Design and Preparation of Imidazole Ionic Liquid-Based Magnetic Polymers and Its Adsorption on Sunset Yellow Dye
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials and Characteristics
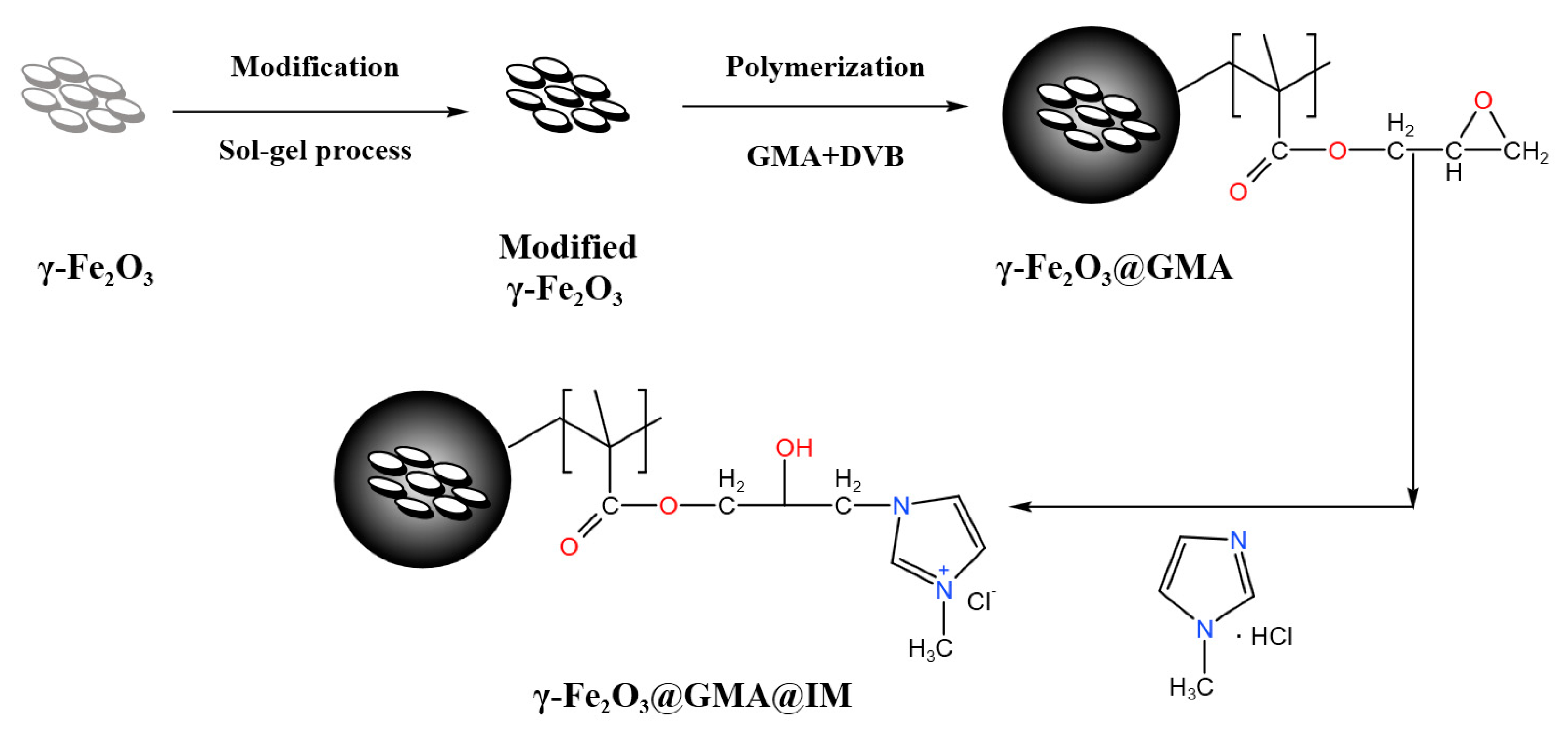
2.2. Preparation of Magnetic Polymers
2.2.1. Surface Modification of γ-Fe2O3
2.2.2. Preparation of γ-Fe2O3@GMA
2.2.3. Preparation of γ-Fe2O3@GMA@IM
2.3. Batch Adsorption Studies
2.3.1. Contrast Experiment
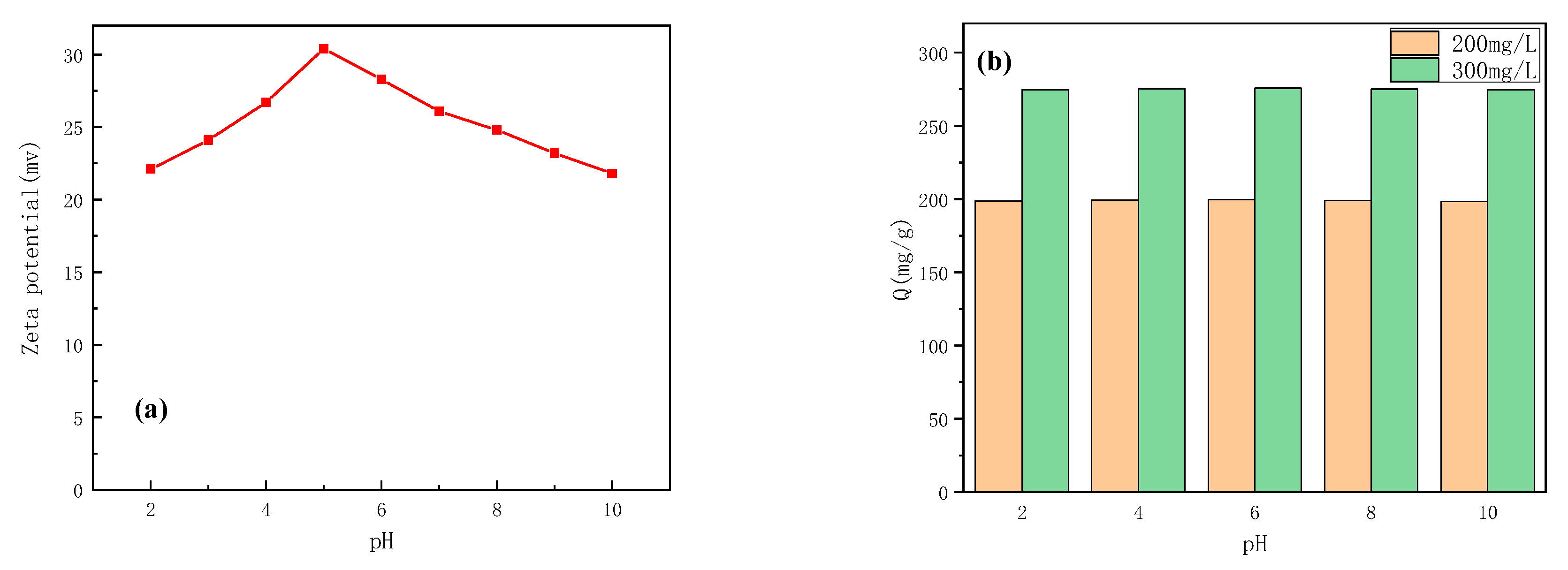
2.3.2. Effect of pH
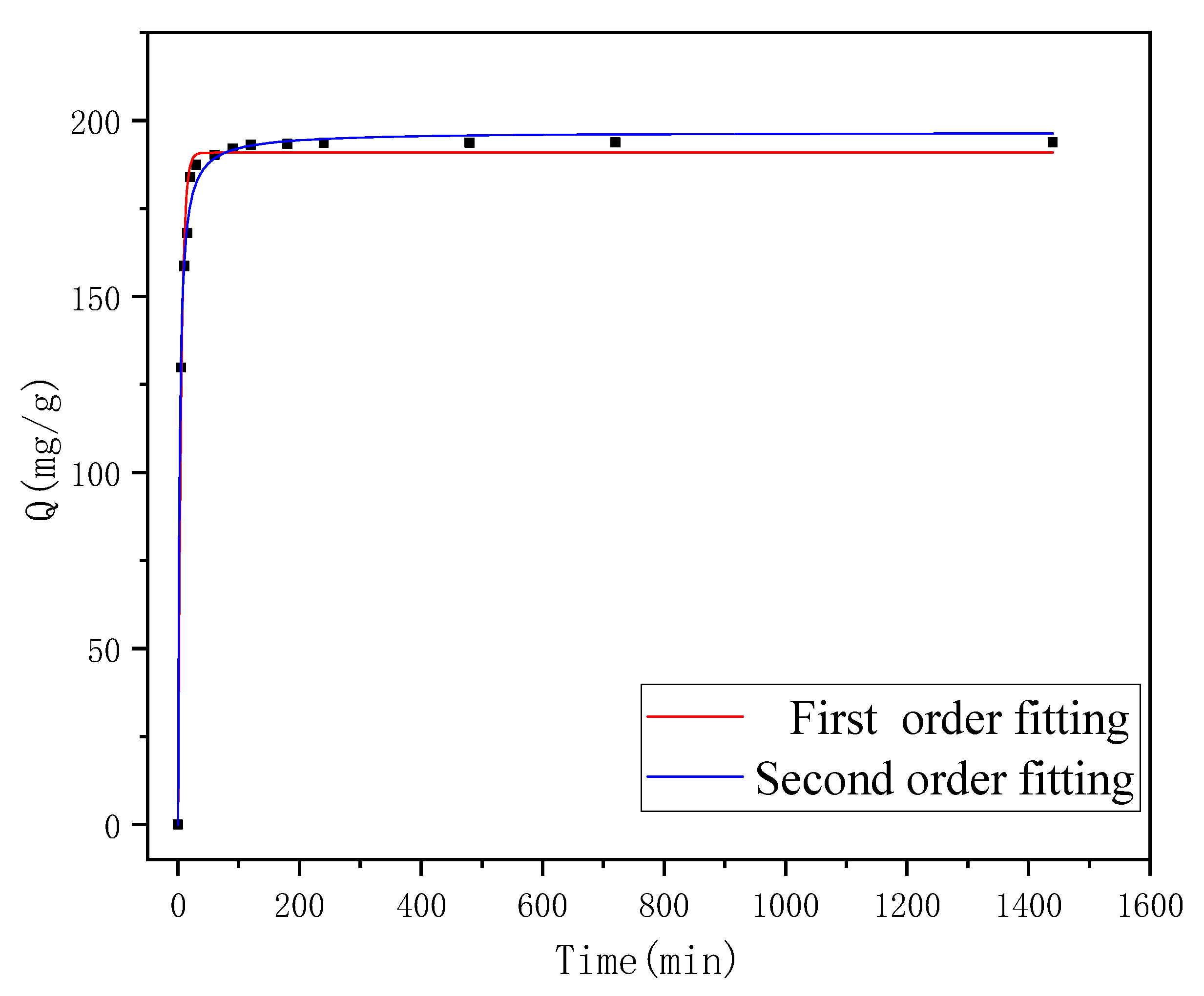
2.3.3. Adsorption Kinetics
2.3.4. Adsorption Isotherm
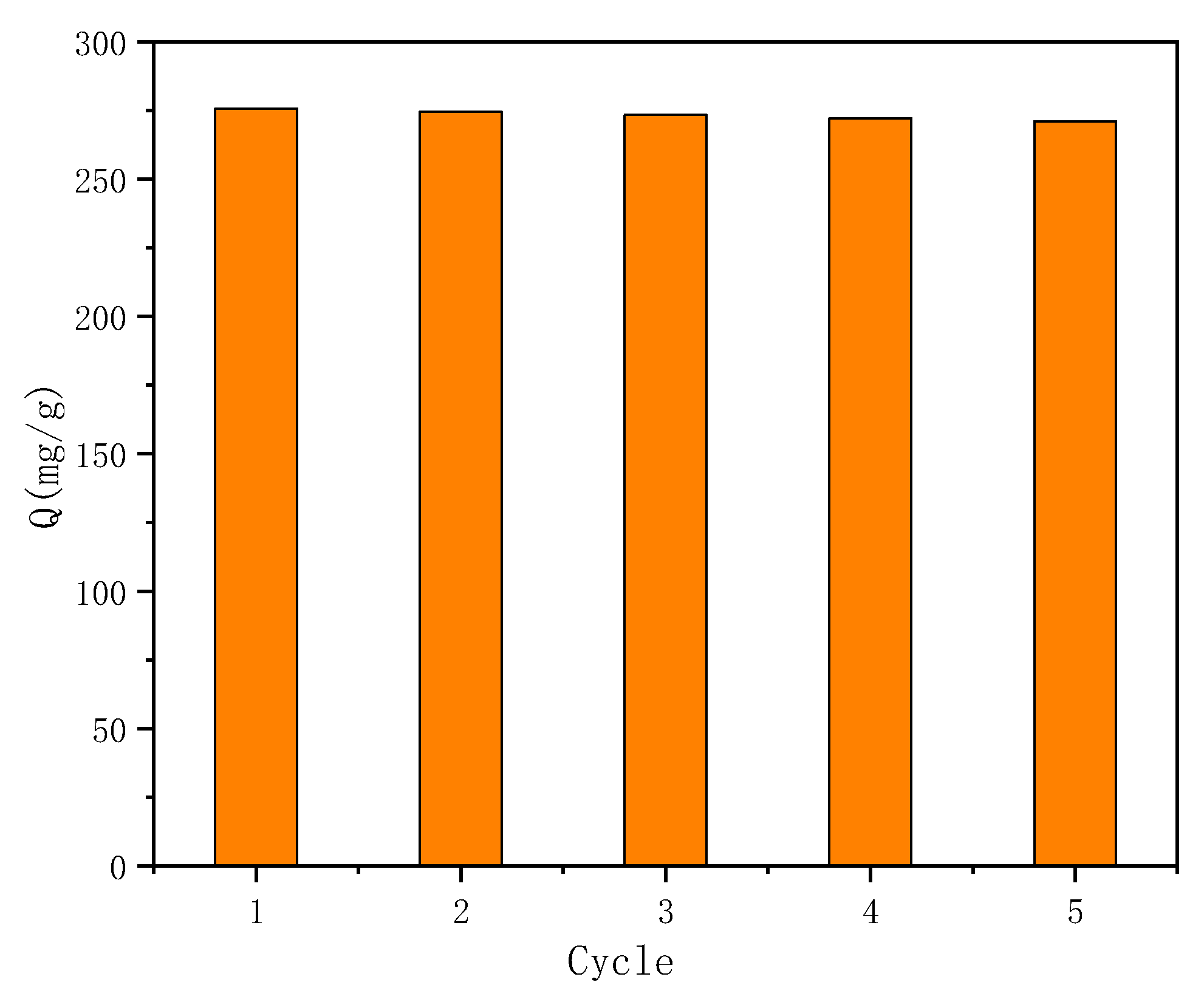
2.3.5. Regeneration Experiment
3. Results and Discussion
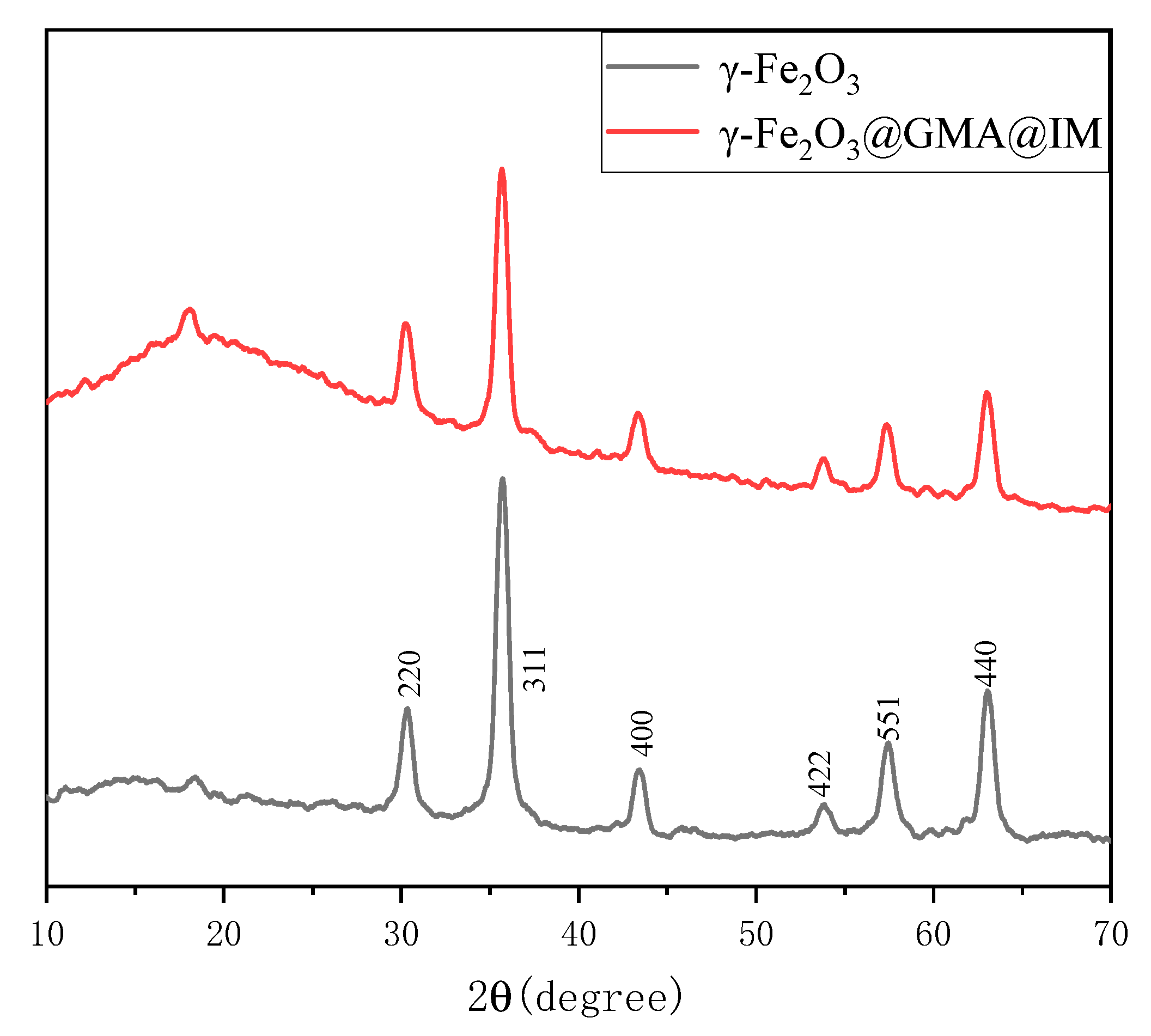
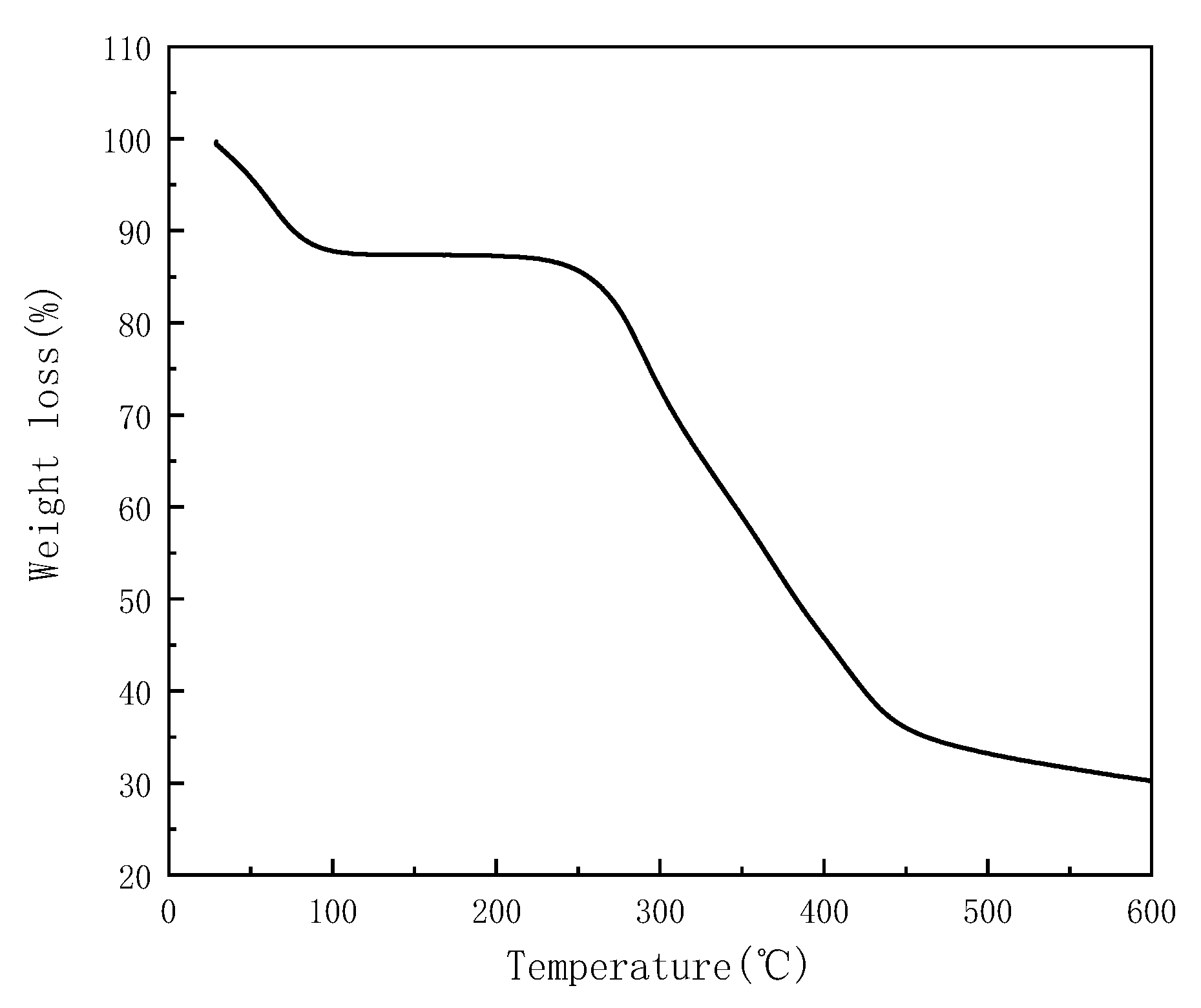
3.1. Description
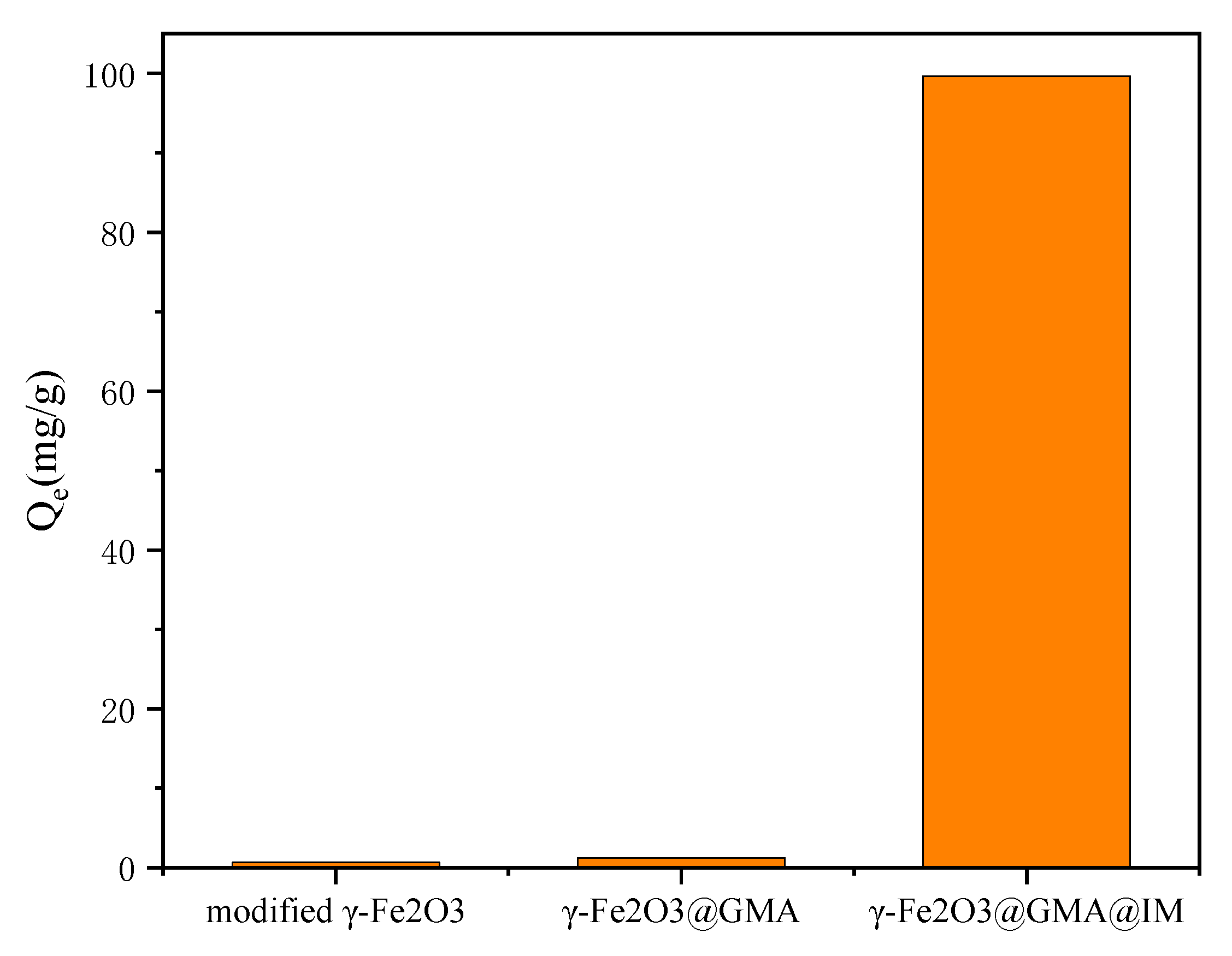
3.2. Comparative Experimental Analysis
3.3. The Influence of pH
3.4. Adsorption Kinetics
3.5. Adsorption Isotherm
3.6. Repeatability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhongale, P.V.; Joshi, S.S.; Mali, N.A. Selective monoalkylation of hydroquinone in the presence of SO3H-functionalized ionic liquids as catalysts. Chem. Pap. 2020, 74, 4461–4471. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Neves, M.C.; Trindade, T.; Marrucho, I.M.; Freire, M.G. Supported ionic liquids as efficient materials to re-move non-steroidal anti-inflammatory drugs from aqueous media. Chem. Eng. J. 2020, 381, 122616. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, L.; Ao, X.; Guo, Y. Study on the synthesis of dual-chain ionic liquids and their application in the extraction of flavonoids. J. Chromatogr. A 2020, 1628, 461446. [Google Scholar] [CrossRef] [PubMed]
- Maniam, K.; Paul, S. Progress in Electrodeposition of Zinc and Zinc Nickel Alloys Using Ionic Liquids. Appl. Sci. 2020, 10, 5321. [Google Scholar] [CrossRef]
- Molodkina, E.B.; Ehrenburg, M.R.; Broekmann, P.; Rudnev, A.V. Electrodeposition of chromium on single-crystal electrodes from solutions of Cr(II) and Cr(III) salts in ionic liquids. J. Electroanal. Chem. 2020, 860, 113892. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Zhou, J.; Zhu, Z.; Zhang, Z.; Li, Y.; Wang, L.; Zhang, J. Polystyrene-supported novel imidazolium ionic liquids: Highly efficient catalyst for the fixation of carbon dioxide under atmospheric pressure. Fuel 2021, 305, 121495. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Khan, M.R. Fabrication of magnetic nanoparticles supported ionic liquid catalyst for trans-esterification of vegetable oil to produce biodiesel. J. Mol. Liq. 2021, 330, 115648. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, G.; Huang, Q.; Dou, J.; Dai, L.; Deng, F.; Liu, M.; Li, X.; Zhang, X.; Wei, Y. Facile synthesis of ionic liquid modified silica nanoparticles for fast removal of anionic organic dyes with extremely high adsorption capacity. J. Mol. Liq. 2022, 347, 117966. [Google Scholar] [CrossRef]
- Hosseinzadegan, S.; Nischkauer, W.; Bica-Schröder, K.; Limbeck, A. FI-ICP-OES determination of Pb in drinking water after pre-concentration using magnetic nanoparticles coated with ionic liquid. Microchem. J. 2019, 146, 339–344. [Google Scholar] [CrossRef]
- Tang, T.; Cao, S.; Xi, C.; Chen, Z. Multifunctional magnetic chitosan-graphene oxide-ionic liquid ternary nanohybrid: An efficient adsorbent of alkaloids. Carbohydr. Polym. 2021, 255, 117338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Y.; Yang, X.-A.; Jin, C.-Z.; Zhang, W.-B. Ultrasound-assisted dispersive solid phase extraction for promoting enrichment of ng L−1 level Hg2+ on ionic liquid coated magnetic materials. Anal. Chim. Acta 2021, 1181, 338906. [Google Scholar] [CrossRef] [PubMed]
- Latifeh, F.; Yamini, Y.; Seidi, S. Ionic liquid-modified silica-coated magnetic nanoparticles: Promising adsorbents for ultra-fast extraction of paraquat from aqueous solution. Environ. Sci. Pollut. Res. 2016, 23, 4411–4421. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, R.S.; Mahmoodi, N.O. Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes; sunset yellow and Congo red. J. Clean. Prod. 2018, 179, 235–245. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Roosta, M.; Ghaedi, M.; Sahraei, R.; Purkait, M. Ultrasonic assisted removal of sunset yellow from aqueous solution by zinc hydroxide nanoparticle loaded activated carbon: Optimized experimental design. Mater. Sci. Eng. C 2015, 52, 82–89. [Google Scholar] [CrossRef]
- Xu, F.; Qi, X.-Y.; Kong, Q.; Shu, L.; Miao, M.-S.; Xu, S.; Du, Y.-D.; Wang, Q.; Liu, Q.; Ma, S.-S. Adsorption of sunset yellow by luffa sponge, modified luffa and activated carbon from luffa sponge. Desalin. Water Treat. 2017, 96, 86–96. [Google Scholar] [CrossRef]
- Coros, M.; Socaci, C.; Pruneanu, S.; Pogacean, F.; Rosu, M.-C.; Turza, A.; Magerusan, L. Thermally reduced graphene oxide as green and easily available adsorbent for Sunset yellow decontamination. Environ. Res. 2020, 182, 109047. [Google Scholar] [CrossRef]
- Foroughirad, S.; Haddadi-Asl, V.; Khosravi, A.; Salami-Kalajahi, M. Synthesis of magnetic nanoparticles-decorated halloysite nanotubes/poly([2-(acryloyloxy)ethyl]trimethylammonium chloride) hybrid nanoparticles for removal of Sunset Yellow from water. J. Polym. Res. 2020, 27, 320. [Google Scholar] [CrossRef]
- Yakout, A.A.; Mahmoud, M.E. Fabrication of magnetite-functionalized-graphene oxide and hexadecyltrimethyl ammonium bromide nanocomposite for efficient nanosorption of sunset yellow. Mater. Sci. Eng. C 2018, 92, 287–296. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Y.; Li, A.; Zhou, Q.; Zhou, W.; Jin, J. Two novel multi-functional magnetic adsorbents for effective removal of hydrophilic and hydrophobic nitroaromatic compounds. J. Hazard. Mater. 2015, 294, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Li, W.; Li, Y.; Wang, G.; Li, A.; Li, H. Efficient removal of acid dyes using permanent magnetic resin and its preliminary investigation for advanced treatment of dyeing effluents. J. Clean. Prod. 2020, 251, 119694. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F.; Pei, M.; Guo, W.; Liu, G.; Du, S. Synthesis of a Cationic Polymer-Bentonite Composite Utilizing a Simple and Green Process for the Adsorption of Acid Orange 7 from Aqueous Solution. J. Macromol. Sci. Part B 2019, 58, 794–809. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Hua, M.; Zhang, G.; Li, W.; Shuang, C.; Li, A. Preparation of Permanent Magnetic Resin Crosslinking by Diallyl Itaconate and Its Adsorptive and Anti-fouling Behaviors for Humic Acid Removal. Sci. Rep. 2017, 7, 17103. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhou, Q.; Li, A.; Shuang, C.; Shi, Q.; Zhang, M. Preparation of a novel magnetic microporous adsorbent and its ad-sorption behavior of p-nitrophenol and chlorotetracycline. J. Hazard. Mater. 2014, 266, 84–93. [Google Scholar] [CrossRef]
- Shuang, C.; Li, P.; Li, A.; Zhou, Q.; Zhang, M.; Zhou, Y. Quaternized magnetic microspheres for the efficient removal of re-active dyes. Water Res. 2012, 46, 4417–4426. [Google Scholar] [CrossRef]




| Parameters | γ-Fe2O3@GMA@IM |
|---|---|
| Matrix structure | Acrylic |
| Water content (%) | 55–60% |
| Diameter (μm) | 50 |
| Average pore size (nm) | 3.421 |
| BET specific surface area (m2/g) | 14.703 |
| Total pore volume (cm3/g) | 0.042 |
| Adsorbate | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| k1 | qe | R12 | k2 | qe | R22 | |
| FCF | 0.1966 | 190.8 | 0.988 | 0.00215 | 196.7 | 0.996 |
| Adsorbate | T (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| KL | Qmax | R2 | n | KF | R2 | ||
| FCF | 25 °C | 0.661 | 369.9 | 0.809 | 6.27 | 162.45 | 0.971 |
| 35 °C | 1.146 | 376.4 | 0.830 | 6.76 | 178.23 | 0.960 | |
| 45 °C | 3.257 | 378.6 | 0.783 | 7.52 | 197.20 | 0.934 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Shi, Y.; Cui, Y.; Zhao, F.; Chen, M. Design and Preparation of Imidazole Ionic Liquid-Based Magnetic Polymers and Its Adsorption on Sunset Yellow Dye. Materials 2022, 15, 2628. https://doi.org/10.3390/ma15072628
Liu Y, Shi Y, Cui Y, Zhao F, Chen M. Design and Preparation of Imidazole Ionic Liquid-Based Magnetic Polymers and Its Adsorption on Sunset Yellow Dye. Materials. 2022; 15(7):2628. https://doi.org/10.3390/ma15072628
Chicago/Turabian StyleLiu, Yafei, Yiyu Shi, Yan Cui, Fen Zhao, and Mindong Chen. 2022. "Design and Preparation of Imidazole Ionic Liquid-Based Magnetic Polymers and Its Adsorption on Sunset Yellow Dye" Materials 15, no. 7: 2628. https://doi.org/10.3390/ma15072628
APA StyleLiu, Y., Shi, Y., Cui, Y., Zhao, F., & Chen, M. (2022). Design and Preparation of Imidazole Ionic Liquid-Based Magnetic Polymers and Its Adsorption on Sunset Yellow Dye. Materials, 15(7), 2628. https://doi.org/10.3390/ma15072628






