Fabrication of Nanoparticle/Polymer Composite Photocatalytic Membrane for Domestic Sewage In Situ Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
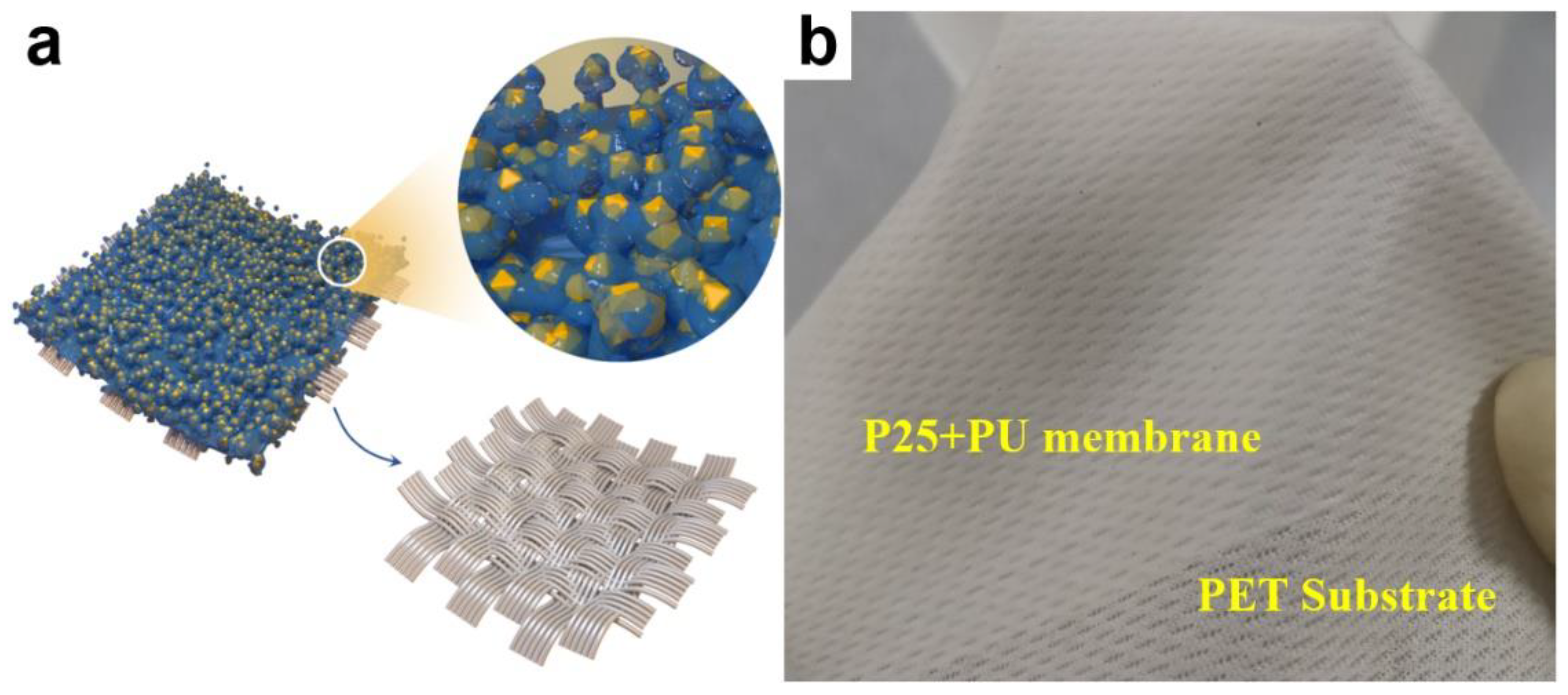
2.2. Fabrication of the Photocatalytic Membranes
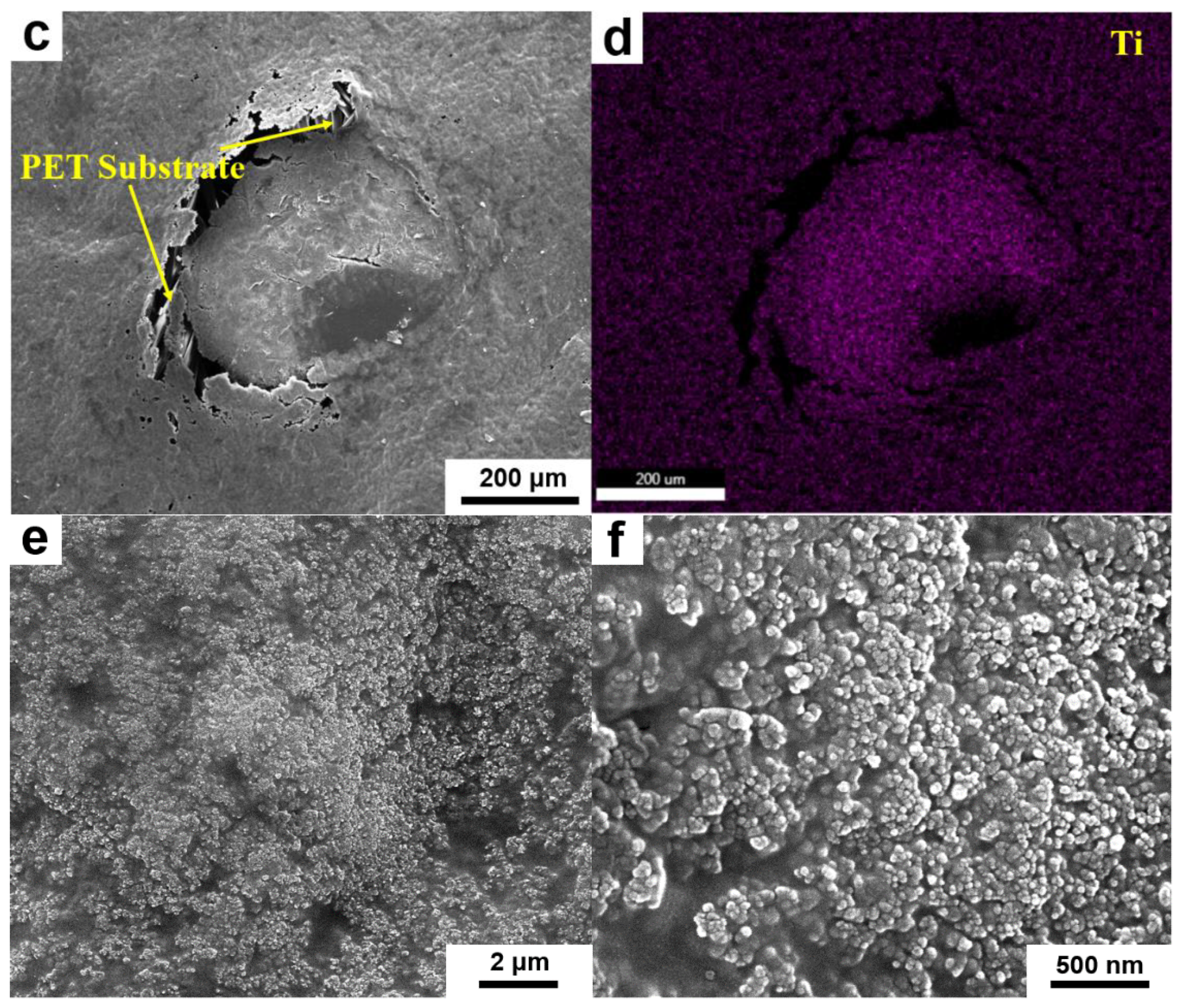
2.3. Characterizations
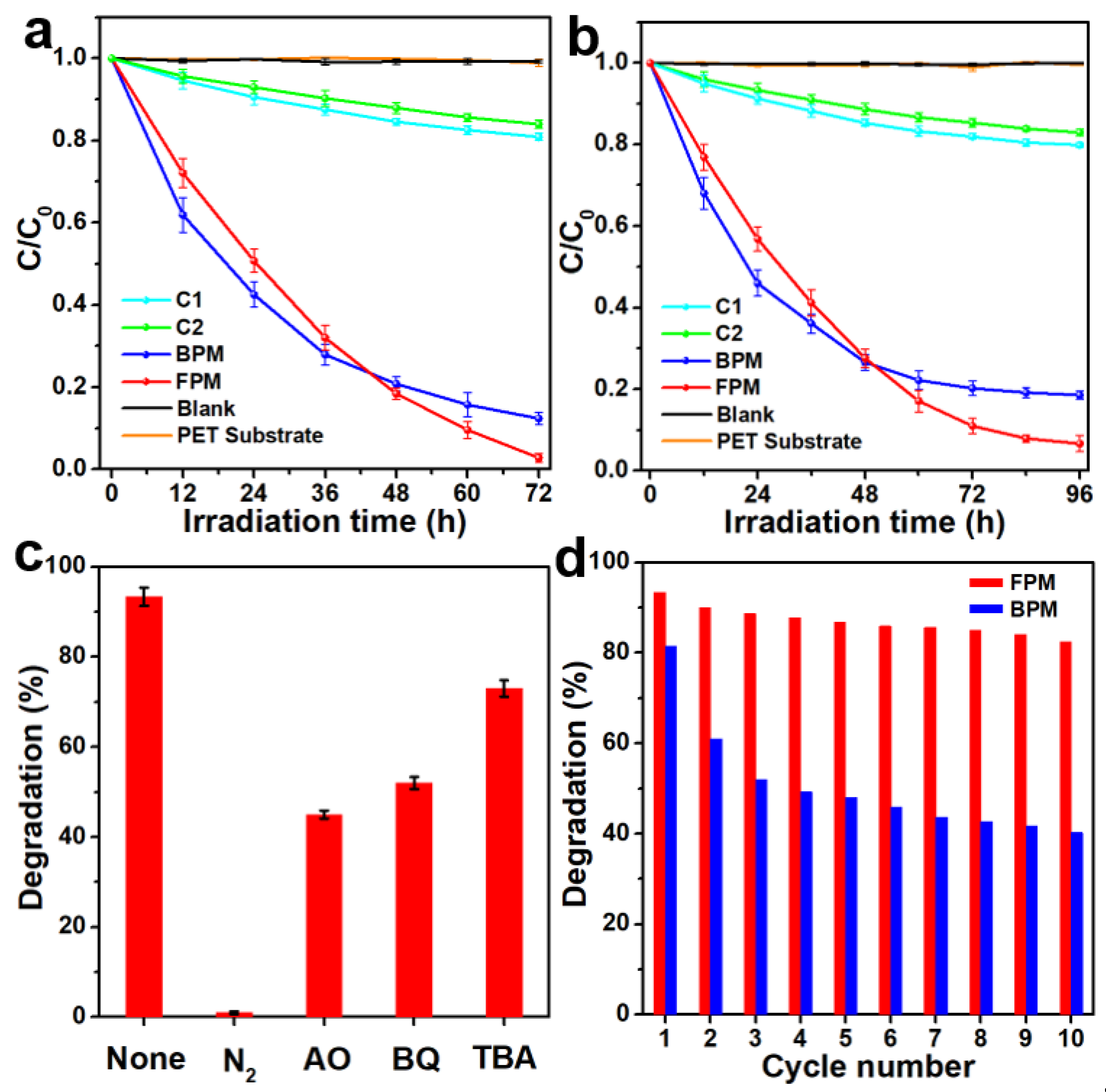
2.4. Photocatalytic Measurements
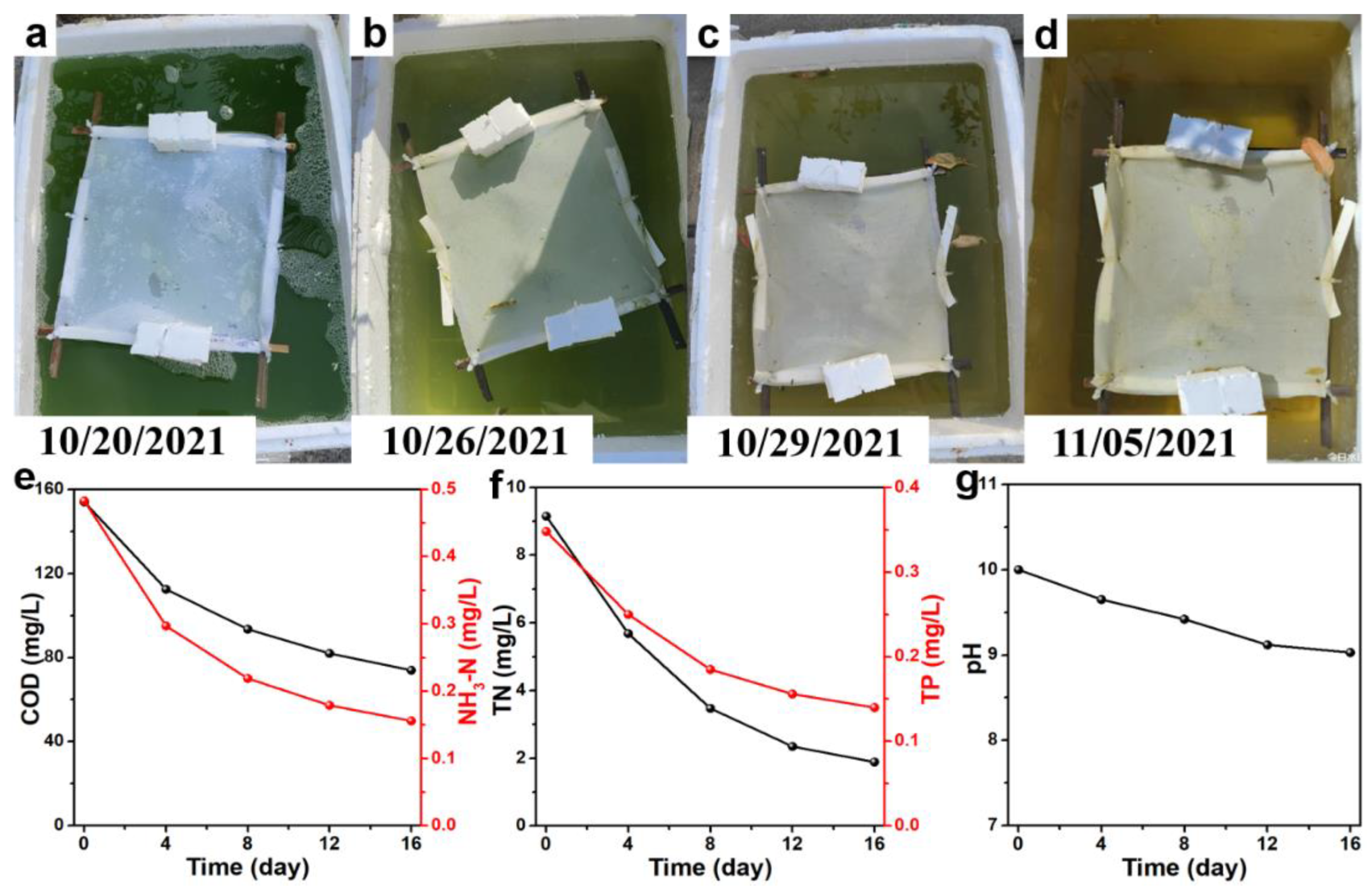
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodges, B.; Cates, E.; Kim, J. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotech. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-light triggered photocatalytic performance of ZnO.SiO2 nanocomposite for water pollutant compound methyl orange dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Ayodhya, D.; Veerabhadram, G. A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today Energy 2018, 9, 83–113. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and fabrication of g-C3N4-based materials and their application in elimination of pollutants. Sci. Total Environ. 2020, 731, 139054. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.H.H.; Omer, K.M.; Mahyar, A.; Miessner, H.; Mueller, S.; Moeller, D. Application of photocatalytic falling film reactor to elucidate the degradation pathways of pharmaceutical diclofenac and ibuprofen in aqueous solutions. Coatings 2019, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, P.J.J.; Chan, C.K.; Elimelech, M.; Halas, N.J.; Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotech. 2018, 13, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Liu, Z.; Zeng, R.; Chen, L.; Feng, X.; Jiang, L. Enhanced photocatalytic reaction at air-liquid-solid joint interfaces. J. Am. Chem. Soc. 2017, 139, 12402–12405. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, R.; Li, T.; Komarneni, S.; Liu, B. Advances in recyclable and superior photocatalytic fibers: Material, construction, application and future perspective. Compos. Part B Eng. 2021, 205, 108512. [Google Scholar] [CrossRef]
- Yu, H.; Jiao, Z.; Hu, H.; Lu, G.; Ye, J.; Bi, Y. Fabrication of Ag3PO4-PAN composite nanofibers for photocatalytic applications. CrystEngComm 2013, 15, 4802–4805. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Seid, M.G.; Choe, J.W.; Kim, E.; Chung, Y.C.; Cho, K.; Lee, C.; Hong, S. Highly reusable TiO2 nanoparticle photocatalyst by direct immobilization on steel mesh via PVDF coating, electrospraying, and thermal fixation. Chem. Eng. J. 2016, 306, 344–351. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Li, H.; Cui, Z.; Hou, Y.; Shi, W.; Jiang, K.; Qu, L.; Zhang, Y. A novel synthesis of oleophylic Fe2O3/polystyrene fibers by γ-Ray irradiation for the enhanced photocatalysis of 4-chlorophenol and 4-nitrophenol degradation. J. Hazard. Mater. 2019, 379, 120806. [Google Scholar] [CrossRef] [PubMed]
- Kanth, N.; Xu, W.; Prasad, U.; Ravichandran, D.; Kannan, A.M.; Song, K. PMMA-TiO2 fibers for the photocatalytic degradation of water pollutants. Nanomaterials 2020, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Cates, E.L. Photocatalytic water treatment: So where are we going with this? Environ. Sci. Technol. 2017, 51, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Vali, A.; Malayeri, H.Z.; Azizi, M.; Choi, H. DPV-assisted understanding of TiO2 photocatalytic decomposition of aspirin by identifying the role of produced reactive species. Appl. Catal. B Environ. 2020, 266, 118646. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wu, T.; Que, W. Fabrication of Nanoparticle/Polymer Composite Photocatalytic Membrane for Domestic Sewage In Situ Treatment. Materials 2022, 15, 2466. https://doi.org/10.3390/ma15072466
Yang Y, Wu T, Que W. Fabrication of Nanoparticle/Polymer Composite Photocatalytic Membrane for Domestic Sewage In Situ Treatment. Materials. 2022; 15(7):2466. https://doi.org/10.3390/ma15072466
Chicago/Turabian StyleYang, Yawei, Tao Wu, and Wenxiu Que. 2022. "Fabrication of Nanoparticle/Polymer Composite Photocatalytic Membrane for Domestic Sewage In Situ Treatment" Materials 15, no. 7: 2466. https://doi.org/10.3390/ma15072466
APA StyleYang, Y., Wu, T., & Que, W. (2022). Fabrication of Nanoparticle/Polymer Composite Photocatalytic Membrane for Domestic Sewage In Situ Treatment. Materials, 15(7), 2466. https://doi.org/10.3390/ma15072466






