Autologous Dentin Graft after Impacted Mandibular Third Molar Extraction to Prevent Periodontal Pocket Formation—A Split-Mouth Pilot Study
Abstract
1. Introduction
2. Materials and Methods
- Subjects requiring the extraction of partially or totally impacted third molars, with horizontal or mesioangular position, due to recurrent pericoronitis, destructive caries of the third molar, destructive caries of the second molar not otherwise treatable except after extraction of the third molar, periodontal defects distal to the second molar;
- Radiographic or clinical evidence of bone loss between the distal aspect of M2 and the adjacent M3. The assignment to the experimental or control group was carried out by a software-controlled randomization procedure;
- Patients ≥ 18 years of age;
- Presence of mandibular second molar adjacent to the third molar to be extracted;
- Willingness and ability to give written informed consent;
- Non-smoking or light smoking patients (≤10 cigarettes per day);
- Willingness to return for follow-up examinations;
- Good general health.
- Uncontrolled systemic diseases/conditions (e.g., pregnancy or lactation, drug or chemical reagents hypersensitivity, diabetes, metabolic bone diseases, history of malignancy);
- Heavy smokers (>11 cigarettes/day);
- Long-term steroidal drug therapy;
- Unwillingness to return for follow-up appointments.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.T.; Hum, L.; Chen, Y.W. The effect of regenerative periodontal therapy in preventing periodontal defects after the extraction of third molars: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2016, 147, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jo, Y.Y.; Kim, J.Y.; Oh, J.H.; Yang, B.E.; Kim, S.G. Retrospective comparative clinical study for silk mat application into extraction socket. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 16. [Google Scholar] [CrossRef]
- Camps-Font, O.; Caro-Bonfill, C.; Sánchez-Garcés, M.À.; Gay-Escoda, C. Periodontal Regenerative Therapy for Preventing Bone Defects Distal to Mandibular Second Molars After Surgical Removal of Impacted Third Molars: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Oral Maxillofac. Surg. 2018, 76, 2482–2514. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.T.; Dodson, T.B. Risk of periodontal defects after third molar surgery: An exercise in evidence-based clinical decision-making. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Mazzaglia, G. Effect of removing an impacted mandibular third molar on the periodontal status of the mandibular second molar. J. Oral Maxillofac. Surg. 2011, 69, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Karapataki, S.; Hugoson, A.; Kugelberg, C.F. Healing following GTR treatment of bone defects distal to mandibular 2nd molars after surgical removal of impacted 3rd molars. J. Clin. Periodontol. 2000, 7, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Aloy-Prósper, A.; García-Mira, B.; Larrazabal-Morón, C.; Peñarrocha-Diago, M. Distal probing depth and attachment level of lower second molars following surgical extraction of lower third molars: A literature review. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e755–e759. [Google Scholar] [CrossRef][Green Version]
- Barbato, L.; Kalemaj, Z.; Buti, J.; Baccini, M.; La Marca, M.; Duvina, M.; Tonelli, P. Effect of Surgical Intervention for Removal of Mandibular Third Molar on Periodontal Healing of Adjacent Mandibular Second Molar: A Systematic Review and Bayesian Network Meta-Analysis. J. Periodontol. 2016, 87, 291–302. [Google Scholar] [CrossRef]
- Mellonig, J.T. Autogenous and allogeneic bone grafts in periodontal therapy. Crit. Rev. Oral Biol. Med. 2016, 3, 333–352. [Google Scholar] [CrossRef]
- Serafini, G.; Lollobrigida, M.; Fortunato, L.; Mazzucchi, G.; Lamazza, L.; Di Nardo, D.; Vozza, I.; Riminucci, M.; De Biase, A. Postextractive Alveolar Ridge Preservation Using L-PRF: Clinical and Histological Evaluation. Case Rep. Dent. 2020, 2020, 5073519. [Google Scholar] [CrossRef]
- Chiriac, G.; Herten, M.; Schwarz, F.; Rothamel, D.; Becker, J. Autogenous bone chips: Influence of a new piezoelectric device (Piezosurgery) on chip morphology, cell viability and differentiation. J. Clin. Periodontol. 2005, 32, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.; Byeon, S.G.; Lee, J.H.; Um, H.J.; Lim, I.U. Development of a novel bone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, C.; Qiao, X.; Yang, B.; Yu, M.; Guo, W.; Tian, W. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Bang, G.; Urist, M.R. Bone induction in excavation chambers in matrix of decalcified dentin. Arch. Surg. 1967, 94, 781–789. [Google Scholar] [CrossRef]
- Butler, W.T.; Mikulski, A.; Urist, M.R. Noncollagenous proteins of a rat dentin matrix processing bone morphogenetic activity. J. Dent. Res. 1977, 56, 228–232. [Google Scholar] [CrossRef]
- Um, I.W.; Ku, J.K.; Kim, Y.K.; Lee, B.K. Histological Review of Demineralized Dentin Matrix as a Carrier of rhBMP-2. Tissue Eng. Part B Rev. 2020, 26, 284–293. [Google Scholar] [CrossRef]
- Bessho, K.; Tagawa, T.; Murata, M. Purification of rabbit bone morphogenetic protein derived from bone, dentin, and wound tissue after tooth extraction. J. Oral Maxillofac. Surg. 1990, 48, 162–169. [Google Scholar] [CrossRef]
- Morotome, Y.; Goseki-Sone, M.; Ishikawa, I.; Oida, S. Gene expression of growth and differentiation factors -5, -6, and -7 in developing bovine tooth at the root forming stage. Biochem. Biophys. Res. Commun. 1988, 244, 85–90. [Google Scholar] [CrossRef]
- Urist, M.R.; Strates, B.S. Bone morphogenetic protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef]
- Sampath, T.K.; Reddi, A.H. Discovery of bone morphogenetic proteins—A historical perspective. Bone 2020, 140, 115548. [Google Scholar] [CrossRef]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human dentin-matrix-derived bone morphogenetic protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Nampo, T.; Watahiki, J.; Enomoto, A. A new method for alveolar bone repair using extracted teeth for the graft material. J. Periodontol. 2010, 81, 264–1272. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, G. Contemporary interpretation of probing depth assessments: Diagnostic and therapeutic implications. A literature review. J. Periodontol. 1997, 68, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Newbrun, E. Indices to measure gingival bleeding. J. Periodontol. 1996, 67, 555–561. [Google Scholar] [CrossRef]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Di Nardo, D.; Mazzucchi, G.; Lollobrigida, M.; Passariello, C.; Guarnieri, R.; Galli, M.; De Biase, A.; Testarelli, L. Immediate or delayed retrieval of the displaced third molar: A review. J. Clin. Exp. Dent. 2019, 11, 55–61. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Cegarra Del Pino, P.; Sapoznikov, L.; Delgado Ruiz, R.A.; Fernández-Domínguez, M.; Gehrke, S.A. A new procedure for processing extracted teeth for immediate grafting in post-extraction sockets. An experimental study in American Fox Hound dogs. Ann. Anat. 2018, 217, 14–23. [Google Scholar] [CrossRef]
- Laurito, D.; Lollobrigida, M.; Graziani, F.; Guerra, F.; Vestri, A.; De Biase, A. Periodontal Effects of a Transposed Versus a Conventional Flap in Mandibular Third Molar Extractions. J. Craniofac. Surg. 2016, 27, 708–711. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Maté-Sánchez de Val, J.E.; RamosOltra, M.L. The use of tooth particles as a biomaterial in post-extraction Sockets. Experimental Study in Dogs. Dent. J. 2018, 6, 12. [Google Scholar] [CrossRef]
- Demetter, R.S.; Calahan, B.G.; Mealey, B.L. Histologic evaluation of wound healing after ridge preservation with cortical, cancellous, and combined cortico-cancellous freeze-dried bone allograft: A randomized controlled clinical trial. J. Periodontol. 2017, 88, 860–868. [Google Scholar] [CrossRef]
- Eskow, A.J.; Mealey, B.L. Evaluation of healing following tooth extraction with ridge preservation using cortical versus cancellous freeze-dried bone allograft. J. Periodontol. 2014, 85, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A.; Mealey, B.L. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J. Periodontol. 2012, 83, 329–336. [Google Scholar] [CrossRef]
- Jambhekar, S.; Kernen, F.; Bidra, A.S. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: A systematic review of randomized controlled clinical trials. J. Prosthet. Dent. 2015, 113, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Amler, M.H. The sequence of tissue regeneration in human extraction wounds. Oral Surg. Oral Med. Oral Pathol. 1969, 27, 309–318. [Google Scholar] [CrossRef]
- De Biase, A.; Mazzucchi, G.; Di Nardo, D.; Lollobrigida, M.; Serafini, G.; Testarelli, L. Prevention of Periodontal Pocket Formation after Mandibular Third Molar Extraction Using Dentin Autologous Graft: A Split Mouth Case Report. Case Rep. Dent. 2020, 31, 1762862. [Google Scholar] [CrossRef]
- Peng, K.Y.; Tseng, Y.C.; Shen, E.C.; Chiu, S.C.; Fu, E.; Huang, Y.W. Mandibular second molar periodontal status after third molar extraction. J. Periodontol. 2001, 72, 1647–1651. [Google Scholar] [CrossRef]
- Kugelberg, C.F. Periodontal healing two and four years after impacted lower third molar surgery. A comparative retrospective study. Int. J. Oral Maxillofac. Surg. 1990, 19, 341–345. [Google Scholar] [CrossRef]
- Sammartino, G.; Tia, M.; Bucci, T.; Wang, H.L. Prevention of mandibular third molar extraction-associated periodontal defects: A comparative study. J. Periodontol. 2009, 80, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.S.; Marei, H.F.; Alagl, A.S. Does grafting of third molar extraction sockets enhance periodontal measures in 30- to 35-year-old patients? J. Oral Maxillofac. Surg. 2012, 70, 757–764. [Google Scholar] [CrossRef]
- Leventis, J.; Minas, L. Treatment of Osseous Defects after Mandibular Third Molar Removal with a Resorbable Alloplastic Grafting Material: A Case Series with 1- to 2-Year Follow-Up. Materials 2020, 13, 4688. [Google Scholar] [CrossRef]
- Mazzucchi, G.; Lollobrigida, M.; Laurito, D.; Di Nardo, D.; Berlutti, F.; Passariello, C.; Serafini, G.; Testarelli, L.; De Biase, A. Microbiological and FE-SEM assessment of d-PTFE membrane exposed to oral environment after alveolar socket preservation managed with granular nc-HA. J. Contemp. Dent. Pract. 2020, 21, 404–409. [Google Scholar] [CrossRef] [PubMed]



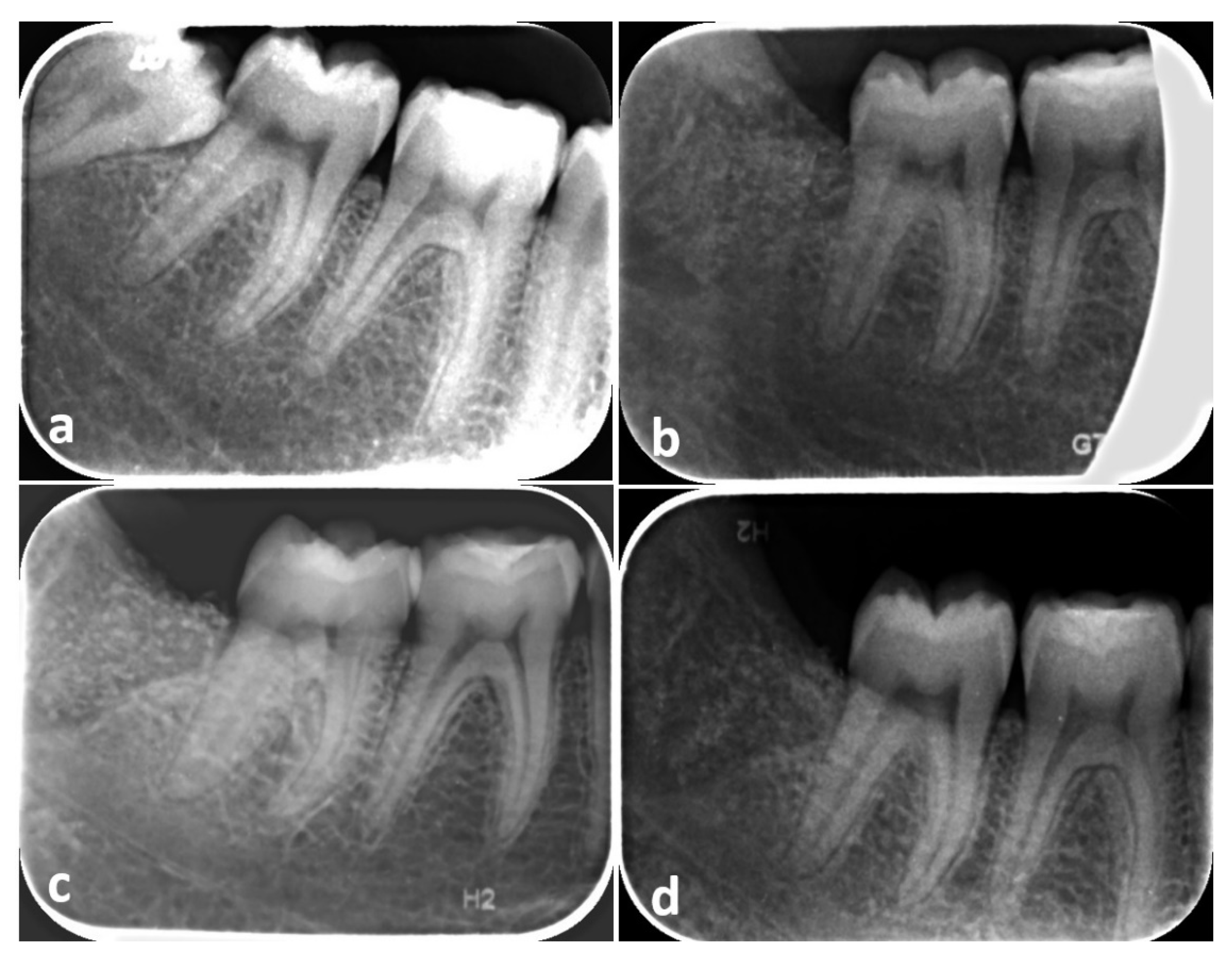


| Case | Gender | Age | Smoker | Type of Inclusion | Experimental Site PPD | Control Site PPD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esperimental Site | Control Site | T1 | T90 | T180 | T1 | T90 | T180 | |||||
| 1 | F | 29 | No | Semi–Impacted | Semi–Impacted | Distobuccal | 4 | 4 | 3 | 4 | 4 | 4 |
| Middistal | 4 | 3 | 3 | 4 | 4 | 4 | ||||||
| Distolingual | 3 | 3 | 3 | 2 | 3 | 2 | ||||||
| 2 | M | 18 | No | Semi–Impacted | Semi–Impacted | Distobuccal | 4 | 2 | 2 | 3 | 4 | 4 |
| Middistal | 6 | 4 | 3 | 3 | 4 | 4 | ||||||
| Distolingual | 4 | 2 | 2 | 3 | 3 | 2 | ||||||
| 3 | F | 44 | Light | Semi–Impacted | Semi–Impacted | Distobuccal | 6 | 2 | 3 | 5 | 3 | 3 |
| Middistal | 6 | 4 | 4 | 8 | 5 | 4 | ||||||
| Distolingual | 6 | 4 | 3 | 4 | 3 | 3 | ||||||
| 4 | M | 37 | No | Sem–Impacted | Semi–Impacted | Distobuccal | 5 | 3 | 2 | 3 | 5 | 3 |
| Middistal | 7 | 4 | 4 | 6 | 5 | 4 | ||||||
| Distolingual | 4 | 2 | 2 | 2 | 3 | 2 | ||||||
| 5 | M | 26 | Light | Semi–Impacted | Semi–Impacted | Distobuccal | 3 | 1 | 1 | 6 | 4 | 4 |
| Middistal | 5 | 3 | 4 | 3 | 4 | 3 | ||||||
| Distolingual | 3 | 2 | 4 | 4 | 4 | 4 | ||||||
| 6 | F | 39 | No | Semi–Impacted | Semi–Impacted | Distobuccal | 4 | 3 | 3 | 7 | 6 | 5 |
| Middistal | 6 | 4 | 4 | 5 | 5 | 7 | ||||||
| Distolingual | 4 | 4 | 3 | 4 | 4 | 4 | ||||||
| 7 | M | 31 | Light | Semi–Impacted | Semi–Impacted | Distobuccal | 3 | 1 | 1 | 6 | 5 | 4 |
| Middistal | 5 | 3 | 4 | 8 | 5 | 5 | ||||||
| Distolingual | 3 | 2 | 4 | 3 | 3 | 3 | ||||||
| 8 | F | 29 | No | Semi–Impacted | Sem–Impacted | Distobuccal | 5 | 2 | 4 | 4 | 3 | 3 |
| Middistal | 4 | 3 | 4 | 4 | 3 | 4 | ||||||
| Distolingual | 4 | 2 | 2 | 4 | 3 | 3 | ||||||
| 9 | F | 33 | No | Semi–Impacted | Impacted | Distobuccal | 4 | 4 | 5 | 7 | 6 | 5 |
| Middistal | 4 | 4 | 4 | 6 | 6 | 4 | ||||||
| Distolingual | 4 | 4 | 3 | 7 | 6 | 4 | ||||||
| 10 | F | 41 | No | Semi–Impacted | Semi–Impacted | Distobuccal | 6 | 5 | 4 | 4 | 4 | 3 |
| Middistal | 5 | 5 | 4 | 4 | 5 | 5 | ||||||
| Distolingual | 3 | 3 | 3 | 5 | 4 | 4 | ||||||
| Mean | 4.53 ± 1.13 | 3.06 ± 1.08 | 3.16 ± 0.98 | 4.6 ± 1.67 | 4.2 ± 1.03 | 3.76 ± 1.04 | ||||||
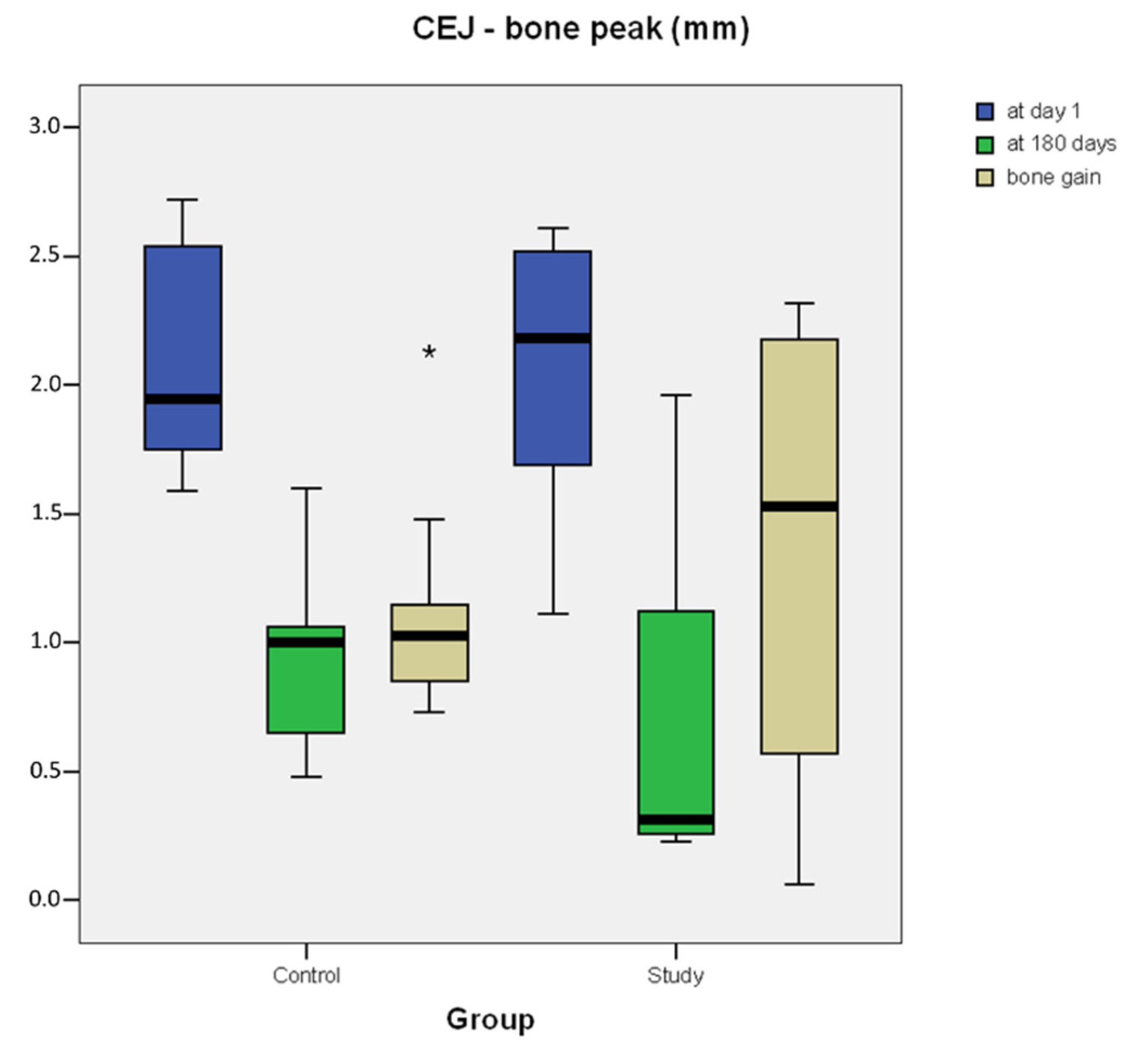
| Case | Experimental Site | Control Site | ||||
|---|---|---|---|---|---|---|
| CEJ–Bone Peak (mm) | CEJ–Bone Peak (mm) | |||||
| T1 | T180 | BG | T1 | T180 | BG | |
| 1 | 1.11 | 0.24 | 0.87 | 1.59 | 0.59 | 1 |
| 2 | 2.61 | 0.29 | 2.32 | 1.59 | 0.65 | 0.94 |
| 3 | 1.24 | 0.23 | 1.01 | 1.78 | 0.98 | 0.8 |
| 4 | 2.52 | 0.34 | 2.18 | 2.38 | 1.23 | 1.15 |
| 5 | 1.70 | 1.13 | 0.57 | 2.61 | 0.48 | 2.13 |
| 6 | 2.44 | 0.39 | 2.05 | 2.72 | 1.6 | 1.12 |
| 7 | 2.02 | 1.96 | 0.06 | 2.54 | 1.06 | 1.48 |
| 8 | 1.69 | 1.12 | 0.57 | 1.87 | 1.02 | 0.85 |
| 9 | 2.35 | 0.26 | 2.09 | 2.02 | 0.97 | 1.05 |
| 10 | 2.58 | 0.27 | 2.31 | 1.75 | 1.02 | 0.73 |
| Mean | 2.026 ± 0.56 | 0.623 ± 0.58 | 1.403 ± 0.87 | 2.085 ± 0.43 | 0.96 ± 0.33 | 1.125 ± 0.41 |
| Median (interquartile range) | 2.185 (0.8075) | 0.315 (0.675) | 1.53 (1.1525) | 1.945 (0.7425) | 1 (0.32) | 1.025 (0.27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzucchi, G.; Lollobrigida, M.; Lamazza, L.; Serafini, G.; Di Nardo, D.; Testarelli, L.; De Biase, A. Autologous Dentin Graft after Impacted Mandibular Third Molar Extraction to Prevent Periodontal Pocket Formation—A Split-Mouth Pilot Study. Materials 2022, 15, 1431. https://doi.org/10.3390/ma15041431
Mazzucchi G, Lollobrigida M, Lamazza L, Serafini G, Di Nardo D, Testarelli L, De Biase A. Autologous Dentin Graft after Impacted Mandibular Third Molar Extraction to Prevent Periodontal Pocket Formation—A Split-Mouth Pilot Study. Materials. 2022; 15(4):1431. https://doi.org/10.3390/ma15041431
Chicago/Turabian StyleMazzucchi, Giulia, Marco Lollobrigida, Luca Lamazza, Giorgio Serafini, Dario Di Nardo, Luca Testarelli, and Alberto De Biase. 2022. "Autologous Dentin Graft after Impacted Mandibular Third Molar Extraction to Prevent Periodontal Pocket Formation—A Split-Mouth Pilot Study" Materials 15, no. 4: 1431. https://doi.org/10.3390/ma15041431
APA StyleMazzucchi, G., Lollobrigida, M., Lamazza, L., Serafini, G., Di Nardo, D., Testarelli, L., & De Biase, A. (2022). Autologous Dentin Graft after Impacted Mandibular Third Molar Extraction to Prevent Periodontal Pocket Formation—A Split-Mouth Pilot Study. Materials, 15(4), 1431. https://doi.org/10.3390/ma15041431








