Effective Anti-Oxidation Repair Coating for C/C Brake Materials Comprising Lead-Borosilicate and Bismuth-Borosilicate Glass
Abstract
1. Introduction
2. Materials and Methods
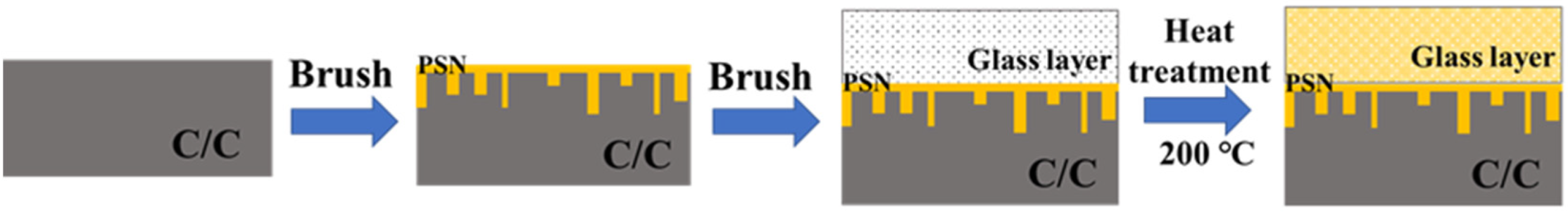
2.1. Preparation
2.2. Tests and Characterization
3. Results and Discussion
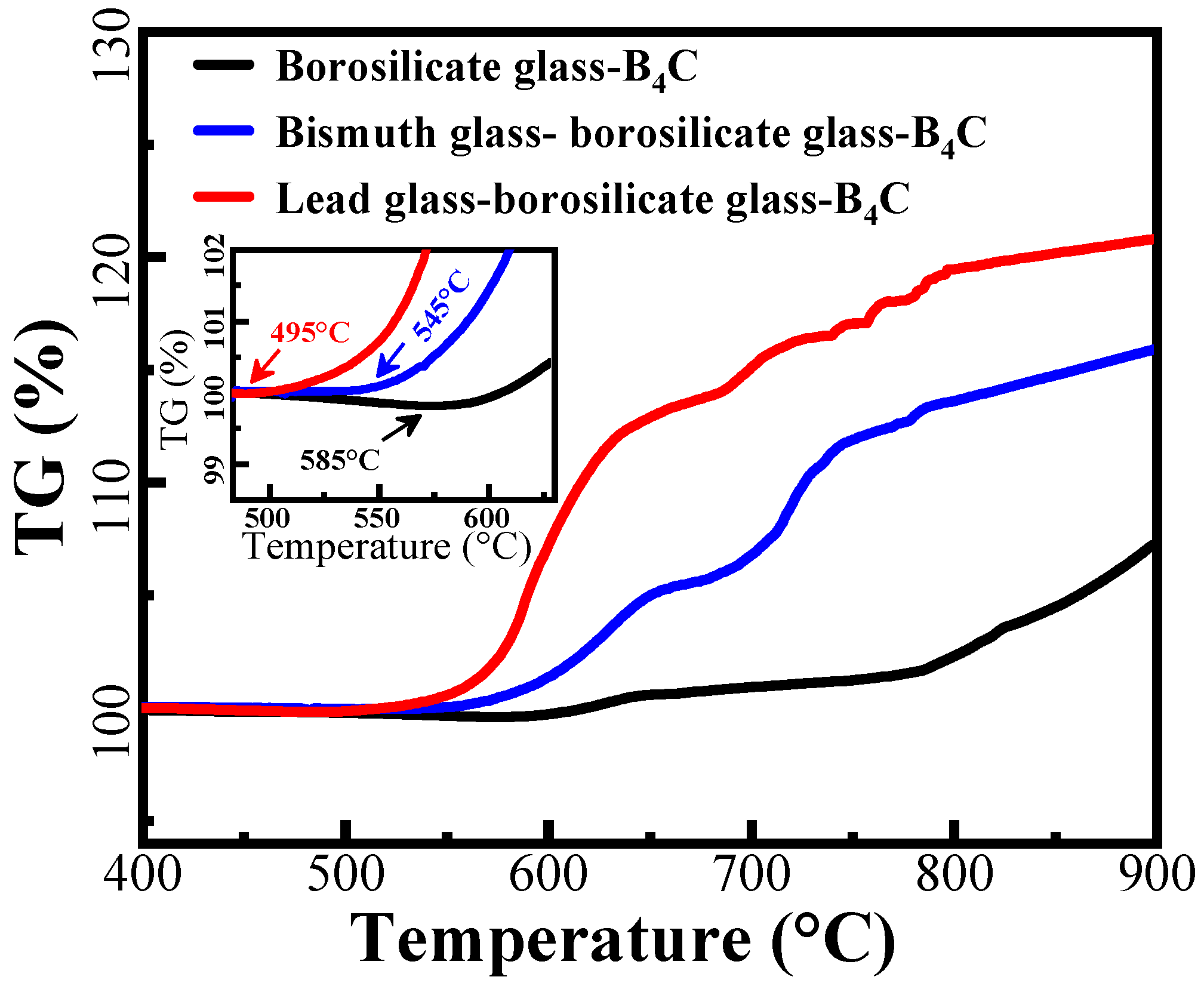
3.1. Thermal Characteristics of the Glass Powders
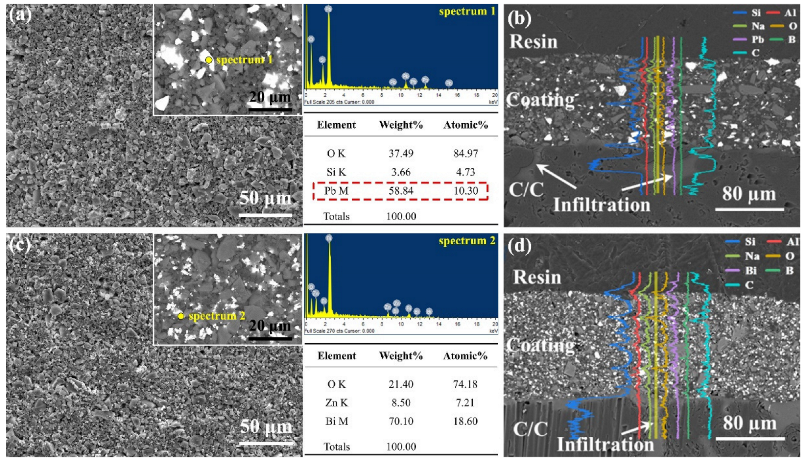
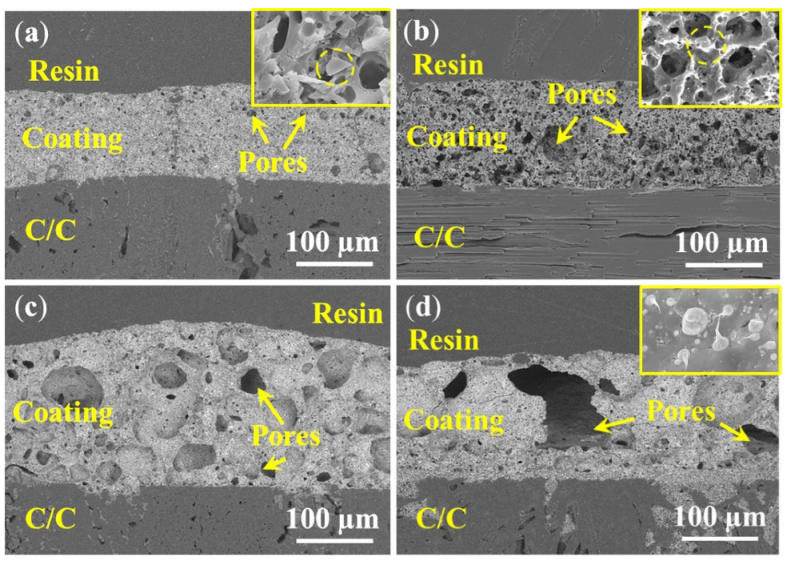
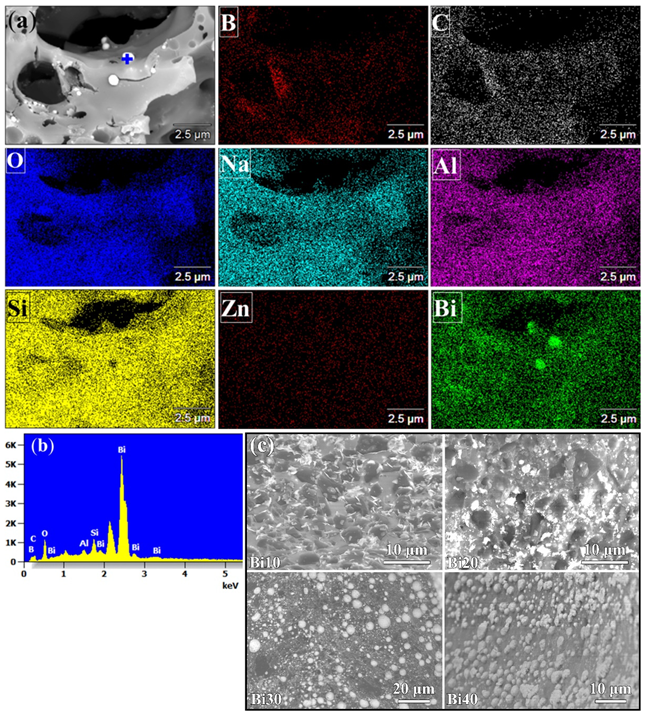
3.2. Microstructure before Oxidation
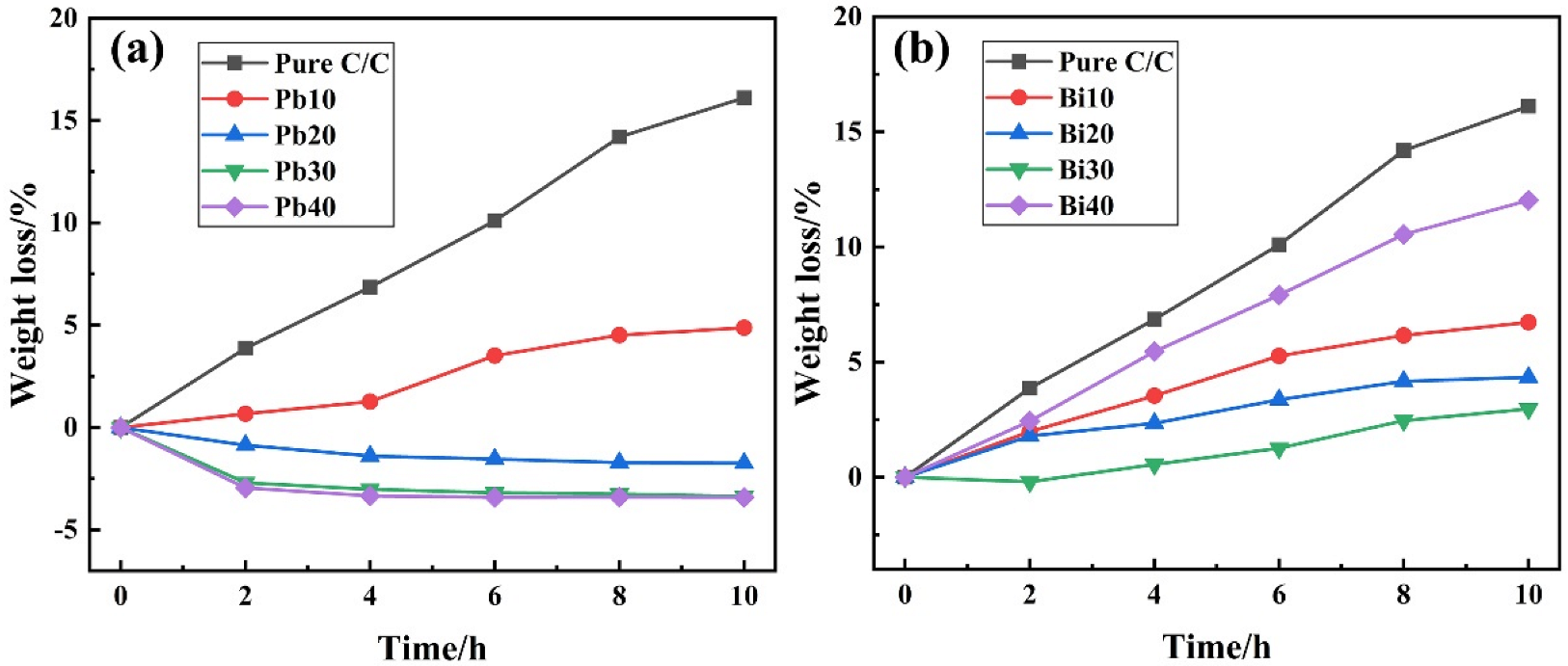
3.3. Oxidation Behaviors at 500 °C
3.4. Morphology after Oxidation
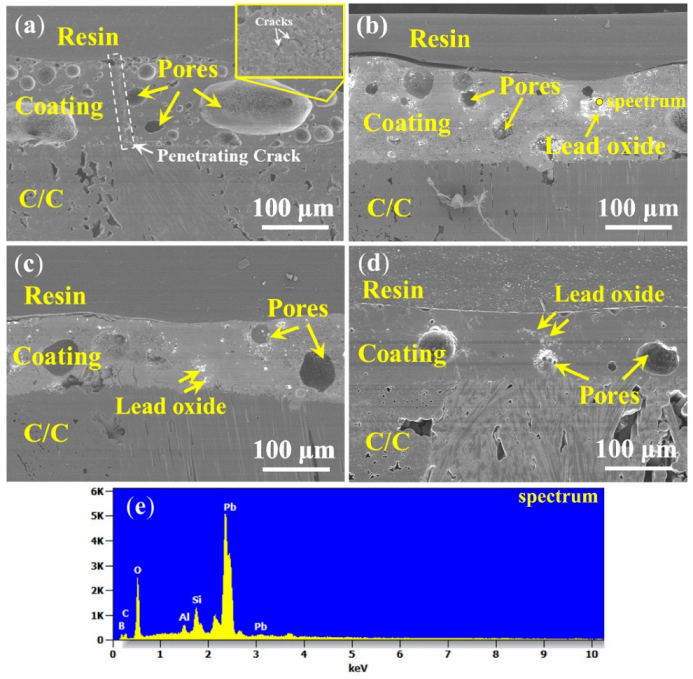
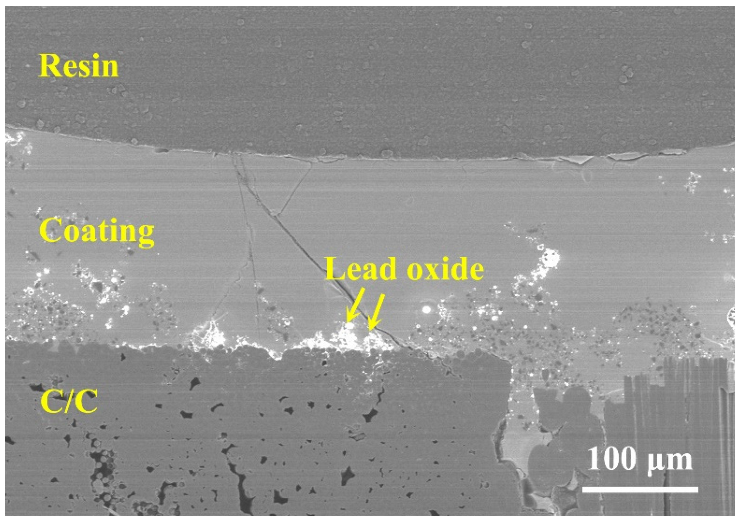
3.4.1. PSN/Lead–Borosilicate Glass–B4C Coatings
3.4.2. PSN/Bismuth–Borosilicate Glass–B4C Coatings
3.5. Oxidation Behavior under Different Temperatures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, S.W.; Zhang, L.T.; Xu, Y.D.; Cheng, L.F.; Lou, J.J.; Zhang, J.Z.; Yu, L. Microstructure and properties of 3D needle-punched carbon/silicon carbide brake materials. Compos. Sci. Technol. 2007, 67, 2390–2398. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, B.Y.; Li, J.H.; Xu, H.J. Friction behaviors of carbon/carbon composites with different pyrolytic carbon textures. Carbon 2006, 44, 463–467. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, I. Finite element analysis of transient thermoelastic behaviors in disk brakes. Wear 2004, 257, 47–58. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Zhang, L.T.; Zeng, Q.F.; Fan, S.Q.; Cheng, L.F. Simulated three-dimensional transient temperature field during aircraft braking for C/SiC composite brake disc. Mater. Des. 2011, 32, 2590–2595. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, C.G. Research of the transient temperature tield and friction properties on disc brakes. Adv. Mat. Res. 2013, 756–759, 4331–4335. [Google Scholar]
- Shemet, V.Z.; Pomytkin, A.P.; Neshpor, V.S. High-temperature oxidation behaviour of carbon materials in air. Carbon 1993, 31, 1–6. [Google Scholar] [CrossRef]
- Schulte-Fischedick, J.; Schmidt, J.; Tamme, R.; Kröner, U.; Arnold, J.; Zeiffer, B. Oxidation behaviour of C/C–SiC coated with SiC–B4C–SiC–cordierite oxidation protection system. Mater. Sci. Eng. A 2004, 386, 428–434. [Google Scholar] [CrossRef]
- Fu, Q.G.; Li, H.J.; Shi, X.H.; Li, K.Z.; Wang, C.; Huang, M. Double-layer oxidation protective SiC/glass coatings for carbon/carbon composites. Surf. Coat. Technol. 2006, 200, 3473–3477. [Google Scholar]
- Feng, T.; Li, H.; Shi, X.; Yang, X.; Wang, S.; He, Z. Multi-layer CVD-SiC/MoSi2–CrSi2–Si/B-modified SiC oxidation protective coating for carbon/carbon composites. Vacuum 2013, 96, 52–58. [Google Scholar] [CrossRef]
- Tian, C.Y.; Liu, H.L. Fabrication of self-healing ceramic coatings against oxidation for carbon/carbon composites using polysilazane and B4C filler. Key Eng. Mater. 2010, 434–435, 455–458. [Google Scholar] [CrossRef]
- Huang, J.F.; Wang, B.; Li, H.J.; Liu, M.; Cao, L.Y.; Yao, C.Y. A MoSi2/SiC oxidation protective coating for carbon/carbon composites. Corros. Sci. 2011, 53, 834–839. [Google Scholar] [CrossRef]
- Fu, Q.; Zou, X.; Chu, Y.; Li, H.; Zou, J.; Gu, C. A multilayer MoSi2–SiC–B coating to protect SiC-coated carbon/carbon composites against oxidation. Vacuum 2012, 86, 1960–1963. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Curry, D.M. Oxidation microstructure studies of reinforced carbon/carbon. Carbon 2006, 44, 1142–1150. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Roth, D.J.; Rauser, R.W.; Cawley, J.D.; Curry, D.M. Oxidation through coating cracks of SiC-protected carbon/carbon. Surf. Coat. Technol. 2008, 203, 372–383. [Google Scholar] [CrossRef][Green Version]
- Ozcan, S.; Filip, P. Wear of carbon fiber reinforced carbon matrix composites: Study of abrasive, oxidative wear and influence of humidity. Carbon 2013, 62, 240–247. [Google Scholar] [CrossRef]
- Fan, S.W.; Ma, X.; Li, Z.; Hu, J.; Xie, Z.; Deng, J.L.; Zhang, L.T.; Cheng, L.F. Design and optimization of oxidation resistant coating for C/C aircraft brake materials. Ceram. Int. 2018, 44, 175–182. [Google Scholar] [CrossRef]
- Lin, Y.C.; Ruiz, E.M.; Rateick, R.G.; McGinn, P.J.; Mukasyan, A.S. One-step synthesis of a multi-functional anti-oxidation protective layer on the surface of carbon/carbon composites. Carbon 2012, 50, 557–565. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.X.; Fan, J.C. Simulation study of temperature field and stress field of disc brake based on direct coupling method. Mater. Sci. Forum 2009, 628–629, 287–292. [Google Scholar] [CrossRef]
- Isola, C.; Appendino, P.; Bosco, F.; Ferraris, M.; Salvo, M. Protective glass coating for carbon-carbon composites. Carbon 1998, 36, 1213–1218. [Google Scholar] [CrossRef]
- Deng, J.L.; Hu, K.Y.; Lu, B.F.; Zheng, B.H.; Fan, S.W.; Zhang, L.T.; Cheng, L.F. Influence of B4C on oxidation resistance of PSN/borosilicate glass-B4C field-based repair coating of C/C aircraft brake materials at 700–900 °C. Ceram. Int. 2019, 45, 20860–20872. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, H.J.; Hu, Z.X.; Ren, J.C.; Li, K.Z. Microstructure and oxidation resistance of Si–Mo–B coating for C/SiC coated carbon/carbon composites. Corros. Sci. 2013, 72, 150–155. [Google Scholar] [CrossRef]
- Wang, K.W.; Luo, L.; Lu, Y.H.; Yang, J.; Wang, Y.G. In-field reparation of the damaged coatings for C/C composites. Ceram. Int. 2015, 41, 7549–7555. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Liu, J.; Fan, S.; Cheng, L.F. Fabrication of oxidation protective coatings on C/C–SiC brake materials at room temperature. Surf. Coat. Technol. 2012, 207, 467–471. [Google Scholar] [CrossRef]



| Glass Powders | Main Components | Average Size (μm) | Softening Temperature (°C) |
|---|---|---|---|
| Lead glass | SiO2, B2O3, PbO | 3 | 380–400 |
| Bismuth glass | BiO2, B2O3, ZnO | 3 | 450–470 |
| Borosilicate glass | SiO2, B2O3, Na2O, Al2O3 | 5 | 650–680 |
| 500 °C | 600 °C | 700 °C | 800 °C | 900 °C | |
|---|---|---|---|---|---|
| Weight loss (%) | −0.94 | −1.51 | −1.29 | −2.22 | −2.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, M.; Xia, X.; Deng, J.; Hu, K.; Luan, C.; Ma, X.; Fan, S.; Wang, P. Effective Anti-Oxidation Repair Coating for C/C Brake Materials Comprising Lead-Borosilicate and Bismuth-Borosilicate Glass. Materials 2022, 15, 2827. https://doi.org/10.3390/ma15082827
Deng M, Xia X, Deng J, Hu K, Luan C, Ma X, Fan S, Wang P. Effective Anti-Oxidation Repair Coating for C/C Brake Materials Comprising Lead-Borosilicate and Bismuth-Borosilicate Glass. Materials. 2022; 15(8):2827. https://doi.org/10.3390/ma15082827
Chicago/Turabian StyleDeng, Mengjia, Xiaoyu Xia, Juanli Deng, Kaiyue Hu, Chenghua Luan, Xu Ma, Shangwu Fan, and Peng Wang. 2022. "Effective Anti-Oxidation Repair Coating for C/C Brake Materials Comprising Lead-Borosilicate and Bismuth-Borosilicate Glass" Materials 15, no. 8: 2827. https://doi.org/10.3390/ma15082827
APA StyleDeng, M., Xia, X., Deng, J., Hu, K., Luan, C., Ma, X., Fan, S., & Wang, P. (2022). Effective Anti-Oxidation Repair Coating for C/C Brake Materials Comprising Lead-Borosilicate and Bismuth-Borosilicate Glass. Materials, 15(8), 2827. https://doi.org/10.3390/ma15082827






