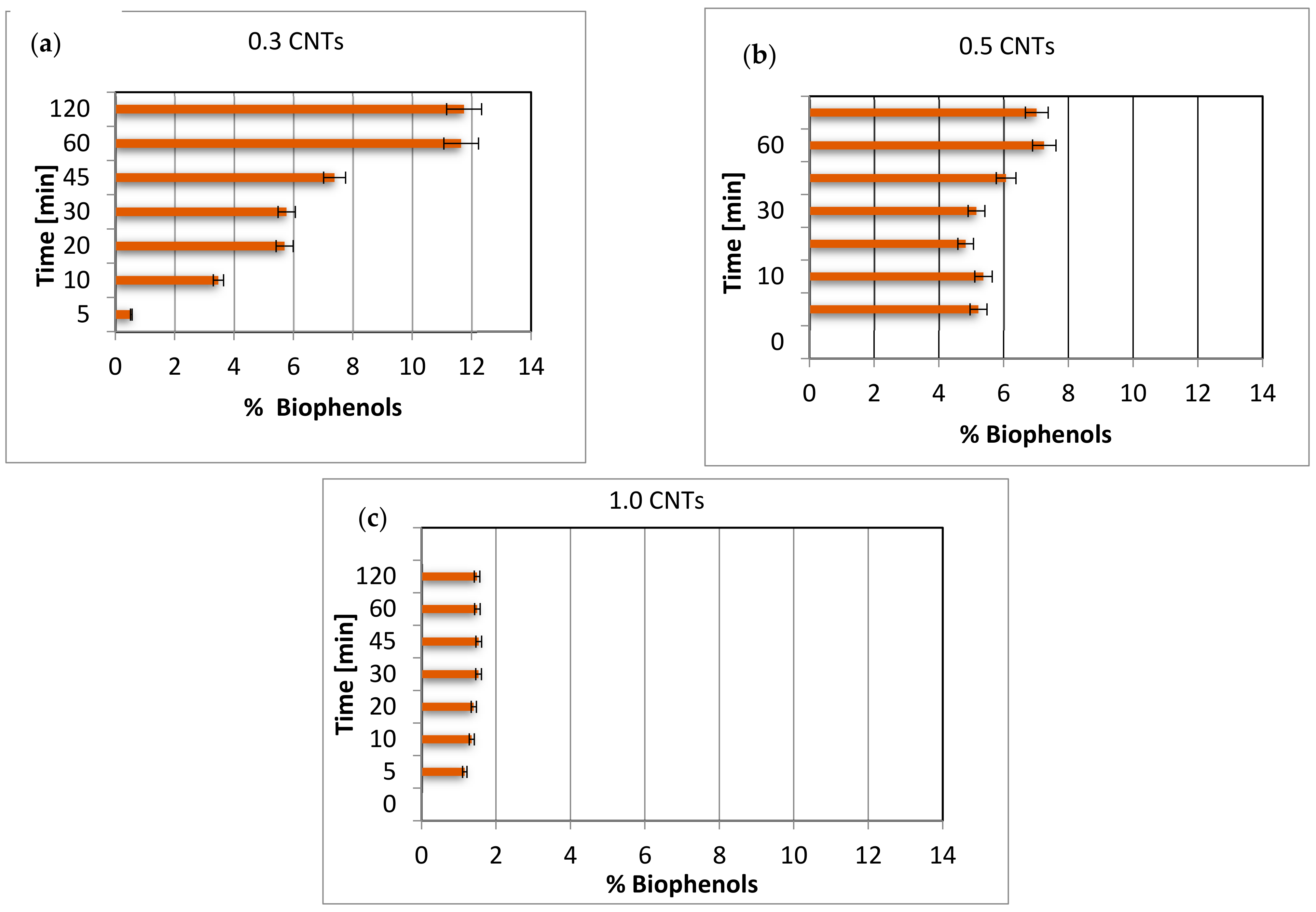
3.3. Amount of Biophenols Retained after Adsorption Tests
3.4. Percentage of Biophenols in the Total Adsorbed Mass
3.5. Study on Reaction Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery based on olive biomass. State of the art and future trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Qdais, H.A.; Alshraideh, H. Selection of management option for solid waste from olive oil industry using the analytical hierarchy process. J. Mater. Cycles Waste Manag. 2014, 18, 1–9. [Google Scholar] [CrossRef]
- Elkacmi, R.; Kamil, N.; Bennajah, M. Separation and purification of high purity products from three different olive mill wastewater samples. J. Environ. Chem. Eng. 2017, 5, 829–837. [Google Scholar] [CrossRef]
- Capasso, R.; Evidente, A.; Schivo, L.; Orru, G.; Marcialis, M.A.; Cristinzio, G. Antibacterial polyphenols from olive oil mill wastewaters. J. Appl. Bacteriol. 1995, 79, 393–398. [Google Scholar] [CrossRef]
- Ramos-Cormenzana, A.; Juárez-Jiménez, B.; Garcia-Pareja, M.P. Antimicrobial activity of olive mill waste-waters (alpechin) and biotransformed olive oil mill wastewater. Int. Biodet. Biodeg. 1996, 38, 283–290. [Google Scholar] [CrossRef]
- Papanikolaou, C.; Melliou, E.; Magiatis, P. Olive Oil Phenols. In Functional Foods; Intech Open: London, UK, 2019; pp. 9–13. [Google Scholar]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive biophenols reduces alzheimer’s pathology in sh-sy5y cells and appswe mice. Int. J. Mol. Sci. 2018, 20, 125. [Google Scholar] [CrossRef] [Green Version]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) biophenols: A nutriceutical against oxidative stress in sh-sy5y cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef] [Green Version]
- Obied, H.K.; Prenzler, P.D.; Omar, S.H.; Ismael, R.; Servili, M.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S. Chapter six—Pharmacology of olive biophenols. In Advances in Molecular Toxicology; Fishbein, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, pp. 195–242. [Google Scholar]
- Papadopoulou, A.; Petrotos, K.; Stagos, D.; Gerasopoulos, K.; Maimaris, A.; Makris, H.; Kafantaris, I.; Makri, S.; Kerasioti, E.; Halabalaki, M.; et al. Enhancement of antioxidant mechanisms and reduction of oxidative stress in chickens after the administration of drinking water enriched with polyphenolic powder from olive mill waste waters. Oxid. Med. Cell. Longev. 2017, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra virgin olive oil and cardiovascular diseases: Benefits for human health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef]
- Valls, R.M.; Farras, M.; Suarez, M.; Fernandez-Castillejo, S.; Fito, M.; Konstantinidou, V.; Fuentes, F.; Lopez-Miranda, J.; Giralt, M.; Covas, M.I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [Green Version]
- Goh, K.; Karahan, H.E.; Wei, L.; Bae, T.-H.; Fane, A.G.; Wang, R.; Chen, Y. Carbon nanomaterials for advancing separation membranes: A strategic perspective. Carbon 2016, 109, 694–710. [Google Scholar] [CrossRef]
- De Luca, P.; Foglia, P.; Siciliano, C.; B.Nagy, J.; Macario, A. Water contaminated by industrial textile dye: Study on decolorization process. Environments 2019, 6, 101. [Google Scholar] [CrossRef] [Green Version]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of Non-Conventional Extraction Methods: Toward a Sustainable and Green Production of Valuable Compounds from Mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- De Luca, P.; Nappo, G.; Siciliano, C.; B.Nagy, J. The role of carbon nanotubes and cobalt in the synthesis of pellets of titanium silicates. J. Porous Mater. 2018, 25, 283–296. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.-H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef]
- Iijima, S. Carbon nanotubes: Past, present, and future. Phys. B Condens. Matter 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Popov, V. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R-Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Bandura, L.; Franus, M.; Madej, J.; Kołodynska, D.; Hubicki, Z. Zeolites in Phenol Removal in the Presence of Cu(II) Ions—Comparison of Sorption Properties after Chitosan Modification. Materials 2020, 13, 643. [Google Scholar] [CrossRef] [Green Version]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Konieczny, K.; Rajca, M.; Bodzek, M.; Kwiecinska, A. Water treatment using hybrid method of coagulation and low-pressure membrane filtration. Environ. Protect. Eng. 2009, 35, 5–22. [Google Scholar]
- Qdais, H.A.; Moussa, H. Removal of Heavy Metals from Waste Water by Membrane Processes a Comparative Study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Ma, Z.; Lei, T.; Ji, X.; Gao, X.; Gao, C. Submerged Membrane Bioreactor for Vegetable Oil Wastewater Treatment. Chem. Eng. Technol. 2015, 38, 101–109. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Hui, K.S.; Chao, C.Y.H.; Kot, S.C. Removal of mixed heavy ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef]
- De Luca, P.; Poulsen, T.G.; Salituro, A.; Tedeschi, A.; Vuono, D.; Kònya, Z.; Madaràsz, D.; B.Nagy, J. Evaluation and comparison of the ammonia adsorption capacity of titanosilicates ETS-4 and ETS-10 and aluminotitanosilicates ETAS-4 and ETAS-10. J. Therm. Anal. Calorim. 2015, 122, 3, 1257–1267. [Google Scholar] [CrossRef]
- De Raffele, G.; Aloise, A.; De Luca, P.; Vuono, D.; Tagarelli, A.; B.Nagy, J. Kinetic and thermodynamic effects during the adsorption of heavy metals on ETS-4 and ETS-10 microporous materials. J. Porous Mater. 2016, 23, 389–400. [Google Scholar] [CrossRef]
- De Luca, P.; Mastroianni, C.; B.Nagy, J. Synthesis of self-bonded pellets of ETS-4 phase by new methodology of preparation. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 374, p. 012003. [Google Scholar]
- De Luca, P.; Siciliano, C.; Macario, A.; B.Nagy, J. The Role of Carbon Nanotube Pretreatments in the Adsorption of Benzoic Acid. Materials 2021, 14, 2118. [Google Scholar] [CrossRef]
- De Luca, P.; Chiodo, A.; Macario, A.; Siciliano, C.; B.Nagy, J. Semi-Continuous Adsorption Processes with Multi-Walled Carbon Nanotubes for the Treatment of Water Contaminated by an Organic Textile Dye. Appl. Sci. 2021, 11, 1687. [Google Scholar] [CrossRef]
- De Luca, P.; B.Nagy, J. Treatment of Water Contaminated with Reactive Black-5 Dye by Carbon Nanotubes. Materials 2020, 13, 5508. [Google Scholar] [CrossRef]
- De Benedetto, C.; Macario, A.; Siciliano, C.; B.Nagy, J.; De Luca, P. Adsorption of reactive blue 116 dye and reactive yellow 81 dye from aqueous solutions by multi-walled carbon nanotubes. Materials 2020, 13, 2757. [Google Scholar] [CrossRef]
- Lico, D.; Vuono, D.; Siciliano, C.; B.Nagy, J.; De Luca, P. Removal on unleaded gasoline from water by multi-walled carbon nanotubes. J. Environ. Manag. 2019, 237, 636–643. [Google Scholar] [CrossRef]
- Hrncirik, K.; Fritsche, S. Comparability and reliability of different techniques for the determination of phenolic compounds in virgin olive oil. Eur. J. Lipid Sci. Technol. 2004, 106, 540–549. [Google Scholar] [CrossRef]
- Tsimidou, M.; Papadopoulos, G.; Boskou, D. Determination of phenolic compounds in virgin olive oil by reversed-phase HPLC with emphasis on UV de-tection. Food Chem. 1992, 44, 53–60. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K. Critical review: Phenolic compounds in olives. Analyst 1998, 123, 31R–44R. [Google Scholar] [CrossRef]
| Recovery [%] | |||
|---|---|---|---|
| Time [min] | System 1 (0.3 CNTs) | System 2 (0.5 CNTs) | System 3 (1.0 CNTs) |
| 5 | 1.52 | 23.31 | 37.63 |
| 10 | 9.73 | 25.65 | 44.12 |
| 20 | 17.03 | 34.12 | 46.28 |
| 30 | 17.23 | 38.51 | 50.61 |
| 45 | 23.72 | 47.97 | 51.90 |
| 60 | 43.48 | 59.80 | 51.49 |
| 120 | 44.09 | 58.78 | 51.42 |
| System | Time [min] | CNTs [g] | Biophenols Adsorbed [g] | Adsorbate Total [g] | gbiophenol ads/gtotal adsorbate |
|---|---|---|---|---|---|
| 1 | 60 | 15 | 0.88 | 2.02 | 0.436 |
| 2 | 60 | 25 | 4.42 | 61 | 0.072 |
| 3 | 60 | 50 | 3.81 | 254 | 0.015 |
| System | Range Adsorption Time | ||
|---|---|---|---|
| 0–5 [min] | 5–60 [min] | 60–120 [min] | |
| (1) 0.3 gCNTs/20 mL OVWs | 0.0005 | 0.001 | 2 × 10−5 |
| (2) 0.5 gCNTs/20 mL OVWs | 0.0069 | 0.001 | 2 × 10−5 |
| (3) 1.0 gCNTs/20 mL OVWs | 0.0111 | 0.0003 | 2 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, P.; Macario, A.; Siciliano, C.; B.Nagy, J. Recovery of Biophenols from Olive Vegetation Waters by Carbon Nanotubes. Materials 2022, 15, 2893. https://doi.org/10.3390/ma15082893
De Luca P, Macario A, Siciliano C, B.Nagy J. Recovery of Biophenols from Olive Vegetation Waters by Carbon Nanotubes. Materials. 2022; 15(8):2893. https://doi.org/10.3390/ma15082893
Chicago/Turabian StyleDe Luca, Pierantonio, Anastasia Macario, Carlo Siciliano, and Janos B.Nagy. 2022. "Recovery of Biophenols from Olive Vegetation Waters by Carbon Nanotubes" Materials 15, no. 8: 2893. https://doi.org/10.3390/ma15082893
APA StyleDe Luca, P., Macario, A., Siciliano, C., & B.Nagy, J. (2022). Recovery of Biophenols from Olive Vegetation Waters by Carbon Nanotubes. Materials, 15(8), 2893. https://doi.org/10.3390/ma15082893









