Efficient Recycling Blast Furnace Slag by Constructing Ti-Embedded Layered Double Hydroxide as Visible-Light-Driven Photocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
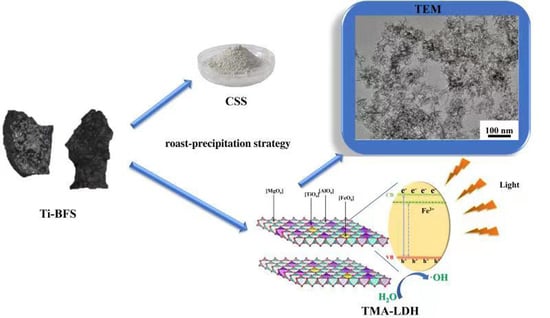
2.2. Recycling Process of Ti-BFS
2.3. Characterizations
2.4. Photocatalytic Activity Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Y.F.; Song, N.N.; Yang, Y.F.; Sun, L.M.; Hu, P.; Wang, J.S. Recent progress of efficient utilization of titanium-bearing blast furnace slag. Int. J. Min. Met. Mater. 2022, 29, 22–31. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, L.N.; Chen, D.S.; Wang, W.J.; Liu, Y.H.; Zhao, H.X.; Qi, T. A method for recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite. Int. J. Miner. Met. Mater. 2018, 25, 131–144. [Google Scholar] [CrossRef]
- Gong, P.J.; Xie, J.L.; Wua, H.T.; Zhang, Y.P.; Cheng, X.K. Novel CeZrTiAl catalyst for NH3-SCR of NOx based on Ti-bearing BFS. J. Environ. Chem. Eng. 2021, 9, 105233. [Google Scholar] [CrossRef]
- Dong, H.G.; Jiang, T.; Guo, Y.F.; Chen, J.L.; Fan, X.X. Upgrading a Ti-slag by a roast-leach process. Hydrometallurgy 2012, 113–114, 119–121. [Google Scholar] [CrossRef]
- Safdar, F.; Zhang, Y.; Zheng, S.L.; Chen, X.; Sun, P.; Zhang, Y.; Li, P. Recovery of TiO2-enriched material from vanadium titano-magnetite concentrates by partial carbon reduction and mild acid leaching. Hydrometallurgy 2020, 193, 105324. [Google Scholar] [CrossRef]
- Du, Y.; Gao, J.T.; Lan, X.; Guo, Z.C. Recovery of rutile from Ti-bearing blast furnace slag through phase transformation and super-gravity separation for dielectric material. Ceram. Int. 2020, 46, 9885–9893. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, H.H.; Wang, J.; Pang, Z.D.; Pei, G.S.; Yan, Z.M.; Mao, H.X.; Qiu, G.B.; Lv, X.W. Influence of TiO2 on viscosity, phase composition and structure of chromium-containing high-titanium blast furnace slag. J. Mater. Res. Technol. 2021, 12, 1615–1622. [Google Scholar] [CrossRef]
- Bian, X.A.; Zhang, S.; Zhao, Y.X.; Shi, R.; Zhang, T.R. Layered double hydroxide-based photocatalytic materials toward renewable solar fuels production. InfoMat 2021, 3, 719–738. [Google Scholar] [CrossRef]
- Yang, W.W.; Cai, W.Q.; Zhou, J.X.; Dang, C.X.; Peng, X.; Chen, Y.T.; Wei, X.C.; Bo, S.L.; Liang, S.L.; Luo, Z.J. Mussel-inspired MgAl-LDH/carbon fiber film modified by polydopamine for highly efficient removal of Pb2+. J. Environ. Chem. Eng. 2021, 9, 106634. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Yu, Y.; Zhou, J.Z.; Liu, J.Y.; Chi, Y.; Xu, Z.P.; Qian, G.R. Effective removal of pyrophosphate by Ca-Fe-LDH and its mechanism. Chem. Eng. J. 2012, 179, 72–79. [Google Scholar] [CrossRef]
- Li, Y.W.; Gao, W.; Peng, M.; Zhang, J.B.; Sun, J.L.; Xu, Y.; Hong, S.; Liu, X.; Liu, X.W.; Wei, M.; et al. Interfacial Fe5C2-Cu catalysts toward low-pressure syngas conversion to long-chain alcohols. Nat. Commun. 2020, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.F.; Liang, J.X.; Yao, J.; Zhao, Y.T.; Meng, Q.; He, G.Y.; Chen, H.Q. Synthesis of Ce-doped NiAl LDH/RGO composite as an efficient photocatalyst for photocatalytic degradation of ciprofloxacin. J. Environ. Chem. Eng. 2021, 9, 105405. [Google Scholar] [CrossRef]
- Hu, J.; Tang, X.M.; Dai, Q.; Liu, Z.Q.; Zhang, H.M.; Zheng, A.M.; Yuan, Z.Z.; Li, X.F. Layered double hydroxide membrane with high hydroxide conductivity and ion selectivity for energy storage device. Nat. Commun. 2021, 12, 3409. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.F.; Chen, M.; Yan, Y.; Wu, L.M. Nickel-cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Hoyo, C.D. Layered double hydroxides and human health: An overview. Appl. Clay Sci. 2007, 36, 103–121. [Google Scholar]
- Gao, R.; Mei, X.; Yan, D.P.; Liang, R.Z.; Wei, M. Nano-photosensitizer based on layered double hydroxide and isophthalic acid for singlet oxygenation and photodynamic therapy. Nat. Commun. 2018, 9, 2798. [Google Scholar] [CrossRef] [Green Version]
- Dib, M.; Ouchetto, H.; Akhramez, S.; Fadili, H.; Essoumhi, A.; Ouchetto, K.; Hafid, A.; Sajieddine, M.; Khouili, M. Preparation of Mg/Al-LDH nanomaterials and its application in the condensation of 3-amino-1-phenyl-2-pyrazolin-5-one with aromatic aldehyde. Mater. Today 2020, 22, 104–107. [Google Scholar] [CrossRef]
- Huang, C.; Nie, J.; Xu, Z.; Zhang, X.; Tang, J.; Wang, B.; Huang, J.; Du, C.; Chen, J. One-step hydrothermal synthesized 3D P–MoO3/FeCo LDH heterostructure electrocatalysts on Ni foam for high-efficiency oxygen evolution electrocatalysis. Int. J. Hydrog. Energy 2021, 46, 12992–13000. [Google Scholar] [CrossRef]
- Kumari, P.; Das, R.K.; Pal, B. Enhanced photocatalytic degradation of eco-toxic pharmaceutical waste diclofenac sodium by anion loaded Cu-Al LDH⋅Bi2O3 composites. J. Taiwan Inst. Chem. Eng. 2021, 129, 227–236. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Vieira, D.E.L.; Salak, A.N.; Ferreira, M.G.S.; Ferreira, A.; Kareiva, A. A comparative study of co-precipitation and sol-gel synthetic approaches to fabricate cerium-substituted Mg-Al layered double hydroxides with luminescence properties. Appl. Clay Sci. 2017, 143, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.P.; Li, Y.; Kong, Q.P.; Lan, Y.L. From wastes to functions: Preparation of layered double hydroxides from industrial waste and its removal performance towards phosphates. Environ. Sci. Pollut. Res. 2021, 29, 11893–11906. [Google Scholar] [CrossRef] [PubMed]
- Pavela, O.; Stamatea, A.; Zăvoianua, R.; Bucurb, I.; Bîrjegac, R.; Angelescua, E.; Pârvulescua, V. Mechano-chemical versus co-precipitation for the preparation of Y-modified LDHs for cyclohexene oxidation and Claisen-Schmidt condensations. Appl. Catal. A Gen. 2020, 605, 117797. [Google Scholar] [CrossRef]
- Zhang, P.; Qian, G.; Xu, Z.; Shi, H.; Ruan, X.; Yang, J.; Frost, R. Effective adsorption of sodium dodecylsulfate (SDS) by hydrocalumite (CaAl-LDH-Cl) induced by self-dissolution and re-precipitation mechanism. J. Colloid. Interf. Sci. 2012, 367, 264–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Xing, H.; Zhang, S.; Ren, Q.; Pan, L.; Zhang, K.; Bu, W.; Zheng, X.; Zhou, L.; Peng, W. A Gd-doped Mg-Al-LDH/Au nanocomposite for CT/MR bimodal imagings and simultaneous drug delivery. Biomaterials 2013, 34, 3390–3401. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, R.; Liu, N.; Dai, C.; Yu, G.; Wang, N.; Chen, B. In situ Ce-doped catalyst derived from NiCeAl-LDHs with enhanced low-temperature performance for CO2 methanation. Appl. Surf. Sci. 2022, 579, 152204. [Google Scholar] [CrossRef]
- Hadj-Abdelkader, N.E.H.; Beltrao-Nunesa, A.P.; Belkhademb, F.; Benselkab, N.; Roya, R.; Azzouz, A. New insights in MgAl and MgFe-LDH affinity towards carbon dioxide—Role of the hydrophilic character on CO2 retention strength. Appl. Clay Sci. 2020, 198, 105829. [Google Scholar] [CrossRef]
- Wu, X.L.; Tan, X.L.; Yang, S.T.; Wen, T.; Guo, H.L.; Wang, X.K.; Xu, A.W. Coexistence of adsorption and coagulation processes of both arsenate and NOM from contaminated groundwater by nanocrystallined Mg/Al layered double hydroxides. Water Res. 2013, 47, 4159–4168. [Google Scholar] [CrossRef]
- He, J.; Shi, H.M.; Shu, X.; Li, M.L. On the nature of Ti(IV)-pillared layered metal hydroxides prepared from green, water-soluble Ti-peroxide. AIChE J. 2010, 56, 1352–1362. [Google Scholar] [CrossRef]
- Luo, X.G.; Lei, X.J.; Cai, N.; Xie, X.P.; Xue, Y.N.; Yu, F.Q. Removal of heavy metal ions from water by magnetic cellulose-based beads with embedded chemically modified magnetite nanoparticles and activated carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Vyalikh, A.; Costa, F.R.; Wagenknecht, U.; Heinrich, G.; Massiot, D.; Scheler, U. From layered double hydroxides to layered double hydroxide-based nanocompositess-a solid-state NMR Study. J. Phys. Chem. C 2009, 113, 21308–21313. [Google Scholar] [CrossRef]
- Licheron, M.; Montouillout, V.; Millot, F.; Neuville, D.R. Raman and 27Al NMR structure investigations of aluminate glasses: (1−x)Al2O3−x MO, with M = Ca, Sr, Ba and 0.5 < x < 0.75). J. Non-Cryst. Solids 2011, 357, 2796–2801. [Google Scholar]
- Arco, M.; Fernández, A.; Martín, C.; Rives, V. Solubility and release of fenbufen intercalated in Mg, Al and Mg, Al, Fe layered double hydroxides (LDH): The effect of eudragit® S 100 covering. J. Solid State Chem. 2010, 183, 3002–3009. [Google Scholar] [CrossRef]
- Rahman, M.T.; Kameda, T.; Kumagai, S.; Yoshioka, T. A novel method to delaminate nitrate-intercalated Mg-Al layered double hydroxides in water and application in heavy metals removal from waste water. Chemosphere 2018, 203, 281–290. [Google Scholar] [CrossRef]
- Du, X.C.; Huang, J.W.; Zhang, J.J.; Yan, Y.C.; Wu, C.Y.; Hu, Y.; Yan, C.Y.; Lei, T.Y.; Chen, W.; Fan, C.; et al. Modulating electronic structures of inorganic nanomaterials for efficient electrocatalytic water splitting. Angew. Chem. Int. Ed. 2019, 58, 4484–4502. [Google Scholar] [CrossRef]
- Wu, Y.S.; Liu, X.J.; Han, D.D.; Song, X.Y.; Shi, L.; Song, Y.; Niu, S.W.; Xie, Y.F.; Cai, J.Y.; Wu, S.Y.; et al. Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nat. Commun. 2018, 9, 1425. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Yuan, J.; Guo, Y.; Luo, X.G. In situ nano-assembly of Mg/Al LDH embedded on phosphorylated cellulose microspheres for tetracycline hydrochloride removal. Cellulose 2020, 28, 301–316. [Google Scholar] [CrossRef]
- Lu, R.J.; Xu, X.; Chang, J.P.; Zhu, Y.; Xu, S.L.; Zhang, F.Z. Improvement of photocatalytic activity of TiO2 nanoparticles on selectively reconstructed layered double hydroxide. Appl. Catal. B Environ. 2012, 111–112, 389–396. [Google Scholar] [CrossRef]
- Duan, S.; Chen, S.Q.; Wang, T.Y.; Li, S.Z.; Liu, J.Y.; Liang, J.S.; Xie, H.Q.; Han, J.T.; Jiao, S.H.; Cao, R.G.; et al. Elemental selenium enables enhanced water oxidation electrocatalysis of NiFe layered double hydroxides. Nanoscale 2019, 11, 17376–17383. [Google Scholar] [CrossRef]
- Wang, Z.C.; Fang, P.F.; Kumar, P.; Wang, W.W.; Liu, B.; Li, J. Controlled growth of LDH films with enhanced photocatalytic activity in a mixed wastewater treatment. Nanomaterials 2019, 9, 807. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Zhu, Z.; Zheng, J.; Liu, Z.; Wang, Q.; Yan, Y.S. Ultrathin magnetic Mg-Al LDH photocatalyst for enhanced CO2 reduction: Fabrication and mechanism. J. Colloid. Interf. Sci. 2019, 555, 1–10. [Google Scholar] [CrossRef]
- Shi, Y.L.; Li, J.Q.; Zhang, B.Y.; Lv, S.Y.; Wang, T.; Liu, X. Tuning electronic structure of CoNi LDHs via surface Fe doping for achieving effective oxygen evolution reaction. Appl. Surf. Sci. 2021, 565, 150506. [Google Scholar] [CrossRef]
- Khodam, F.; Amani-Ghadim, H.R.; Aber, S.; Amani-Ghadim, A.R.; Ahadzadeh, I. Neodymium doped mixed metal oxide derived from CoAl-layered double hydroxide: Considerable enhancement in visible light photocatalytic activity. J. Ind. Eng. Chem. 2018, 68, 311–324. [Google Scholar] [CrossRef]
- Wang, X.Y.; Tuo, Y.X.; Zhou, Y.; Wang, D.; Wang, S.T.; Zhang, J. Ta-doping triggered electronic structural engineering and strain effect in NiFe LDH for enhanced water oxidation. Chem. Eng. J. 2021, 403, 126297. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.H.; Jeon, H.J.; Choi, K.M.; Lee, J.W.; Kang, J.K. Titanium-embedded layered double hydroxides as highly efficient water oxidation photocatalysts under visible light. Energy Environ. Sci. 2011, 4, 914–920. [Google Scholar] [CrossRef]
- Zhou, M.; Han, D.; Liu, X.; Ma, C.; Wang, H.; Tang, Y.; Huo, P.; Shi, W.; Yan, Y.; Yang, J. Enhanced visible light photocatalytic activity of alkaline earth metal ions-doped CdSe/rGO photocatalysts synthesized by hydrothermal method. Appl. Catal. B Environ. 2015, 172–173, 174–184. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, L.; Wang, J.; Zhu, Y.; Pu, Y.; Dai, W. Photocatalytic degradation of tetracycline antibiotics using three-dimensional network structure perylene diimide supramolecular organic photocatalyst under visible-light irradiation. Appl. Catal. B Environ. 2020, 277, 119122. [Google Scholar] [CrossRef]
- Jing, H.; Ou, R.; Yu, B.; Zhao, Y.; Lu, Y.; Huo, M.; Huo, H.; Wang, X. Engineering of g-C3N4 nanoparticles/WO3 hollow microspheres photocatalyst with Z-scheme heterostructure for boosting tetracycline hydrochloride degradation. Sep. Purif. Technol. 2021, 255, 117646. [Google Scholar] [CrossRef]










| Composition | CaO | SiO2 | TiO2 | MgO | Al2O3 |
|---|---|---|---|---|---|
| wt. % | 28.91 | 26.16 | 21.45 | 9.05 | 14.43 |
| Compositional Element | CSS (wt. %) | TMA-LDH (wt. %) | Recovery Efficiency (wt. %) |
|---|---|---|---|
| Ca | 55.67 | 5.71 | 88.11 |
| Si | 37.59 | 1.74 | 96.00 |
| Ti | 5.30 | 52.19 | 95.26 |
| Al | 0.05 | 17.83 | 97.14 |
| Mg | 1.39 | 22.53 | 91.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, N.; Cai, Y.; Sun, L.; Hu, P.; Zhou, Q.; Wu, J.; Wang, J. Efficient Recycling Blast Furnace Slag by Constructing Ti-Embedded Layered Double Hydroxide as Visible-Light-Driven Photocatalyst. Materials 2022, 15, 1514. https://doi.org/10.3390/ma15041514
Song N, Cai Y, Sun L, Hu P, Zhou Q, Wu J, Wang J. Efficient Recycling Blast Furnace Slag by Constructing Ti-Embedded Layered Double Hydroxide as Visible-Light-Driven Photocatalyst. Materials. 2022; 15(4):1514. https://doi.org/10.3390/ma15041514
Chicago/Turabian StyleSong, Ningning, Yongfeng Cai, Lingmin Sun, Peng Hu, Qinqin Zhou, Junshu Wu, and Jinshu Wang. 2022. "Efficient Recycling Blast Furnace Slag by Constructing Ti-Embedded Layered Double Hydroxide as Visible-Light-Driven Photocatalyst" Materials 15, no. 4: 1514. https://doi.org/10.3390/ma15041514
APA StyleSong, N., Cai, Y., Sun, L., Hu, P., Zhou, Q., Wu, J., & Wang, J. (2022). Efficient Recycling Blast Furnace Slag by Constructing Ti-Embedded Layered Double Hydroxide as Visible-Light-Driven Photocatalyst. Materials, 15(4), 1514. https://doi.org/10.3390/ma15041514