Evaluation of Mechanical and Electrical Performance of Aging Resistance ZTA Composites Reinforced with Graphene Oxide Consolidated by SPS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene Oxide Preparation
2.2. Powder Processing and Sintering
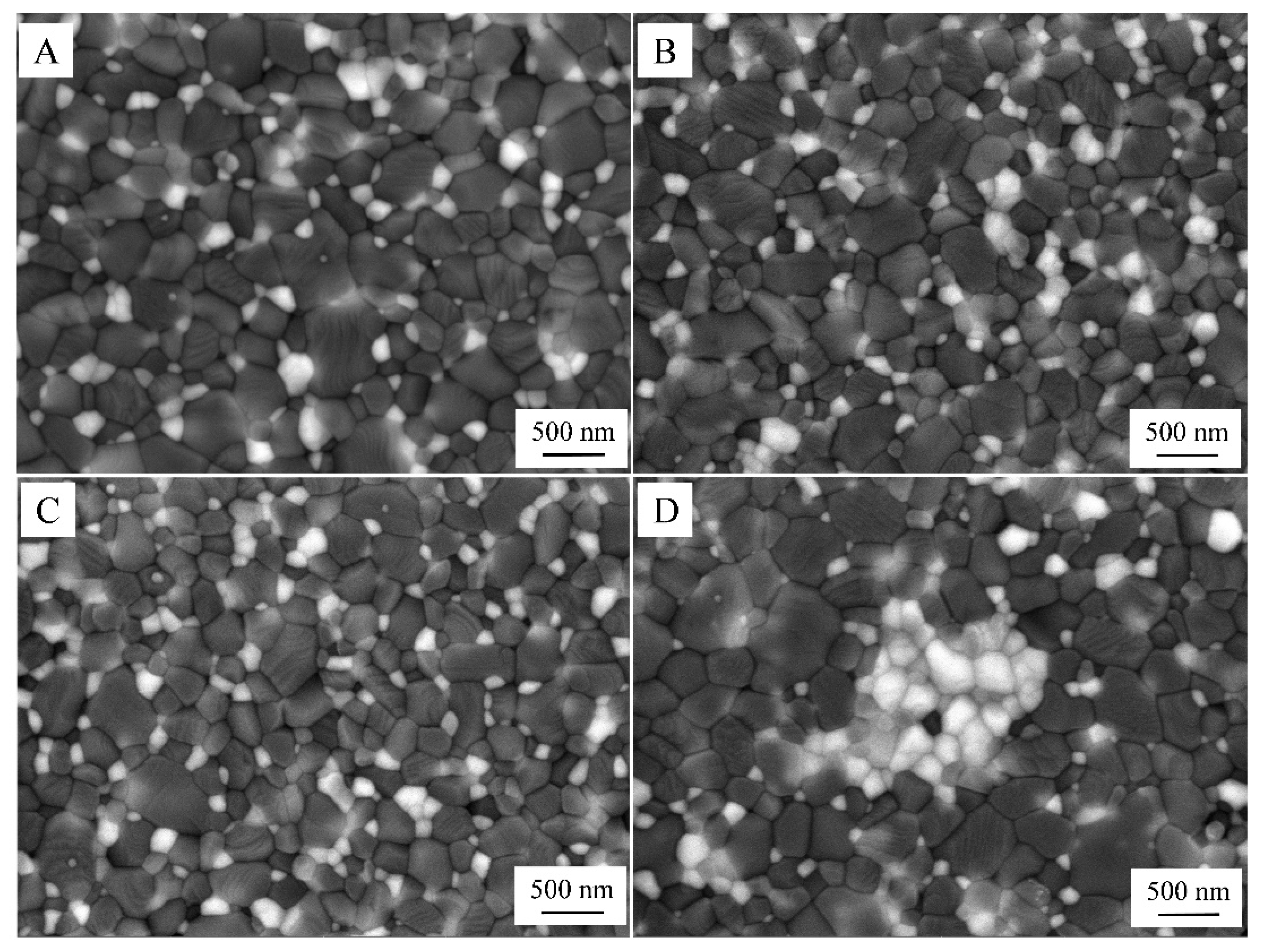
2.3. Microstructural Characterization
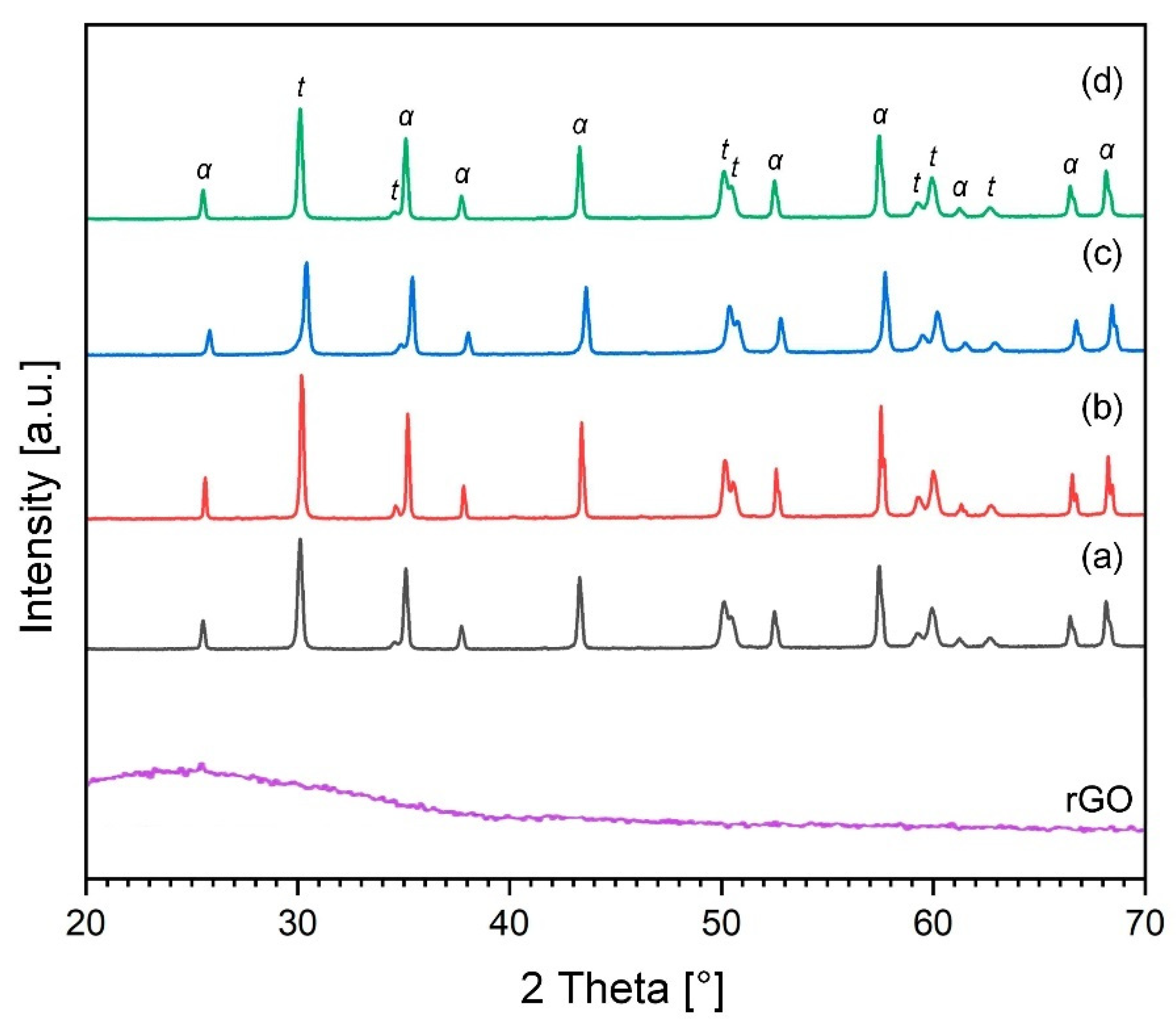
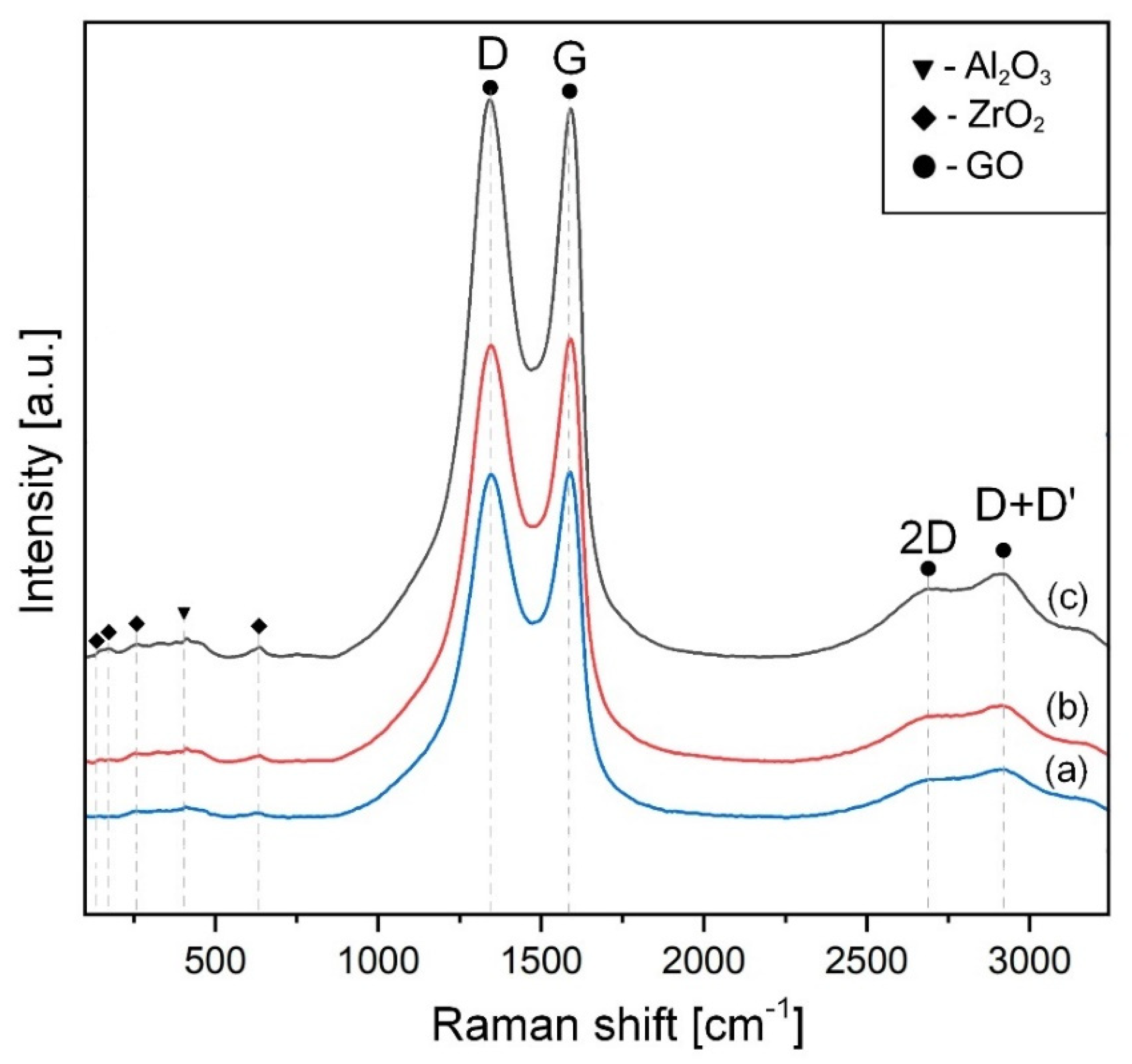
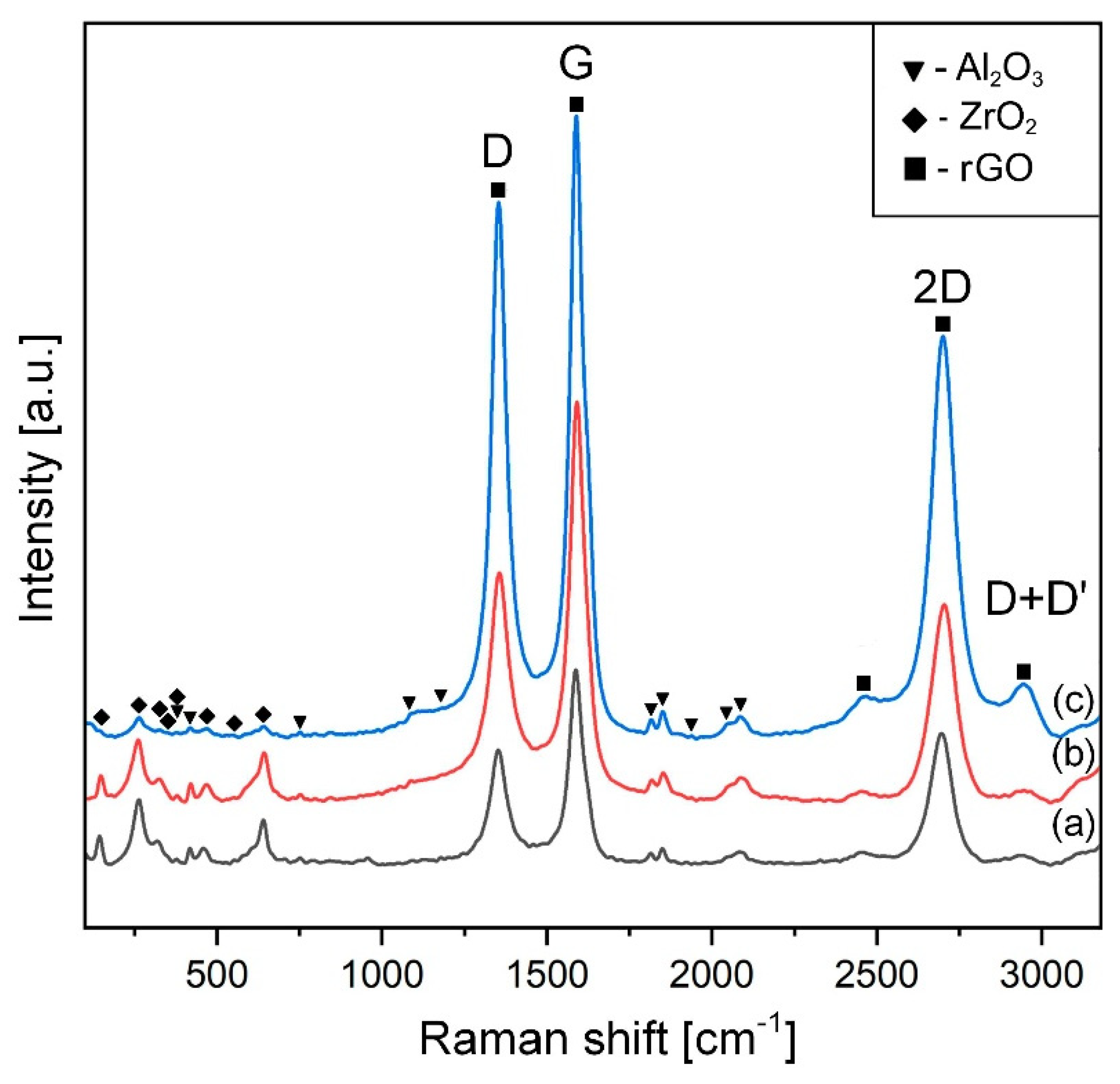
2.4. X-ray Diffraction (XRD), Raman Characterization
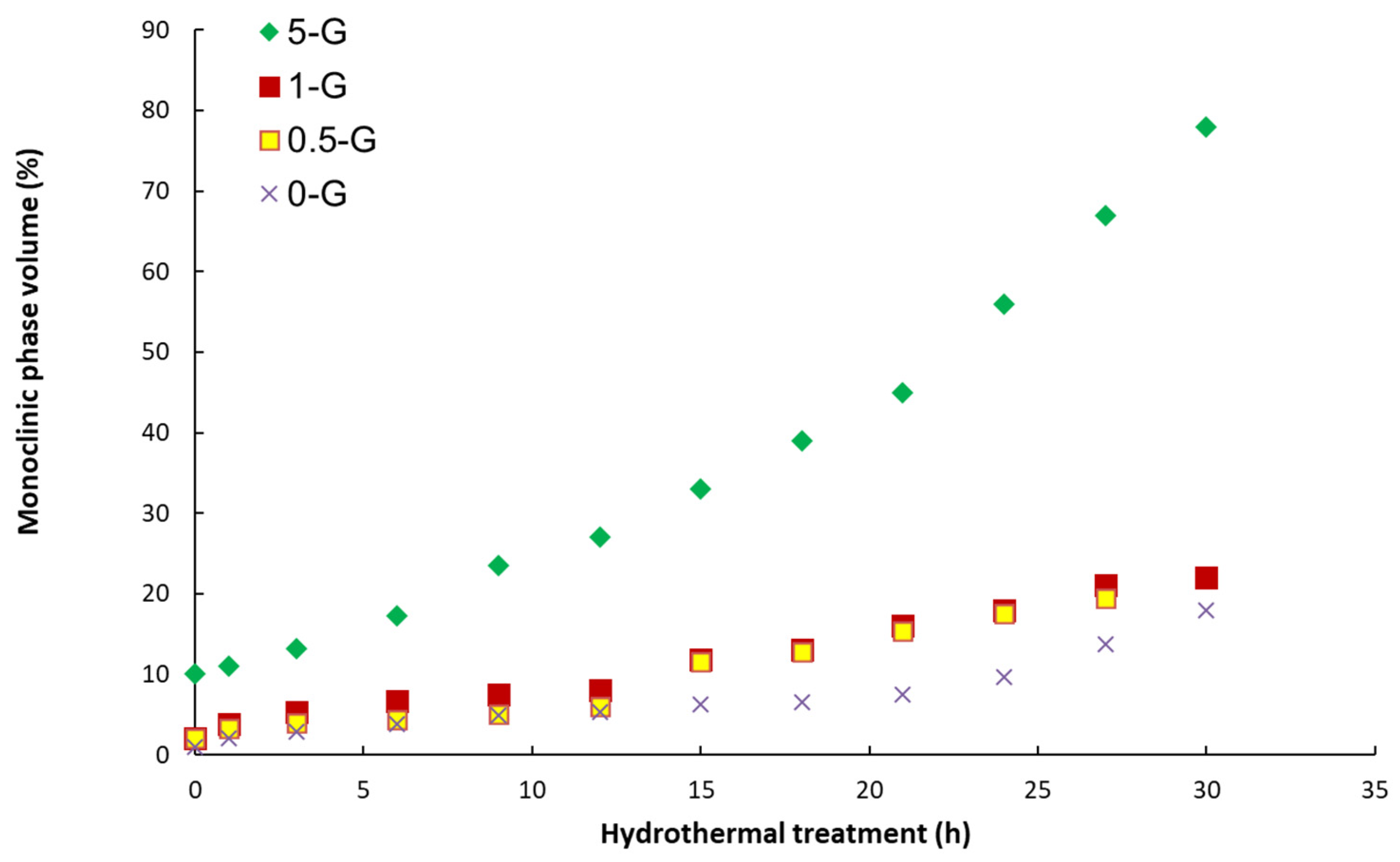
2.5. Aging Experiments
2.6. Mechanical Properties Characterization
2.7. Measurement of Electrical Resistance
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuan, W.H.; Chen, R.Z.; Wang, T.C.; Cheng, C.H.; Kuo, P.S. Mechanical properties of Al2O3/ZrO2 composites. J. Eur. Ceram. Soc. 2002, 22, 2827–2833. [Google Scholar] [CrossRef]
- Meng, F.; Liu, C.; Zhang, F.; Tian, Z.; Huang, W. Densification and mechanical properties of fine-grained Al2O3–ZrO2 composites consolidated by spark plasma sintering. J. Alloys Compd. 2012, 512, 63–67. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Wu, H.; Huang, R.; He, R.; Jiang, Q.; Wu, S. Research into the mechanical properties, sintering mechanism and microstructure evolution of Al2O3-ZrO2 composites fabricated by a stereolithography-based 3D printing method. Mater. Chem. Phys. 2018, 207, 1–10. [Google Scholar] [CrossRef]
- Piconi, C. Alumina. In Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 73–94. [Google Scholar] [CrossRef]
- Nettleship, I.; Stevens, R. Tetragonal zirconia polycrystal (TZP)—A review. Int. J. High Technol. Ceram. 1987, 3, 1–32. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Ezatpour, H.R.; Beygi, H. Microstructure and mechanical properties of Al–Al2O3 micro and nano composites fabricated by stir casting. Mater. Sci. Eng. 2011, 528, 8765–8771. [Google Scholar] [CrossRef]
- Li, S.; Wei, C.; Wang, P.; Gao, P.; Zhou, L.; Wen, G. Fabrication of ZrO2 whisker modified ZrO2 ceramics by oscillatory pressure sintering. Ceram. Int. 2020, 46, 17684–17690. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Bartolomé, J.F. Processing and mechanical properties of new hierarchical metal-graphene flakes reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3491–3497. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Clarke, I.C.; Manaka, M.; Green, D.D.; Williams, P.; Pezzotti, G.; Kim, Y.H.; Ries, M.; Sugano, N. Current status of zirconia used in total hip implants. J. Bone Jt. Surg. 2003, 85, 73–84. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-temperature degradation of zirconia and implications for biomedical implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Masonis, J.L.; Bourne, R.B.; Ries, M.D.; McCalden, R.W.; Salehi, A.; Kelman, D.C. Zirconia femoral head fractures. J. Arthroplast. 2004, 19, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.M.; Vohra, S.; Catledge, S.A.; McClenny, M.D.; Lemons, J.; Moore, K.D. Examination of surface and material properties of explanted zirconia femoral heads. J. Arthroplast. 2007, 9, 30–34. [Google Scholar]
- Maccauro, G.; Piconi, C.; Burger, W.; Pilloni, L.; De Santis, E.; Muratori, F.; Learmonth, I.D. Fracture of a Y-TZP ceramic femoral head. Bone Jt. Surg. 2004, 86, 1192–1196. [Google Scholar] [CrossRef]
- Insley, G.M.; Streicher, R.M. Next generation ceramics based on zirconia toughened alumina for hip joint prostheses. Key Eng. Mater. 2004, 254, 675–678. [Google Scholar] [CrossRef]
- Nakanishi, T.; Sasaki, M.; Ikeda, J.; Miyaji, F.; Kondo, M. Mechanical and phase stability of zirconia toughened alumina. Key Eng. Mater. 2007, 330–332, 1267–1270. [Google Scholar] [CrossRef]
- Hori, S.; Yoshimura, M.; Somiya, S. Strength-toughness relations in sintered and isostatically hot-process ZrO2-toughened Al2O3. J. Am. Ceram. Soc. 1986, 69, 169–172. [Google Scholar] [CrossRef]
- Magnani, G.; Brillante, A. Effect of the composition and sintering process on mechanical properties and residual stresses in zirconia–alumina composites. J. Eur. Ceram. Soc. 2005, 25, 3383–3392. [Google Scholar] [CrossRef]
- Begand, S.; Oberbach, T.; Glien, W. ATZ—A new material with a high potential in joint replacement. Key Eng. Mater. 2005, 284, 983–986. [Google Scholar] [CrossRef]
- Pecharromán, C.; Bartolomé, J.F.; Requena, J.; Moya, J.S.; Deville, S.; Chevalier, J.; Torrecillas, R. Percolative Mechanism of Aging in Zirconia-Containing Ceramics for Medical Applications. Adv. Mater. 2003, 15, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Suárez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R.; Kessel, H.U.; Hennicke, J.; Kirchner, R.; Kessel, T. Challenges and opportunities for spark plasma sintering: A key technology for a new generation of materials. Sinter. Appl. 2013, 13, 319–342. [Google Scholar]
- Wang, L.; Zhang, J.; Jiang, W. Recent development in reactive synthesis of nanostructured bulk materials by spark plasma sintering. Int. J. Refract. Met. Hard 2013, 39, 103–112. [Google Scholar] [CrossRef]
- Grigoriev, S.; Volosova, M.; Peretyagin, P.; Seleznev, A.; Okunkova, A.; Smirnov, A. The Effect of TiC Additives on Mechanical and Electrical Properties of Al2O3 Ceramic. Appl. Sci. 2018, 8, 2385. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, A.; Peretyagin, P.; Bartolomé, J.F. Wire electrical discharge machining of 3Y-TZP/Ta ceramic-metal composites. J. Alloys Compd. 2018, 739, 62–68. [Google Scholar] [CrossRef]
- Díaz, L.A.; Fernández, A.; Okunkova, A.; Solís, W.; Peretyagin, P.; Gotor Martínez, F.J.; Torrecillas, R. Electro conductive alumina nanocomposites from different alumina-carbides mixtures. MATEC Web Conf. 2016, 65, 02003. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.; Liu, Y.; Diao, R.; Xu, C.; Li, X.; Cai, B.; Zhang, Y. Influence of electrical resistivity and machining parameters on electrical discharge machining performance of engineering ceramics. PLoS ONE 2014, 9, e110775. [Google Scholar] [CrossRef]
- Grigoriev, S.; Peretyagin, P.; Smirnov, A.; Solís, W.; Díaz, L.A.; Fernández, A.; Torrecillas, R. Effect of graphene addition on the mechanical and electrical properties of Al2O3-SiCw ceramics. J. Eur. Ceram. Soc. 2017, 37, 2473–2479. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Solís Pinargote, N.W.; Gershman, I.; Bartolomé, J.F. Wear Behavior of Graphene-Reinforced Alumina–Silicon Carbide Whisker Nanocomposite. Nanomaterials 2019, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Miranzo, P.; Ramírez, C.; Román-Manso, B.; Garzón, L.; Gutiérrez, H.R.; Terrones, M.; Ocal, C.; Osendi, M.I.; Belmonte, M. In situ processing of electrically conducting graphene/SiC nanocomposites. J. Eur. Ceram. Soc. 2013, 33, 1665. [Google Scholar] [CrossRef]
- Yazdani, B.; Xia, Y.; Ahmad, I.; Zhu, Y. Graphene and carbon nanotube (GNT)-reinforced alumina nanocomposites. J. Eur. Ceram. Soc. 2015, 35, 179. [Google Scholar] [CrossRef]
- Lahiri, D.; Khaleghi, E.; Bakshi, S.R.; Li, W.; Olevsky, E.A.; Agarwal, A. Graphene-induced strengthening in spark plasma sintered tantalum carbide–nanotube composite. Scr. Mater. 2013, 68, 285. [Google Scholar] [CrossRef]
- Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. Toughening in graphene ceramic composites. ACS Nano 2011, 5, 3182. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Vereschaka, A.A.; Vereschaka, A.S.; Kutin, A.A. Cutting tools made of layered composite ceramics with nano-scale multilayered coatings. Procedia CIRP 2012, 1, 301–306. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Oh, Y.; Yang, Y.; Lim, Y. Unoxidized graphene/alumina nanocomposite: Fracture-and wear-resistance effects of graphene on alumina matrix. Sci. Rep. 2014, 4, 5176. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Botas, C.; Perez-Mas, A.M.; Alvarez, P.; Santamaria, R.; Granda, M.; Blanco, C.; Menendez, R. Optimization of the size and yield of graphene oxide sheets in the exfoliation step. Carbon 2013, 63, 576–578. [Google Scholar] [CrossRef] [Green Version]
- Garvie, R.C.; Nicholson, P.S. Phase Analysis in Zirconia Systems. J. Am. Ceram. Soc. 1972, 55, 303–305. [Google Scholar] [CrossRef]
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration Curve for Quantitative Analysis of the Monoclinic Tetragonal ZrO2 System by X-ray Diffraction. J. Am. Ceram. Soc. 1984, 67, 119–121. [Google Scholar]
- Miranzo, P.; Moya, J.S. Elastic/plastic indentation in ceramics: A fracture toughness determination method. Ceram. Int. 1984, 10, 147–152. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Wurst, J.C.; Nelson, J.A. Lineal intercept technique for measuring grain size in two-phase polycrystalline ceramics. J. Am. Ceram. Soc. 1972, 55, 109–111. [Google Scholar] [CrossRef]
- ISO 13356; Implants for Surgery—Ceramic Materials Based on Yttria-Stabilized Tetragonal Zirconia (Y-TZP). ISO: Geneva, Switzerland, 2008.



| Property/Characteristic | Alumina [4] | Zirconia [5] |
|---|---|---|
| Composition | Al2O3 | Y–TZP |
| Flexural strength | 400–580 MPa | 700–1500 MPa |
| Vickers hardness | 18.0–23.0 GPa | 11.0–12.5 GPa |
| Fracture toughness | 3.3–4.2 MPa·m1/2 | 4.5–20 MPa·m1/2 |
| Elastic modulus | 380 GPa | 210–233 GPa |
| Mean grain size | <1.8 µm | 0.1–0.6 µm |
| Thermal expansion coefficient | 8 × 10−6/°C | 11 × 10−6/°C |
| Density | 3.98 g/cm3 | 6.00–6.05 g/cm3 |
| Sample | Density (%ρth) | Vickers Hardness HV (GPa) | Fracture Toughness Kc (MPa∙m1/2) | Flexural Strength σf (MPa) |
|---|---|---|---|---|
| 0-G | 99.9 | 16.8 ± 0.2 | 5.2 ± 0.3 | 847 ± 30 |
| 0.5-G | 99.8 | 17.6 ± 0.3 | 6.8 ± 0.3 | 876 ± 43 |
| 1-G | 99.4 | 17.2 ± 0.3 | 5.3 ± 0.1 | 832 ± 27 |
| 5-G | 99.1 | 16.1 ± 0.1 | 5.2 ± 0.2 | 786 ± 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriev, S.; Smirnov, A.; Pinargote, N.W.S.; Yanushevich, O.; Kriheli, N.; Kramar, O.; Pristinskiy, Y.; Peretyagin, P. Evaluation of Mechanical and Electrical Performance of Aging Resistance ZTA Composites Reinforced with Graphene Oxide Consolidated by SPS. Materials 2022, 15, 2419. https://doi.org/10.3390/ma15072419
Grigoriev S, Smirnov A, Pinargote NWS, Yanushevich O, Kriheli N, Kramar O, Pristinskiy Y, Peretyagin P. Evaluation of Mechanical and Electrical Performance of Aging Resistance ZTA Composites Reinforced with Graphene Oxide Consolidated by SPS. Materials. 2022; 15(7):2419. https://doi.org/10.3390/ma15072419
Chicago/Turabian StyleGrigoriev, Sergey, Anton Smirnov, Nestor Washington Solis Pinargote, Oleg Yanushevich, Natella Kriheli, Olga Kramar, Yuri Pristinskiy, and Pavel Peretyagin. 2022. "Evaluation of Mechanical and Electrical Performance of Aging Resistance ZTA Composites Reinforced with Graphene Oxide Consolidated by SPS" Materials 15, no. 7: 2419. https://doi.org/10.3390/ma15072419
APA StyleGrigoriev, S., Smirnov, A., Pinargote, N. W. S., Yanushevich, O., Kriheli, N., Kramar, O., Pristinskiy, Y., & Peretyagin, P. (2022). Evaluation of Mechanical and Electrical Performance of Aging Resistance ZTA Composites Reinforced with Graphene Oxide Consolidated by SPS. Materials, 15(7), 2419. https://doi.org/10.3390/ma15072419








