Experimental and Molecular Dynamics Simulation Study on Sol–Gel Conversion Process of Aluminum Carboxylate System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Preparation of Aluminum Sol and Gel Fiber
2.2.2. Characterizations
2.3. Computational Details
2.3.1. Confirm the Aluminum Sol Component
2.3.2. Construction of Aluminum Sol Models
2.3.3. Molecular Dynamic Simulation
3. Results and Discussion
3.1. Morphology of Aluminum Sol and Gel
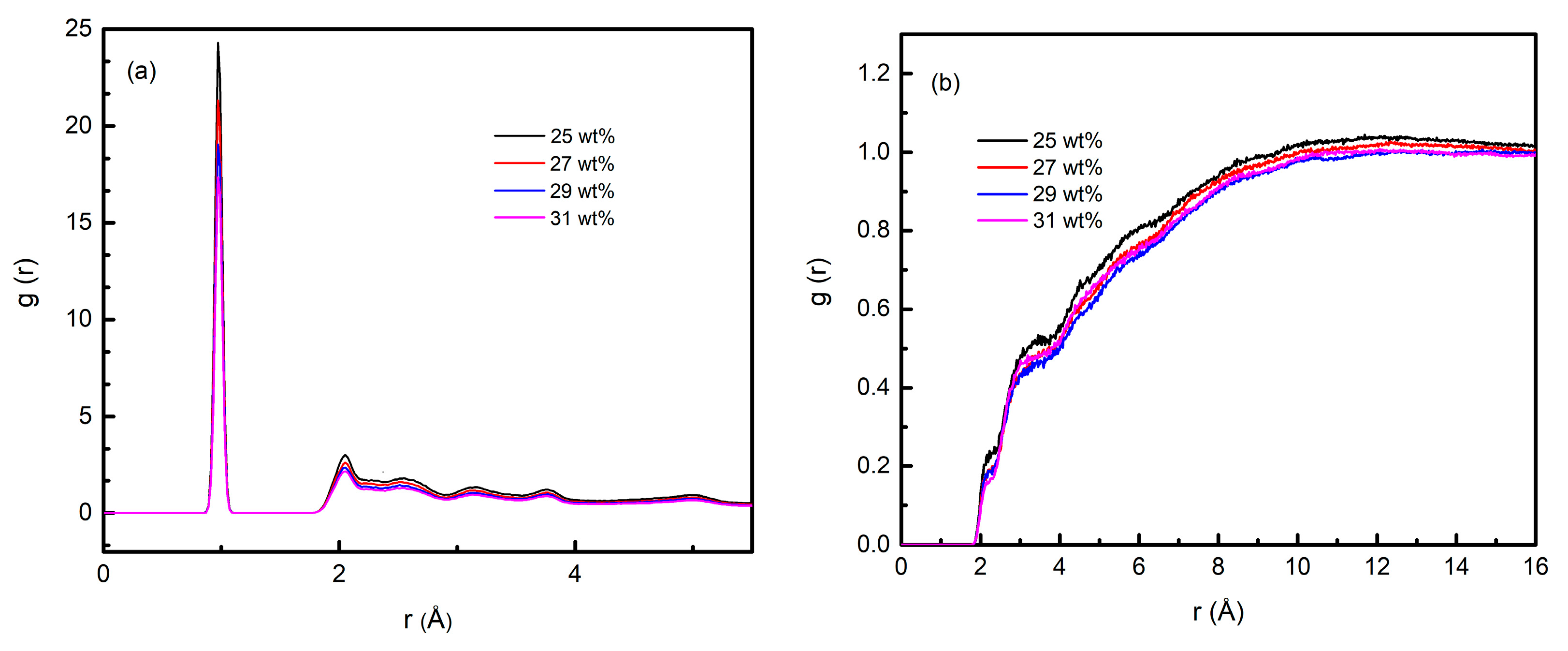
3.2. Structural Evolution of Aluminum Sol and Gel
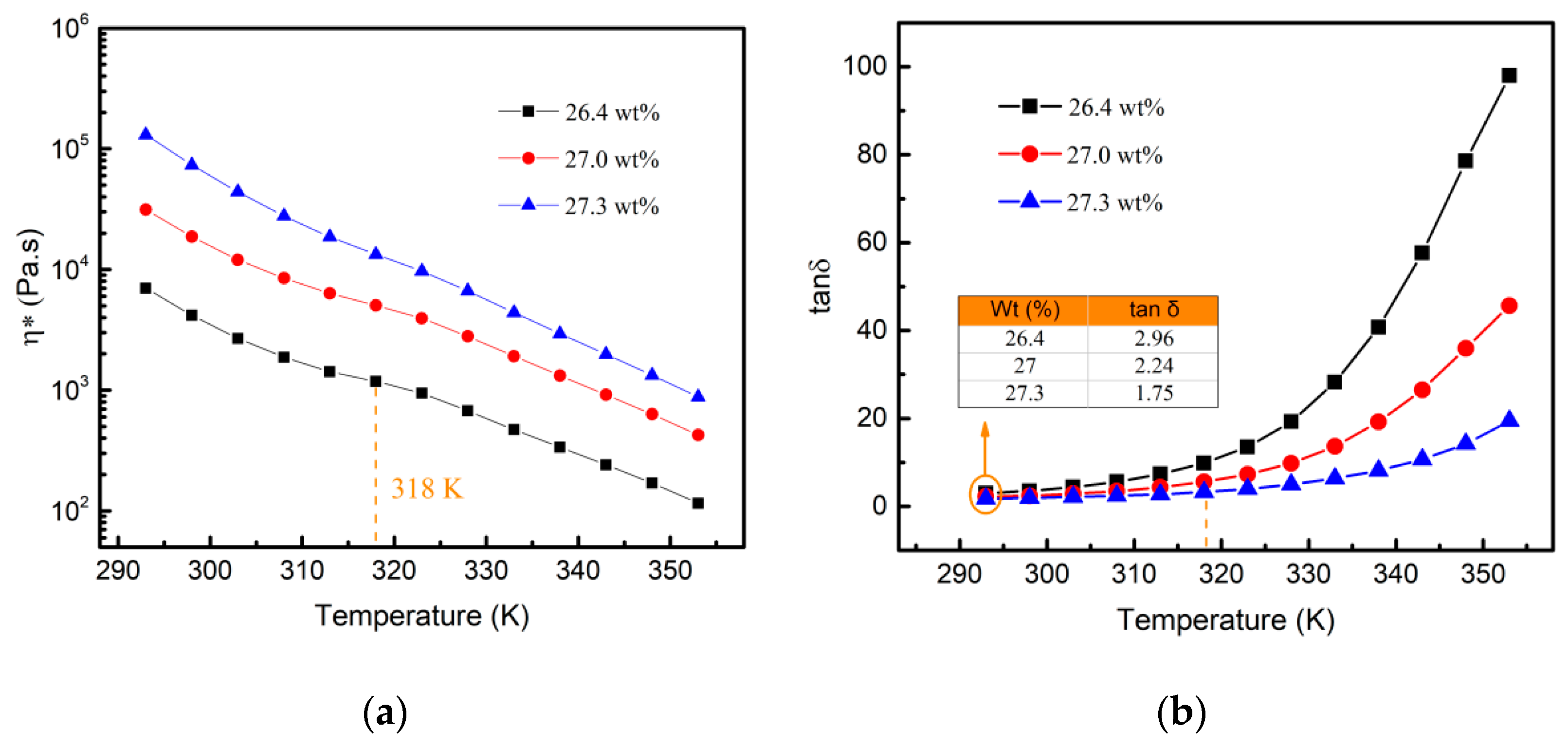
3.3. Rheological Behaviors of Aluminum Sols with High Viscosity
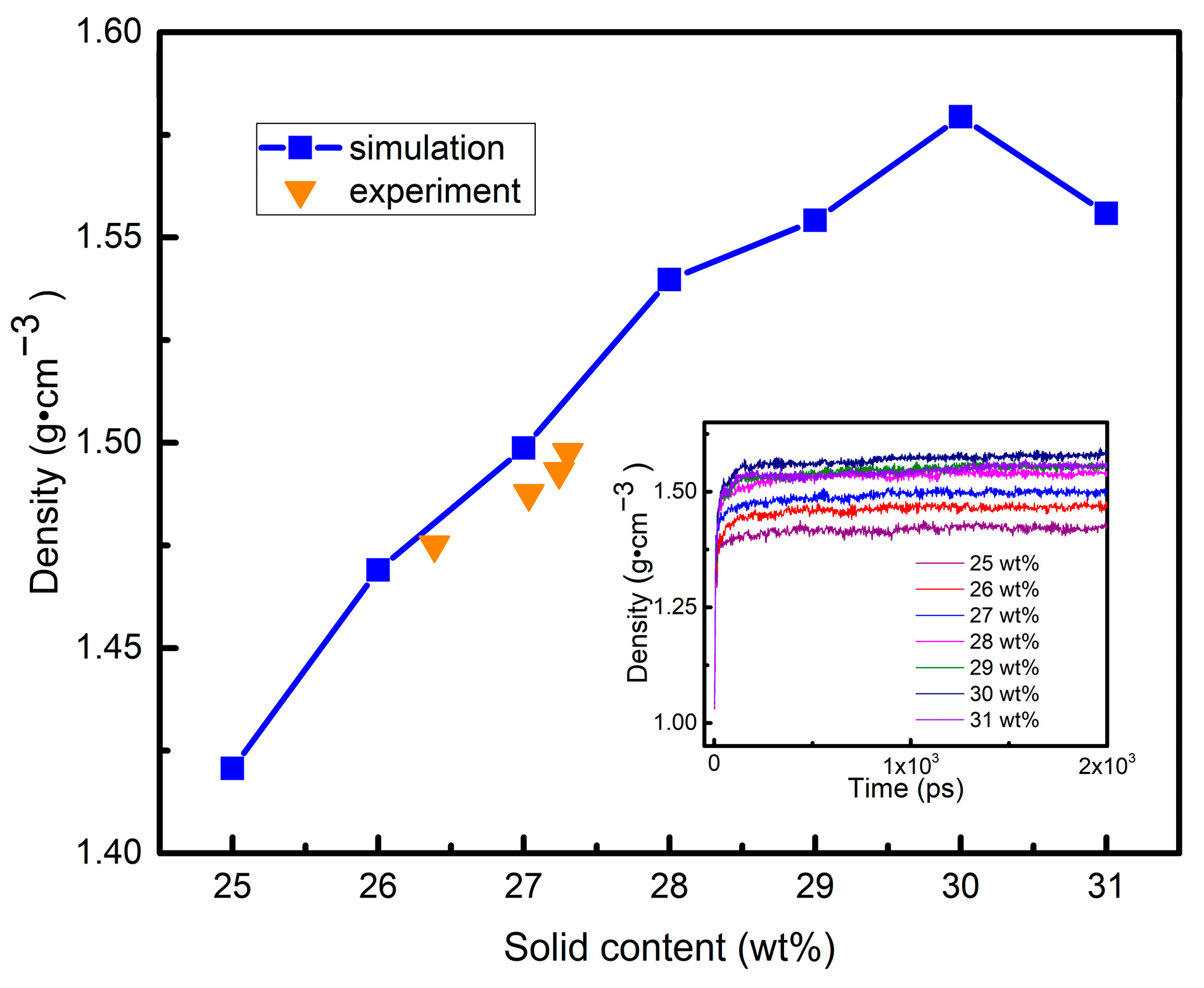
3.4. Simulation of Conformational Variation in the Sol–Gel Process
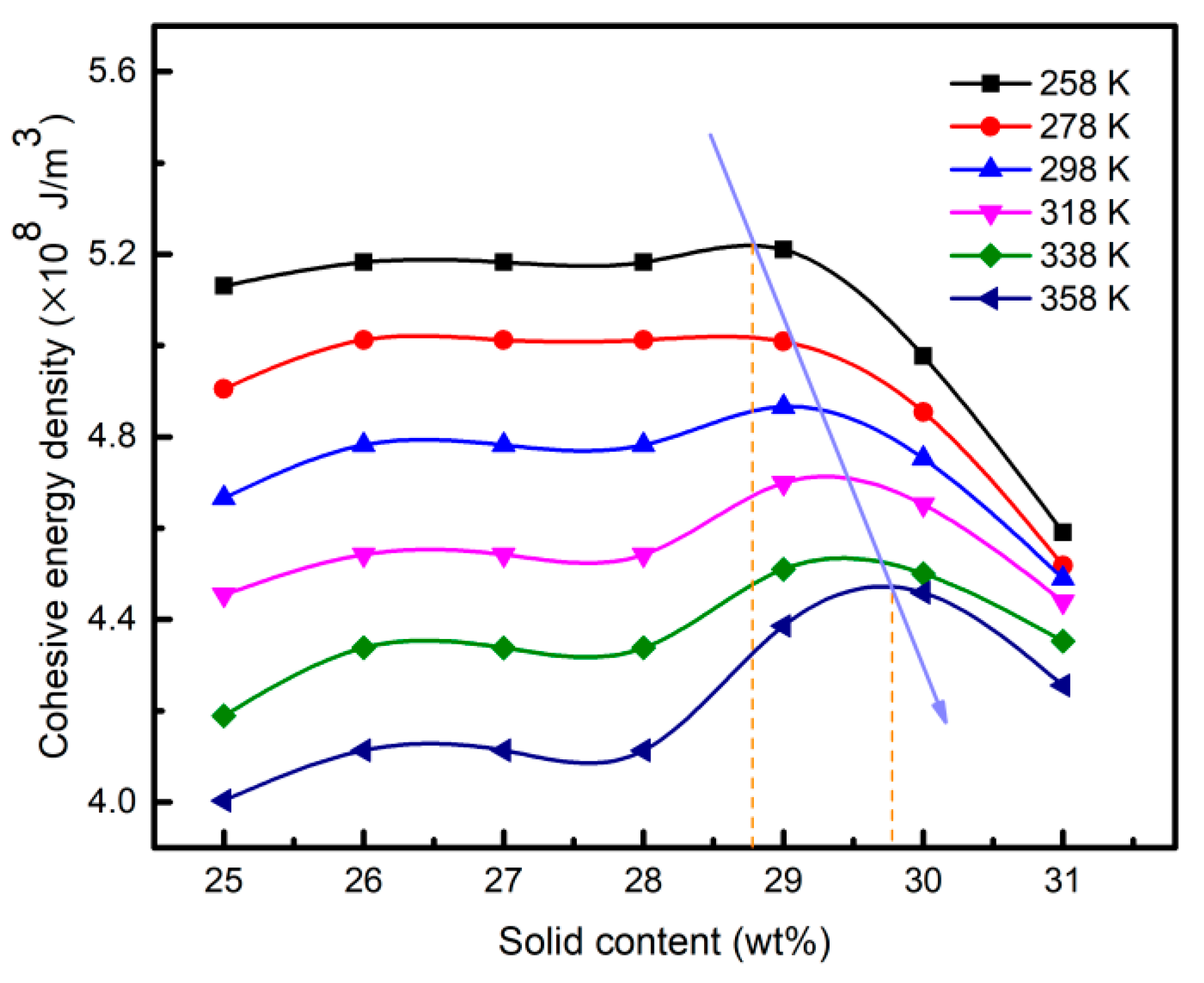
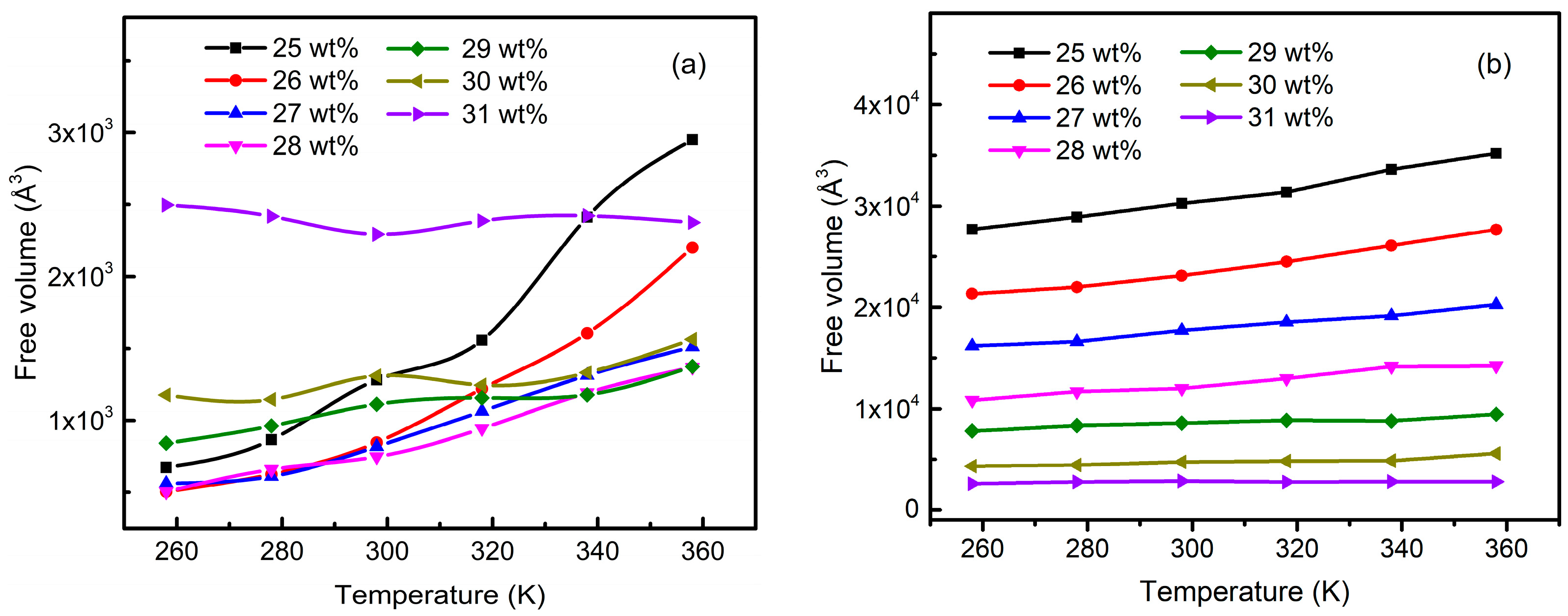
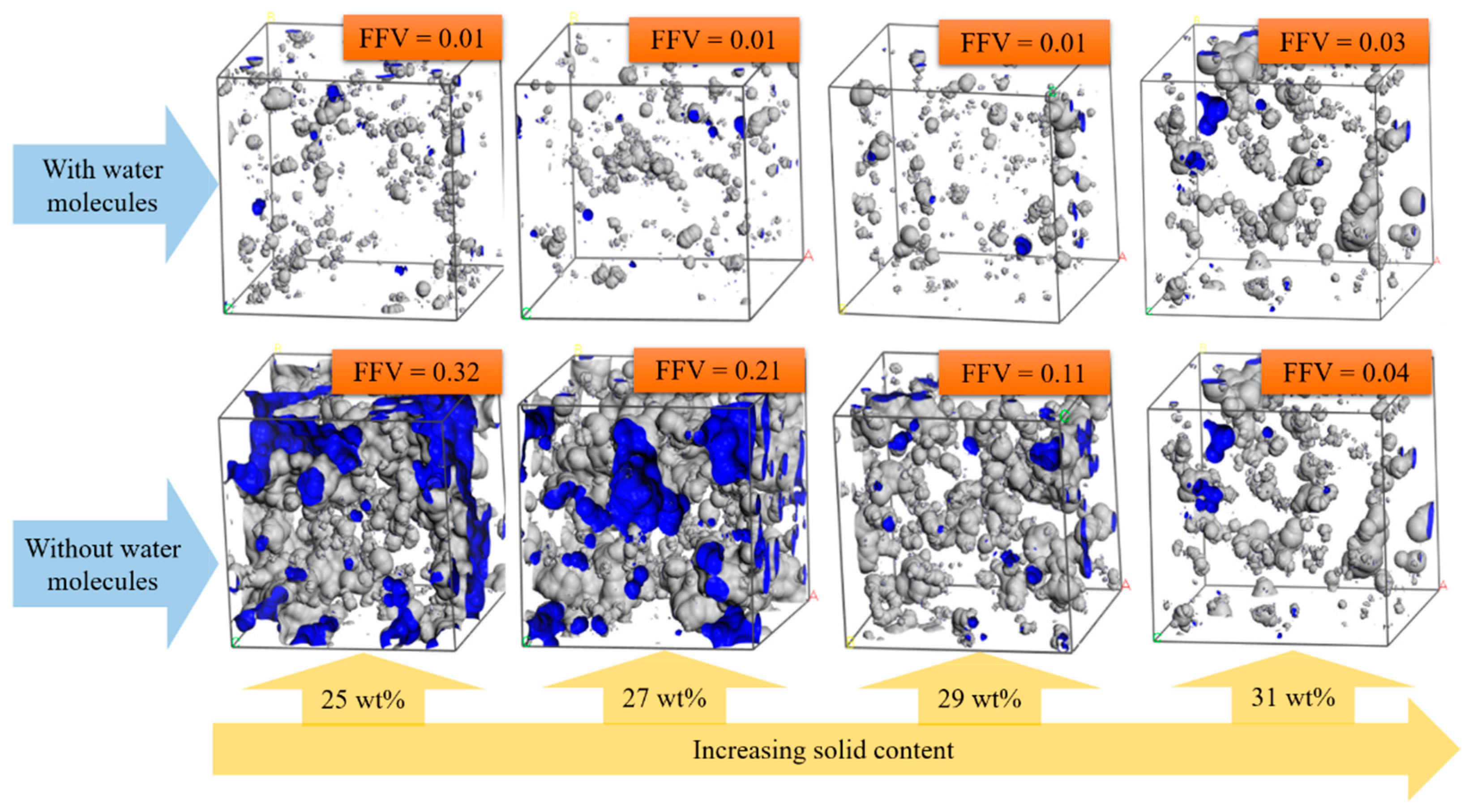
3.5. Free Volume Characteristics of Aluminum Sols and Gels
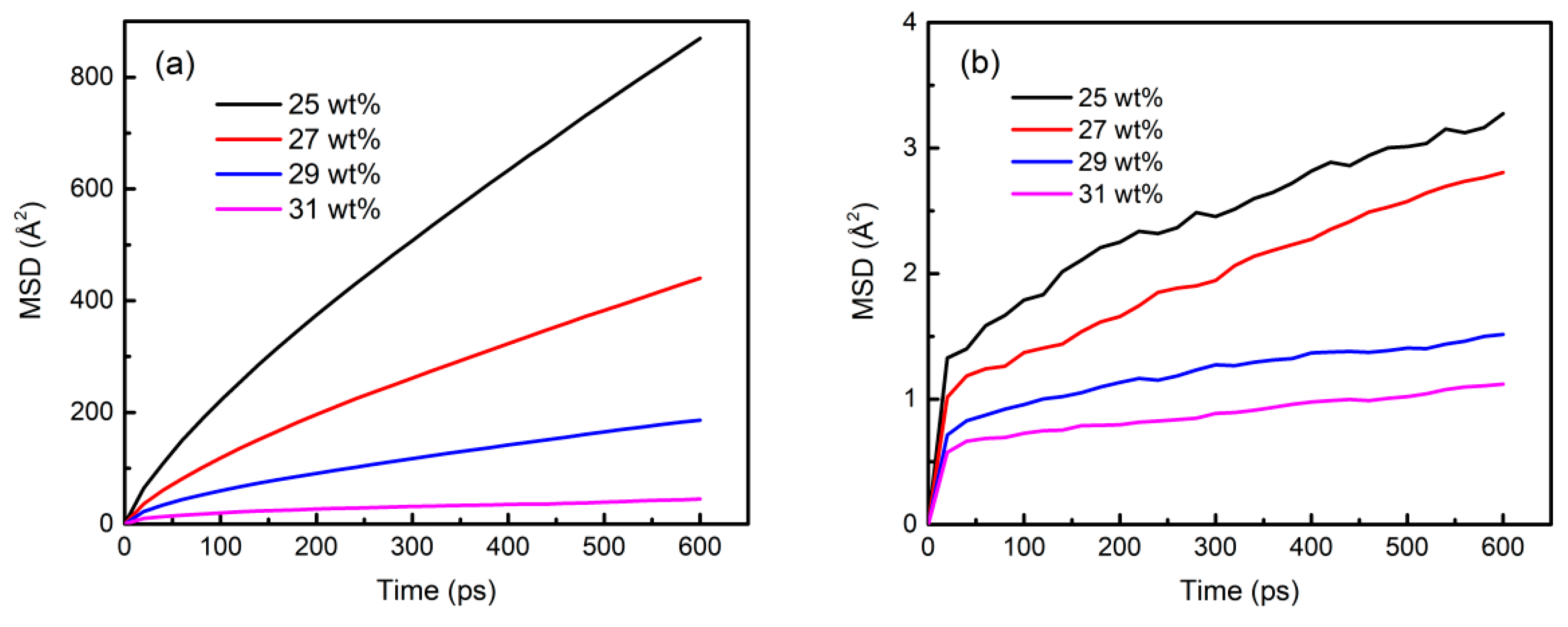
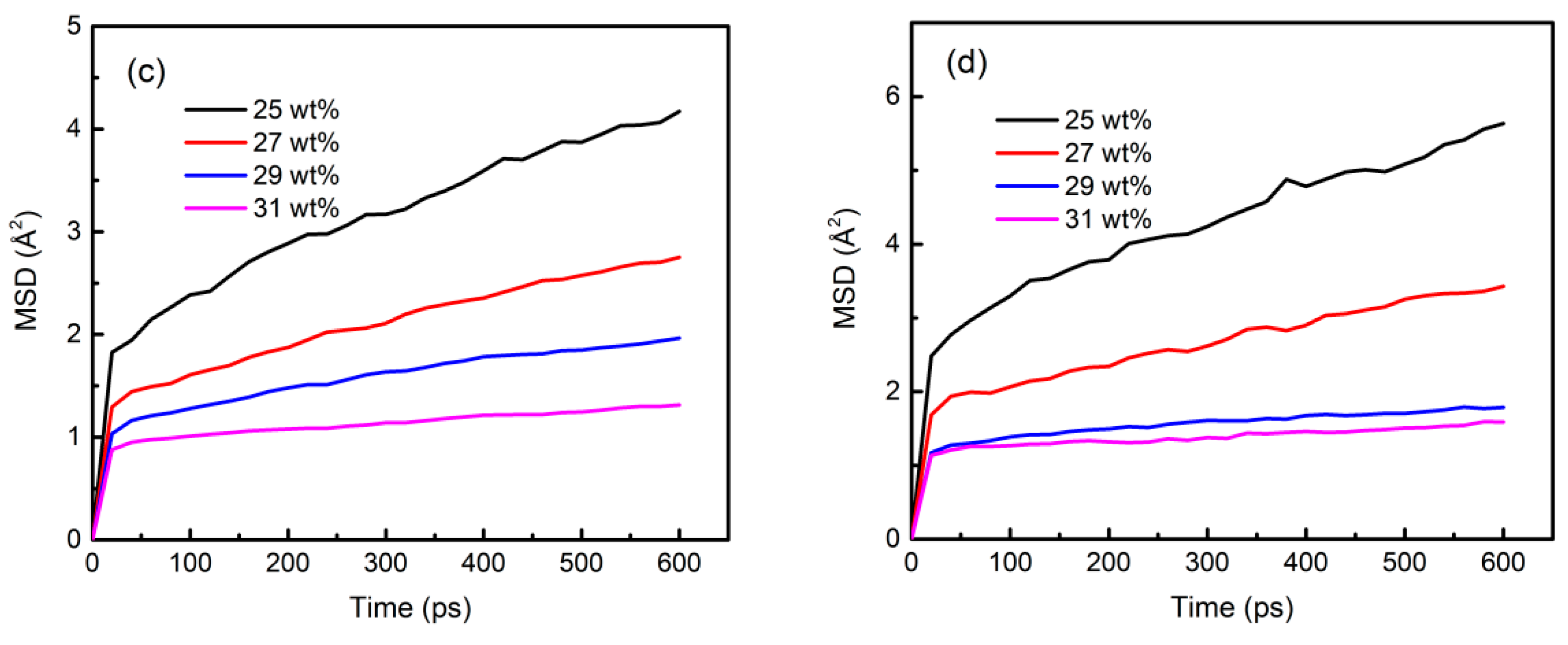
3.6. Component Migration Characteristics in the Sol–Gel Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. DREIDING Parameters
| Element Symbol | Type | Description | Hybridization |
|---|---|---|---|
| Al | Al3 | Aluminum | Tetrahedral |
| C | C_3 | Carbon, sp3 | Tetrahedral |
| C | C_R | Carbon, aromatic | Trigonal |
| H | H_ | Hydrogen | No hybridization |
| H | H___A | Hydrogen, involved in hydrogen bonds | No hybridization |
| O | O_2 | Oxygen, sp2 | Trigonal |
| O | O_3 | Oxygen, sp3 | Tetrahedral |
| O | O_R | Oxygen, aromatic | Trigonal |
References
- Ruggles-Wrenn, M.B.; Ozer, M. Creep behavior of NextelTM 720/alumina-mullite ceramic composite with ±45° fiber orientation at 1200 °C. Mater. Sci. Eng. A 2010, 527, 5326–5334. [Google Scholar] [CrossRef]
- Tariq, M.; Hanif, F.; Ashraf, A. Overview of Nextel TM based structures for Space applications. J. Space Technol. 2011, 1, 88–94. [Google Scholar]
- Jiang, R.; Sun, X.; Liu, H.; Liu, Y.; Mao, W. Microstructure and mechanical properties improvement of the NextelTM 610 fiber reinforced alumina composite. J. Eur. Ceram. Soc. 2021, 41, 5394–5399. [Google Scholar] [CrossRef]
- Guel, N.; Hamam, Z.; Godin, N.; Reynaud, P.; Caty, O.; Bouillon, F.; Paillassa, A. Data merging of ae sensors with different frequency resolution for the detection and identification of damage in oxide-based ceramic matrix composites. Materials 2020, 13, 4691. [Google Scholar] [CrossRef]
- Rutkowska, I.; Marchewka, J.; Jeleń, P.; Odziomek, M.; Korpyś, M.; Paczkowska, J.; Sitarz, M. Chemical and structural characterization of amorphous and crystalline alumina obtained by alternative sol-gel preparation routes. Materials 2021, 14, 1761. [Google Scholar] [CrossRef]
- Zhang, H.; Hang, Y.; Qin, Y.; Yang, J.; Wang, B. Synthesis and characterization of sol-gel derived continuous spinning alumina based fibers with silica nano-powders. J. Eur. Ceram. Soc. 2014, 34, 465–473. [Google Scholar] [CrossRef]
- Chen, X.; Gu, L. Spinnablity and structure characterization of mullite fibers via sol-gel-ceramic route. J. Non-Cryst. Solids 2009, 355, 2415–2421. [Google Scholar] [CrossRef]
- Ádám, P.; Temesi, O.; Dankházi, Z.; Voniatis, C.; Rohonczy, J.; Sinkó, K. Various colloid systems for drawing of aluminum oxide fibers. Ceram. Int. 2021, 48, 5499–5508. [Google Scholar] [CrossRef]
- Chen, X.; Gu, L. Structural evolution of sol-gel derived mullite fibers with different solid contents during sintering. J. Mater. Process. Technol. 2009, 209, 3991–3998. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, W.; Yao, S.; Wang, J.; Ma, Y. Comparing the phase transformation of continuous alumina fiber and xerogels derived from the same precursor. J. Sol-Gel Sci. Technol. 2021, 99, 169–177. [Google Scholar] [CrossRef]
- Duan, N.; Zhang, X.; Lu, C.; Zhang, Y.; Li, C.; Xiong, J. Effect of rheological properties of AlOOH sol on the preparation of Al2O3 nanofiltration membrane by sol-gel method. Ceram. Int. 2022, 48, 6528–6538. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Ma, Y.; Liu, W.; Yao, S. Preparation of continuous alumina fiber with nano grains by the addition of iron sol. Materials 2020, 13, 5442. [Google Scholar] [CrossRef]
- Liang, C.; Liu, W.; Liu, Q.; Gao, Y.; Liu, J.; Wang, J.; Yao, S.; Ma, Y. The formation of core-sheath structure and its effects on thermal decomposition and crystallization of alumina fibers. Ceram. Int. 2021, 47, 5145–5155. [Google Scholar] [CrossRef]
- Nie, Y.; Zhan, H.; Kou, L.; Gu, Y. Atomistic Insights on the Rheological Property of Polycaprolactone Composites with the Addition of Graphene. Adv. Mater. Technol. 2021, 2100507. [Google Scholar] [CrossRef]
- Yu, D.; Mao, G.; Cai, H.; Wang, S.; Liu, J.; Pan, P.; Bao, Y. Free volume characteristics of 2,2-bistrifluoromethyl-4,5-difluoro-1,3-dioxole-co-tetrafluoroethylene copolymers: Effect of composition and molecular weight. J. Polym. Sci. 2021, 59, 754–763. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, D.J.; Ye, Q.X.; Xiao, K.; Hao, L.S.; Nan, Y.Q. CO2/N2-switchable sol–gel transition based on NaDC/NaCl solution: Experiments and molecular dynamics simulations. J. Mol. Liq. 2021, 325, 115193. [Google Scholar] [CrossRef]
- Wen, L.; Xu, J.; Yang, Q.; Zhang, F.; Li, F.; Zhang, L. Gelation process of nanosilica sol and its mechanism: Molecular dynamics simulation. Chem. Eng. Sci. 2020, 216, 115538. [Google Scholar] [CrossRef]
- Huang, W.; Dalal, I.S.; Larson, R.G. Analysis of solvation and gelation behavior of methylcellulose using atomistic molecular dynamics simulations. J. Phys. Chem. B 2014, 118, 13992–14008. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.; Chen, D. Mechanisms of Hydrolysis–Oligomerization of Aluminum Alkoxide Al(OC3H7) 3. J. Phys. Chem. A 2011, 115, 4719–4728. [Google Scholar] [CrossRef]
- Cheng, X.; Ding, W.; Liu, Y.; Chen, D. Mechanistic investigations of Al(OH)3 oligomerization mechanisms. J. Mol. Model. 2013, 19, 1565–1572. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Luo, T.; Cheng, M.; Liu, Q.; Wang, J.; Yao, S.; Ma, Y. Effect of formic-acid-to-acetic-acid ratio on the structure and spinnability of aqueous aluminium sol of alumina fibre. Ceram. Int. 2021, 47, 26034–26041. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Wang, J.; Yao, S.; Ma, Y. A density functional theory study on the structure formation of Al(III) carboxylate complexes in aqueous aluminum sols. Int. J. Quantum Chem. 2021, 121, e26430. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Ma, Y. DFT Studies on the Al-Speciation and Its Structure in Aqueous Aluminum Sol Formed by Aluminum Formoacetate. J. Phys. Chem. B 2019, 123, 9167–9179. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, W.; Ma, Y. Influence of H3O+ on the structure formation of oligomers in aluminium sols prepared from basic aluminium acetate: Experiments and computations. J. Mol. Liq. 2019, 289, 111052. [Google Scholar] [CrossRef]
- Boyd, P.G.; Moosavi, S.M.; Witman, M.; Smit, B. Force-Field Prediction of Materials Properties in Metal-Organic Frameworks. J. Phys. Chem. Lett. 2017, 8, 357–363. [Google Scholar] [CrossRef]
- Haouas, M.; Taulelle, F.; Martineau, C. Recent advances in application of 27Al NMR spectroscopy to materials science. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 94–95, 11–36. [Google Scholar] [CrossRef]
- Liu, L.; Cai, W.; Dang, C.; Han, B.; Chen, Y.; Yi, R.; Fan, J.; Zhou, J.; Wei, J. One-step vapor-phase assisted hydrothermal synthesis of functionalized carbons: Effects of surface groups on their physicochemical properties and adsorption performance for Cr(VI). Appl. Surf. Sci. 2020, 528, 146984. [Google Scholar] [CrossRef]
- Persson, P.; Karlsson, M.; Öhman, L.O. Coordination of acetate to Al(III) in aqueous solution and at the water-aluminum hydroxide interface: A potentiometric and attenuated total reflectance FTIR study. Geochim. Cosmochim. Acta 1998, 62, 3657–3668. [Google Scholar] [CrossRef]
- Ghaderi, S.; Ramazani, S.A.; Haddadi, S.A. Applications of highly salt and highly temperature resistance terpolymer of acrylamide/styrene/maleic anhydride monomers as a rheological modifier: Rheological and corrosion protection properties studies. J. Mol. Liq. 2019, 294, 111635. [Google Scholar] [CrossRef]
- Mills, J.N.; Wagner, N.J.; Mondal, P. Relating chemical composition, structure, and rheology in alkali-activated aluminosilicate gels. J. Am. Ceram. Soc. 2021, 104, 572–583. [Google Scholar] [CrossRef]
- Lakkegowda, Y.; Ammannappa, R.; Ananthamurthy, S. Investigations on rheological properties and gelation of tasar regenerated silk fibroin solution. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The rheology of gelatin hydrogels modified by κ-carrageenan. Lwt 2015, 63, 612–619. [Google Scholar] [CrossRef]
- Gan, Y.; Cheng, Q.; Wang, Z.; Yang, J.; Sun, W.; Liu, Y. Molecular dynamics simulation of the microscopic mechanisms of the dissolution, diffusion and aggregation processes for waxy crystals in crude oil mixtures. J. Pet. Sci. Eng. 2019, 179, 56–69. [Google Scholar] [CrossRef]
- Lin, D.; Li, R.; Li, T.; Zi, Y.; Qi, S.; Wu, D. Effects of pre-imidization on rheological behaviors of polyamic acid solution and thermal mechanical properties of polyimide film: An experiment and molecular dynamics simulation. J. Mater. Sci. 2021, 56, 14518–14530. [Google Scholar] [CrossRef]
- Lin, L.; Kedzierski, M.A. Density and viscosity of a polyol ester lubricant: Measurement and molecular dynamics simulation. Int. J. Refrig. 2020, 118, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.K.; Manik, G. A combined theoretical and experimental investigation on the wettability of MWCNT filled PVAc-g-PDMS easy-clean coating. Prog. Org. Coat. 2021, 151, 106092. [Google Scholar] [CrossRef]

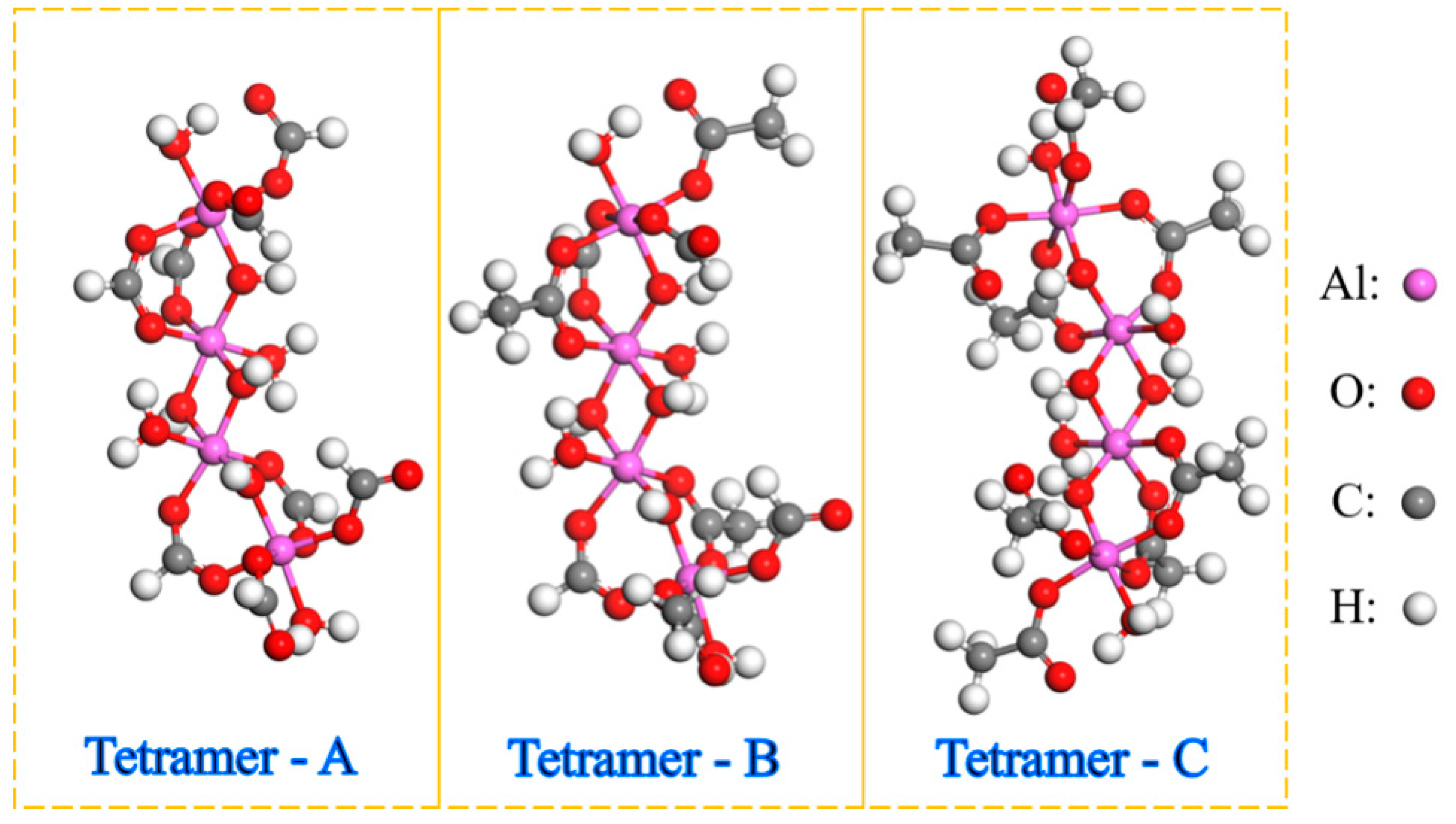
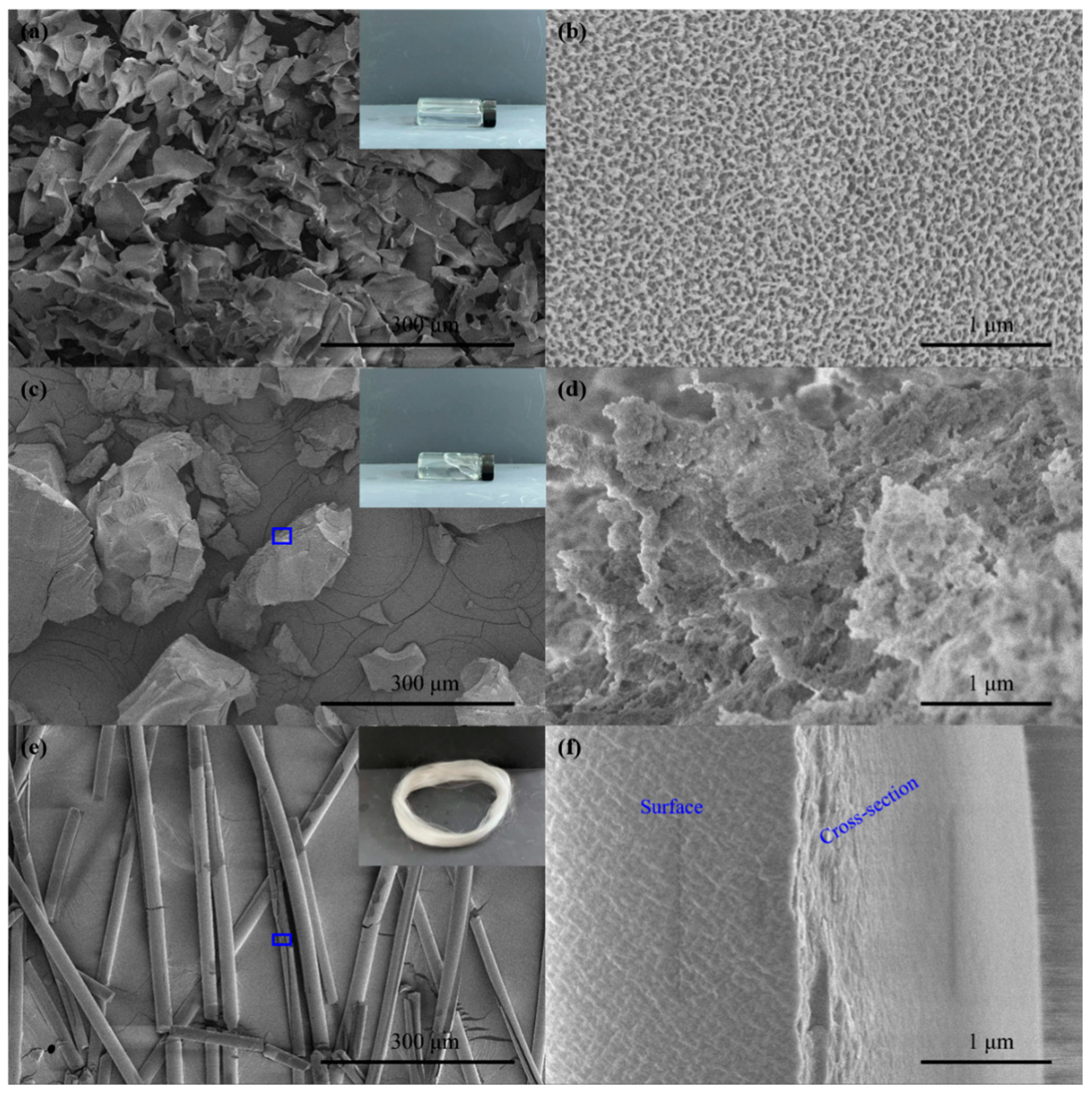
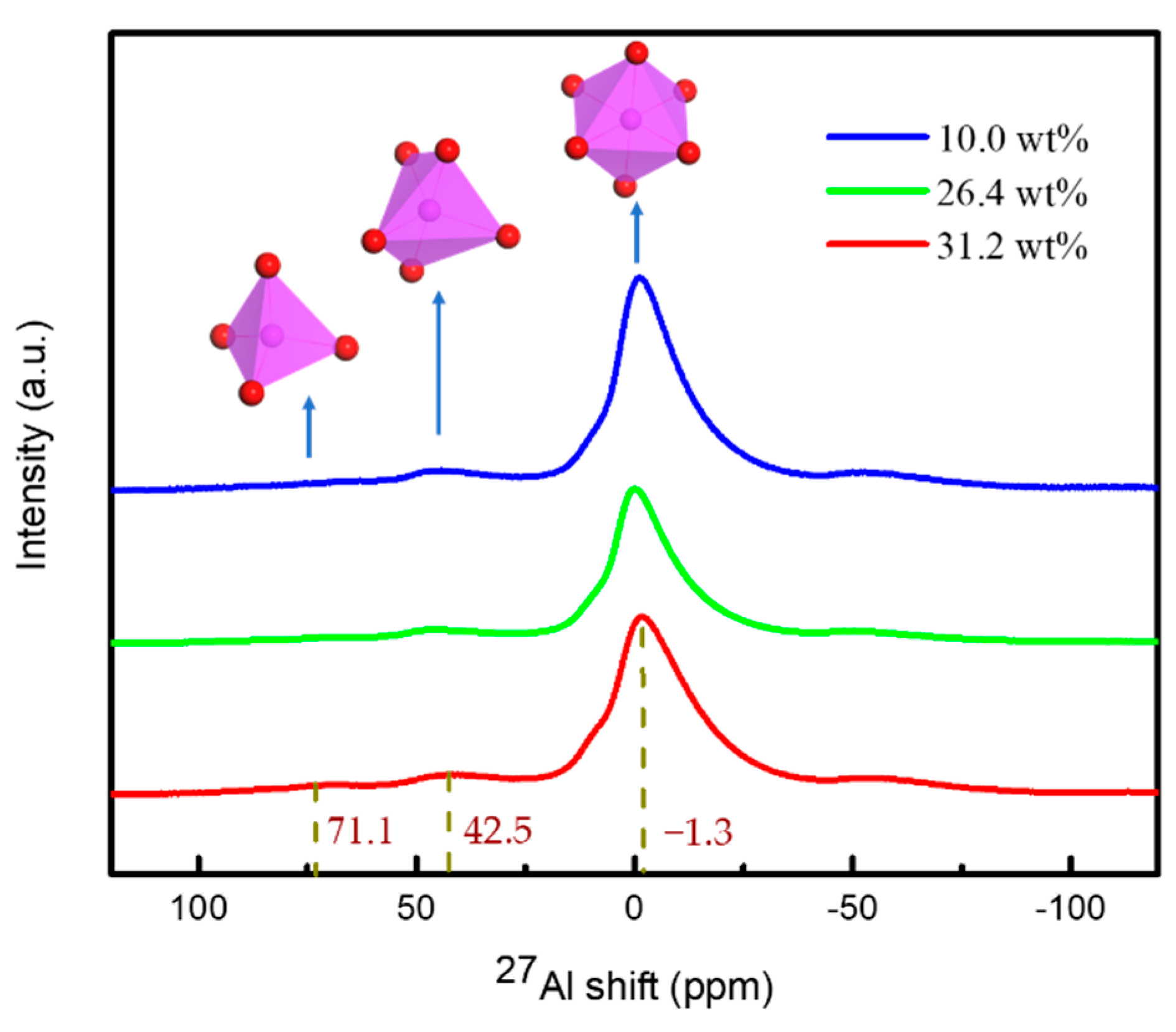
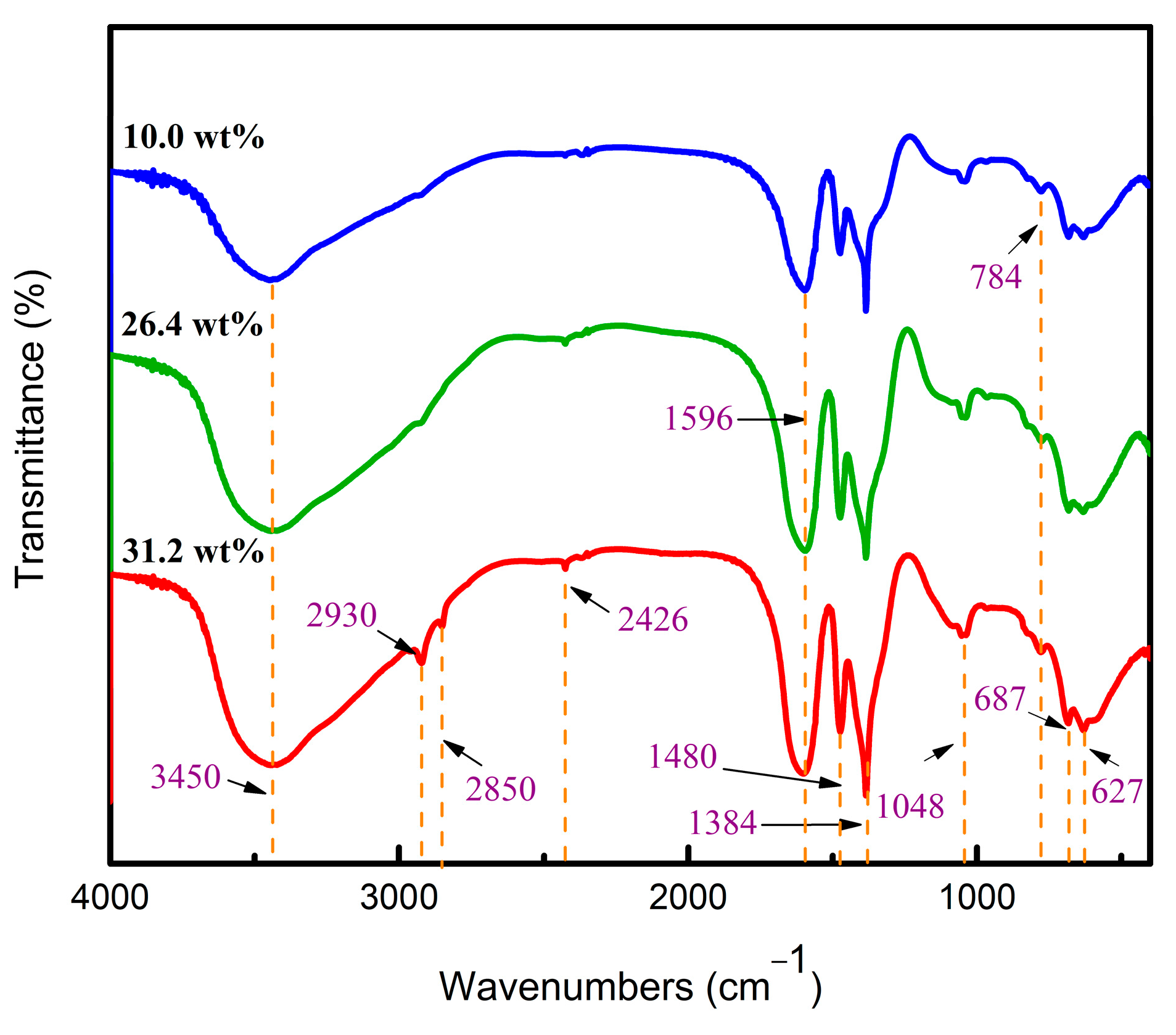
| A:B:C | ENon-Bond /kcal∙mol−1 | △E /kcal∙mol−1 | Epo /kcal∙mol−1 | △E /kcal∙mol−1 |
|---|---|---|---|---|
| 4:15:1 | −3752.0 | 0.0 | 69,112.5 | 0.0 |
| 5:13:2 | −3695.0 | 57.1 | 69,211.1 | 98.6 |
| 6:11:3 | −3658.3 | 93.7 | 69,259.5 | 147.0 |
| 7:9:4 | −3641.7 | 110.3 | 69,289.3 | 176.8 |
| 8:7:5 | −3630.9 | 121.1 | 69,318.9 | 206.4 |
| 9:5:6 | −3602.0 | 150.1 | 69,374.0 | 261.5 |
| 10:3:7 | −3584.6 | 167.4 | 69,424.3 | 311.8 |
| 11:1:8 | −3584.2 | 167.8 | 69,437.5 | 325.0 |
| Cell Dimension () (Å3) | Cell Volume (Å3) | W Volume (Å3) | |
|---|---|---|---|
| 25.0 wt% | 50.92 × 45.50 × 42.17 | 94,851.285 | 28,997.936 |
| 26.0 wt% | 46.71 × 40.38 × 46.79 | 88,147.821 | 22,257.313 |
| 27.0 wt% | 43.88 × 41.42 × 45.97 | 83,540.026 | 16,893.963 |
| 28.0 wt% | 40.96 × 43.91 × 45.20 | 78,364.597 | 11,247.907 |
| 29.0 wt% | 39.59 × 45.32 × 43.52 | 75,342.807 | 7470.678 |
| 30.0 wt% | 36.89 × 45.35 × 43.57 | 71,531.849 | 3437.288 |
| 31.0 wt% | 43.16 × 39.97 × 41.09 | 70,466.882 | 270.524 |
| Diffusion Coefficient/Å2∙ps−1 | ||||
|---|---|---|---|---|
| W | A | B | C | |
| 25.0 wt% | 0.20086 | 0.000475 | 0.000549 | 0.000698 |
| 27.0 wt% | 0.09936 | 0.000468 | 0.000342 | 0.000436 |
| 29.0 wt% | 0.03860 | 0.000128 | 0.000173 | 0.000108 |
| 31.0 wt% | 0.00735 | 0.000124 | 0.000096 | 0.000102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Ma, Y.; Yao, S.; Wang, J.; Liu, W. Experimental and Molecular Dynamics Simulation Study on Sol–Gel Conversion Process of Aluminum Carboxylate System. Materials 2022, 15, 2704. https://doi.org/10.3390/ma15072704
Luo T, Ma Y, Yao S, Wang J, Liu W. Experimental and Molecular Dynamics Simulation Study on Sol–Gel Conversion Process of Aluminum Carboxylate System. Materials. 2022; 15(7):2704. https://doi.org/10.3390/ma15072704
Chicago/Turabian StyleLuo, Tao, Yunzhu Ma, Shuwei Yao, Juan Wang, and Wensheng Liu. 2022. "Experimental and Molecular Dynamics Simulation Study on Sol–Gel Conversion Process of Aluminum Carboxylate System" Materials 15, no. 7: 2704. https://doi.org/10.3390/ma15072704
APA StyleLuo, T., Ma, Y., Yao, S., Wang, J., & Liu, W. (2022). Experimental and Molecular Dynamics Simulation Study on Sol–Gel Conversion Process of Aluminum Carboxylate System. Materials, 15(7), 2704. https://doi.org/10.3390/ma15072704





