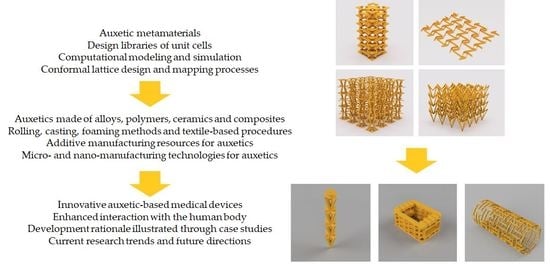
Auxetic Metamaterials for Biomedical Devices: Current Situation, Main Challenges, and Research Trends
Abstract
:1. Introduction
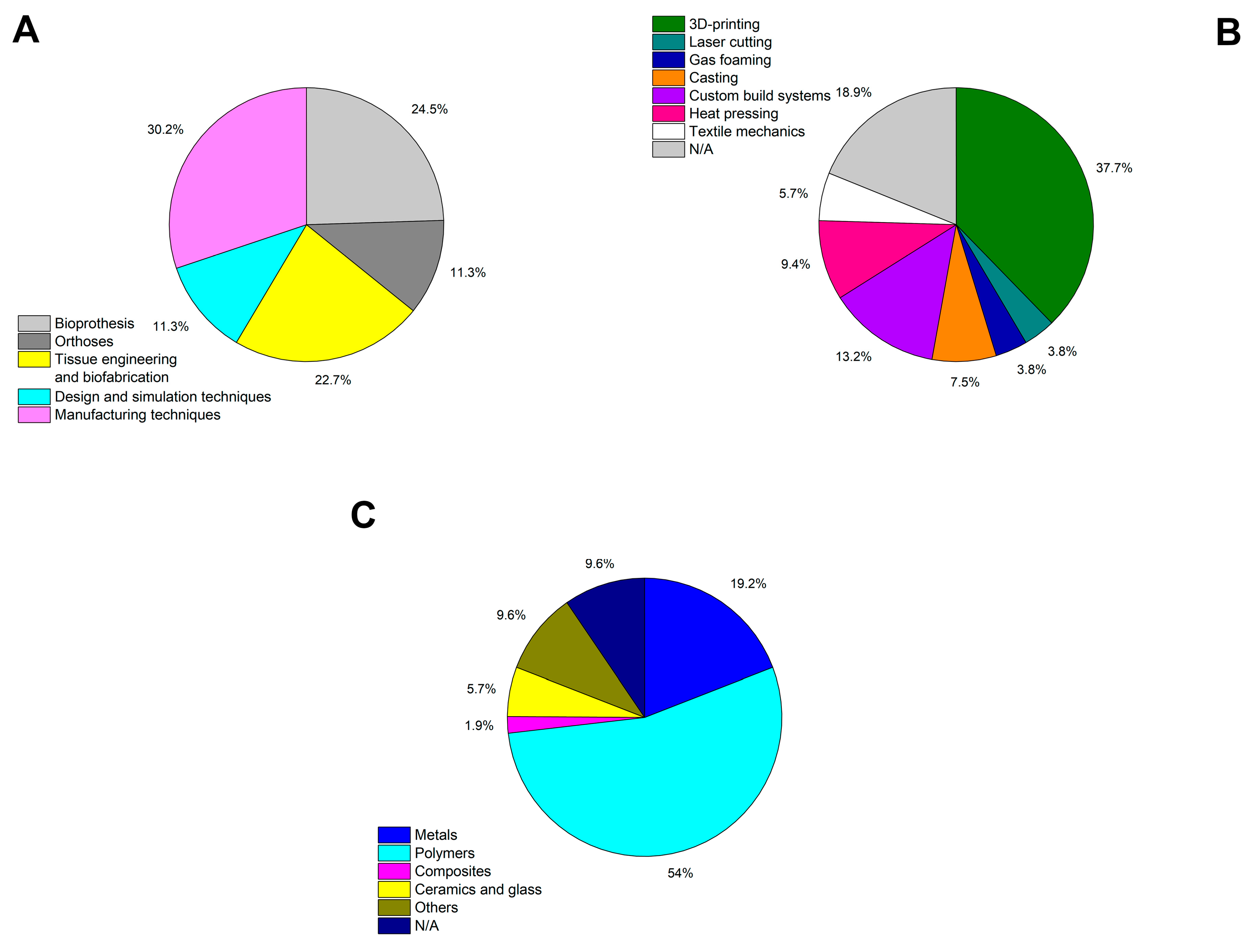
2. Overview of Medical Applications for Auxetics
2.1. Bioprostheses
2.1.1. Spinal Surgery
2.1.2. Stents
2.1.3. Hip Implant Stems
2.1.4. Fixation for Long Bones
2.1.5. Cardiac Patches
2.1.6. Nasopharyngeal Swabs
2.2. Orthoses
2.2.1. Orthoses, Bandages, Orthopaedic Insoles
2.2.2. Sport Protection
2.3. Tissue Engineering and Biofabrication
2.3.1. Scaffolds
2.3.2. Auxetic Structures and Membranes for In Vitro Medical Devices
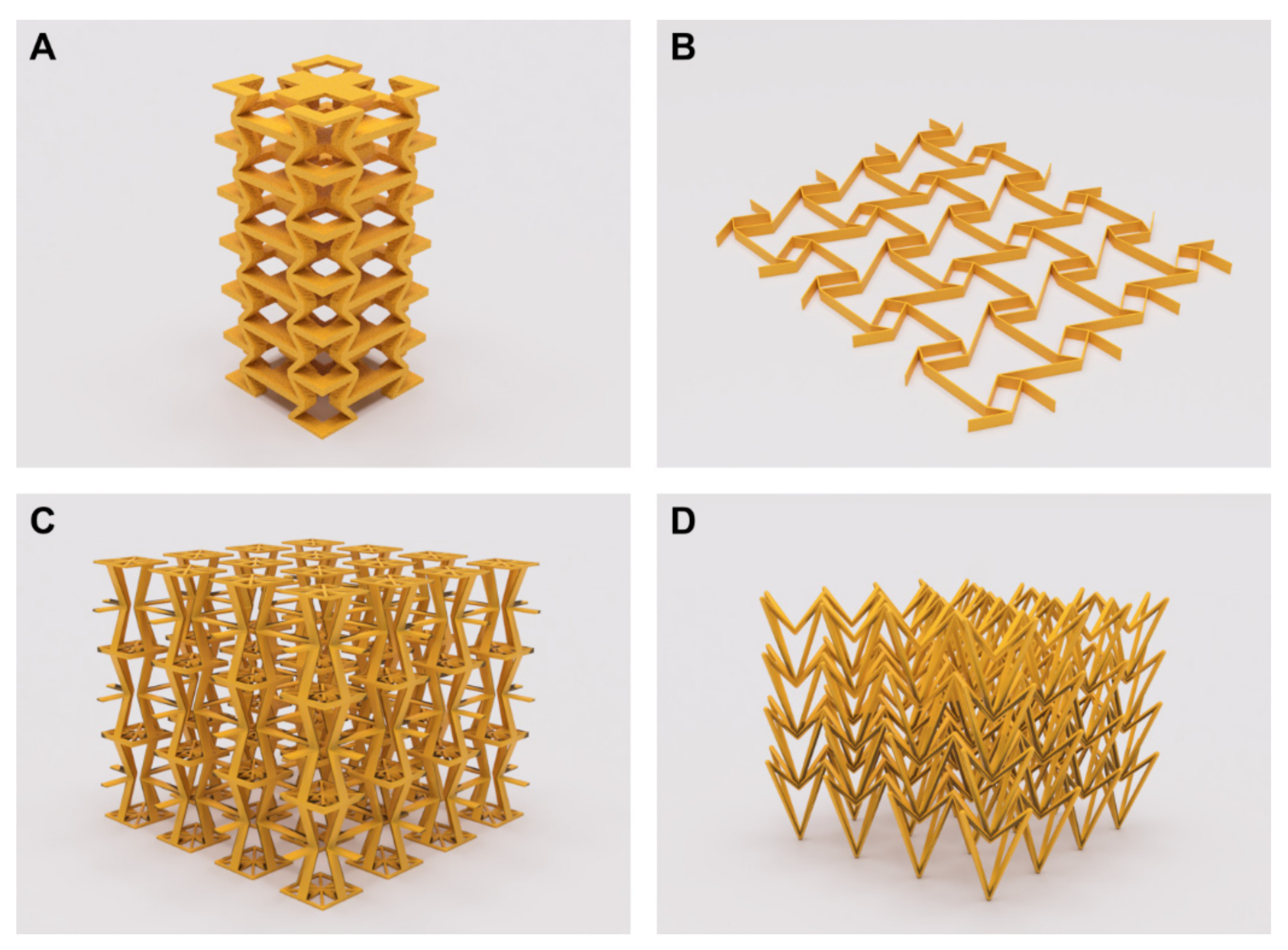
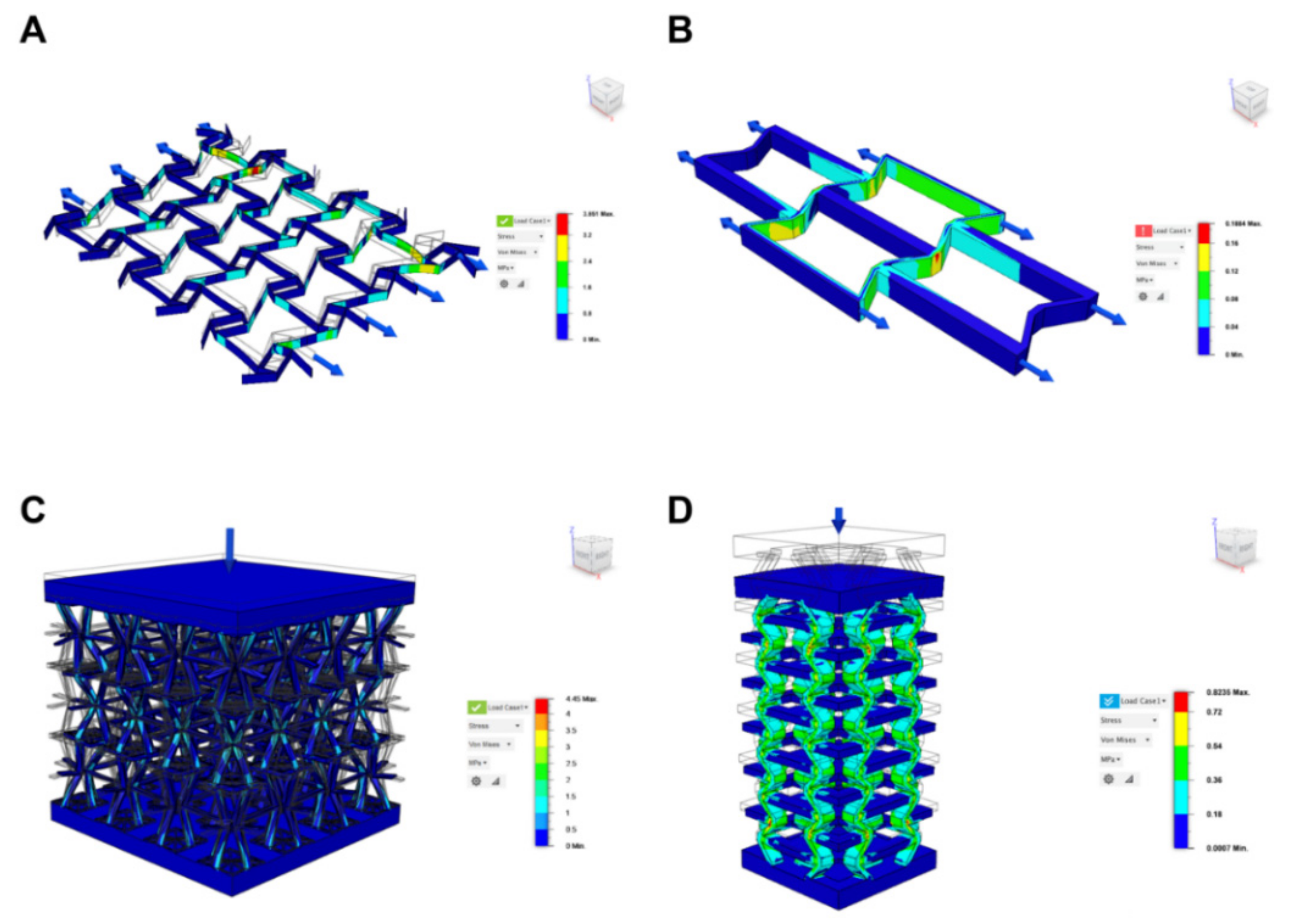
3. Design and Modelling of Auxetic Geometries and Implants
3.1. Supporting Simulation and Topology Optimization Resources
3.2. Computational Prediction and AI-Aided Design of New Auxetic Geometries
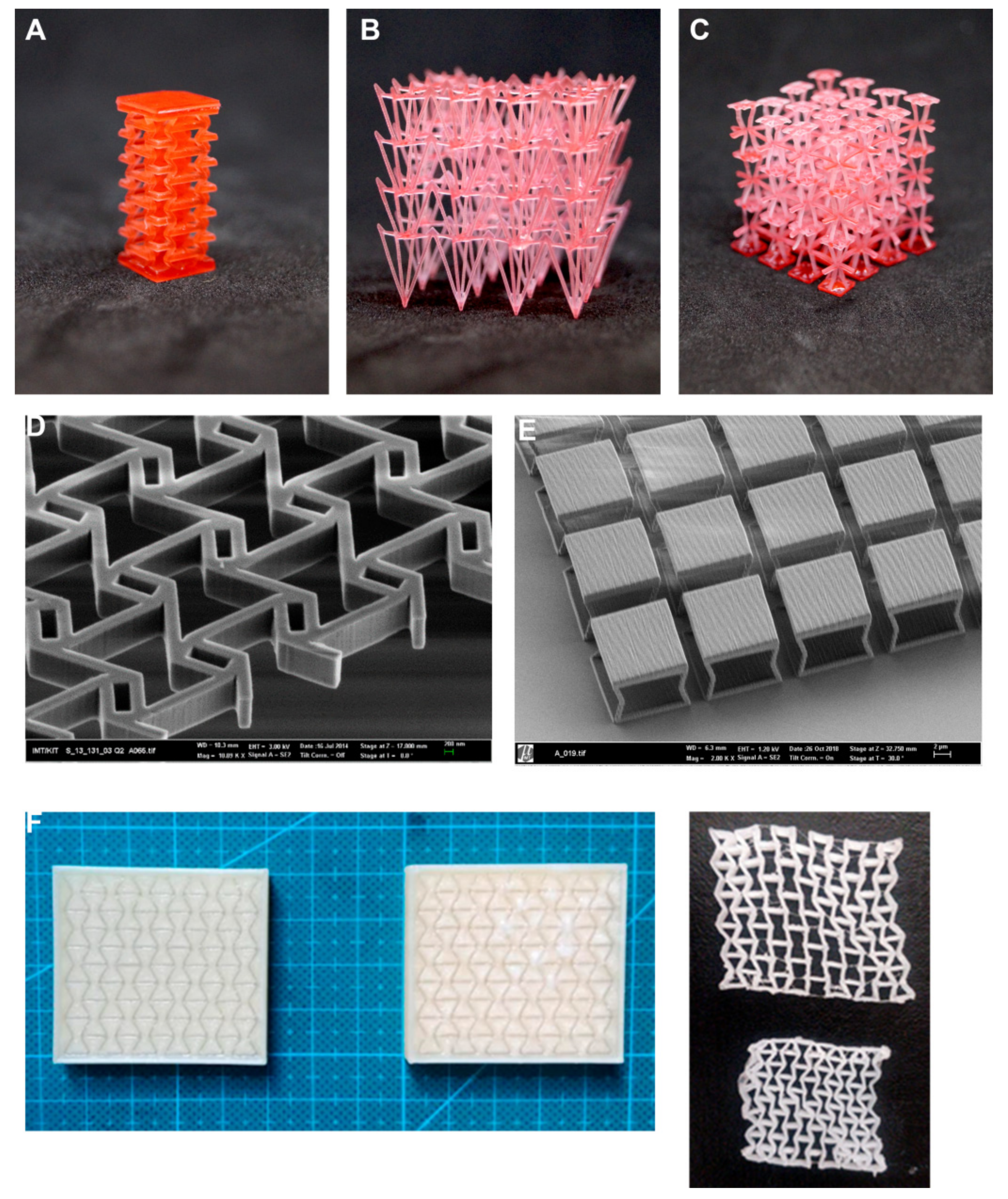
4. Prototyping and Manufacturing Methods
4.1. Traditional Methods
4.1.1. Rolling, Casting, and Foaming Methods
4.1.2. Textile Based Processes
4.2. Additive Manufacturing
4.3. Micro/Nanomanufacturing Methods, Multi-Scale and Multi-Material Approaches
5. Summary of Design Rationale and Development
- (1)
- Are solutions already employed for solving the concrete optimal medical needs?
- (2)
- Would an auxetic geometry provide potential benefits?
- (3)
- Is it possible to transform state-of-the-art solutions into innovative auxetic-based designs?
- (4)
- Should the design be oriented to personalization or mass production?
6. Future Trends and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Research Type | Research Objective | |
|---|---|---|---|
| 1 | Martz et al. [10]—Design of an artificial intervertebral disc exhibiting a negative Poisson’s ratio | Spinal surgery | Investigate the feasibility of an artificial intervertebral lumbar disc to eliminate problems of bulge |
| 2 | Baker et al. [11]—Auxetic spinal implants: consideration of negative Poisson’s ratio in the design of an artificial intervertebral disc | Spinal surgery | Investigate possibility of eliminating the damage to the surrounding nerves by the artificial intervertebral disc |
| 3 | Yao et al. [12] –Biomechanical design and analysis of auxetic pedicle screw to resist loosening | Spinal surgery | Design of auxetic pedicle screw to improve the biomechanical interaction of the surrounding bone and screw |
| 4 | Amin et al. [13]—Auxetic coronary stent endoprosthesis: fabrication and structural analysis | Stents | Development of a coronary stent for increasing mechanical adhesion with the arterial wall |
| 5 | Liua et al. [14]—A flexible porous chiral auxetic tracheal stent with ciliated epithelium | Stents | Development of a tracheal stent for expanding the ventilation cross-section and enhance the anti-migration force of the stent |
| 6 | Wua et al. [15]—Mechanical properties of anti-tetrachiral auxetic stents | Stents | Study of coronary stent interaction with an arterial model |
| 7 | Kolken et al. [16]—Rationally designed meta-implants: a combination of auxetic and conventional meta-biomaterials | Hip stems implant (THR) | Development the efficient hybrid implants for increasing implant longevity |
| 8 | Ghavidelnia et al. [21]—Femur auxetic Meta-Implants with tuned micromotion distribution | Hip stems implant (THR) | Development of a hip stems implant with gradient auxetic structure to reduce micromotions and stress shielding |
| 9 | Mehmood et al. [22]—Auxetic polymeric bone plate as internal fixator for long bone fractures: Design, fabrication and structural analysis | Fixation for long bones | Development of an auxetic bone plate to reduce stress shielding and increase micromotions |
| 10 | Kapnisi et al. [30]—Auxetic cardiac patches with tuneable mechanical and conductive properties toward treating myocardial infarction | Cardiac patches | Developed auxetic conductive cardiac patches for the treatment of myocardial infarction (MI) |
| 11 | Arjunan et al. [32]—3D printed auxetic nasopharyngeal swabs for COVID-19 sample collection | Nasopharyngeal swabs | Design of nasopharyngeal swabs to reduce the stress on the surrounding tissues in the nasal cavity during swab collection |
| 12 | Panico et al. [33]—Development of a biomedical neck brace through tailored auxetic shapes | Orthoses, bandages, orthopaedic insoles | Development of a neck brace for orthopaedic purposes |
| 13 | Hinrichs et al. [35]—Active auxetic heel support for Achilles tendon therapy, Senior Design Project Report | Orthoses, bandages, orthopaedic insoles | Development of flexible mesh materials with the ability to adjust their flexibility and strength to simulate and support muscles and tendons |
| 14 | Pattinson et al. [36]—Additive manufacturing of biomechanically tailored meshes for compliant wearable and implantable devices | Orthoses, bandages, orthopaedic insoles | Presenting approach to digital fabrication of biomechanically tailored auxetic mesh materials (ankle brace) using AM |
| 15 | Mottram et al. [37]—US Patent for cranial remoulding orthosis and method of manufacture thereof (Patent # 10,695,211) | Orthoses, bandages, orthopaedic insoles | The purpose of the invention is to eliminate some of the disadvantages of existing orthoses, as well as to create an economical, effective, and hygienic orthosis for correcting head deformities |
| 16 | Moroney et al. [38]—Application of auxetic material for protective sports apparel | Sport protection | Investigate auxetic materials potential for enhanced wearer functionality through properties of synclastic curvature and biaxial expansion |
| 17 | Chen et al. [39]—Cyclic tensile stimulation enrichment of Schwann cell-laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering | Scaffolds | Development auxetic hydrogel scaffolds and studied cyclic tensile stimulation effects on the neural differentiation capabilities of human Schwann cells |
| 18 | Flamourakis et al. [40]—Laser-made 3D Auxetic Metamaterial Scaffolds for Tissue Engineering Applications | Scaffolds | Development adaptable auxetic scaffolds for tissue engineering applications |
| 19 | Yan et al. [41]—Pluripotent stem cell expansion and neural differentiation in 3-D scaffolds of tuneable Poisson’s ratio | Scaffolds | Evaluate the ability of the 3-D auxetic scaffolds with tuneable biophysical properties (E and ν) to influence PSC expansion and neural differentiation |
| 20 | Song et al. [42]—Vascular differentiation from pluripotent stem cells in 3-D auxetic scaffolds | Scaffolds | Demonstrated 3D auxetic scaffolds for vascular differentiation and provides a platform to study the influence of biophysical microenvironments on differentiation of pluripotent stem cells |
| 21 | Díaz Lantada et al. [43]—Auxetic tissue engineering scaffolds with nanometric features and resonances in the megahertz range | Scaffolds | Presenting an approach for the development of auxetics based on the use of deep reactive ion etching (DRIE) |
| 22 | Soman et al. [45]—Spatial tuning of negative and positive Poisson’s ratio in a multi-layer scaffold | Scaffolds | Developing of hybrid scaffolds for integration with human mesenchymal stem cells |
| 23 | Pagliara et al. [46]—Auxetic nuclei in embryonic stem cells exiting pluripotency Nature Materials | Auxetic structures and membranes for in vitro medical devices | Study of the mechanical phenotype of the metastable phenotype of the transition of ESCs the study of the nuclear response to compressive and tensile forces |
| 24 | Warner et al. [48]—3D-printed biomaterials with regional auxetic properties | Scaffolds | Developing scaffolds to aid in tendon-to-muscle tissue regeneration, i.e., appropriate scale for clinical tissue replacement, unit-cell architectures capable of supporting aggregate cell growth, and tuneable auxetic kinematics with actuation and mechanical energy storage capabilities that mimic tendon behaviour |
| 25 | Xue et al. [49]—Design of self-expanding auxetic stents using topological optimization | Stents/Simulation and topology optimization | Develop a topology optimization method |
| 26 | Auricchio et al. [50]—A novel layered topology of auxetic materials based on the tetrachiral honeycomb microstructure | Simulation and topology optimization | Design, test and compare bi-tetrachiral structure with tetrachiral structure, using both solid and beam lattice models |
| 27 | Wilt et al. [51]—Accelerating auxetic metamaterial design with Deep Learning | Simulation and topology optimization | Develop an auxetic material design process based on predictions by FEA and deep learning |
| 28 | Nguyen et al. [52]—Conformal lattice structure design and fabrication | Computational prediction and AI-aided design | Develop a method of CAD-generation of MSLS for complex-shaped parts |
| 29 | Engelbrecht et al. [53]—Design of meso-scale cellular structure for rapid manufacturing | Computational prediction and AI-aided design | Develop a method to generate one or more layers of meso-scale cellular structure for a given surface |
| 30 | Chu et al. [54]—A comparison of synthesis methods for cellular structures with application to additive manufacturing | Computational prediction and AI-aided design of new auxetic | Comparison of two synthesis methods, Particle Swarm Optimization (PSO) and least-squares minimization (LSM), for the design of components comprised of cellular structures |
| 31 | Dagdelen et al. [57]—Computational prediction of new auxetic materials | Simulation and topology optimization | Develop a database screening process to find non-organic crystalline materials with auxetic properties |
| 32 | Vinay et al. [58]—Fabrication and Testing of Auxetic Foams for Rehabilitation Applications | Traditional methods of manufacturing auxetics | Exploring auxetic foams for suitability in various rehabilitation applications |
| 33 | Plant et al. [59]—Injection mouldable rate stiffening re-entrant cell arrays for wearable impact protection | Traditional methods of manufacturing auxetics/Sport protection | Describing design and testing of affordable personal protection using an intrinsically impact-mitigating auxetic structure, moulded from rate stiffening thermoplastic blend |
| 34 | Ghaedizadeh et al. [60]—Tuning the performance of metallic auxetic metamaterials by using buckling and plasticity | Traditional methods of manufacturing auxetics | Guideline for the design of 2D metallic auxetics for various applications |
| 35 | Taylor et al. [61]—Low Porosity Metallic Periodic Structures with Negative Poisson’s Ratio | Traditional methods of manufacturing auxetics | Investigate the effect of the hole aspect ratio on the macroscopic Poisson’s ratio |
| 36 | Grujicic et al. [62]—multi-physics modelling of the fabrication and dynamic performance of all-metal auxetic-hexagonal sandwich-structures | Rolling, casting, and foaming methods of manufacturing auxetics | Investigate the effect of the prior processing and the resulting microstructure on the performance of all-metal sandwich-structures with an auxetic-hexagonal core |
| 38 | Ali et al. [29]—Auxetic polyurethane stents and stent-grafts for the palliative treatment of squamous cell carcinomas of the proximal and mid oesophagus: a novel fabrication route | Stents, rolling, casting, 3D printing and foaming methods of manufacturing auxetics | Development of a small diameter auxetic stent to the palliative treatment of squamous cell carcinomas of the oesophagus and for the prevention of dysphagia |
| 39 | Scarpa et al. [63]—Auxetic compliant flexible PU foams: static and dynamic properties | Rolling, casting, and foaming methods of manufacturing auxetics | Manufacturing of auxetic thermoplastic polyurethane foams |
| 40 | Jiang et al. [64]—A study of tubular braided structure with negative Poisson’s ratio behaviour | Textile based processes manufacturing of auxetics | Development of auxetic production process by modifying the pipe braiding technology. |
| 41 | Sloan et al. [65]—The helical auxetic yarn—A novel structure for composites and textiles; geometry, manufacture, and mechanical properties | Textile based processes manufacturing of auxetics | Describing the manufacture of monofilament HAYs and mechanical characterization process in detail and identify the mechanism behind the observed auxetic behaviour |
| 42 | Bhattacharya et al. [66]—The variation in Poisson’s ratio caused by interactions between core and wrap in helical composite auxetic yarns | Textile based processes manufacturing of auxetics | Study of the helical auxetic yarn via characterization of a wide range of polymeric fibres and yarns |
| 43 | Zhou et al. [67]—Auxetic composites made of 3D textile structure and polyurethane foam | Textile based processes manufacturing of auxetics | Development 3D auxetic textile composites for different potential applications |
| 44 | Vyavahare et al. [75]—Re-entrant auxetic structures fabricated by fused deposition modelling: An experimental study of influence of process parameters under compressive loading | 3D-printing methods (FDM, SLM, SLS, stereolithographic methods) manufacturing of auxetics | Investigating the influence of process parameters under compressive loading of auxetic structures |
| 45 | Alomarah et al. [76]—An investigation of in-plane tensile properties of re-entrant chiral auxetic structure | 3D-printing methods (FDM, SLM, SLS, stereolithographic methods) manufacturing of auxetics | Investigation of mechanical properties a new auxetic structure, named RCA |
| 46 | Geng et al. [77]—Mechanical properties of Selective Laser Sintering (SLS) additive manufactured chiral auxetic cylindrical stent | 3D-printing methods (FDM, SLM, SLS, stereolithographic methods) manufacturing of auxetics/Stents | Fabrication of hybrid chiral stent with auxetic properties by additive manufacturing technique. Including investigation In-plane theoretical and experimental mechanical properties of stents |
| 47 | Kolken et al. [78]—Mechanical performance of auxetic meta-biomaterial | 3D-printing methods (FDM, SLM, SLS, stereolithographic methods) manufacturing of auxetics | Investigation mechanical properties of auxetic meta-biomaterials |
| 48 | Xue et al. [79]—Compressive property of Al-based auxetic lattice structures fabricated by 3-D printing combined with investment casting | 3D-printing methods (FDM, SLM, SLS, stereolithographic methods) manufacturing of auxetics | Investigation relationship between the structure and properties of auxetic structure |
| 49 | Cheng et al. [82]—3D printing-directed auxetic Kevlar aerogel architectures with multiple functionalization options | 3D-printing methods (FDM, SLM, SLS, stereolithographic methods) manufacturing of auxetics | Designing and fabrication of aerogel auxetic architectures |
| 50 | Tang et al. [84]—Highly tailorable electromechanical properties of auxetic piezoelectric ceramics with ultra-low porosity | 3D-printing methods (FDM, SLM, SLS, stereolithographic methods) manufacturing of auxetics/Simulation and topology optimization | Investigation of electromechanical properties of auxetic piezoelectric ceramic with ultra-low porosity |
References
- Lakes, R. Foam Structures with a Negative Poisson’s Ratio. Science 1987, 235, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K. Two-dimensional isotropic system with a negative poisson ratio. Phys. Lett. A 1989, 137, 60–64. [Google Scholar] [CrossRef]
- Evans, K.E. Auxetic polymers: A new range of materials. Endeavour 1991, 15, 170–174. [Google Scholar] [CrossRef]
- Grima, J.N.; Evans, K.E. Auxetic behavior from rotating squares. J. Mater. Sci. Lett. 2000, 19, 1563–1565. [Google Scholar] [CrossRef]
- Sanami, M. Auxetic Materials for Biomedical Applications. Ph.D. Thesis, University of Bolton, Bolton, UK, 2015. [Google Scholar]
- Scarpa, F. Auxetic materials for bioprostheses [In the Spotlight]. IEEE Signal Process. Mag. 2008, 25, 126–128. [Google Scholar] [CrossRef]
- Mardling, P.; Alderson, A.; Jordan-Mahy, N.; Le Maitre, C.L. The use of auxetic materials in tissue engineering. Biomater. Sci. 2020, 8, 2074–2083. [Google Scholar] [CrossRef]
- Sanami, M.; Ravirala, N.; Alderson, K.; Alderson, A. Auxetic Materials for Sports Applications. Procedia Eng. 2014, 72, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Duncan, O.; Shepherd, T.; Moroney, C.; Foster, L.; Venkatraman, P.D.; Winwood, K.; Allen, T.; Alderson, A. Review of Auxetic Materials for Sports Applications: Expanding Options in Comfort and Protection. Appl. Sci. 2018, 8, 941. [Google Scholar] [CrossRef] [Green Version]
- Martz, E.O.; Lakes, R.S.; Goel, V.K.; Park, J.B. Design of an Artificial Intervertebral Disc Exhibiting a Negative Poisson’s Ratio. Cell. Polym. 2005, 24, 127–138. [Google Scholar] [CrossRef]
- Baker, C.E. Auxetic Spinal Implants: Consideration of Negative Poisson’s Ratio in the Design of an Artificial Intervertebral Disc. Master’s Thesis, University of Toledo, Toledo, OH, USA, 2011. [Google Scholar]
- Yao, Y.; Yuan, H.; Huang, H.; Liu, J.; Wang, L.; Fan, Y. Biomechanical design and analysis of auxetic pedicle screw to resist loosening. Comput. Biol. Med. 2021, 133, 104386. [Google Scholar] [CrossRef]
- Amin, F.; Ali, M.N.; Ansari, U.; Mir, M.; Minhas, M.A.; Shahid, W. Auxetic Coronary Stent Endoprosthesis: Fabrication and Structural Analysis. J. Appl. Biomater. Funct. Mater. 2015, 13, 127–135. [Google Scholar] [CrossRef]
- Liu, J.; Yao, X.; Wang, Z.; Ye, J.; Luan, C.; He, Y.; Lin, H.; Fu, J. A flexible porous chiral auxetic tracheal stent with ciliated epithelium. Acta Biomater. 2021, 124, 153–165. [Google Scholar] [CrossRef]
- Wu, W.; Song, X.; Liang, J.; Xia, R.; Qian, G.; Fang, D. Mechanical properties of anti-tetrachiral auxetic stents. Compos. Struct. 2018, 185, 381–392. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; Janbaz, S.; Leeflang, S.M.A.; Lietaert, K.; Weinans, H.H.; Zadpoor, A.A. Rationally designed meta-implants: A combination of auxetic and conventional meta-biomaterials. Mater. Horiz. 2018, 5, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Risk Factors for Aseptic Loosening Following Total Hip Arthroplasty IntechOpen. Available online: https://www.intechopen.com/chapters/26867 (accessed on 5 February 2022).
- Prudhon, J.L.; Desmarchelier, R.; Hamadouche, M.; Delaunay, C.; Verdier, R. Is dual mobility associated with an increased risk of revision for infection? Matched cohort of 231 cases of dual-mobility cups and 231 fixed cups. HIP Int. 2018, 28, 200–204. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kamath, A.F.; Ong, K.; Lau, E.; Kurtz, S.; Chan, V.; Vail, T.P.; Rubash, H.; Berry, D.J. Comparative Epidemiology of Revision Arthroplasty: Failed THA Poses Greater Clinical and Economic Burdens Than Failed TKA. Clin. Orthop. Relat. Res. 2015, 473, 2131–2138. [Google Scholar] [CrossRef] [Green Version]
- Winter, W.; Klein, D.; Karl, M. Micromotion of Dental Implants: Basic Mechanical Considerations. J. Med. Eng. 2013, 2013, 265412. [Google Scholar] [CrossRef] [Green Version]
- Ghavidelnia, N.; Bodaghi, M.; Hedayati, R. Femur Auxetic Meta-Implants with Tuned Micromotion Distribution. Materials 2020, 14, 114. [Google Scholar] [CrossRef]
- Mehmood, S.; Ali, M.N.; Ansari, U.; Mir, M.; Khan, M.A. Auxetic polymeric bone plate as internal fixator for long bone fractures: Design, fabrication and structural analysis. Technol. Health Care 2015, 23, 819–833. [Google Scholar] [CrossRef]
- Aro, H.T.; Chao, E.Y.S. Bone-Healing Patterns Affected by Loading, Fracture Fragment Stability, Fracture Type, and Fracture Site Compression. Clin. Orthop. Relat. Res. 1993, 293, 8–17. [Google Scholar] [CrossRef]
- Goodship, A.; Kenwright, J. The influence of induced micromovement upon the healing of experimental tibial fractures. J. Bone Jt. Surgery. Br. Vol. 1985, 67, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Goodship, A.E.; Kelly, D.J.; Rigby, H.S.; Watkins, P.E.; Kenwright, J. The Effect of Different Regimes of Axial Micromovement on the Healing of Experimental Tibial Fractures. In Biomechanics: Basic and Applied Research: Selected Proceedings of the Fifth Meeting of the European Society of Biomechanics, 8–10 September 1986; Berlin, F.R.G., Bergmann, G., Kölbel, R., Rohlmann, A., Eds.; Developments in Biomechanics; Springer: Dordrecht, The Netherlands, 1987; pp. 441–446. ISBN 978-94-009-3355-2. [Google Scholar]
- Kassis, B.; Glorion, C.; Tabib, W.; Blanchard, O.; Pouliquen, J.-C. Callus Response to Micromovement After Elongation in the Rabbit. J. Pediatr. Orthop. 1996, 16, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Kenwright, J.; Goodship, A.; Kelly, D.; Newman, J.; Harris, J.; Richardson, J.; Evans, M.; Spriggins, A.; Burrough, S.; Rowley, D. Effect of Controlled Axial Micromovement on Healing of Tibial Fractures. Lancet 1986, 2, 1185–1187. [Google Scholar] [CrossRef]
- Yamaji, T.; Ando, K.; Wolf, S.; Augat, P.; Claes, L. The effect of micromovement on callus formation. J. Orthop. Sci. 2001, 6, 571–575. [Google Scholar] [CrossRef]
- Ali, M.N.; Rehman, I.U. Auxetic polyurethane stents and stent-grafts for the palliative treatment of squamous cell carcinomas of the proximal and mid oesophagus: A novel fabrication route. J. Manuf. Syst. 2015, 37, 375–395. [Google Scholar] [CrossRef]
- Kapnisi, M.; Mansfield, C.; Marijon, C.; Guex, A.G.; Perbellini, F.; Bardi, I.; Humphrey, E.J.; Puetzer, J.L.; Mawad, D.; Koutsogeorgis, D.; et al. Auxetic Cardiac Patches with Tunable Mechanical and Conductive Properties toward Treating Myocardial Infarction. Adv. Funct. Mater. 2018, 28, 1800618. [Google Scholar] [CrossRef] [Green Version]
- Mawad, D.; Mansfield, C.; Lauto, A.; Perbellini, F.; Nelson, G.W.; Tonkin, J.; Bello, S.O.; Carrad, D.J.; Micolich, A.P.; Mahat, M.M.; et al. A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2016, 2, e1601007. [Google Scholar] [CrossRef] [Green Version]
- Arjunan, A.; Zahid, S.; Baroutaji, A.; Robinson, J. 3D printed auxetic nasopharyngeal swabs for COVID-19 sample collection. J. Mech. Behav. Biomed. Mater. 2021, 114, 104175. [Google Scholar] [CrossRef]
- Panico, M.; Langella, C.; Santulli, C. Development of a Biomedical Neckbrace through Tailored Auxetic Shapes. Ital. J. Sci. Eng. 2017, 1, 105–117. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature; Henry Holt and Company: New York, NY, USA, 1982; ISBN 978-0-7167-1186-5. [Google Scholar]
- Hinrichs, A.; Malukhina, K.; Sharma, I.; Vierra, M. Active Auxetic Heel Support for Achilles Tendon Therapy. Senior’s Thesis, Santa Clara University, Santa Clara, CA, USA, 2018. [Google Scholar]
- Pattinson, S.W.; Huber, M.E.; Kim, S.; Lee, J.; Grunsfeld, S.; Roberts, R.; Dreifus, G.; Meier, C.; Liu, L.; Hogan, N.; et al. Additive Manufacturing of Biomechanically Tailored Meshes for Compliant Wearable and Implantable Devices. Adv. Funct. Mater. 2019, 29, 1901815. [Google Scholar] [CrossRef] [Green Version]
- Mottram, S.; Rosicky, J. Cranial Remoulding Orthosis and Method of Manufacture Thereof. CA2996246C, 16 February 2021. [Google Scholar]
- Moroney, C.; Alderson, A.; Allen, T.; Sanami, M.; Venkatraman, P. The Application of Auxetic Material for Protective Sports Apparel. Proceedings 2018, 2, 251. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-W.; Wang, K.; Ho, C.-C.; Kao, C.-T.; Ng, H.Y.; Shie, M.-Y. Cyclic tensile stimulation enrichment of Schwann cell-laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering. Mater. Des. 2020, 195, 108982. [Google Scholar] [CrossRef]
- Flamourakis, G.; Spanos, I.; Vangelatos, Z.; Manganas, P.; Papadimitriou, L.; Grigoropoulos, C.; Ranella, A.; Farsari, M. Laser-made 3D Auxetic Metamaterial Scaffolds for Tissue Engineering Applications. Macromol. Mater. Eng. 2020, 305, 2000238. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Song, L.; Zeng, C. Pluripotent stem cell expansion and neural differentiation in 3-D scaffolds of tunable Poisson’s ratio. Acta Biomater. 2017, 49, 192–203. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Ahmed, M.; Li, Y.; Zeng, C. Vascular differentiation from pluripotent stem cells in 3-D auxetic scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, 1679–1689. [Google Scholar] [CrossRef]
- Díaz Lantada, A.; Muslija, A.; García-Ruíz, J.P. Auxetic tissue engineering scaffolds with nanometric features and resonances in the megahertz range. Smart Mater. Struct. 2015, 24, 055013. [Google Scholar] [CrossRef]
- Clyne, A.M.; Swaminathan, S.; Lantada, A.D. Biofabrication strategies for creating microvascular complexity. Biofabrication 2019, 11, 032001. [Google Scholar] [CrossRef]
- Soman, P.; Lee, J.W.; Phadke, A.; Varghese, S.; Chen, S. Spatial tuning of negative and positive Poisson’s ratio in a multi-layer scaffold. Acta Biomater. 2012, 8, 2587–2594. [Google Scholar] [CrossRef] [Green Version]
- Auxetic Nuclei in Embryonic Stem Cells Exiting Pluripotency Nature Materials. Available online: https://www.nature.com/articles/nmat3943 (accessed on 5 February 2022).
- MCB1 CellScale. Available online: https://www.cellscale.com/products/mcb1/ (accessed on 5 February 2022).
- Warner, J.J.; Gillies, A.R.; Hwang, H.H.; Zhang, H.; Lieber, R.; Chen, S. 3D-printed biomaterials with regional auxetic properties. J. Mech. Behav. Biomed. Mater. 2017, 76, 145–152. [Google Scholar] [CrossRef]
- Xue, H.; Luo, Z.; Brown, T.; Beier, S. Design of Self-Expanding Auxetic Stents Using Topology Optimization. Front. Bioeng. Biotechnol. 2020, 8, 736. [Google Scholar] [CrossRef]
- Auricchio, F.; Bacigalupo, A.; Gambarotta, L.; Lepidi, M.; Morganti, S.; Vadalà, F. A novel layered topology of auxetic materials based on the tetrachiral honeycomb microstructure. Mater. Des. 2019, 179, 107883. [Google Scholar] [CrossRef]
- Wilt, J.K.; Yang, C.X.; Gu, G.X. Accelerating Auxetic Metamaterial Design with Deep Learning. Adv. Eng. Mater. 2020, 22, 1901266. [Google Scholar] [CrossRef]
- Nguyen, J.; Park, S.-I.; Rosen, D.W.; Folgar, L.; Williams, J. Conformal Lattice Structure Design and Fabrication. Available online: https://repositories.lib.utexas.edu/bitstream/handle/2152/88400/2012-10-Nguyen.pdf?sequence=2 (accessed on 1 January 2022).
- Engelbrecht, S. Design of Meso-Scale Cellular Structure for Rapid Manufacturing. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2009. [Google Scholar]
- Chu, J.; Engelbrecht, S.; Graf, G.; Rosen, D.W. A comparison of synthesis methods for cellular structures with application to additive manufacturing. Rapid Prototyp. J. 2010, 16, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Chen, Y.; Rosen, D.W. A Hybrid Geometric Modeling Method for Large Scale Conformal Cellular Structures. In Proceedings of the ASME 2005 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. Volume 3: 25th Computers and Information in Engineering Conference, Parts A and B, Long Beach, CA, USA, 24–28 September 2005; pp. 421–427. [Google Scholar] [CrossRef] [Green Version]
- Gibson, I.; Rosen, D.W.; Stucker, B. Introduction and Basic Principles. In Additive Manufacturing Technologies: Rapid Prototyping to Direct Digital Manufacturing; Gibson, I., Rosen, D.W., Stucker, B., Eds.; Springer: Boston, MA, USA, 2010; pp. 20–35. ISBN 978-1-4419-1120-9. [Google Scholar]
- Dagdelen, J.; Montoya, J.; De Jong, M.; Persson, K. Computational prediction of new auxetic materials. Nat. Commun. 2017, 8, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinay, V.C.; Varma, D.S.M. Fabrication and Testing of Auxetic Foams for Rehabilitation Applications. J. Indian Inst. Sci. 2019, 99, 511–518. [Google Scholar] [CrossRef]
- Plant, D.; Leevers, P. Injection moldable rate stiffening re-entrant cell arrays for wearable impact protection. Polym. Eng. Sci. 2020, 60, 1546–1555. [Google Scholar] [CrossRef]
- Ghaedizadeh, A.; Shen, J.; Ren, X.; Xie, Y.M. Tuning the Performance of Metallic Auxetic Metamaterials by Using Buckling and Plasticity. Materials 2016, 9, 54. [Google Scholar] [CrossRef]
- Taylor, M.; Francesconi, L.; Gerendás, M.; Shanian, A.; Carson, C.; Bertoldi, K. Low Porosity Metallic Periodic Structures with Negative Poisson’s Ratio. Adv. Mater. 2014, 26, 2365–2370. [Google Scholar] [CrossRef] [Green Version]
- Grujicic, M.; Galgalikar, R.; Snipes, J.; Yavari, R.; Ramaswami, S. Multi-physics modeling of the fabrication and dynamic performance of all-metal auxetic-hexagonal sandwich-structures. Mater. Des. 2013, 51, 113–130. [Google Scholar] [CrossRef]
- Scarpa, F.; Pastorino, P.; Garelli, A.; Patsias, S.; Ruzzene, M. Auxetic compliant flexible PU foams: Static and dynamic properties. Phys. Status Solidi B 2005, 242, 681–694. [Google Scholar] [CrossRef]
- Jiang, N.; Hu, H. A study of tubular braided structure with negative Poisson’s ratio behavior. Text. Res. J. 2018, 88, 2810–2824. [Google Scholar] [CrossRef]
- Sloan, M.R.; Wright, J.R.; Evans, K.E. The helical auxetic yarn—A novel structure for composites and textiles; geometry, manufacture and mechanical properties. Mech. Mater. 2011, 43, 476–486. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Zhang, G.; Ghita, O.; Evans, K. The variation in Poisson’s ratio caused by interactions between core and wrap in helical composite auxetic yarns. Compos. Sci. Technol. 2014, 102, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Jiang, L.; Hu, H. Auxetic composites made of 3D textile structure and polyurethane foam. Phys. Status Solidi 2016, 253, 1331–1341. [Google Scholar] [CrossRef]
- Kadic, M.; Bückmann, T.; Stenger, N.; Thiel, M.; Wegener, M. On the practicability of pentamode mechanical metamaterials. Appl. Phys. Lett. 2012, 100, 191901. [Google Scholar] [CrossRef] [Green Version]
- Askari, M.; Hutchins, D.A.; Thomas, P.J.; Astolfi, L.; Watson, R.L.; Abdi, M.; Ricci, M.; Laureti, S.; Nie, L.; Freear, S.; et al. Additive manufacturing of metamaterials: A review. Addit. Manuf. 2020, 36, 101562. [Google Scholar] [CrossRef]
- Kadic, M.; Milton, G.W.; Van Hecke, M.; Wegener, M. 3D metamaterials. Nat. Rev. Phys. 2019, 1, 198–210. [Google Scholar] [CrossRef]
- Lantada, A.D.; Morgado, P.L. Rapid Prototyping for Biomedical Engineering: Current Capabilities and Challenges. Annu. Rev. Biomed. Eng. 2012, 14, 73–96. [Google Scholar] [CrossRef] [Green Version]
- Klein, F.; Richter, B.; Striebel, T.; Franz, C.M.; von Freymann, G.; Wegener, M.; Bastmeyer, M. Two-Component Polymer Scaffolds for Controlled Three-Dimensional Cell Culture. Adv. Mater. 2011, 23, 1341–1345. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Joseph, A.; Mahesh, V.; Harursampath, D. On the application of additive manufacturing methods for auxetic structures: A review. Adv. Manuf. 2021, 9, 342–368. [Google Scholar] [CrossRef]
- Vyavahare, S.; Kumar, S. Re-entrant auxetic structures fabricated by fused deposition modeling: An experimental study of influence of process parameters under compressive loading. Polym. Eng. Sci. 2020, 60, 3183–3196. [Google Scholar] [CrossRef]
- Alomarah, A.; Ruan, D.; Masood, S.; Sbarski, I.; Faisal, B. An investigation of in-plane tensile properties of re-entrant chiral auxetic structure. Int. J. Adv. Manuf. Technol. 2018, 96, 2013–2029. [Google Scholar] [CrossRef]
- Geng, L.C.; Ruan, X.L.; Wu, W.W.; Xia, R.; Fang, D.N. Mechanical Properties of Selective Laser Sintering (SLS) Additive Manufactured Chiral Auxetic Cylindrical Stent. Exp. Mech. 2019, 59, 913–925. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; Lietaert, K.; van der Sloten, T.; Pouran, B.; Meynen, A.; Van Loock, G.; Weinans, H.; Scheys, L.; Zadpoor, A. Mechanical performance of auxetic meta-biomaterials. J. Mech. Behav. Biomed. Mater. 2020, 104, 103658. [Google Scholar] [CrossRef]
- Xue, Y.Y.; Wang, X.F.; Wang, W.; Zhong, X.K.; Han, F.H. Compressive property of Al-based auxetic lattice structures fabricated by 3-D printing combined with investment casting. Mater. Sci. Eng. A 2018, 722, 255–262. [Google Scholar] [CrossRef]
- Schwentenwein, M.; Schneider, P.; Homa, J. Lithography-Based Ceramic Manufacturing: A Novel Technique for Additive Manufacturing of High-Performance Ceramics. Adv. Sci. Technol. 2014, 88, 60–64. [Google Scholar] [CrossRef]
- Díaz Lantada, A.; Romero, A.D.B.; Schwentenwein, M.; Jellinek, C.; Homa, J. Lithography-based ceramic manufacture (LCM) of auxetic structures: Present capabilities and challenges. Smart Mater. Struct. 2016, 25, 54015. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Liu, Y.; Lyu, J.; Lu, Q.; Zhang, X.; Song, W. 3D printing-directed auxetic Kevlar aerogel architectures with multiple functionalization options. J. Mater. Chem. A 2020, 8, 14243–14253. [Google Scholar] [CrossRef]
- Tang, H.; Jiang, X.; Ling, L.; Li, L.; Hu, Y. Highly tailorable electromechanical properties of auxetic piezoelectric ceramics with ultra-low porosity. J. Am. Ceram. Soc. 2020, 103, 6330–6347. [Google Scholar] [CrossRef]
- Bertoldi, K.; Taylor, M.; Shanian, A.; Gerendas, M.; Carson, C. Low Porosity Auxetic Sheet. U.S. Patent 20160025344A1, 28 January 2016. [Google Scholar]
- Xu, B.; Arias, F.; Brittain, S.T.; Zhao, X.-M.; Grzybowski, B.; Torquato, S.; Whitesides, G.M. Making Negative Poisson’s Ratio Microstructures by Soft Lithography. Adv. Mater. 1999, 11, 1186–1189. [Google Scholar] [CrossRef]
- Muslija, A.; Díaz Lantada, A. Deep reactive ion etching of auxetic structures: Present capabilities and challenges. Smart Mater. Struct. 2014, 23, 87001. [Google Scholar] [CrossRef] [Green Version]
- Hengsbach, S.; Díaz Lantada, A. Direct laser writing of auxetic structures: Present capabilities and challenges. Smart Mater. Struct. 2014, 23, 085033. [Google Scholar] [CrossRef]
- Saxena, K.K.; Das, R.; Calius, E.P. 3D Printable Multimaterial Cellular Auxetics with Tunable Stiff-Ness. arXiv 2017, arXiv:1707.04486. [Google Scholar]
- Bader, C.; Kolb, D.; Weaver, J.C.; Sharma, S.; Hosny, A.; Costa, J.; Oxman, N. Making data matter: Voxel printing for the digital fabrication of data across scales and domains. Sci. Adv. 2018, 4, eaas8652. [Google Scholar] [CrossRef] [Green Version]
- Bhullar, S.K.; Rana, D.; Lekesiz, H.; Bedeloglu, A.C.; Ko, J.; Cho, Y.; Aytac, Z.; Uyar, T.; Jun, M.; Ramalingam, M. Design and fabrication of auxetic PCL nanofiber membranes for biomedical applications. Mater. Sci. Eng. C 2017, 81, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Xie, C.; Gao, Q.; Zhou, X.; Li, G.; Du, J.; He, Y. Fabrication of multi-scale and tunable auxetic scaffolds for tissue engineering. Mater. Des. 2021, 197, 109277. [Google Scholar] [CrossRef]
- Lvov, V.A.; Senatov, F.S.; Stepashkin, A.A.; Veveris, A.A.; Pavlov, M.D.; Komissarov, A.A. Low-cycle fatigue behavior of 3D-printed metallic auxetic structure. Mater. Today Proc. 2020, 33, 1979–1983. [Google Scholar] [CrossRef]
- Álvarez Elipe, J.C.; Díaz Lantada, A. Comparative study of auxetic geometries by means of computer-aided design and engineering. Smart Mater. Struct. 2012, 21, 105004. [Google Scholar] [CrossRef]
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Arch. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Zhou, W.; Qiao, Z.; Zare, E.N.; Huang, J.; Zheng, X.; Sun, X.; Shao, M.; Wang, H.; Wang, X.; Chen, D.; et al. 4D-Printed Dynamic Materials in Biomedical Applications: Chemistry, Challenges, and Their Future Perspectives in the Clinical Sector. J. Med. Chem. 2020, 63, 8003–8024. [Google Scholar] [CrossRef]
- Nguyen, P.Q.; Courchesne, N.-M.D.; Duraj-Thatte, A.; Praveschotinunt, P.; Joshi, N.S. Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials. Adv. Mater. 2018, 30, e1704847. [Google Scholar] [CrossRef]
- Prescott, T.J.; Lepora, N.; Verschure, P.F.M.J. Living Machines: A Handbook of Research in Biomimetics and Biohybrid Systems; Prescott, T.J., Lepora, N., Verschure, P.F.M.J., Eds.; Oxford University Press: Oxford, UK, 2018; ISBN 978-0-19-967492-3. [Google Scholar]
- Lin, Z.; Jiang, T.; Shang, J. The emerging technology of biohybrid micro-robots: A review. Bio Design Manuf. 2022, 5, 107–132. [Google Scholar] [CrossRef]
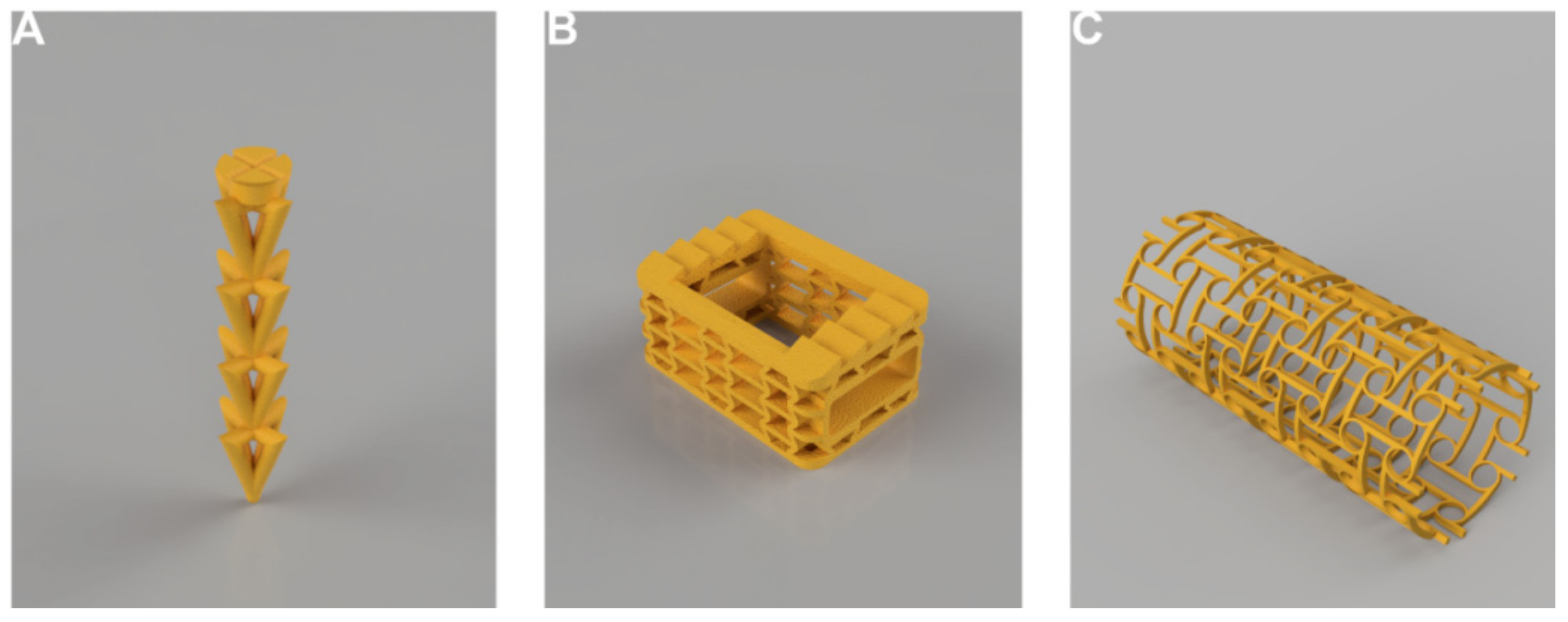
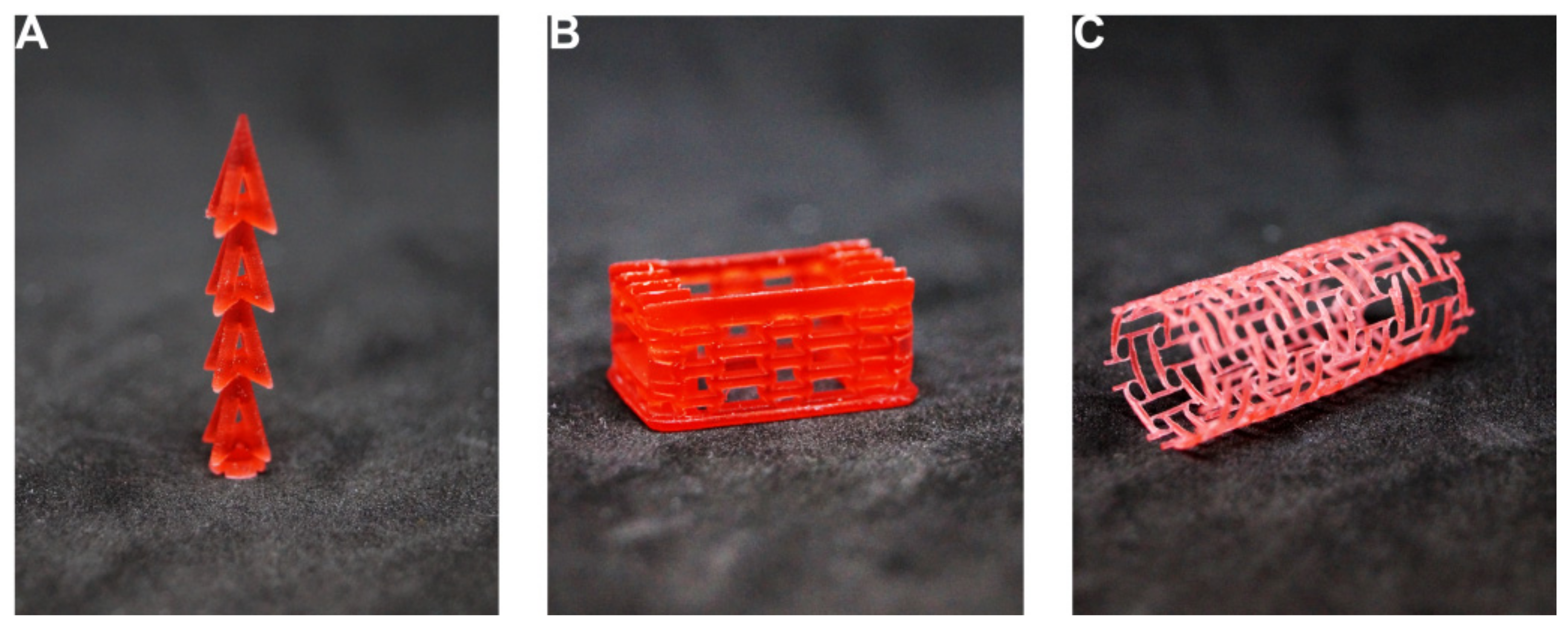
| Patient | |
| Evaluation of the medical problem. The medical team with support of biomedical technology designers and developers analyse if a mass-produced medical device or a custom-made solution is advisable. The potential benefits of auxetic geometries for enhanced implantability, improved biomechanical performance, or promoted ergonomics/aesthetics are discussed. | |
| Medical Imaging | |
| CT/MRI data employed as starting point in DICOM format. Segmentation and processing of the anatomical part of interest. Obtaining a 3D model of the defect or region of interest using “MIMICS-like” software resources (i.e., STL format, as input for CAD modelling and design personalization.For external devices such as orthoses, more affordable and direct optical imaging systems may be employed, even based on smartphones’ cameras and dedicated software, to obtain the geometry. | |
| Choice of Auxetic Geometry | |
| Selection of auxetic geometries from design libraries based on the type of problem and the behaviour of the different auxetics. Open-source libraries may promote healthcare equity. Library of auxetics forloaded products (scaffolds, artificial disks, etc.), for which re-entrant auxetics may work better.Products requiring flexibility (for example for minimal invasion such as stents, or skin and muscular patches or soft tissue engineering), for which chiral and rotating auxetics may work better. | |
| Designing with CAD Systems | |
| Creation of a medical device using standards to design an original product. Adaptation of an existing medical device by incorporation of auxetic features or regions. Matching with a 3D model of the defect for personalized approaches. | Designing auxetic geometries is possible in varied ways and through a combination of different strategies:
|
| FEM Simulations and Biomechanical Optimization | |
| Simulation of simplified mechanical, thermal, fluidic and other tests to determine compliance with required standards and objectives. Evaluation of biomechanical interaction with the body and verification of improved performance of the auxetic devices, as compared with current gold standards. | |
| Manufacturing of prototypes (i.e., 3D printing and reviewed tools) for experimental evaluation | |
| Three-dimensional printing or rapid manufacturing of a prototype employing testing materials or materials used in the manufacture of medical devices allows developers to evaluate the design quality and potentials.Systematic in vitro trials with biomimetic work benches and dummies for checking the simulations and design optimization purposes, testing also the improved designs for safely approaching medical trials. | |
| Validation | |
| Conducting systematic tests according to internationally recognized standards (ISO 10993 for biocompatibility, ISO 14971 and ISO 13485 for risk and quality management etc.).Quality assessment by the surgical planning team in the case of custom-made or patient-specific solution, CE-marking or similar certification depending on applicable regulations for mass-produced devices.Final creation of the custom-made device or production planning, supply chain management and marketing for mass-produced devices. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lvov, V.A.; Senatov, F.S.; Veveris, A.A.; Skrybykina, V.A.; Díaz Lantada, A. Auxetic Metamaterials for Biomedical Devices: Current Situation, Main Challenges, and Research Trends. Materials 2022, 15, 1439. https://doi.org/10.3390/ma15041439
Lvov VA, Senatov FS, Veveris AA, Skrybykina VA, Díaz Lantada A. Auxetic Metamaterials for Biomedical Devices: Current Situation, Main Challenges, and Research Trends. Materials. 2022; 15(4):1439. https://doi.org/10.3390/ma15041439
Chicago/Turabian StyleLvov, Vladislav A., Fedor S. Senatov, Alnis A. Veveris, Vitalina A. Skrybykina, and Andrés Díaz Lantada. 2022. "Auxetic Metamaterials for Biomedical Devices: Current Situation, Main Challenges, and Research Trends" Materials 15, no. 4: 1439. https://doi.org/10.3390/ma15041439
APA StyleLvov, V. A., Senatov, F. S., Veveris, A. A., Skrybykina, V. A., & Díaz Lantada, A. (2022). Auxetic Metamaterials for Biomedical Devices: Current Situation, Main Challenges, and Research Trends. Materials, 15(4), 1439. https://doi.org/10.3390/ma15041439