The Effect of the Type of Activator Anion on the Hydration of Ground Granulated Blast Furnace Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water/Slag Ratio
2.2. Compressive Strength
2.3. Changes in Linear Dimensions
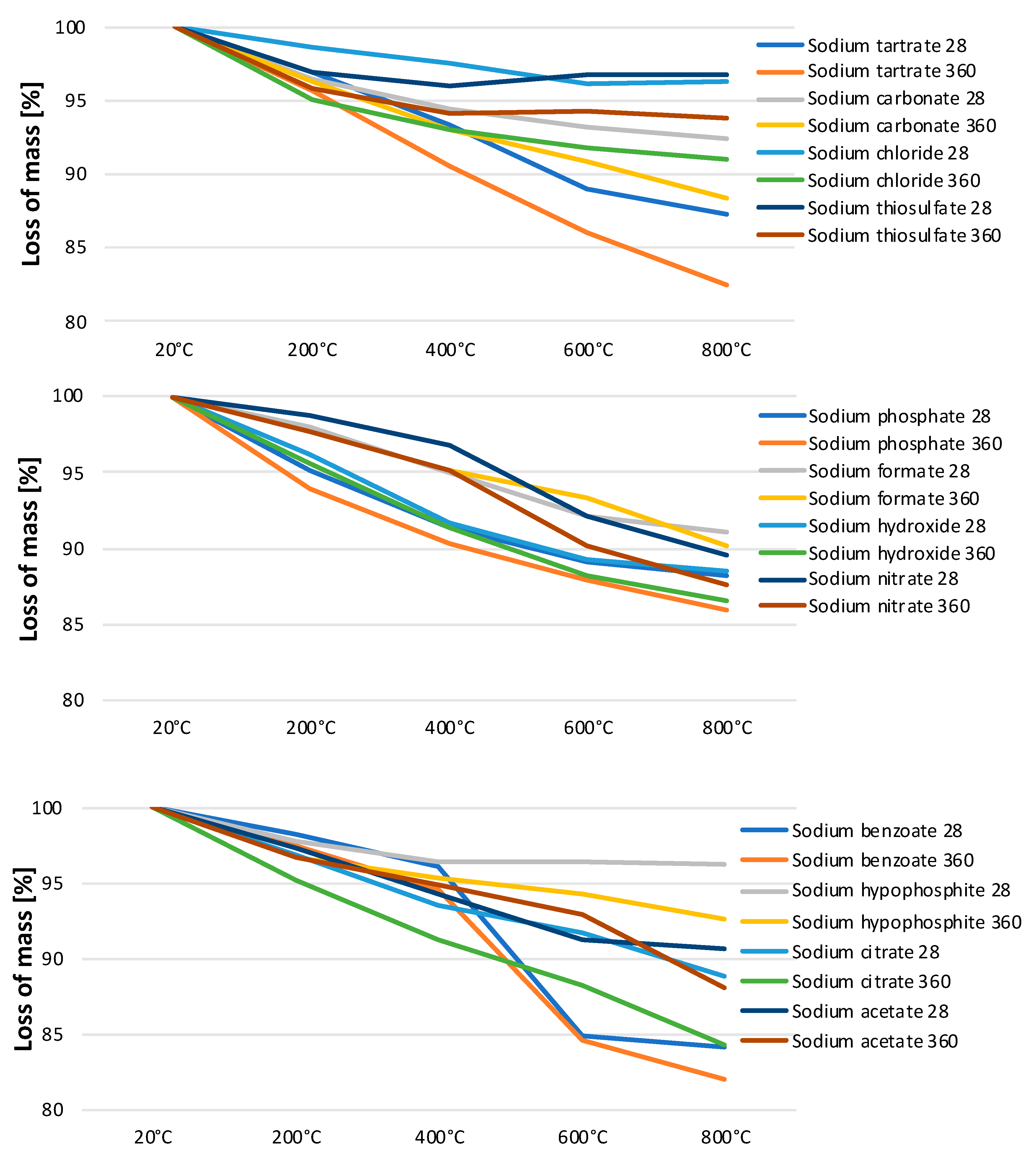
2.4. Loss on Ignition
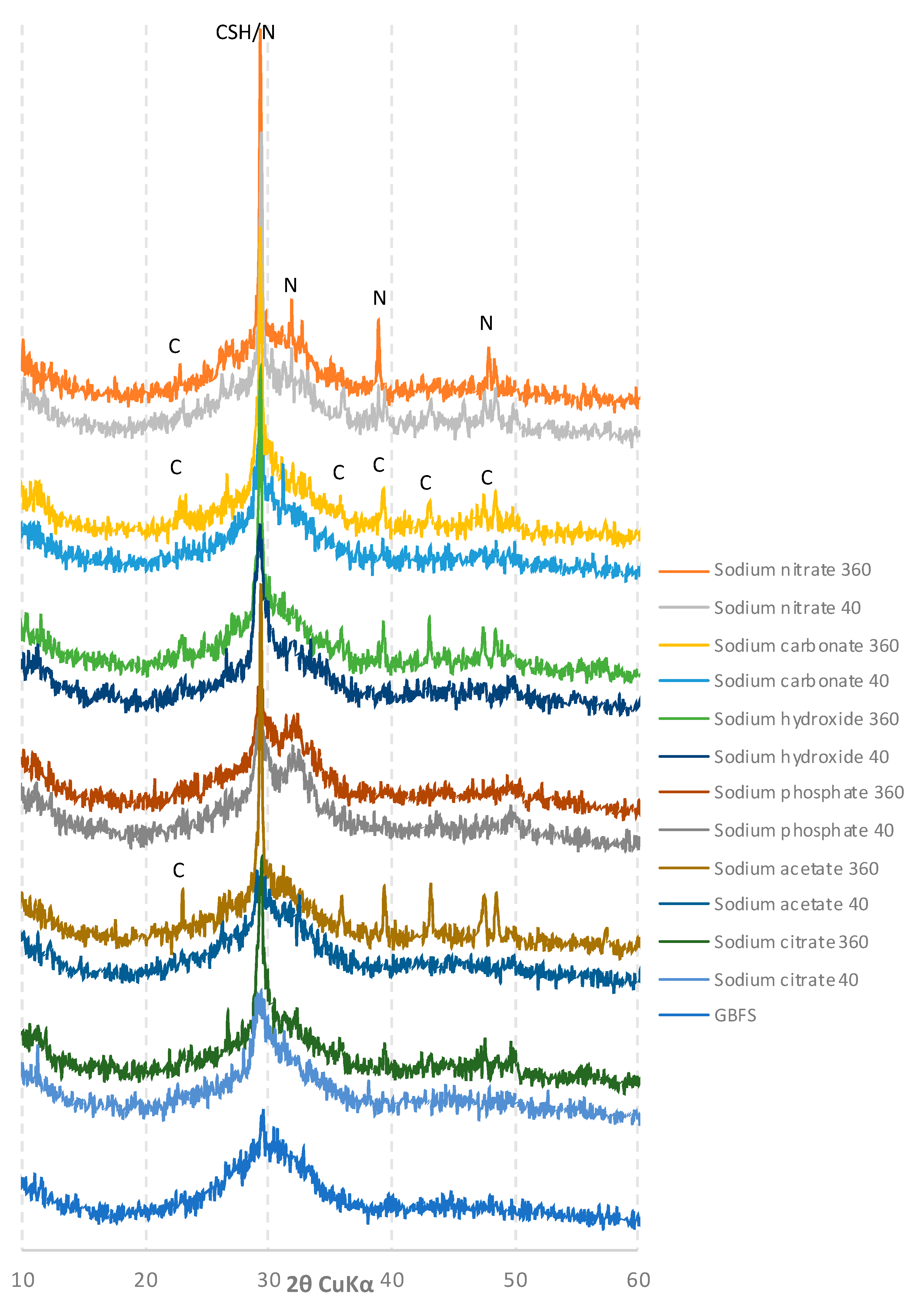
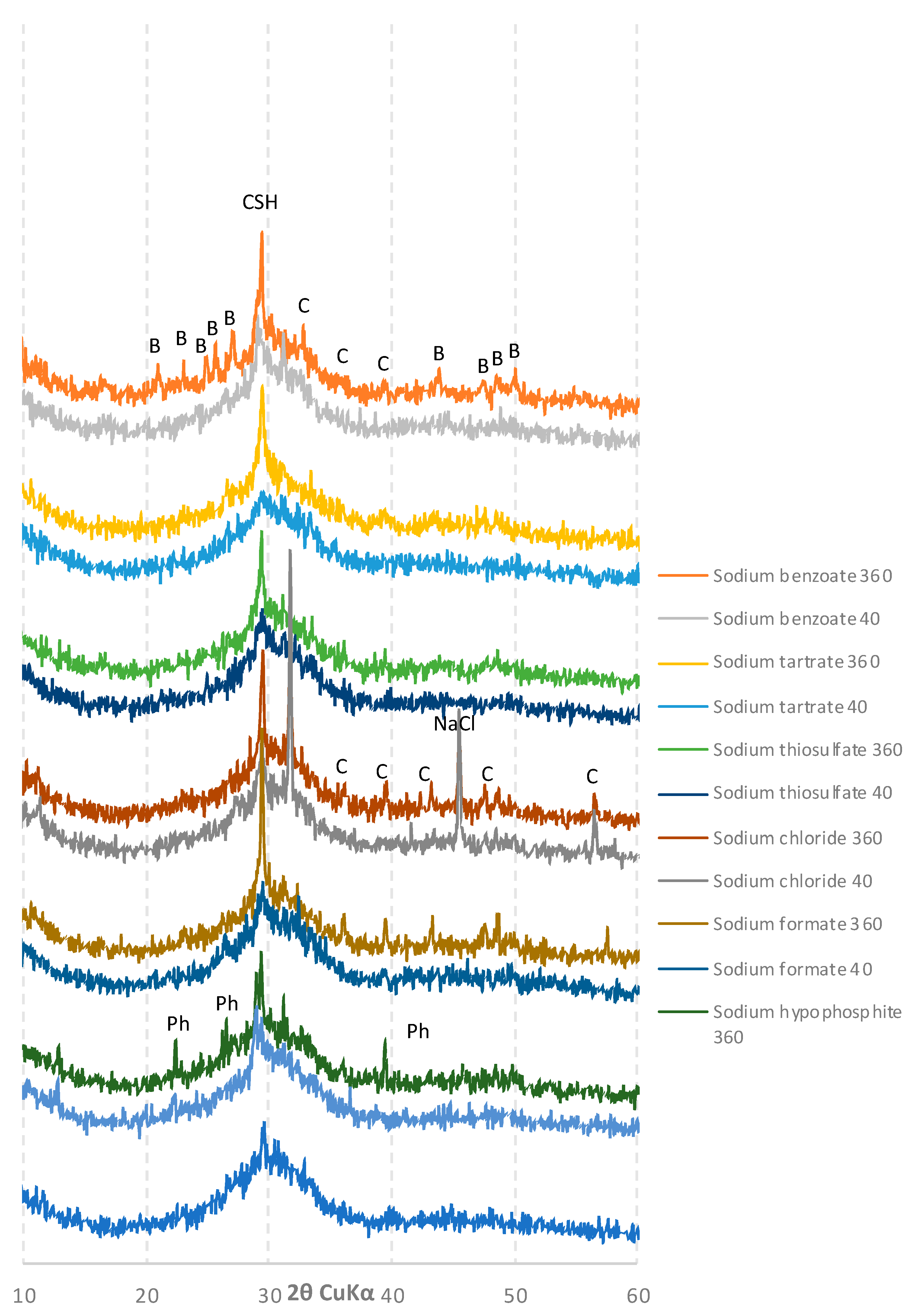
2.5. Phase Composition
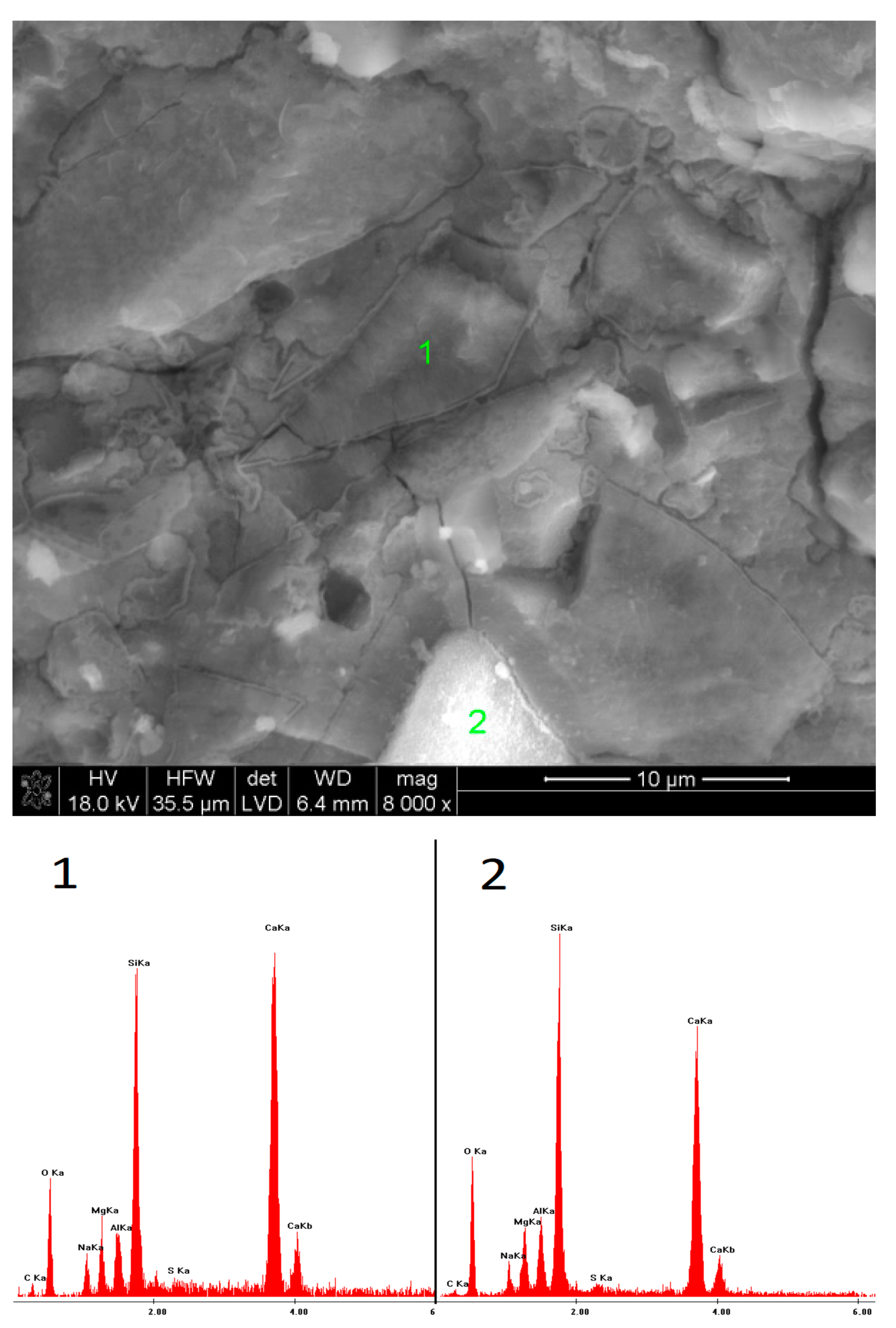
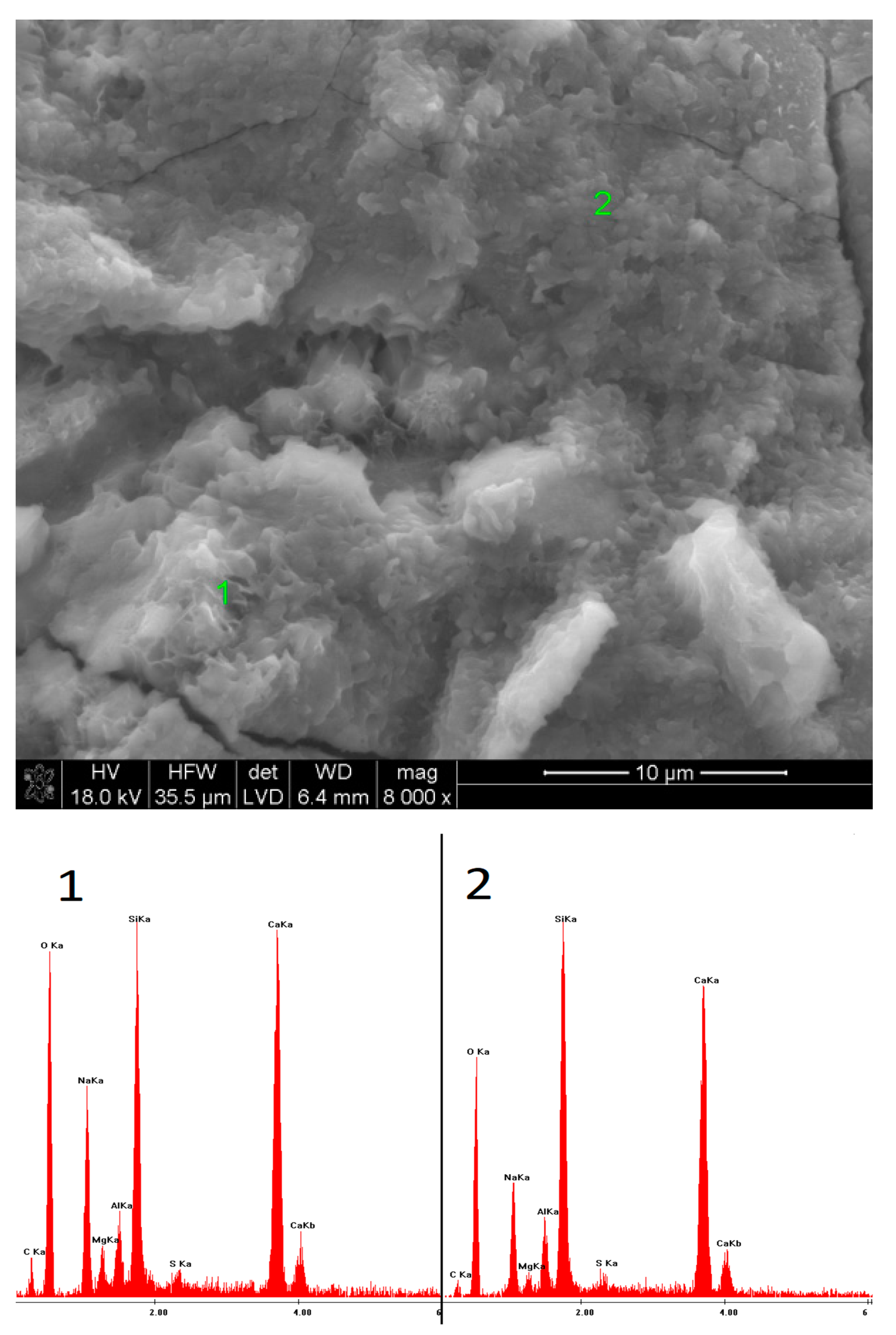
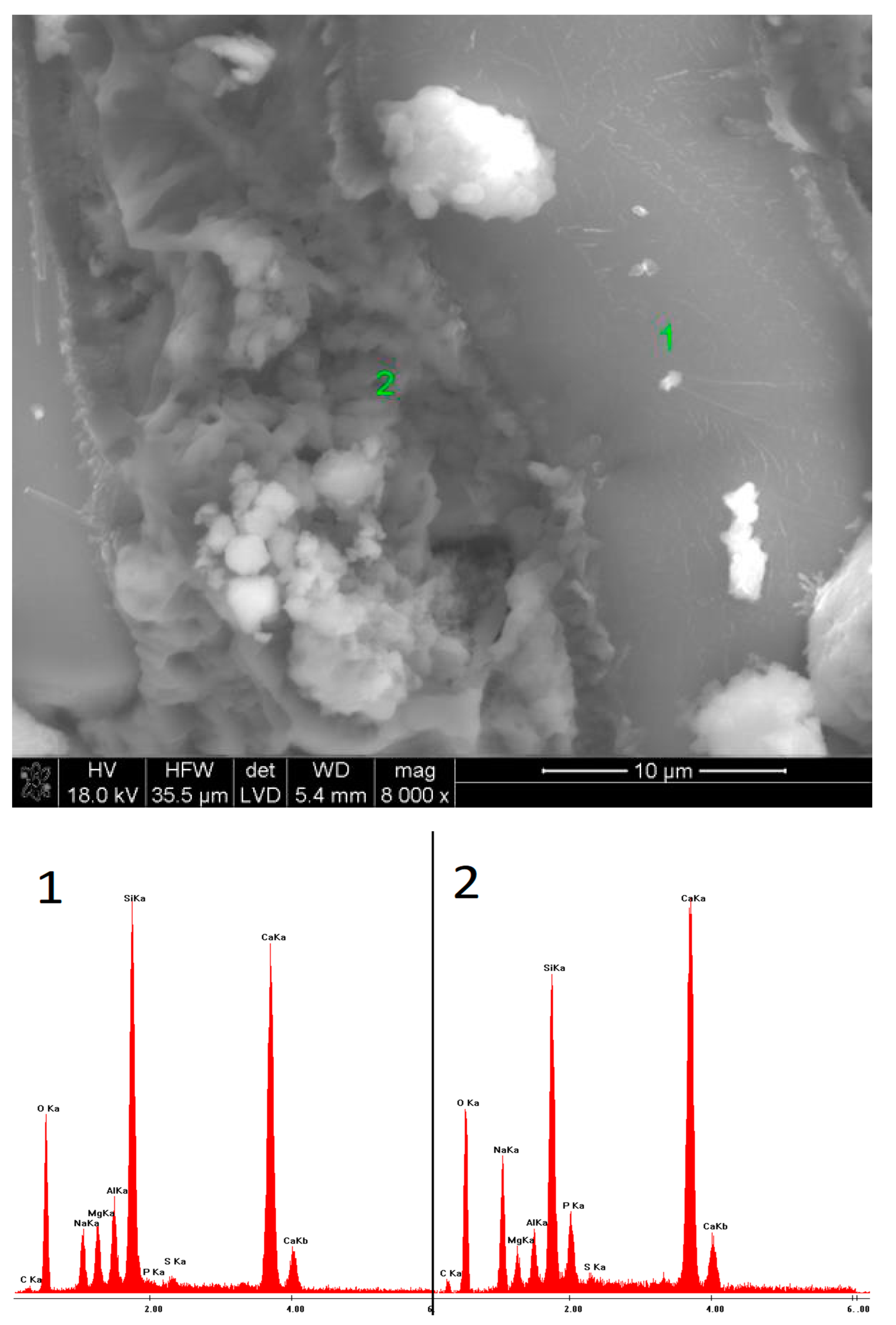
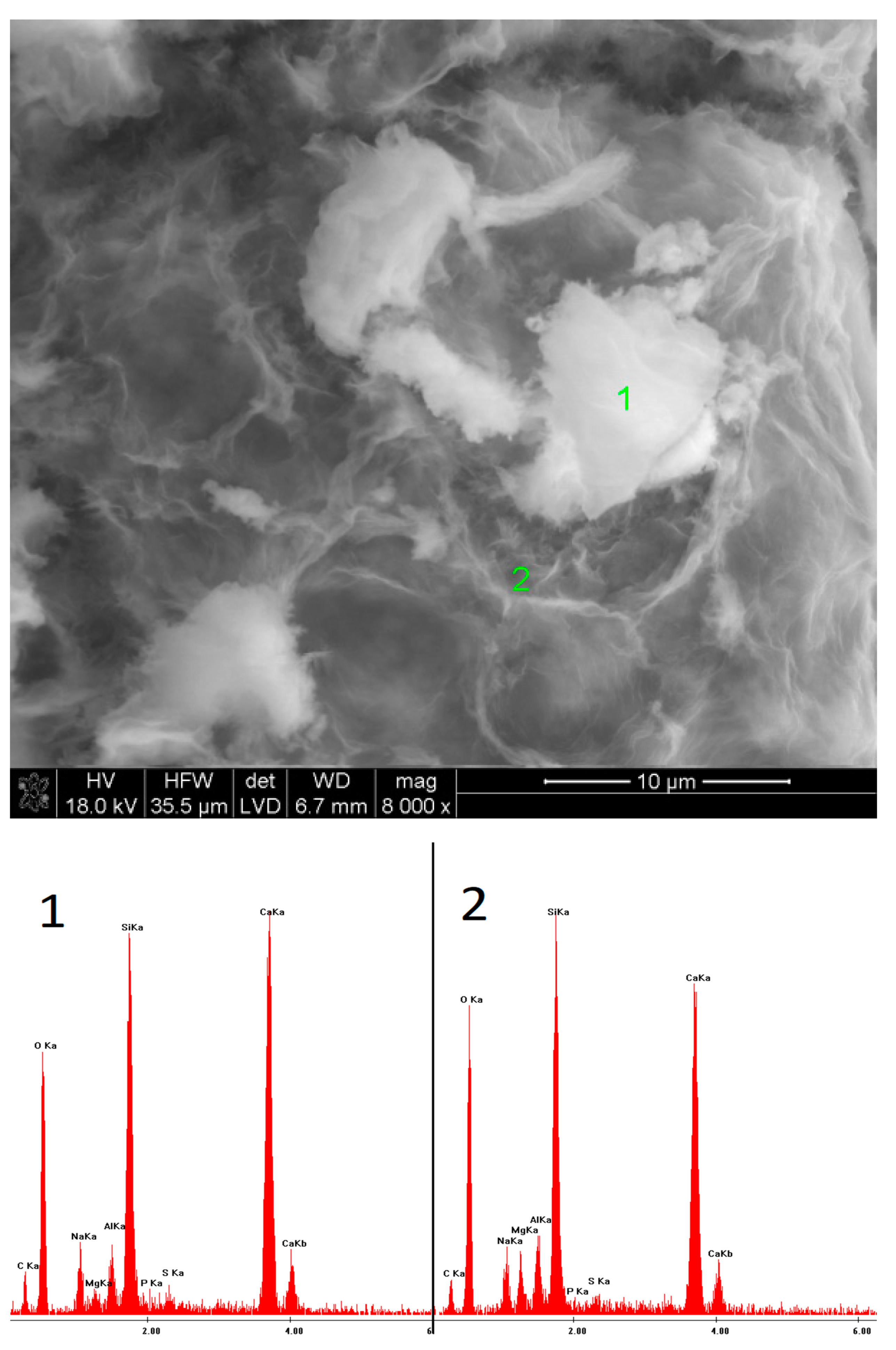
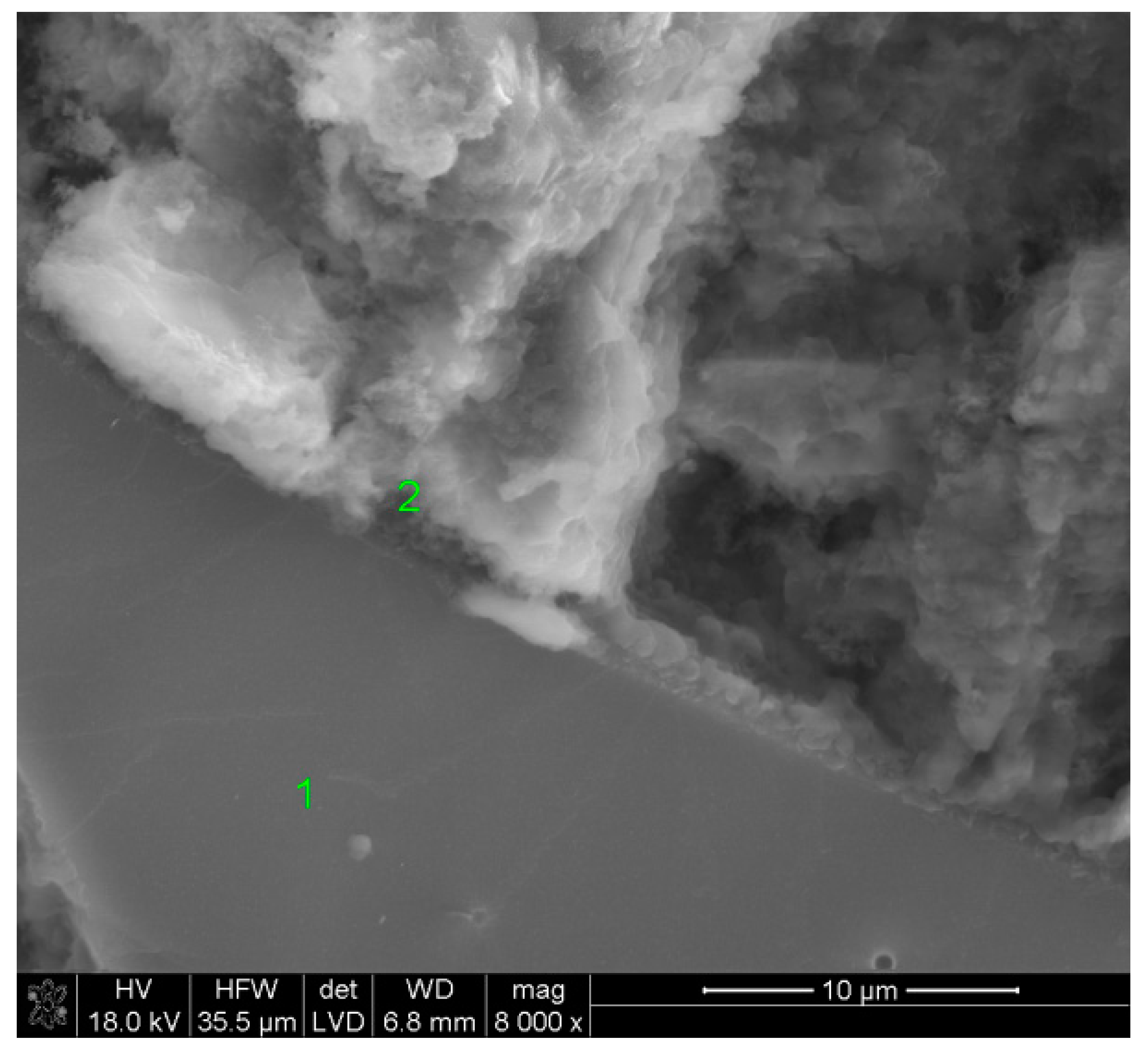
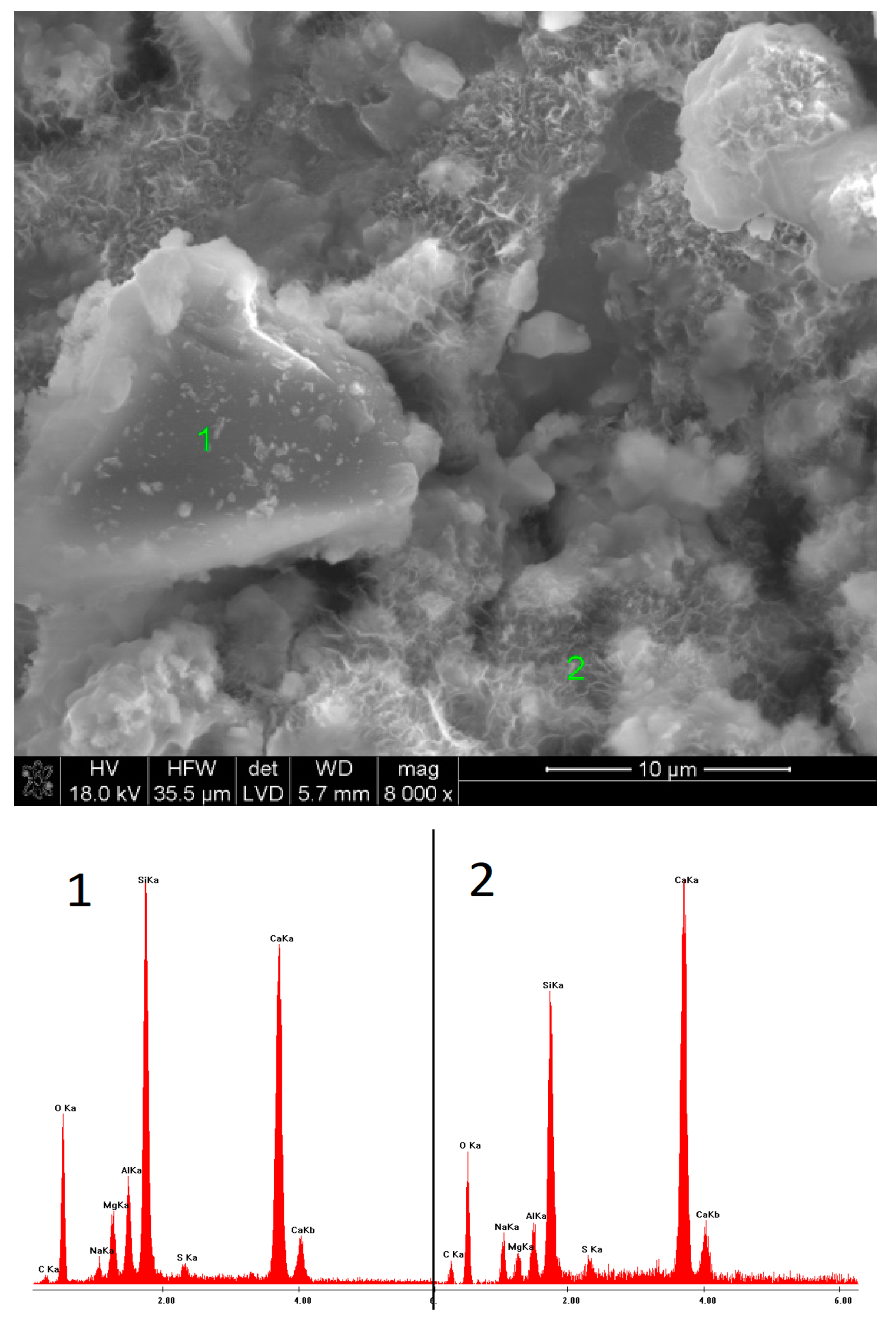
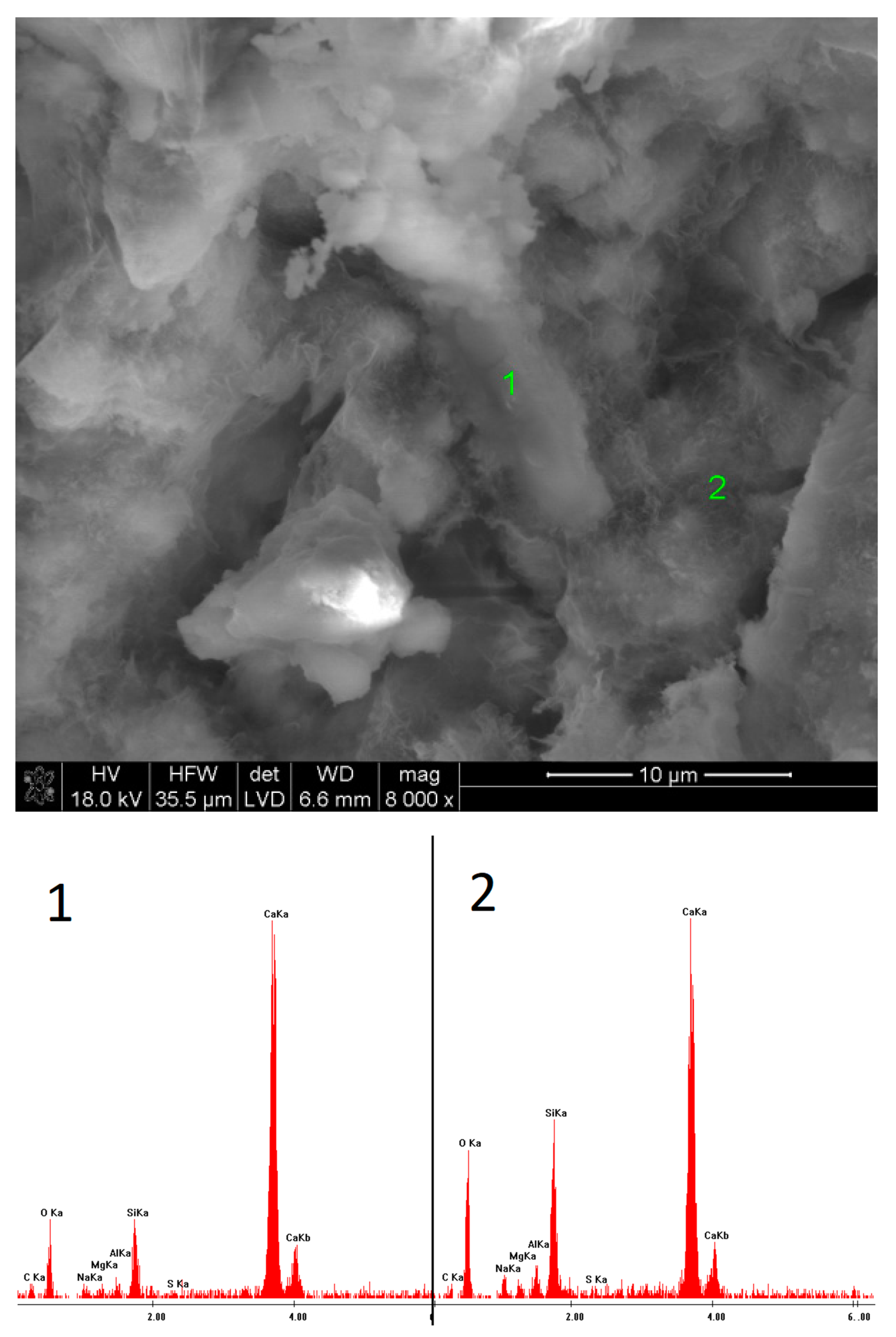
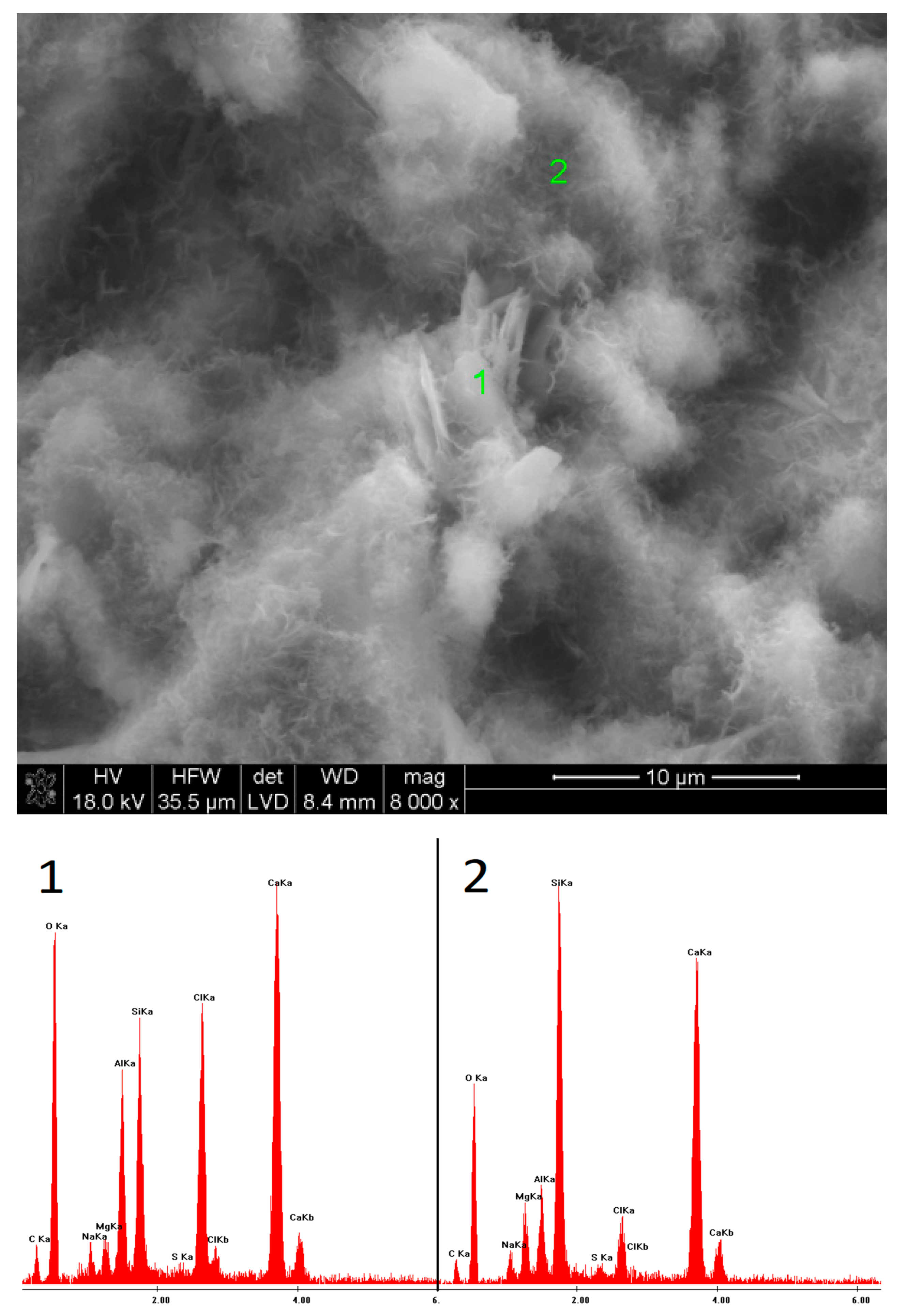
2.6. SEM Observations
3. Results
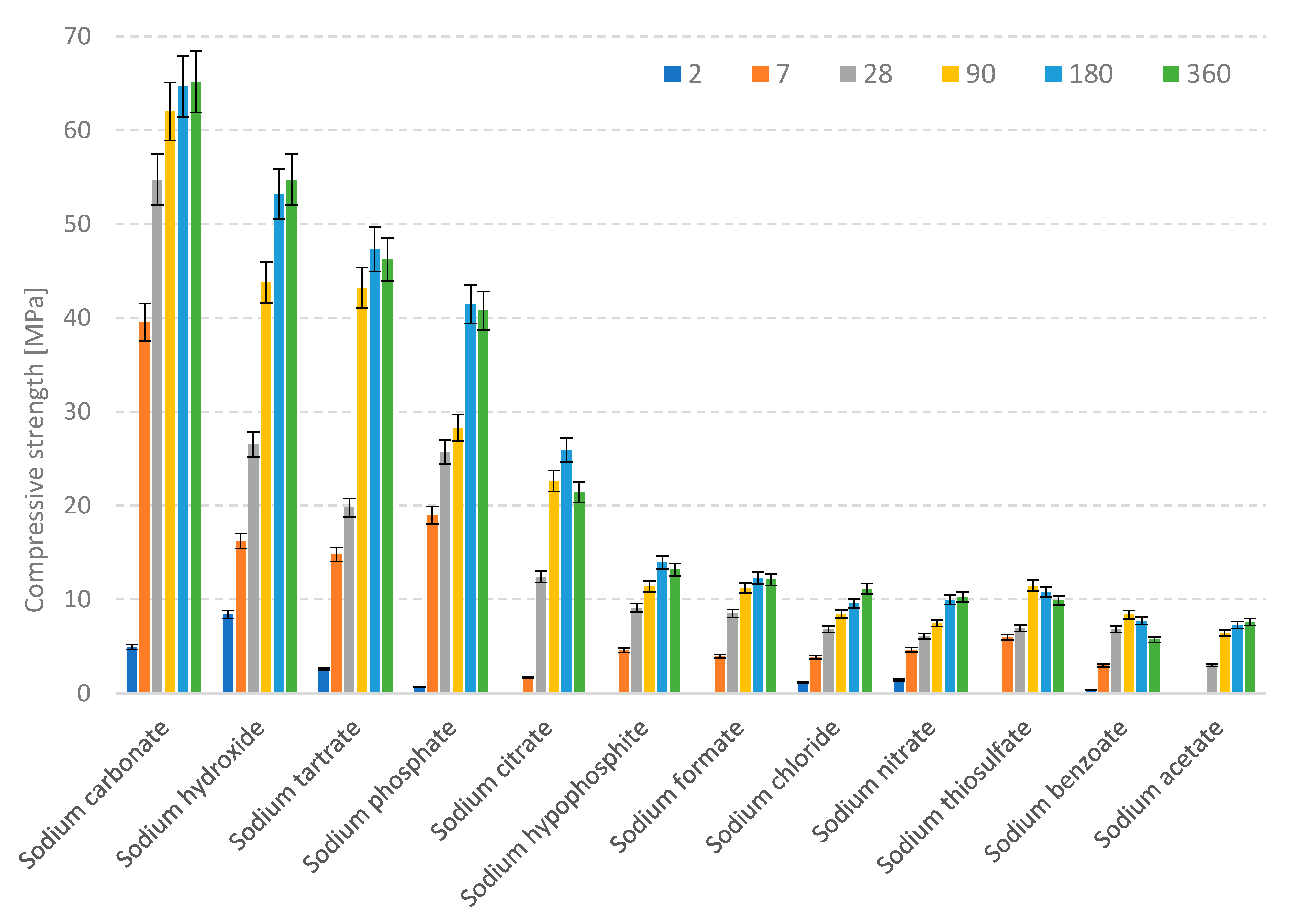
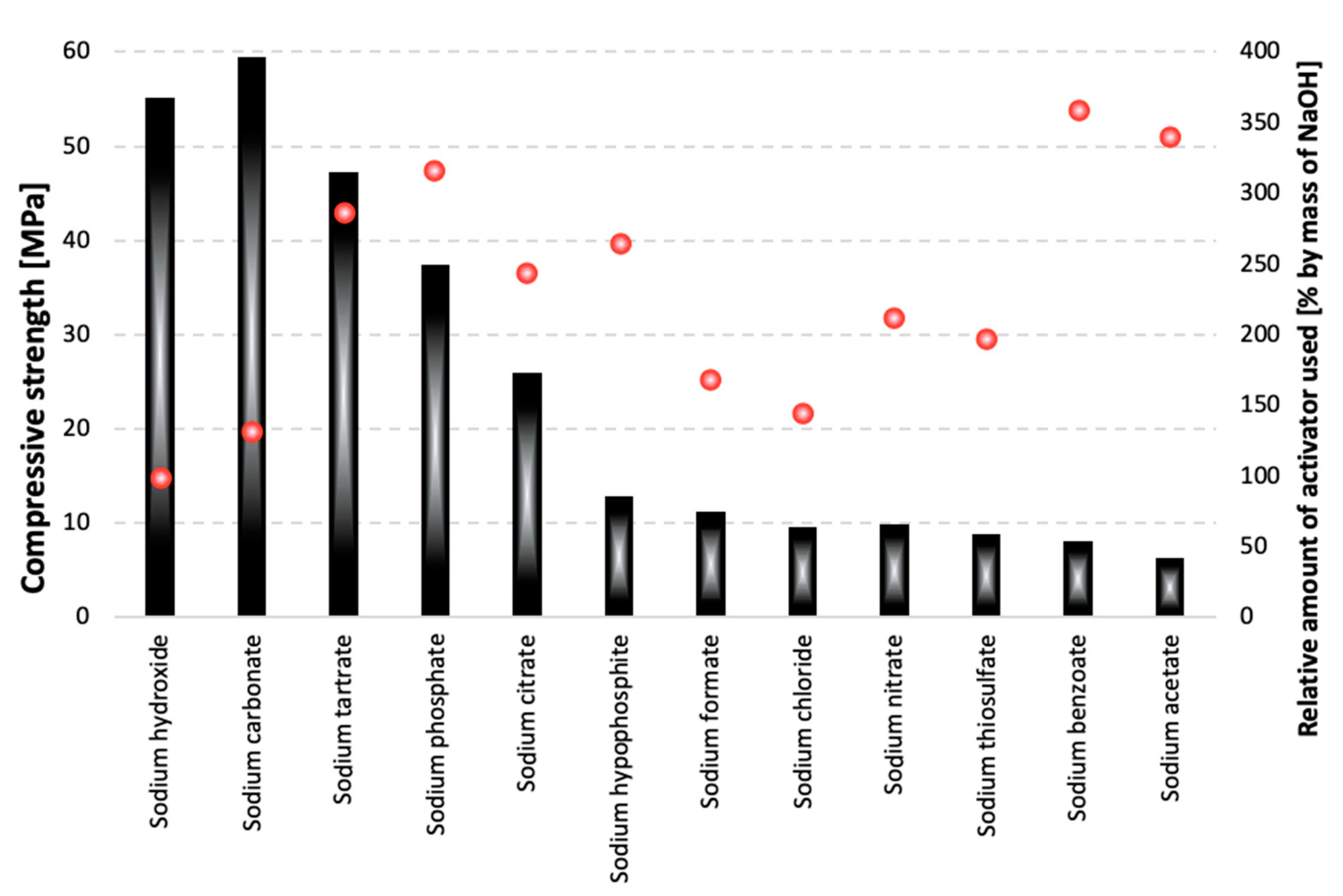
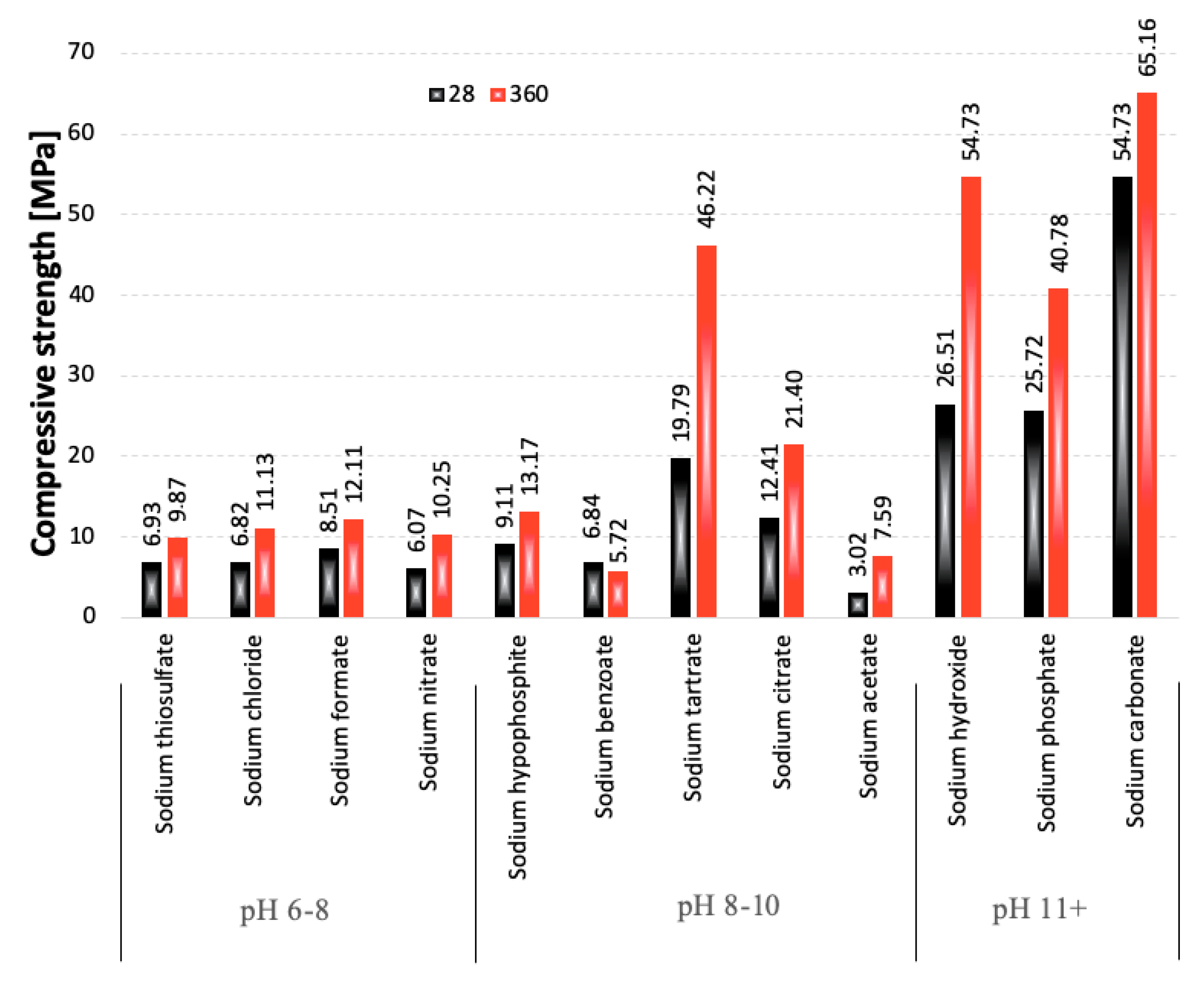
3.1. Compressive Strength
3.2. Changes in Linear Dimensions
3.3. Loss on Ignition
3.4. XRD Analysis
3.5. SEM Observations
4. Conclusions
- Strongly basic compounds are the most effective activators of blast furnace slags.
- Strength development was observed for all of the samples prepared in the study, which proves that sodium activation occurs even at a low or neutral pH.
- Despite the relatively low compressive strength values obtained for pastes activated with low-pH compounds, the microstructure of hydrates formed in the case of, e.g., sodium chloride, was compact, and they were strongly bonded to the surface of the slag grains, which shows potential for further research.
- Slag activation with sodium carbonate provides high early compressive strength values after 7 days of curing and does not entail changes in the linear dimensions of the samples over time that could lead to the destruction of the material.
- Most of the samples cured for 360 days were carbonated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GGBFS | ground granulated blast furnace slag |
| OPC | ordinary Portland cement |
| XRD | X-ray powder diffraction |
| SEM | scanning electron microscopy |
| EDS | energy dispersive spectroscopy |
| W/S | water to slag ratio |
| C-(A)-S-H | calcium (aluminum) silicate hydrates |
| LOI | loss on ignition |
References
- Zbigniew Giergiczny Cement, Kruszywa, Beton w Ofercie Grupy Górażdże; Heidelberg Cement Group—Górażdże 2018. Available online: https://www.gorazdze.pl/pl/system/files_force/assets/document/6f/16/cement_kruszywa_beton_2018_www.pdf?download=1 (accessed on 6 April 2022).
- Machowska, A. Spoiwa Niskoemisyjne i o Niskim Cieple Hydratacji z Wybranych Ubocznych Produktów Przemysłowych jako Potencjalny Składnik Betonów Masywnych i Hydrotechnicznych; II tom serii monografii naukowych Wydziału Instalacji Budowlanych, Hydrotechniki i Inżynierii Środowiska Politechniki Warszawskiej—“CIRCULAR ECONOMY—TECHNOLOGIE”: Warszawa, Poland, 2019; ISBN 978-83-7814-898-2. [Google Scholar]
- Kotwica, Ł.; Chorembała, M.; Kapeluszna, E.; Stępień, P.; Deja, J.; Illikainen, M.; Gołek, Ł. Influence of calcined mine tailings on the properties of alkali activated slag mortars. Key Eng. Mater. 2018, 761, 83–86. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Szudek, W.; Wolka, P.; Zieliński, A. Implementation of alternative mineral additives in low-emission sustainable cement composites. Materials 2021, 14, 6423. [Google Scholar] [CrossRef] [PubMed]
- Kapeluszna, E.; Kotwica, Ł.; Malata, G.; Murzyn, P.; Nocuń-Wczelik, W. The effect of highly reactive pozzolanic material on the early hydration of alite–C3A–gypsum synthetic cement systems. Constr. Build. Mater. 2020, 251, 118879. [Google Scholar] [CrossRef]
- Kotwica, Ł.; Kapeluszna, E.; Stepien, P.; Deja, J.; Illikainen, M.; Gołek, Ł. Influence of sulphides on hydration of ground granulated slag alkali activated mortars and pastes. Key Eng. Mater. 2018, 761, 92–95. [Google Scholar] [CrossRef]
- Spiesz, P.; Rouvas, S.; Brouwers, H.J.H. Utilization of waste glass in translucent and photocatalytic concrete. Constr. Build. Mater. 2016, 128, 436–448. [Google Scholar] [CrossRef]
- Kotwica, Ł.; Chorembała, M.; Kapeluszna, E.; Stępień, P.; Deja, J.; Illikainen, M.; Gołek, Ł. Effect of metakaolinite on properties of alkali activated slag materials. Key Eng. Mater. 2018, 761, 69–72. [Google Scholar] [CrossRef]
- Gołek, Ł. Glass powder and high-calcium fly ash based binders—Long term examinations. J. Clean. Prod. 2019, 220, 493–506. [Google Scholar] [CrossRef]
- Gołek, Ł.; Deja, J.; Sitarz, M.; Fojud, Z. The role of aluminium ions during the slag activation process. Phys. Chem. Glas. Eur. J. Glas. Sci. Technol. Part B 2014, 55, 111–117. [Google Scholar]
- Gołek, Ł.; Kapeluszna, E.; Rzepa, K. Badania aktywności fazy szklistej w odpadach ze spalarni komunalnych i specjalnych/Investigations of the glass activity in municipal and special incinerating plants waste. Cem. Wapno Beton 2017, 22, 77–89. [Google Scholar] [CrossRef]
- Gołek, Ł.; Deja, J.; Sitarz, M. The hydration process of alkali activated calcium aluminosilicate glasses. Phys. Chem. Glas. Eur. J. Glas. Sci. Technol. Part B 2019, 60, 78–90. [Google Scholar] [CrossRef]
- Gołek, Ł.; Kapeluszna, E. Comparison of the properties of alkali activated monticellite and gehlenite glasses | Porównanie właściwości szkieł o składzie monticellitu i gehlenit, aktywowanych dodatkiem naoh. Cem. Wapno Beton 2014, 2014, 416–421. [Google Scholar]
- Gołek, Ł.; Szudek, W.; Błądek, M.; Cięciwa, M. Wpływ dodatku mielonej stłuczki szklanej na wytrzymałość oraz mikrostrukturę zaczynów i zapraw z cementu portlandzkiego | The influence of ground waste glass cullet addition on the compressive Strength and microstructure of portland cement pastes. Cem. Wapno Beton 2020, 25, 480–494. [Google Scholar] [CrossRef]
- Gołek, Ł.; Szudek, W.; Łój, G. Zastosowanie mielonej stłuczki szklanej w przemysłowej produkcji betonowych elementów prefabrykowanych | Utilization of ground waste glass cullet in the industrial production of precast concrete elements. Cem. Wapno Beton 2021, 26, 118–133. [Google Scholar] [CrossRef]
- Szudek, W.; Gołek, Ł.; Malata, G.; Pytel, Z. Influence of waste glass powder addition on the microstructure and mechanical properties of autoclaved building materials. Materials 2022, 15, 434. [Google Scholar] [CrossRef]
- Gołek, Ł.; Guła, A. Wpływ alkalicznie aktywowanego spoiwa żużlowego na wzrost i rozwój roślin | Effect of the alkali-activated Binder on plant growth. Cem. Wapno Beton 2020, 25, 242–254. [Google Scholar]
- Skibicki, S.; Kaszyńska, M.; Wahib, N.; Techman, M.; Federowicz, K.; Zieliński, A.; Wróblewski, T.; Olczyk, N.; Hoffmann, M. Properties of composite modified with limestone powder for 3D concrete printing. In Second RILEM International Conference on Concrete and Digital Fabrication; Bos, F.P., Lucas, S.S., Wolfs, R.J.M., Salet, T.A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 125–134. [Google Scholar]
- Sabau, E.; Udroiu, R.; Bere, P.; Buranský, I.; Miron-Borzan, C.Ş. A novel polymer concrete composite with GFRP waste: Applications, morphology, and porosity characterization. Appl. Sci. 2020, 10, 2060. [Google Scholar] [CrossRef] [Green Version]
- Szydłowski, J.; Szudek, W.; Gołek, Ł. Effect of temperature on the long-term properties of mortars containing waste glass powder and ground granulated blast furnace slag. Cem. Wapno Beton 2021, 26, 264–278. [Google Scholar] [CrossRef]
- Deja, J. Trwałość Zapraw i Betonow Żużlowo-Alkalicznych; Wydawnictwo Naukowe “Akapit”: Kraków, Poland, 2003; ISBN 8389541173. [Google Scholar]
- Kuterasińska, J.; Król, A. Żużel pomiedziowy jako surowiec w produkcji alkalicznie aktywowanych spoiw żużlowych. Pr. Inst. Ceram. Mater. Bud. 2014, 17, 21–36. [Google Scholar]
- Łowińska-Kluge, A. Żużel Pomiedziowy jako Składnik Kompozytów Cementowych o Zwiększonej Trwałości; Wydawnictwo Politechniki Poznańskiej: Piotrowo, Poland, 2008. [Google Scholar]
- Uliasz-Bocheńczyk, A.; Mokrzycki, E. Emisja dwutlenku węgla w przemyśle cementowym (carbon dioxide emission in cement industry). Polityka Energ. 2003, 6, 367–376. [Google Scholar]
- Maraghechi, H.; Salwocki, S.; Rajabipour, F. Utilisation of alkali activated glass powder in binary mixtures with Portland cement, slag, fly ash and hydrated lime. Mater. Struct. Constr. 2017, 50, 1–14. [Google Scholar] [CrossRef]
- Palmero, P.; Formia, A.; Antonaci, P.; Brini, S.; Tulliani, J.M. Geopolymer technology for application-oriented dense and lightened materials. elaboration and characterization. Ceram. Int. 2015, 41, 12967–12979. [Google Scholar] [CrossRef]
- Kim, Y.; Hanif, A.; Usman, M.; Munir, M.J.; Kazmi, S.M.S.; Kim, S. Slag waste incorporation in high early strength concrete as cement replacement: Environmental impact and influence on hydration & durability attributes. J. Clean. Prod. 2018, 172, 3056–3065. [Google Scholar] [CrossRef]
- Peys, A.; White, C.E.; Rahier, H.; Blanpain, B.; Pontikes, Y. Alkali-activation of CaO-FeOx-SiO2 slag: Formation mechanism from in-situ X-ray total scattering. Cem. Concr. Res. 2019, 122, 179–188. [Google Scholar] [CrossRef]
- Środa, B. Dekarbonizacja w przemyśle cementowym-nowe podejście. Bud. Technol. Archit. 2019, 86, 70–73. [Google Scholar]
- Polski Komitet Normalizacyjny, PN-EN 197-1:2012, Cement-Część 1: Skład, Wymagania i Kryteria Zgodności Dotyczące Cementów Powszechnego Użytku, Warszawa 2012. Available online: https://www.bta-czasopismo.pl/wp-content/uploads/2019/05/1919.pdf (accessed on 6 April 2022).
- Górażdże Cement S.A. Granulowany Żużel Wielkopiecowy Składnikiem Cementu i Spoiw Drogowych. 2016. Available online: https://www.gorazdze.pl/pl/system/files_force/assets/document/granulowany_zuzel_wielkopiecowy_skladnikiem_cementu_i_spoiw_drogowych_web.pdf?download=1 (accessed on 6 April 2022).
- Thomas, R.; Ye, H.; Radlinska, A.; Peethamparan, S. Alkali-activated slag cement concrete. Concr. Int. 2016, 38, 33–38. [Google Scholar]
- Małolepszy, J. Hydratacja i Własności Spoiwa Żużlowo-Alkalicznego; Zeszyty Naukowe AGH: Kraków, Poland, 1989; p. 53. [Google Scholar]
- Szweycer, M.; Nagolska, D. Technologia materiałów. In Metalurgia i Odlewnictwo; Wydawnictwo Politechniki Poznańskiej; Politechniki Poznańskiej: Poznań, Poland, 2002. [Google Scholar]
- Malik, M. Wpływ Rodzaju Aktywatora na Hydratację Żużla Wielkopiecowego. Master’s Thesis, AGH-University of Science and Technology, Kraków, Poland, 2019. [Google Scholar]
- EN 12617-4:202; Products and Systems for the Protection and Repair of Concrete Structures-Test Methods-Part 4: Determination of Shrinkage and Expansion. European Committee for Standardization: Brussels, Belgium, 2002.
- Shi, C.; Krivenko, P.V.; Roy, D. Alkali-Activated Cements and Concretes; Taylor & Francis: Abingdon, UK, 2006. [Google Scholar]
- Neville, A.M. Właściwości Betonu; Polski Cement: Kraków, Poland, 2010; ISBN 8391300005. [Google Scholar]
- Owsiak, Z.; Przeorska-imiołek, I. Korozja Wewnętrzna Betonu; Wydawnictwo Politechniki Świętokrzyskiej: Kielce, Poland, 2015; ISSN 1897-2691. Available online: http://bc.tu.kielce.pl/96/1/M66.pdf (accessed on 6 April 2022).
- Bobrowski, A.; Gawlicki, M.; Łagosz, A.; Łój, G.; Nocuń-Wczelik, W. Cement. Metody Badań. Wybrane Kierunki Stosowania; Wydawnictwa AGH: Kraków, Poland, 2015; ISBN 978-83-7464-959-9. [Google Scholar]
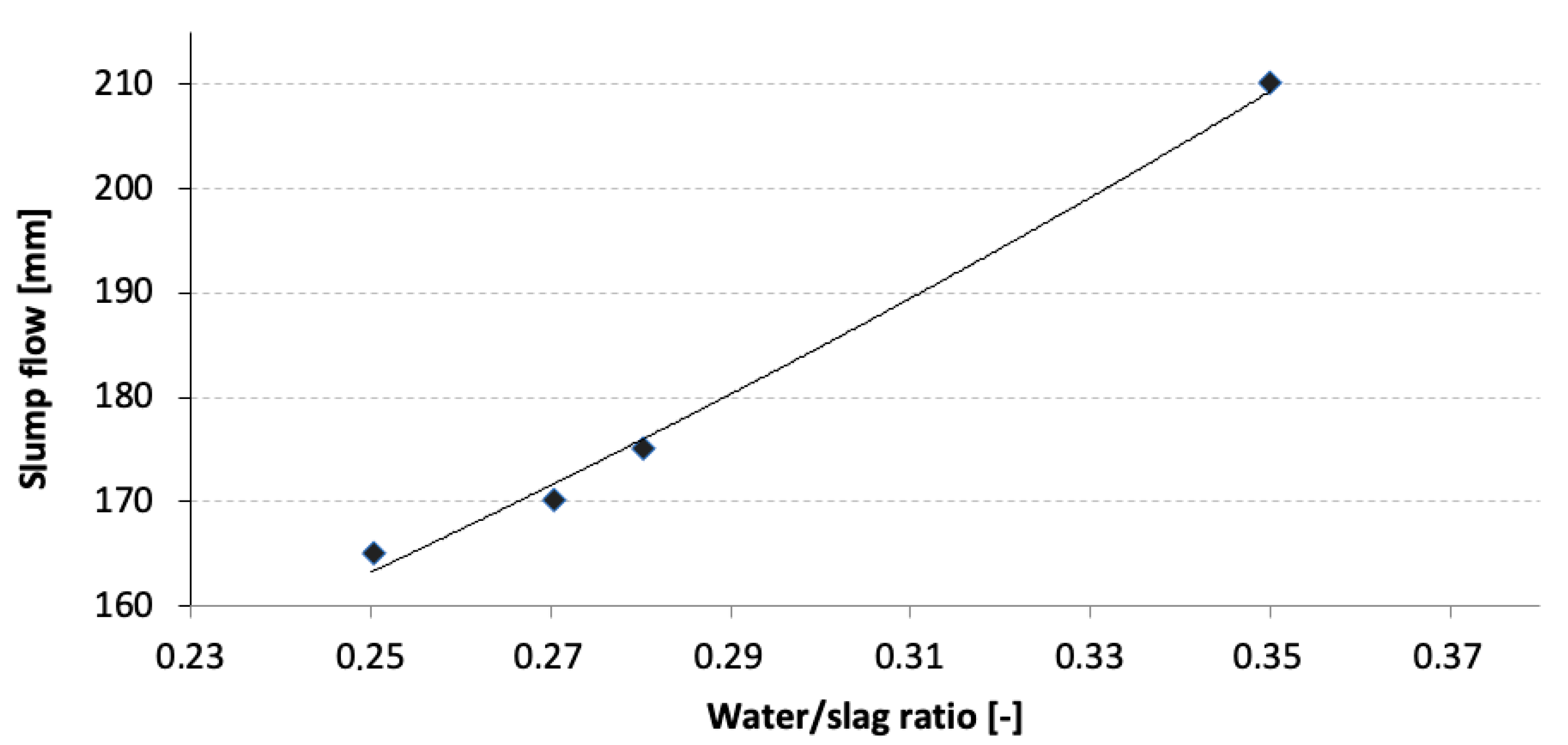






| W/S Ratio [-] | Flow [mm] |
|---|---|
| 0.25 | 165 |
| 0.27 | 170 |
| 0.28 | 175 |
| 0.35 | 210 |
| Compressive Strength [MPa] | ||||||
|---|---|---|---|---|---|---|
| Activator | 2 Days | 7 Days | 28 Days | 90 Days | 180 Days | 360 Days |
| Sodium benzoate (C6H6COONa) | 0.41 | 2.96 | 7.74 | 8.37 | 8.86 | 5.72 |
| Sodium carbonate (Na2CO3) | 4.92 | 39.55 | 34.57 | 68.37 | 59.45 | 53.03 |
| Sodium hypophosphite (NaH2PO2) | 0.00 | 4.59 | 9.11 | 14.38 | 12.94 | 16.97 |
| Sodium tartrate (C4H4Na2O6·2H2O) | 2.59 | 14.78 | 16.79 | 44.07 | 47.31 | 32.02 |
| Sodium citrate (C6H6O7Na3·2H2O) | 0.00 | 1.72 | 8.41 | 20.28 | 26.43 | 19.31 |
| Sodium nitrate (NaNO3) | 1.41 | 4.63 | 6.07 | 7.47 | 9.94 | 10.25 |
| Sodium phosphate (Na3PO4·12H2O) | 0.66 | 18.96 | 30.86 | 35.73 | 44.24 | 44.22 |
| Sodium formate (CHNaO2) | 0.00 | 3.95 | 8.51 | 12.21 | 11.28 | 13.10 |
| Sodium acetate (C2H3NaO2·3H2O) | 0.00 | 0.00 | 3.02 | 6.42 | 6.28 | 6.59 |
| Sodium hydroxide (NaOH) | 8.39 | 16.22 | 26.51 | 43.78 | 55.12 | 49.02 |
| Sodium thiosulfate (Na2O3S2) | 0.00 | 5.96 | 6.93 | 11.46 | 8.78 | 9.87 |
| Sodium chloride (NaCl) | 1.15 | 3.83 | 6.82 | 8.43 | 9.56 | 11.13 |
| Elongation [mm] | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 Days | 3 Days | 7 Days | 14 Days | 28 Days | 90 Days | 360 Days | 420 Days | |
| Sodium tartrate (C4H4Na2O6·2H2O) | 0.000 | 0.000 | 0.000 | 0.010 | 0.050 | 0.160 | 0.240 | 0.310 |
| Sodium carbonate (Na2CO3) | 0.000 | 0.010 | 0.020 | 0.027 | 0.020 | 0.010 | −0.005 | −0.035 |
| Sodium chloride (NaCl) | 0.000 | 0.000 | 0.007 | 0.023 | 0.050 | 0.070 | 0.090 | 0.120 |
| Sodium thiosulfate (Na2O3S2) | 0.000 | −0.010 | 0.000 | 0.020 | 0.040 | 0.040 | 0.040 | 0.050 |
| Sodium phosphate (Na3PO4·12H2O) | 0.000 | 0.020 | 0.050 | 0.043 | 0.050 | 0.090 | 0.140 | 0.180 |
| Sodium formate (CHNaO2) | 0.000 | 0.003 | 0.013 | 0.030 | 0.070 | 0.070 | 0.080 | 0.130 |
| Sodium hydroxide (NaOH) | 0.000 | 0.017 | 0.027 | 0.077 | 0.077 | 0.077 | 0.107 | 0.140 |
| Sodium nitrate (NaNO3) | 0.000 | 0.007 | 0.013 | 0.045 | 0.070 | 0.060 | 0.090 | 0.140 |
| Sodium benzoate (C6H6COONa) | 0.000 | −0.003 | 0.067 | 0.067 | 0.097 | 0.117 | 0.110 | 0.127 |
| Sodium hypophosphite (NaH2PO2) | 0.000 | 0.003 | 0.023 | 0.033 | 0.043 | 0.033 | 0.033 | 0.030 |
| Sodium citrate (C6H6O7Na3·2H2O) | 0.000 | 0.003 | 0.053 | 0.053 | 0.083 | 0.063 | 0.060 | 0.073 |
| Sodium acetate (C2H3NaO2·3H2O) | 0.000 | 0.003 | 0.003 | 0.013 | 0.007 | −0.015 | −0.017 | −0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołek, Ł.P.; Szudek, W.; Malik, M. The Effect of the Type of Activator Anion on the Hydration of Ground Granulated Blast Furnace Slag. Materials 2022, 15, 2835. https://doi.org/10.3390/ma15082835
Gołek ŁP, Szudek W, Malik M. The Effect of the Type of Activator Anion on the Hydration of Ground Granulated Blast Furnace Slag. Materials. 2022; 15(8):2835. https://doi.org/10.3390/ma15082835
Chicago/Turabian StyleGołek, Łukasz P., Wojciech Szudek, and Michał Malik. 2022. "The Effect of the Type of Activator Anion on the Hydration of Ground Granulated Blast Furnace Slag" Materials 15, no. 8: 2835. https://doi.org/10.3390/ma15082835
APA StyleGołek, Ł. P., Szudek, W., & Malik, M. (2022). The Effect of the Type of Activator Anion on the Hydration of Ground Granulated Blast Furnace Slag. Materials, 15(8), 2835. https://doi.org/10.3390/ma15082835






