Progress in Preparation of Sea Urchin-like Micro-/Nanoparticles
Abstract
1. Introduction
2. Preparation of Urchin-like Micro-/Nanoparticles
2.1. Preparation of Solid Urchin-like Micro-/Nanoparticles
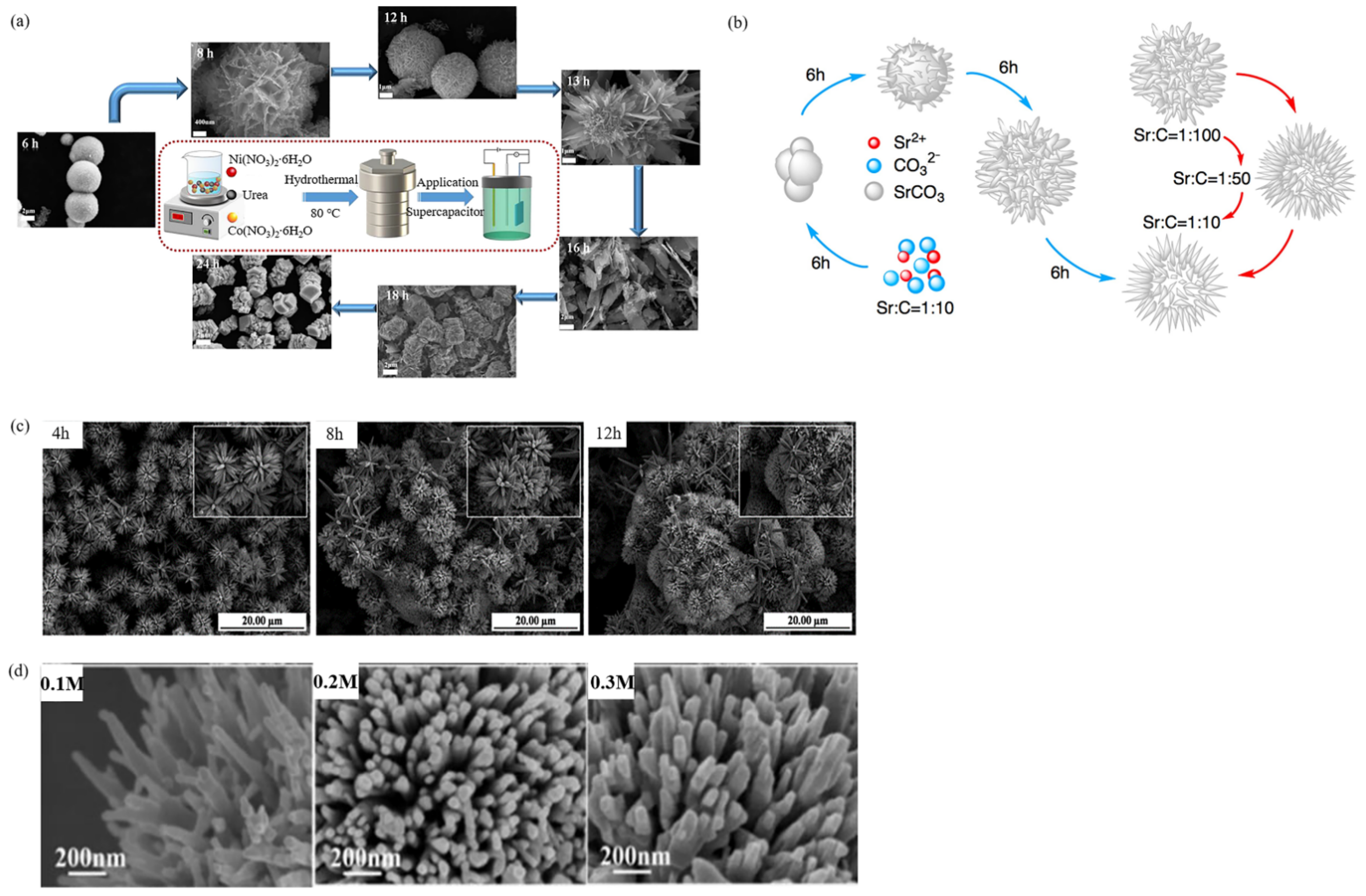
2.1.1. Solvothermal/Hydrothermal Growth
- (1)
- Two-step method
- Template-assisted precursor conversion method
- b.
- Template-free precursor conversion method
- (2)
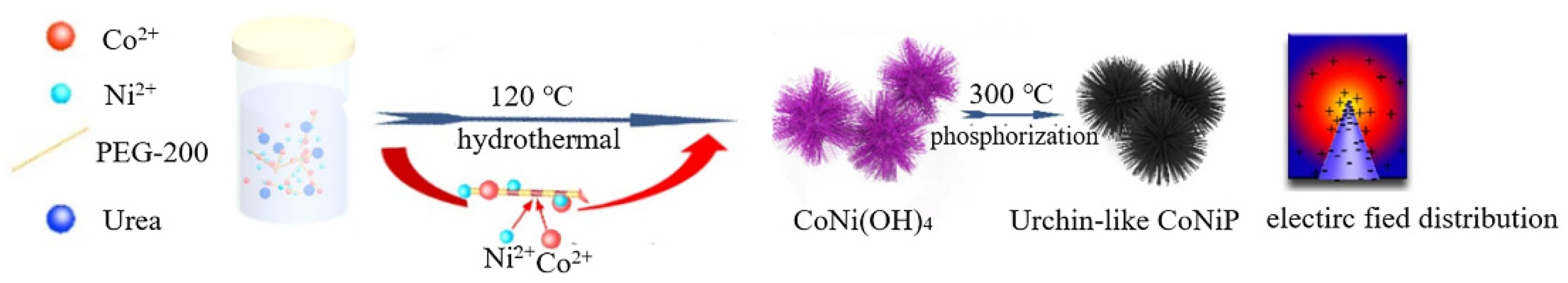
- One-step method
2.1.2. Other Methods
2.2. Preparation of Sea Urchin-like Composite Micro-Nanoparticles
2.2.1. Coated Urchin-like Nanoparticles
2.2.2. Decorative Urchin-like Nanoparticles
2.3. Preparation of Sea Urchin-Shaped Hollow Micro-/Nanoparticles
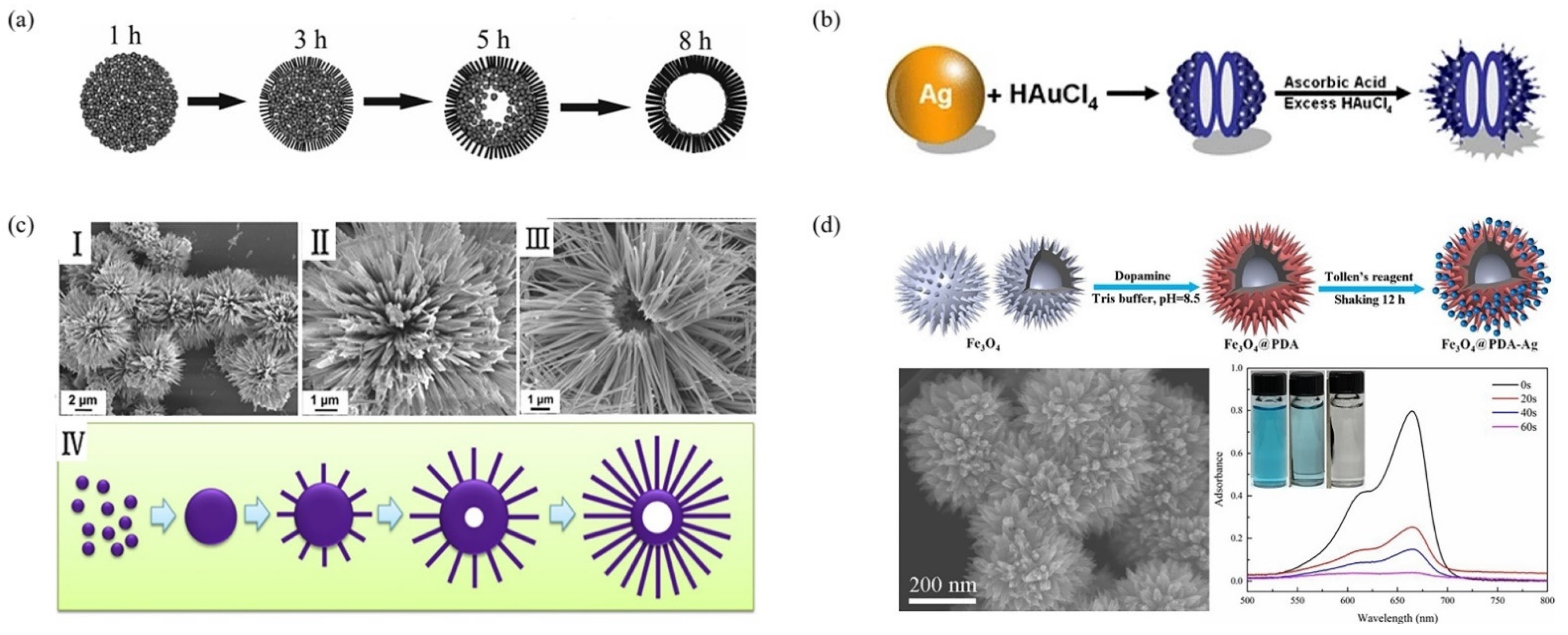
2.3.1. Step-by-Step Method
2.3.2. Synchronizing Method
3. Applications
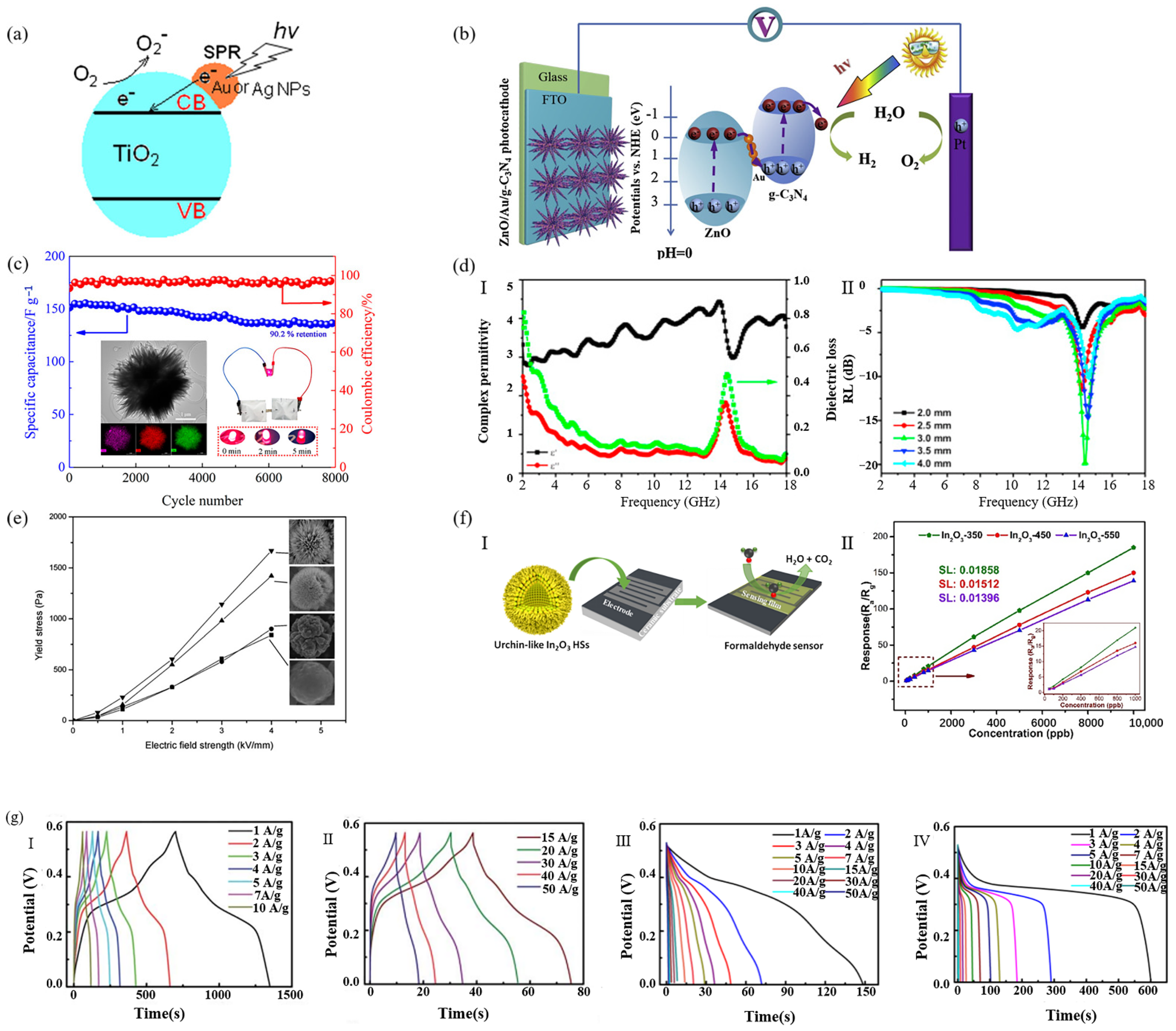
3.1. Photocatalyst
3.2. Electrochemical
3.3. Other Applications
4. Conclusions and Outlook
4.1. Conclusions
4.2. Outlook
- (1)
- The preparation method of urchin-like hollow microspheres is not advanced and is still in the stage of experimental exploration. There are few studies on the preparation of urchin-like inorganic hollow microspheres with itself as the template, which mostly requires a multi-step process, which is time-consuming and laborious. At the same time, the urchin-like hollow microspheres prepared by the existing methods usually have low morphology regularity, a large size, and poor stability. Therefore, developing new and more effective preparation methods to make the reaction conditions mild, controllable, environmentally friendly, and with a low cost, as well as make the prepared sea urchin-like inorganic hollow microspheres have a regular structure, small particle size, and uniformity is one of the main research directions in the future.
- (2)
- At present, the prepared urchin-like hollow microspheres have developed from single shell to a multi-shell structure, and the application performance of the materials has been greatly improved. However, most multi-shell urchin-like hollow composite microspheres are often prepared and play a role alone. How to obtain urchin-like hollow microspheres with a multi-shell at the same time is still facing challenges.
- (3)
- Because the urchin-like hollow microspheres have inner and outer surfaces and one-dimensional nanorods, they have a larger specific surface area and higher quantum yield than solid microspheres. However, at present, the research on the properties of urchin-like hollow microspheres is not in-depth, which has certain limitations for expanding their applications in various fields. Therefore, in-depth study on the correlation between structural parameters and properties of urchin-like hollow microspheres is significant for the development and application of urchin-like hollow microspheres.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 Superstructure-Based Plasmonic Photocatalysts Exhibiting Efficient Charge Separation and Unprecedented Activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Tada, H. Overall water splitting and hydrogen peroxide synthesis by gold nanoparticle-based plasmonic photocatalysts. Nano Adv. 2019, 1, 4238–4245. [Google Scholar] [CrossRef]
- Wang, X.; Kolen’Ko, Y.V.; Bao, X.; Kovnir, K.; Liu, L. One-Step Synthesis of Self-Supported Nickel Phosphide Nanosheet Array Cathodes for Efficient Electrocatalytic Hydrogen Generation. Angew. Chem. 2015, 127, 8306–8310. [Google Scholar] [CrossRef]
- Cobo, S.; Heidkamp, J.; Jacques, P.A.; Fize, J.; Fourmond, V.; Guetaz, L.; Jousselme, B.; Ivanova, V.; Dau, H.; Palacin, S. A Janus cobalt-based catalytic material for electro-splitting of water. Nat. Mater. 2012, 11, 802–807. [Google Scholar] [CrossRef]
- Liu, M.; Pang, Y.; Zhang, B.; De Luna, P.; Voznyy, O.; Xu, J.; Zheng, X.; Dinh, C.T.; Fan, F.; Cao, C. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 2016, 537, 382–386. [Google Scholar] [CrossRef]
- Chen, X.; Jensen, L. Morphology dependent near-field response in atomistic plasmonic nanocavities. Nanoscale 2018, 10, 11410–11417. [Google Scholar] [CrossRef]
- Koh, Y.W.; Lai, C.S.; Du, A.Y.; Tiekink, E.R.; Loh, K.P. Growth of Bismuth Sulfide Nanowire Using Bismuth Trisxanthate Single Source Precursors. Chem. Mater. 2003, 15, 4544–4554. [Google Scholar] [CrossRef]
- Xie, G.; Qiao, Z.P.; Zeng, M.H.; Chen, X.M.; Gao, S.L. A single-source approach to Bi2S3 and Sb2S3 nanorods via a hydrothermal treatment. Cryst. Growth Des. 2004, 4, 513–516. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, J.; Li, S.; Peng, S.; Qian, Y. Synthesis and growth mechanism of Bi2S3 nanoribbons. Chem. Eur. J. 2004, 10, 634–640. [Google Scholar] [CrossRef]
- Ye, C.; Meng, G.; Jiang, Z.; Wang, Y.; Wang, G.; Zhang, L. Rational growth of Bi2S3 nanotubes from quasi-two-dimensional precursors. J. Am. Chem. Soc. 2002, 124, 15180–15181. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, G.; Wang, H.; Zhang, Y.; Li, G. Facile synthesis of Bi2S3 nanowire arrays. Mater. Lett. 2008, 62, 3663–3665. [Google Scholar] [CrossRef]
- Liu, X.; Cui, J.; Zhang, L.; Yu, W.; Guo, F.; Qian, Y. Control to synthesize Bi2S3 nanowires by a simple inorganic-surfactant-assisted solvothermal process. Nanotechnology 2005, 16, 1771. [Google Scholar] [CrossRef]
- Park, S.; Lim, J.-H.; Chung, S.-W.; Mirkin, C.A. Self-assembly of mesoscopic metal-polymer amphiphiles. Science 2004, 303, 348–351. [Google Scholar] [CrossRef]
- Cölfen, H.; Mann, S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew. Chem. Int. Ed. 2003, 42, 2350–2365. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Agarwal, R.; Lieber, C.M. Single-nanowire electrically driven lasers. Nature 2003, 421, 241–245. [Google Scholar] [CrossRef]
- Park, W.; Jun, Y.; Jung, S.; Yi, G.-C. Excitonic emissions observed in ZnO single crystal nanorods. Appl. Phys. Lett. 2003, 82, 964–966. [Google Scholar] [CrossRef]
- Mao, L.-F. Effects of quantum coupling on the performance of metal-oxide-semiconductor field transistors. Pramana 2009, 72, 407–414. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, X.; Lin Wang, Z.; Sun, S. Misfit dislocations in multimetallic core-shelled nanoparticles. Appl. Phys. Lett. 2012, 100, 111603. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Guo, H.; Zhou, X.; Lu, G. Au@In2O3 core-shell composites: A metal-semiconductor heterostructure for gas sensing applications. RSC Adv. 2015, 5, 545–551. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, J.; Cui, M.; Huang, L.; Su, Y. Biomimetic synthesis of novel calcium carbonate heterogeneous dendrites. New J. Chem. 2015, 39, 5309–5315. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Xiang, Q.; Li, H.; Pan, Q.; Xu, P. Brush-like hierarchical ZnO nanostructures: Synthesis, photoluminescence and gas sensor properties. J. Phys. Chem. C 2009, 113, 3430–3435. [Google Scholar] [CrossRef]
- Wu, W.Q.; Xu, Y.F.; Rao, H.S.; Feng, H.L.; Su, C.Y.; Kuang, D.B. Constructing 3D Branched Nanowire Coated Macroporous Metal Oxide Electrodes with Homogeneous or Heterogeneous Compositions for Efficient Solar Cells. Angew. Chem. 2014, 126, 4916–4921. [Google Scholar] [CrossRef]
- Lei, B.X.; Luo, Q.P.; Yu, X.Y. Hierarchical TiO2 flowers built from TiO2 nanotubes for efficient Pt-free based flexible dye-sensitized solar cells. PCCP 2012, 14, 13175–13179. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Shi, Y.; Kuang, Y.; Huang, G.; Zhang, H. Laser fusion–brazing of aluminum alloy to galvanized steel with pure Al filler powder. Opt. Laser Technol. 2015, 66, 1–8. [Google Scholar] [CrossRef]
- Wu, G. High-valence-state nickel-iron phosphonates with urchin-like hierarchical architecture for highly efficient oxygen evolution reaction. J. Alloys Compd. 2021, 861, 158614. [Google Scholar]
- Nguyen-Phan, T.D.; Si, L.; Vovchok, D.; Llorca, J.; Rodriguez, J.A. Three-dimensional ruthenium-doped TiO2 sea urchins for enhanced visible-light-responsive H2 production. PCCP 2016, 18, 15972–15979. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Jose-Yacaman, M. Nanostructures with animal-like shapes. Ind.Eng.chem.Res. 2010, 49, 8289–8309. [Google Scholar] [CrossRef]
- Chang, T.H.; Hsu, C.Y.; Lin, H.C.; Chang, K.H.; Li, Y.Y. Formation of urchin-like CuO structure through thermal oxidation and its field-emission lighting application. J. Alloys Compd. 2015, 644, 324–333. [Google Scholar] [CrossRef]
- Tiya-Djowe, A.; Laminsi, S.; Noupeyi, G.L.; Gaigneaux, E.M. Non-thermal plasma synthesis of sea-urchin like α-FeOOH for the catalytic oxidation of Orange II in aqueous solution. Appl. Catal. B 2015, 176, 99–106. [Google Scholar] [CrossRef]
- Chen, J.; Qin, S.; Song, G.; Xiang, T.; Xin, F.; Yin, X. Shape-controlled solvothermal synthesis of Bi2S3 for photocatalytic reduction of CO2 to methyl formate in methanol. Dalton Trans. 2013, 42, 15133–15138. [Google Scholar] [CrossRef]
- Lee, C.T.; Peng, J.D.; Li, C.T.; Tsai, Y.L.; Vittal, R.; Ho, K.C. Ni3Se4 hollow architectures as catalytic materials for the counter electrodes of dye-sensitized solar cells. Nano Energy 2014, 10, 201–211. [Google Scholar] [CrossRef]
- Jia, H.; Liu, X.; Liang, J.; Xu, B.; Liu, H. Self-assembly of indium phosphide with an urchin-like architecture through a hydrothermal route. Mater.Lett. 2012, 82, 95–98. [Google Scholar]
- Dao, Z.; Xu, M.; Lu, M.; Chen, G.; Tang, D. Urchin-like (gold core)@(platinum shell) nanohybrids: A highly efficient peroxidase-mimetic system for in situ amplified colorimetric immunoassay. Biosens. Bioelectron. 2015, 70, 194–201. [Google Scholar]
- Wu, W.Q.; Xu, Y.F.; Rao, H.S.; Su, C.Y.; Kuang, D.B. A double layered TiO2 photoanode consisting of hierarchical flowers and nanoparticles for high-efficiency dye-sensitized solar cells. Nanoscale 2013, 5, 4362–4369. [Google Scholar] [CrossRef]
- Li, H.; Fei, G.T.; Cui, P.; Jin, Y.; Feng, X.; Chen, C. Urchin-like Co3O4 Nanostructure and Their Electrochemical Behavior in Rechargeable Lithium Ion Battery. Chin. J. Chem. Phys. 2011, 24, 343–347. [Google Scholar] [CrossRef]
- Zeng, F.; Huang, T.; Li, B.; Li, Y.-Y.; Zhang, H.-W.; Wang, Y.-M.; Chai, Y.-F.; Huang, W.-Q.; Huang, G.-F. Novel urchin-like CoNiP as advanced pH-universal electrocatalysts toward hydrogen evolution reaction. J. Phys. D Appl. Phys. 2021, 54, 365502. [Google Scholar] [CrossRef]
- Xu, X.; Mei, X.; Zhao, P.; Peng, S.; Sun, Y.; Hu, X.; Lu, G. One-step synthesis and gas sensing characteristics of urchin-like In2O3. Sens. Actuators. B 2013, 186, 61–66. [Google Scholar] [CrossRef]
- Guoxiu, T.; Jianguo, G.; Wenhua, W.U.; Liangchao, L.I.; Yao, G.; Qiao, H. Preparation and electrochemical properties of urchin-like α-Fe2O3 nanomaterials. Sci. China Technol. Sci. 2010, 53, 1897–1903. [Google Scholar]
- Tong, G.; Wu, W.; Guan, J.; Qian, H.; Yuan, J.; Li, W. Synthesis and characterization of nanosized urchin-like α-Fe2O3 and Fe3O4: Microwave electromagnetic and absorbing properties. J. Alloys Compd. 2011, 509, 4320–4326. [Google Scholar] [CrossRef]
- Bai, L.; Yuan, F.; Peng, H.; Yan, S.; Xi, W.; Li, S. A facile route to sea urchin-like NiO architectures. Mater. Lett. 2007, 61, 1698–1700. [Google Scholar] [CrossRef]
- Hongchao, Y.; Yejun, Z.; Feng, H.; Qiangbin, W. Urchin-like CoP Nanocrystals as Hydrogen Evolution Reaction and Oxygen Reduction Reaction Dual-Electrocatalyst with Superior Stability. Nano Lett. 2015, 15, 7616. [Google Scholar]
- Chen, H.; Jiang, J.; Zhang, L.; Wan, H.; Xia, D. Highly conductive NiCo2S4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 2013, 5, 8879–8883. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Yin, J.; Li, J.; Wu, H. Hydrothermal synthesis of uniform urchin-like γ-MnS architectures and their photocatalytic properties—ScienceDirect. Physica E 2020, 116, 113711. [Google Scholar] [CrossRef]
- Tang, C.; Wang, C.; Su, F.; Zang, C.; Yang, Y.; Zong, Z.; Zhang, Y. Controlled synthesis of urchin-like Bi2S3 via hydrothermal method. Solid State Sci. 2010, 12, 1352–1356. [Google Scholar] [CrossRef]
- Sang, Y.; Dai, G.; Wang, L.; Gao, X.; Fang, C. Hydrothermal Synthesis of Urchin-like Bi2S3 Nanostructures for Superior Visible-light-driven Cr (VI) Removal Capacity. ChemistrySelect 2018, 3, 7123–7128. [Google Scholar] [CrossRef]
- Yan, S.X.; Luo, S.H.; Sun, M.Z.; Wang, Q.; Zhang, Y.H.; Liu, X. Facile hydrothermal synthesis of urchin-like NiCo2O4 as advanced electrochemical pseudocapacitor materials. Int.J. Energy Res. 2021, 45, 20186–20198. [Google Scholar] [CrossRef]
- Wang, Z.; He, G.; Yin, H.; Bai, W.; Ding, D. Corrigendum to ‘Evolution of controllable urchin-like SrCO3 with enhanced electrochemical performance via an alternative processing’. App. Surf. Sci. 2018, 456, 1015. [Google Scholar] [CrossRef]
- Yang, J.; Jia, H.; Lv, X.; Wang, Y. Facile preparation of urchin-like ZnO nanostructures and their photocatalytic performance. Ceram. Int. 2016, 42, 12409–12413. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Pan, L.; Liu, Y.; Huang, L. Controllable synthesis of 3D Urchin-like V2O5 as high-stability for Lithium-ion battery cathodes. Funct. Mat. Lett. 2018, 12, 1950037. [Google Scholar] [CrossRef]
- Aadil, M.; Zulfiqar, S.; Warsi, M.F.; Agboola, P.O.; Shakir, I. Free-standing urchin-like nanoarchitectures of Co3O4 for advanced energy storage applications. J. Mater. Res. Technol. 2020, 9, 12697–12706. [Google Scholar] [CrossRef]
- Miao, C.; Xu, P.; Zhao, J.; Zhu, K.; Cheng, K.; Ye, K.; Yan, J.; Cao, D.; Wang, G.; Zhang, X.F. Binder-Free Hierarchical Urchin-like Manganese–Cobalt Selenide with High Electrochemical Energy Storage Performance. ACS Appl. Energy Mater. 2019, 2, 3595–3604. [Google Scholar] [CrossRef]
- Dou, X. A Facile Synthesis of Urchin-Like ZnMn2O4 Architectures with Enhanced Electrochemical Lithium Storage. ChemistrySelect 2020, 5, 12697–12706. [Google Scholar] [CrossRef]
- Hua, J.; Heqing, Y. Thermal decomposition synthesis of 3D urchin-like α-Fe2O3 superstructures. Mater. Sci. Engi. B 2009, 156, 68–72. [Google Scholar] [CrossRef]
- Mullins, W.W. Morphological stability of a particle growing by diffusion of heat flow. Appl. Phys. 1963, 34, 323–329. [Google Scholar] [CrossRef]
- Hu, H.; Sugawara, K. Preparation of Sea Urchin-Like Nickel Particles under Rapid Wet Chemical Process. J. Chen. Eng. Jpn. 2009, 42, s85–s89. [Google Scholar] [CrossRef][Green Version]
- Gan, Z.; Wei, D.; Wei, W.; Xu, Z.; Zheng, X.; Zhao, A. Facile synthesis and characterization of urchin-like CdSe nanostructures. Mater. Lett. 2010, 64, 1601–1603. [Google Scholar] [CrossRef]
- Ballesteros-Balbuena, M.; Roa-Morales, G.; Vilchis-Nestor, A.R.; Castrejón-Sánchez, V.; Camacho-López, M. Photocatalytic urchin-like and needle-like ZnO nanostructures synthetized by thermal oxidation. Mater. Chem. Phys. 2020, 244, 122703. [Google Scholar] [CrossRef]
- An, Z.; Pan, S.; Zhang, J. Synthesis and Tunable Assembly of Spear-like Nickel Nanocrystallites: From Urchin-like Particles to Prickly Chains. J. Phys. Chem. C 2009, 113, 1346–1351. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, Y.; Liu, Y.; Zheng, C.; Zhao, X. Ultrafast synthesis of urchin-like rutile TiO2 by single-step microwave-assisted method. Nanomaterials 2018, 8, 630. [Google Scholar] [CrossRef]
- Kim, M.J.; Liu, Y.D.; Choi, H.J. Urchin-like polyaniline microspheres fabricated from self-assembly of polyaniline nanowires and their electro-responsive characteristics. Chem. Eng. J. 2014, 235, 186–190. [Google Scholar] [CrossRef]
- Li, L.L.; Li, H.; Chen, T.Y.; Gu, Z.J.; Shen, Q. Fabrication and characterization of urchin-like polyaniline microspheres using lignosulfonate as template. Mater. Sci. Semicond. Process. 2015, 35, 34–37. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, Q.; Yan, Y.; Zhang, L.; Li, C. Synthesis, Characterization and Electrochemical Capacitance of Urchin-Like Hierarchical Polyaniline Microspheres. J. Macromol. Sci. Phys. 2012, 51, 897–905. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, M.H. Synthesis of branched gold nanocrystals by a seeding growth approach. Langmuir 2005, 21, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Bakr, O.M.; Wunsch, B.H.; Stellacci, F. High-Yield Synthesis of Multi-Branched Urchin-Like Gold Nanoparticles. Chem. Mater. 2006, 18, 3297–3301. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Zhang, X.; Liu, Y.; Zhou, D.; Sun, H.; Zhang, H.; Yang, B. Controllable Synthesis of Stable Urchin-like Gold Nanoparticles Using Hydroquinone to Tune the Reactivity of Gold Chloride. J. Phys. Chem. C 2011, 115, 3630–3637. [Google Scholar] [CrossRef]
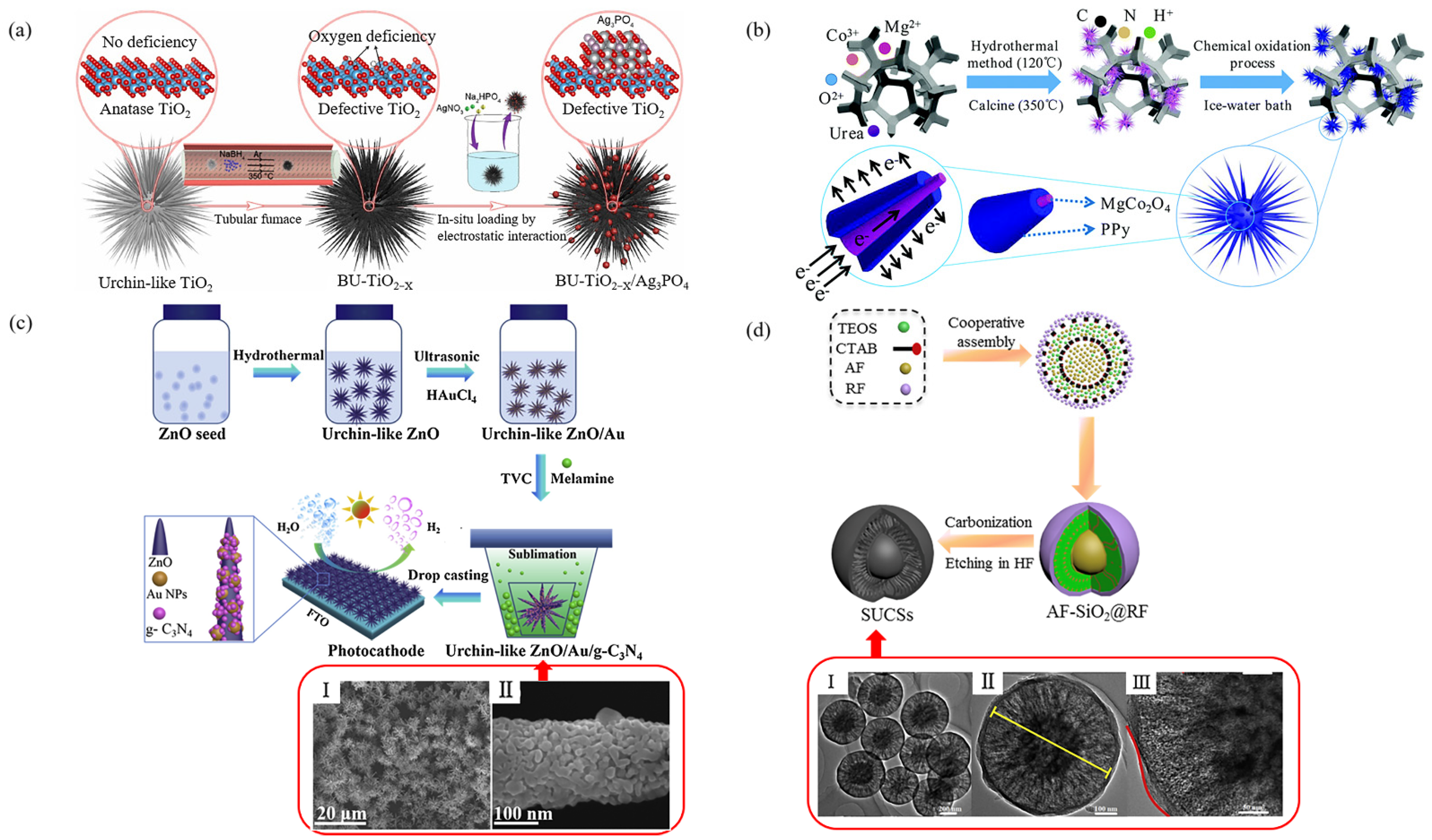
- Xu, Y.; Liu, X.; Zheng, Y.; Li, C.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wu, S. Ag3PO4 decorated black urchin-like defective TiO2 for rapid and long-term bacteria-killing under visible light—ScienceDirect. Bio. Mater. 2021, 6, 1575–1587. [Google Scholar]
- Gao, H.; Wang, X.; Wang, G.; Hao, C.; Zhou, S.; Huang, C. Urchin-like MgCo2O4@PPy Core-Shell Composite Grown on Ni Foam for a High-Performance All-Solid-State Asymmetric Supercapacitor. Nanoscale 2018, 10, 10190–10202. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Y.; Li, H.; Liang, Z.; Jiang, L. A Highly Active Three-Dimensional Z-scheme ZnO/Au/g-C3N4 Photocathode for Efficient Photoelectrochemical Water Splitting. App. Catal. B 2019, 263, 118180. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Li, G.; Liu, L.; Zhang, H.; Wang, G.; Chen, A. Sea urchin-like core/shell hierarchical porous carbon for supercapacitors. J. Alloys Compd. 2017, 719, 438–445. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, Y.; Xiang, Y.; Zhu, J. Construction of Urchinmmike ZnO/TiO2 Direct Zlogcheme System to Improve Charge Separation. Chem. Select 2019, 4, 12963–12970. [Google Scholar]
- Tada, H. Radial TiO2 Nanorod-Based Mesocrystals: Synthesis, Characterization, and Applications. Catalysts 2021, 11, 1298. [Google Scholar]
- Wen, Z.; Li, X.; Rui, Y.; Lei, Q.; Huang, X.; Liu, H.; Zhang, J.; Wang, J.; Ding, T.; Guo, Z. Urchin-like NiO–NiCo2O4 heterostructure microsphere catalysts for enhanced rechargeable non-aqueous Li–O2 batteries. Nanoscale 2018, 11, 50–59. [Google Scholar]
- Xi, G.; Ye, J.; Ma, Q.; Su, N.; Bai, H.; Wang, C. In situ growth of metal particles on 3D urchin-like WO3 nanostructures. J. Am. Che. Soc. 2012, 134, 6508–6511. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; He, J.; Ye, J.; Ge, K.; Zhang, Y.; Yang, Y. Urchin-Like Bi2S3/Ag Nanostructures for Photocatalytic Reduction of Cr (VI). ACS Appl. Nano Mater. 2021, 4, 1260–1269. [Google Scholar] [CrossRef]
- Zeng, Y. Sea urchin-like Al-doped MnO2 nanowires with excellent cycling stability for supercapacitor. Int. J. Electrochem. Sci. 2019, 14, 4350–4360. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Xia, Y. Metal nanostructures with hollow interiors. Adv. Mater. 2003, 15, 641–646. [Google Scholar] [CrossRef]
- Hu, J.; Chen, M.; Fang, X.; Wu, L. Fabrication and application of inorganic hollow spheres. Chem. Soc. Rev. 2011, 40, 5472–5491. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Fabrication of ZnO “dandelions” via a modified Kirkendall process. J. Am. Chem. Soc. 2004, 126, 16744–16746. [Google Scholar] [CrossRef]
- Shen, G.; Bando, Y.; Lee, C.J. Synthesis and evolution of novel hollow ZnO urchins by a simple thermal evaporation process. J. Phys. Chem. B 2005, 109, 10578. [Google Scholar] [CrossRef]
- Gu, Z.; Paranthaman, M.P.; Xu, J.; Pan, Z.W. Aligned ZnO Nanorod Arrays Grown Directly on Zinc Foils and Zinc Spheres by a Low-Temperature Oxidization Method. ACS Nano 2009, 3, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.; Levy-Clement, R.; Bechelany, R.; Michler, R.; Wang, G.Y.; Wang, R.; Philippe, R. Hollow Urchin-like ZnO thin Films by Electrochemical Deposition. Adv. Mater. 2010, 22, 1607–1612. [Google Scholar] [CrossRef]
- Wu, G.; Cheng, Y.; Xie, Q.; Jia, Z.; Xiang, F.; Wu, H. Facile synthesis of urchin-like ZnO hollow spheres with enhanced electromagnetic wave absorption properties. Mater. Lett. 2015, 144, 157–160. [Google Scholar] [CrossRef]
- Tian, Y.F.; Zhou, W.; Yin, B.C.; Ye, B.C. Highly sensitive surface-enhanced Raman scattering detection of adenosine triphosphate based on core-satellite assemblies. Anal. Methods 2017, 9, 6038–6043. [Google Scholar] [CrossRef]
- Yang, M.; Yao, X.; Wang, G.; Ding, H. A simple method to synthesize sea urchin-like polyaniline hollow spheres. Colloids Surf. A Physicochem. Eng. Asp. 2008, 324, 113–116. [Google Scholar] [CrossRef]
- Yan, B.; Cheng, W.; Ma, J.Z. A two-step hydrothermal route for synthesis hollow urchin-like ZnO microspheres. Ceram. Int. 2016, 42, 10289–10296. [Google Scholar]
- Guo, Z.; Wang, Q.; Shen, T.; Kuang, J.; Cao, W. Synthesis of urchin-like and yolk─shell TiO2 microspheres with enhanced photocatalytic properties. Environ.Technol. 2018, 41, 1726–1737. [Google Scholar] [CrossRef]
- Li, B.; Rong, G.; Xie, Y.; Huang, L.; Feng, C. Low-Temperature Synthesis of α-MnO2 Hollow Urchins and Their Application in Rechargeable Li+ Batteries. Inorg. Chem. 2006, 45, 6404–6410. [Google Scholar] [CrossRef]
- Wang, W.; Pang, Y.; Yan, J.; Wang, G.; Suo, H.; Zhao, C.; Xing, S. Facile synthesis of hollow urchin-like gold nanoparticles and their catalytic activity. Gold Bull. 2012, 45, 91–98. [Google Scholar] [CrossRef][Green Version]
- Rui, X.; Tan, H.; Sim, D.; Liu, W.; Xu, C.; Hng, H.H.; Yazami, R.; Lim, T.M.; Yan, Q. Template-free synthesis of urchin-like Co3O4 hollow spheres with good lithium storage properties—ScienceDirect. J. Power Sources 2013, 222, 97–102. [Google Scholar] [CrossRef]
- Cui, K.; Yan, B.; Xie, Y.; Qian, H.; Wang, X.; Huang, Q.; He, Y.; Jin, S.; Zeng, H. Regenerable urchin-like Fe3O4 at PDA-Ag hollow microspheres as catalyst and adsorbent for enhanced removal of organic dyes. J. Hazard. Mater. 2018, 350, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, S.; Liu, W.; Cheng, H.; Chen, S.; Liu, X.; Liu, J.; Tai, Q.; Hu, C. Facile Fabrication of Urchin-like Polyaniline Microspheres for Electrochemical Energy Storage. Electrochim. Acta 2017, 254, 25–35. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Wang, Y.; Yi, Q.; Li, X.; Liu, C. Synthesis of sea urchin-like ZnO by a simple soft template method and its photoelectric properties. Mater. Sci. Semicond. Process. 2014, 27, 1050–1056. [Google Scholar] [CrossRef]
- Du, D.; Cao, M. Ligand-Assisted Hydrothermal Synthesis of Hollow Fe2O3 Urchin-like Microstructures and Their Magnetic Properties. J. Phys. Chem. C 2008, 112, 10754–10758. [Google Scholar] [CrossRef]
- Cheng, Q.; Pavlinek, V.; He, Y.; Yan, Y.; Li, C.; Saha, P. Synthesis and electrorheological characteristics of sea urchin-like TiO2 hollow spheres. Colloid. Polym. Sci. 2011, 289, 799–805. [Google Scholar] [CrossRef]
- Qi, L.; Xiaorui, G.; Tingting, Q.; Fancheng, M. P123 assisted synthesis and characterization of urchin-like γ-Al2O3 hollow microspheres. J. Adv. Ceram. Soc. 2016, 5, 225–231. [Google Scholar]
- Shafique, S.; Yang, S.; Woldu, Y.T.; Wang, Y. Hierarchical synthesis of urchin-like V2O5 hollow spheres and its photodetection properties. Sens. Actuators A 2019, 288, 107–116. [Google Scholar] [CrossRef]
- Li, Q.; Lu, W.; Li, Z.; Ning, J.; Hu, Y. Hierarchical MoS2/NiCo2S4@C urchin-like hollow microspheres for asymmetric supercapacitors. Chem. Eng. J. 2019, 380, 122544. [Google Scholar] [CrossRef]
- Hou, C.; Yue, H.; Fan, Y.; Zhai, Y.; Yu, W.; Sun, Z.; Fan, R.; Feng, D.; Wang, J. Oxygen vacancy derived local build-in electric field in mesoporous hollow Co3O4 microspheres promotes high-performance Li-ion batteries. Mater. Chem. A 2018, 6, 6967–6976. [Google Scholar] [CrossRef]
- Xiang, S.; Xu, S.; Yuan, G.; Min, Y.; Yue, Q.; Gao, B. 3D hierarchical golden wattle-like TiO2 microspheres: Polar acetone-based solvothermal synthesis and enhanced water purification performance. Crystengcomm 2017, 19, 2187–2194. [Google Scholar]
- Liu, H.; Yu, X.; Li, L.; Wang, Z.; Zhao, Z.; Zhang, J.; Guo, W. One-step synthesis of ultrathin nanobelts-assembled urchin-like anatase TiO2 nanostructures for highly efficient photocatalysis. Crystengcomm 2016, 19, 129–136. [Google Scholar]
- Xiang, L.; Zhao, X.; Shang, C.; Yin, J. Au or Ag nanoparticle-decorated 3D urchin-like TiO2 nanostructures: Synthesis, characterization, and enhanced photocatalytic activity. J. Colloid Interface Sci. 2013, 403, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhao, X.; Xiang, L.; Xiang, X.; Zhang, Z. Enhanced electrorheology of suspensions containing sea-urchin-like hierarchical Cr-doped titania particles. Soft Matter. 2009, 5, 4687–4697. [Google Scholar] [CrossRef]
- Tao, Z.; Li, Y.; Zhang, B.; Sun, G.; Xiao, M.; Bala, H.; Cao, J.; Zhang, Z.; Wang, Y. Synthesis of urchin-like In2O3 hollow spheres for selective and quantitative detection of formaldehyde. Sens. Actuators B 2019, 298, 126889. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Wang, X.; Xie, X.; Wang, M.; Zhang, L.; Tian, J. Hierarchical assembly of In2O3 nanoparticles on ZnO hollow nanotubes using carbon fibers as templates: Enhanced photocatalytic and gas-sensing properties. J.Colloid Interface Sci. 2017, 498, 263–270. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, B.; Zhu, G.; Chen, R.; Hu, Y.; Ma, L.; Lv, H.; Wang, Y.; Liang, J.; Tie, Z.; et al. Highly Efficient Retention of Polysulfides in “Sea Urchin”-Like Carbon Nanotube/Nanopolyhedra Superstructures as Cathode Material for Ultralong-Life Lithium–Sulfur Batteries. Nano Lett. 2016, 17, 437–444. [Google Scholar] [CrossRef]
- Guo, B.; Batool, A.; Xie, G.; Boddula, R.; Tian, L.; Jan, S.U.; Gong, J.R. Facile Integration between Si and Catalyst for High-Performance Photoanodes by a Multifunctional Bridging Layer. Nano Lett. 2018, 18, 1516–1521. [Google Scholar] [CrossRef]
- Yang, G.; Ding, H.; Chen, D.; Feng, J.; Hao, Q.; Zhu, Y. Construction of urchin-like ZnIn2S4-Au-TiO2 heterostructure with enhanced activity for photocatalytic hydrogen evolution. Appl. Catal. B 2018, 234, 260–267. [Google Scholar] [CrossRef]
- You, B.; Jiang, N.; Sheng, M.; Bhushan, M.W.; Sun, Y. Hierarchically Porous Urchin-Like Ni2P Superstructures Supported on Nickel Foam as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2015, 6, 714–721. [Google Scholar] [CrossRef]
- Wu, C.; Cai, J.; Zhang, Q.; Zhou, X.; Zhu, Y.; Li, L.; Shen, P.; Zhang, K. Direct growth of urchin-like ZnCo2O4 microspheres assembled from nanowires on nickel foam as high-performance electrodes for supercapacitors. Electrochim. Acta 2015, 169, 202–209. [Google Scholar] [CrossRef]



| Method | Material | Solvent | Surfactant | BET Surface Area | Particle Size (Diameter) | Application | Ref. |
|---|---|---|---|---|---|---|---|
| hydrothermal | Co3O4 | deionized water | CTAB | - | 5–7 µm | lithium ion battery | [36] |
| deionized water | - | 165 m2/g | 3 µm | energy storage | [51] | ||
| CoNiP | deionized water | PEG-2000 | - | 2–5 µm | Hydrogen evolution reaction catalytic | [37] | |
| In2O3 | ethanol | SDS | 58.6 m2/g | 1 µm | O3 gas sensor devices | [38] | |
| NiO | deionized water | - | - | 1 µm | - | [41] | |
| CoP | deionized water | - | - | 5 µm | electrocatalysts | [42] | |
| NiCo2S4 | deionized water | - | 20.33 m2/g | 4 µm | high-rate supercapacitors | [43] | |
| γ-MnS | distilled water, EG | - | 34.55 m2/g | 4–5 µm | photocatalytic | [44] | |
| Bi2S3 | deionized water | - | - | 6–8 µm | - | [45] | |
| DMF, deionized water | - | - | 2 µm | photocatalyst | [46] | ||
| NiFeP | DMF | 118.9 m2/g | 5 µm | photocatalyst | [26] | ||
| NiCo2O4 | deionized water | - | 158.6 m2/g | 4 µm | supercapacitors | [47] | |
| SrCO3 | deionized water | - | - | 4 µm | capacitor | [48] | |
| α-Fe2O3 | deionized water | glucose | 151.2 m2/g | 0.5–1.0 µm | - | [39] | |
| Fe3O4 | deionized water | glucose | - | 0.5–1.0 µm | microwave absorbing | [40] | |
| ZnO | deionized water | - | - | 5–10 µm | Photocatalytic | [49] | |
| MnCo-selenide | deionized water | - | - | 4.28 µm | capacitors | [52] | |
| electrodeposition-hydrothermal | ZnMn2O4 | ethanol and deionizer water | sodium 王citrate | 25.34 m2/g | 500 nm | electrodes | [53] |
| thermal decomposition | α-Fe2O3 | deionized water | - | 60.24 m2/g | 400 nm–2.5 µm | electrodes | [54] |
| seeding growth approach | Au | deionized water | SDS | - | 40 nm | - | [64] |
| hydrolyzing-heat-treating | Au | deionized water | sodium 王citrate | - | 40 nm | - | [65] |
| Au | Citrate, deionized water | hydroquinone | - | 50–200 nm | - | [66] | |
| - | Ni | deionized water | Na2CO3 | 4.29 m2/g | 1.28–2.55 µm | - | [56] |
| - | V2O5 | ethylene glycol, deionized water | - | - | 2–3 µm | - | [50] |
| W/O microemulsion approach | CdSe | n-octane, 1-butanol, deionized water | CTAB | 13.14 m2/g | 2.5–3.5 µm | - | [57] |
| thermal oxidation | ZnO | - | - | - | photocatalytic | [58] | |
| microwave-assisted method | TiO2 | toluene | - | - | 2–3 µm | photocatalytic | [60] |
| self-assembly | Polyaniline | deionized water | - | 24.5 m2/g | 2.5 µm | electrorheological | [61] |
| ethanol | - | - | 10 µm | electrochemical | [63] |
| Material [Ref.] | Inner Diameter | Outer Diameter | Template | BET Surface Area | Application | Reference |
|---|---|---|---|---|---|---|
| ZnO | 3 µm | 4.3 µm | Polystyrene microsphere | - | - | [82] |
| - | 5–6 µm | glucose monohydrate | 36.1 m2/g | EM wave absorption | [83] | |
| 40 µm | 50 µm | - | - | - | [80] | |
| 1 µm | 1.8 µm | - | - | - | [86] | |
| 2 µm | 4 µm | H2 | - | solar cells | [93] | |
| Polyaniline | 280 nm | 400 nm | Hollow polystyrene microsphere | - | - | [85] |
| - | 1.5 mm | sulfonated polystyrene microsphere | - | Electrochemical Energy Storage | [92] | |
| TiO2 | 600 nm | 1 µm | - | 230 m2/g | Photocatalysis | [87] |
| - | 3 µm | O2 | 251.2 m2/g | electrorheological | [95] | |
| TiO2@Ag | 200 nm | 600 nm | SiO2 | - | surface-enhanced Raman scattering sensor | [84] |
| Fe2O3 | 600 nm | 0.9 µm | CO, CO2 | 30.68 m2/g | - | [94] |
| Fe3O4 @PDA-Ag | 200 nm. | 350 nm | - | 48.04 m2/g | catalytic | [91] |
| α-MnO2 | 1.4 µm | 2 µm | - | 132 m2/g | - | [88] |
| Gold | 23–45 nm | 104 nm | Ag nanoparticle | - | [89] | |
| γ-Al2O3 | - | 2.5 µm | P123 | 210.2 m2/g | - | [96] |
| Co3O4 | 1–2 µm | 5–8 µm | - | - | Lithium-ion batteries- | [90] |
| 800 nm | 1.0 µm | - | - | Lithium-ion batteries | [99] | |
| MoS2/NiCo2S4@C | 500 nm | 2 µm | Molybdenum-Glycerate nanospheres | 100.31 m2/g | electrode | [98] |
| V2O5 | 670–730 nm | 3–4 m | - | - | photodetector | [97] |
| Material | BET Surface Area | Particle Size (Diameter) | Solvent | Specific Capacitance | Application | Ref. |
|---|---|---|---|---|---|---|
| BU-TiO2–X/Ag3PO4 | - | - | deionized water | - | Photocatalytic, antibacterial | [67] |
| MgCo2O4@polypyrrole | - | 9–12 µm | deionized water | 1079.6 F/g at 1 A/g | supercapacitor | [68] |
| ZnO/Au/graphitic 王carbon nitride | 45.2 m2/g | 5 µm | deionized water | - | photocathodes | [69] |
| ZnO/TiO2 | 9.5 m2/g | 5 µm | - | - | photocatalytic | [71] |
| carbon | 159.5 m2/g | 550–630 nm | deionized water | 230 F/g at 0.5 A/g | supercapacitors | [70] |
| NiO-NiCo2O4 | - | 5 µm | deionized water | Li–O2 batteries | [73] | |
| Bi2S3/Ag | 2 µm | DMF | photocatalysts | [75] | ||
| metal/WO3 | 102 m2/g | 1.5 µm | - | photocatalytic | [74] | |
| Al-doped MnO2 | - | - | deionized water | 101 F/g at 5 A/g | - | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Xiang, L.; Zhao, X.; Yin, J. Progress in Preparation of Sea Urchin-like Micro-/Nanoparticles. Materials 2022, 15, 2846. https://doi.org/10.3390/ma15082846
Ma R, Xiang L, Zhao X, Yin J. Progress in Preparation of Sea Urchin-like Micro-/Nanoparticles. Materials. 2022; 15(8):2846. https://doi.org/10.3390/ma15082846
Chicago/Turabian StyleMa, Ruijing, Liqin Xiang, Xiaopeng Zhao, and Jianbo Yin. 2022. "Progress in Preparation of Sea Urchin-like Micro-/Nanoparticles" Materials 15, no. 8: 2846. https://doi.org/10.3390/ma15082846
APA StyleMa, R., Xiang, L., Zhao, X., & Yin, J. (2022). Progress in Preparation of Sea Urchin-like Micro-/Nanoparticles. Materials, 15(8), 2846. https://doi.org/10.3390/ma15082846







