Biocompatibility and Antibacterial Action of Salvadora persica Extract as Intracanal Medication (In Vitro and Ex Vivo Experiment)
Abstract
:1. Introduction
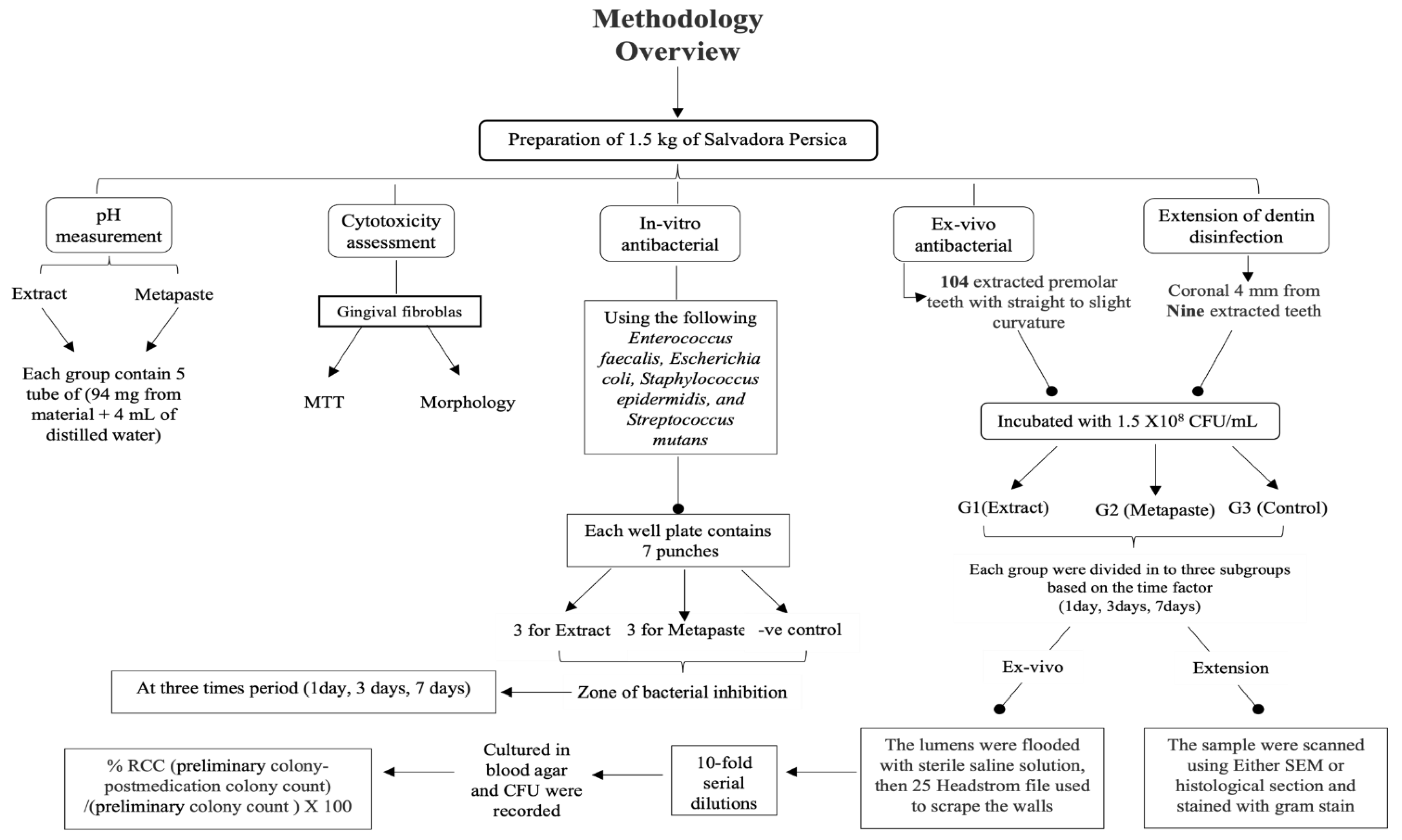
2. Materials and Methods
2.1. Preparation of the S. persica Methanolic Extract
2.2. pH Measurements
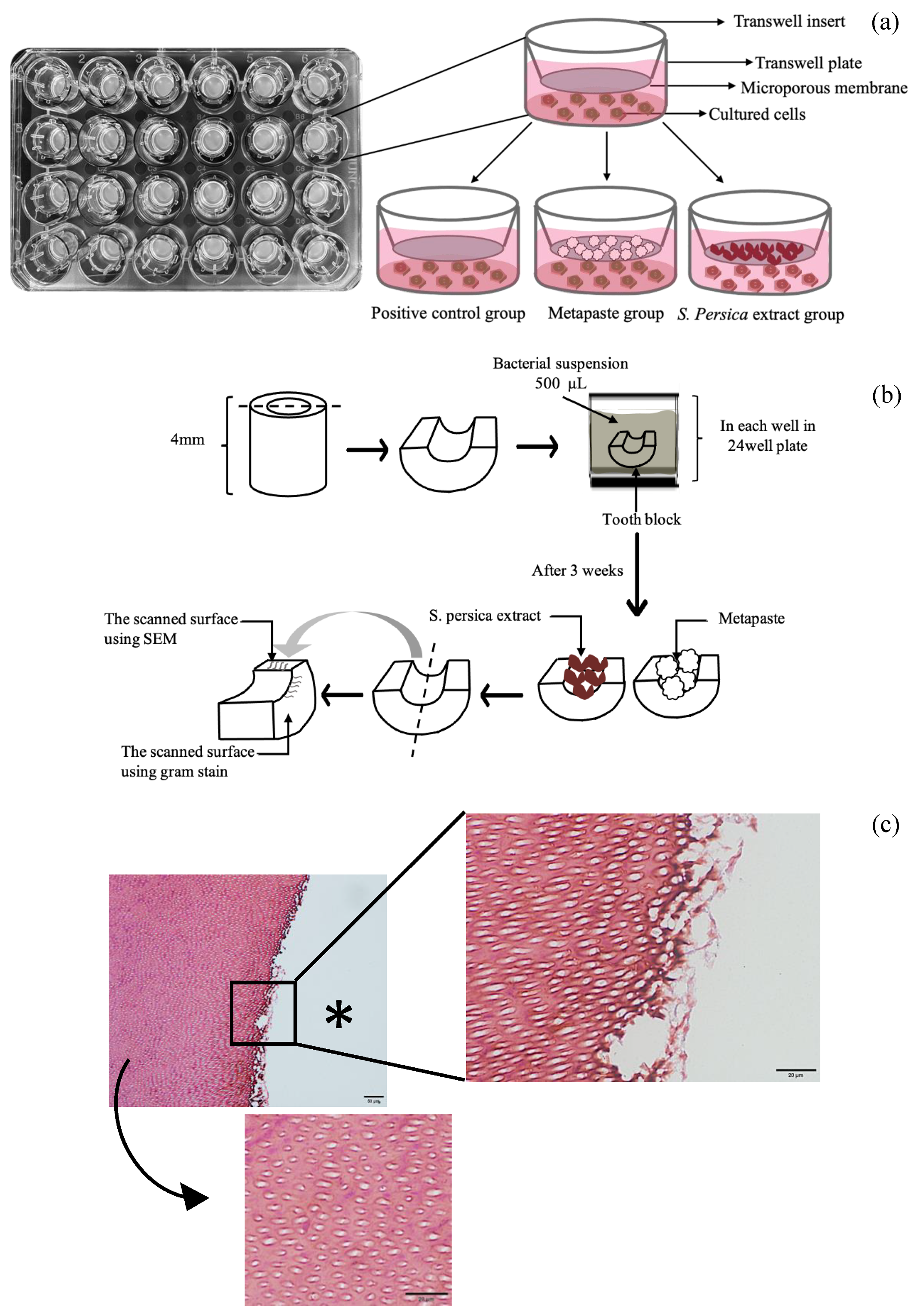
2.3. Biocompatibility Assessment
2.4. Cell Attachment
2.5. Cell Viability
2.6. In Vitro Antibacterial Activity (Agar-Well Diffusion Test)
2.7. Ex Vivo Antibacterial Activity (% Reduction in Colony Count)
2.8. Extension of Dentin Disinfection Activity
2.9. Scanning Electron Microscopy
2.10. Gram Staining Technique
2.11. Statistical Analysis
3. Results
3.1. pH Measurements
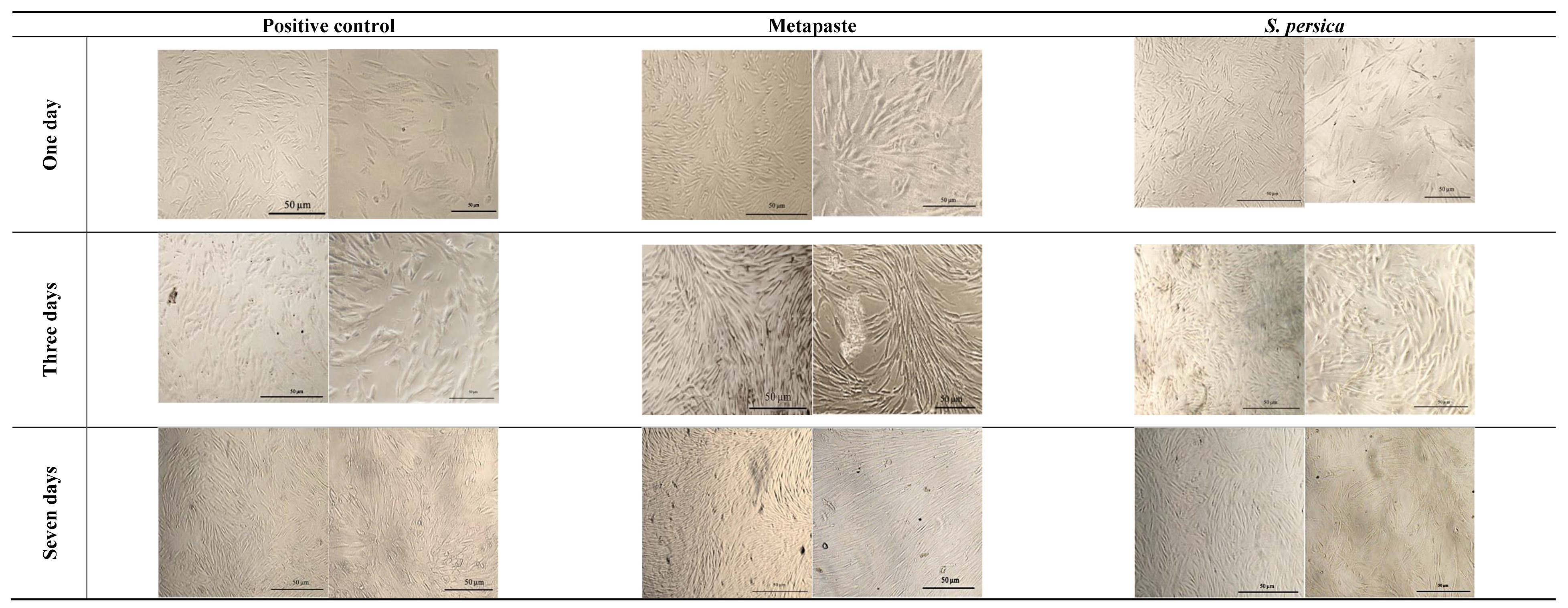
3.2. Biocompatibility Assessment
3.2.1. Cell Morphology
3.2.2. Cell Viability (MTT Assay)
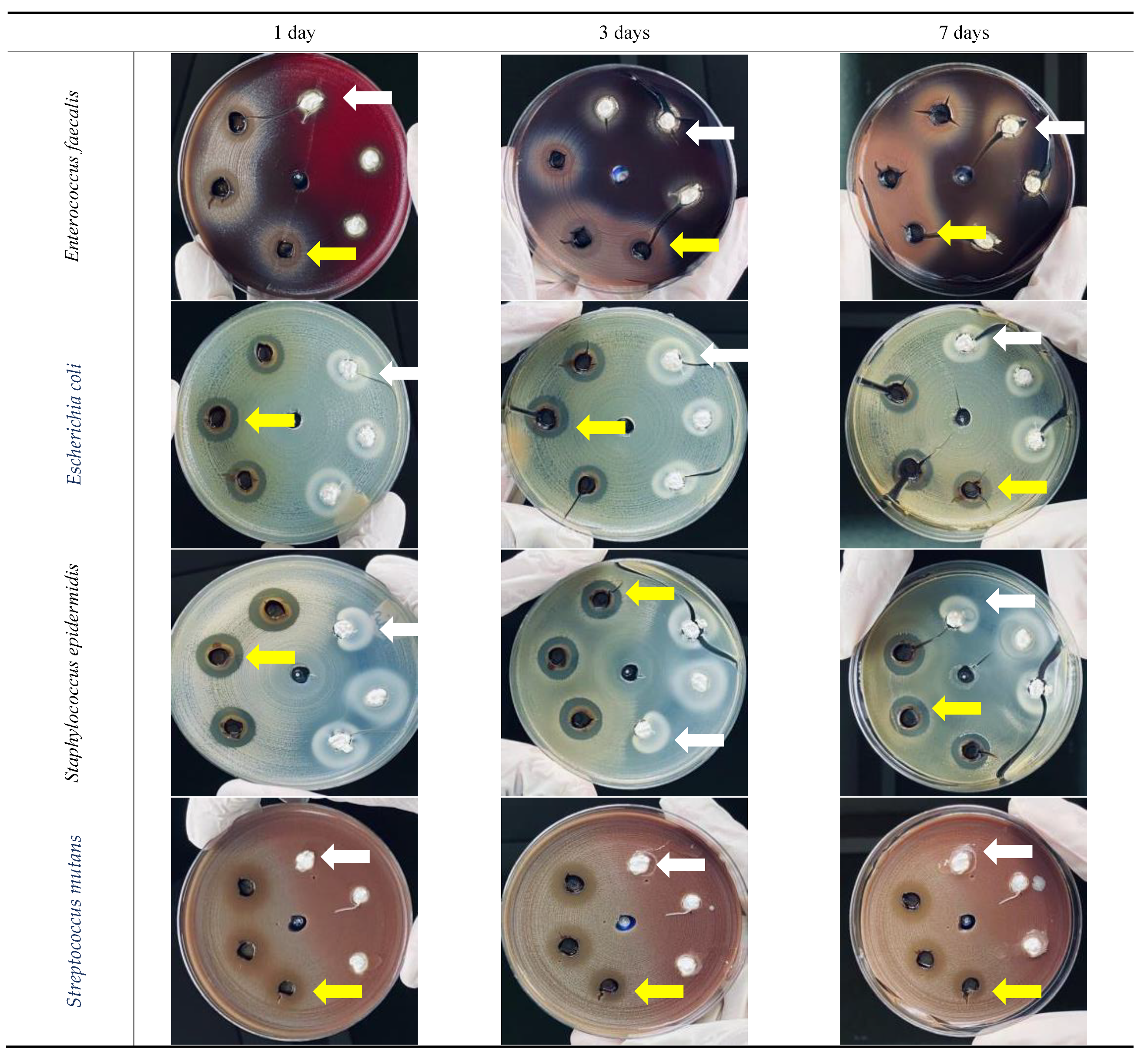
3.3. In Vitro Antibacterial Activity
3.3.1. Enterococcus faecalis
3.3.2. Escherichia coli
3.3.3. Staphylococcus epidermidis
3.3.4. Streptococcus mutans
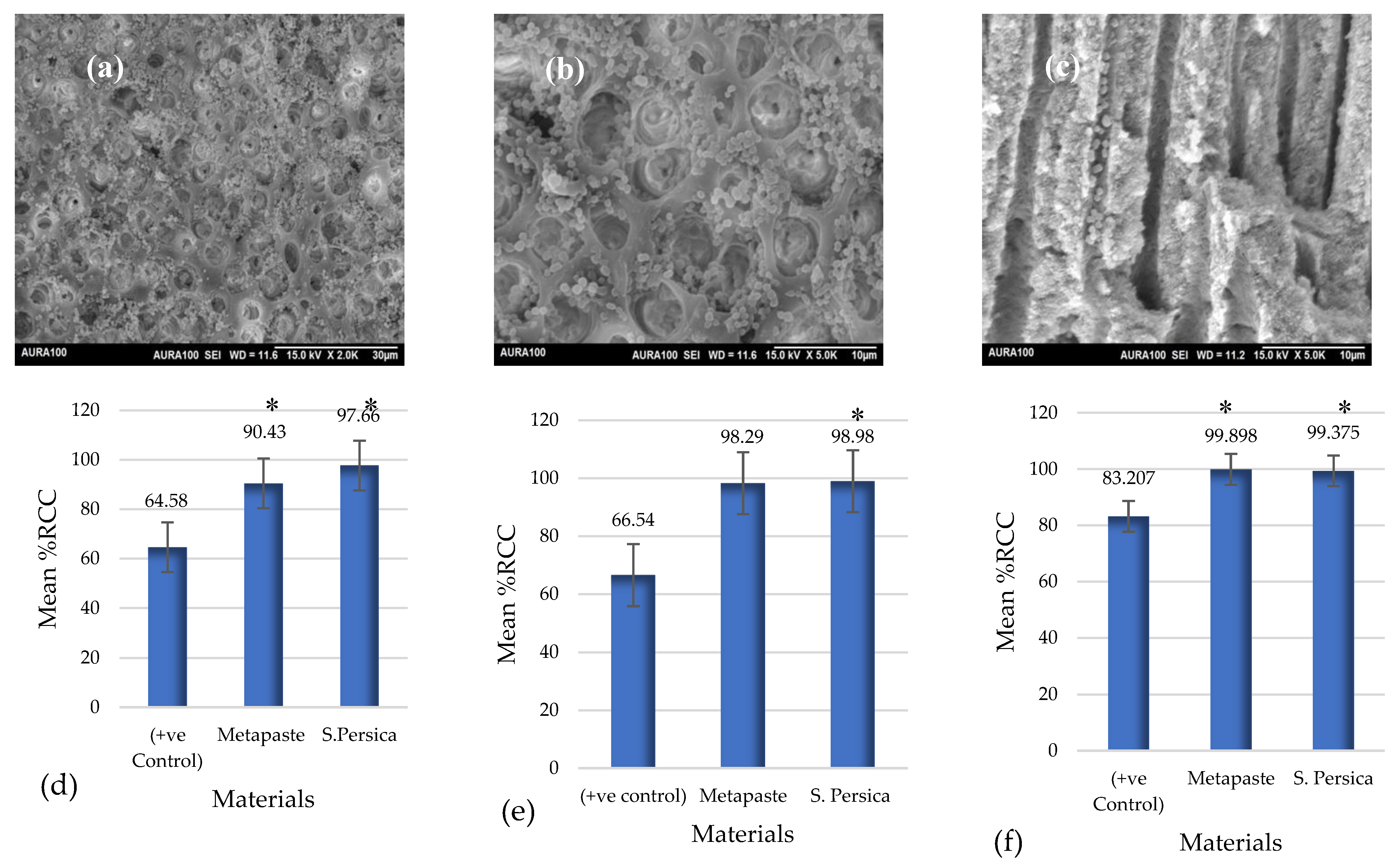
3.4. Ex Vivo Antibacterial Activity
3.5. Extension of Dentin Disinfection Activity
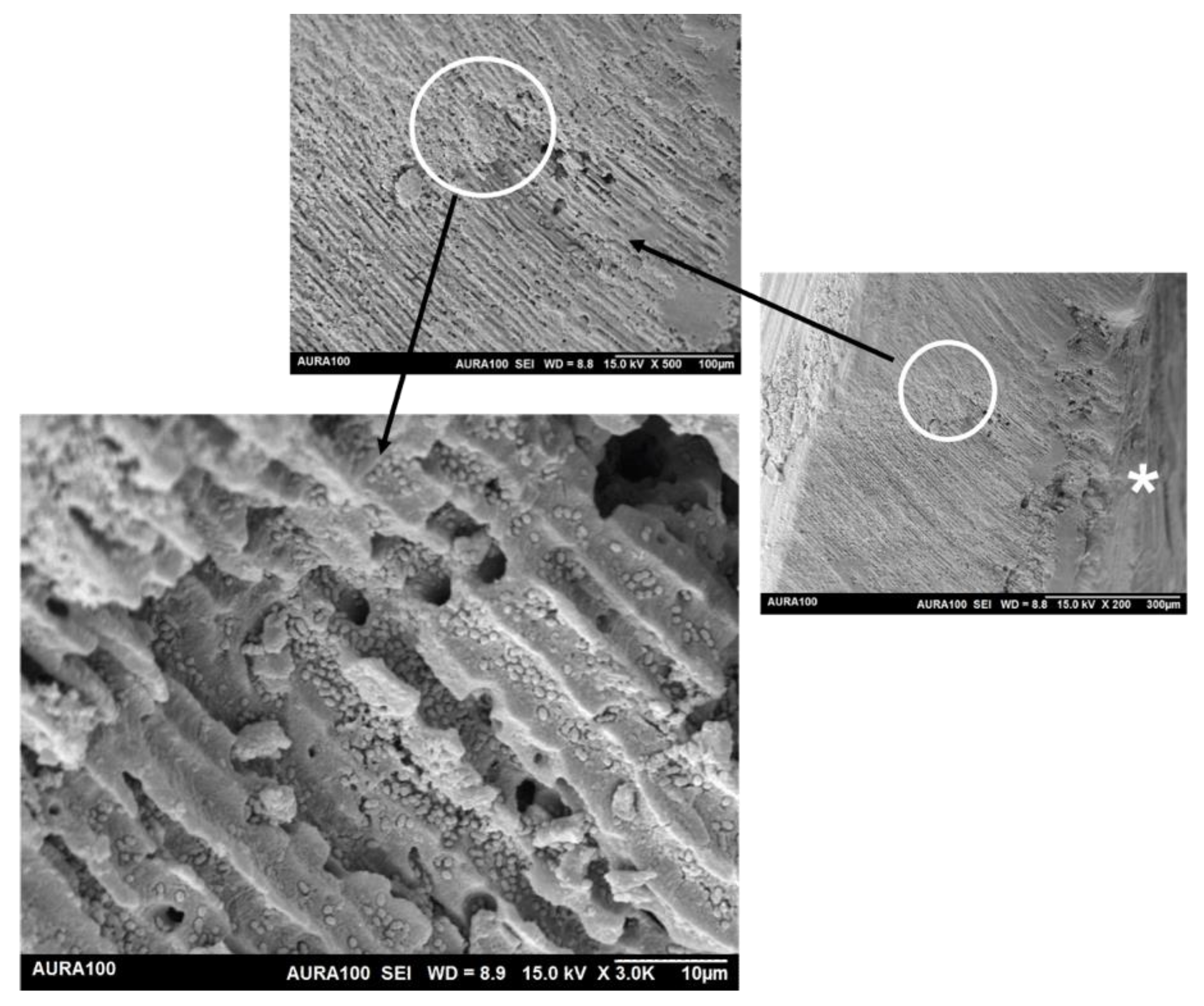
3.5.1. Scanning Electron Microscopy
3.5.2. Gram Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bortoluzzi, E.A.; Carlon, D.; Meghil, M.M.; El-Awady, A.R.; Niu, L.; Bergeron, B.E.; Susin, L.; Cutler, C.W.; Pashley, D.H.; Tay, F.R. Efficacy of 3D conforming nickel titanium rotary instruments in eliminating canal wall bacteria from oval-shaped root canals. J. Dent. 2015, 43, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.A.; Schönenberger, K.; Laib, A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int. Endod. J. 2001, 34, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Paqué, F.; Zehnder, M.; De-Deus, G. Microtomography-based comparison of reciprocating single-file f2 protaper technique versus rotary full sequence. J. Endod. 2011, 37, 1394–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, J.F.J.; Pérez, A.R.; Marceliano-Alves, M.F.; Provenzano, J.C.; Silva, S.G.; Pires, F.R.; Vieira, G.; Rôças, I.N.; Alves, F. What happens to unprepared root canal walls: A correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int. Endod. J. 2018, 51, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Paqué, F.; Al-Jadaa, A.; Kfir, A. Hard-tissue debris accumulation created by conventional rotary versus self-adjusting file instrumentation in mesial root canal systems of mandibular molars. Int. Endod. J. 2012, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Portenier, I.; Haapasalo, H.; Rye, A.; Waltimo, T.; Ørstavik, D.; Haapasalo, M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int. Endod. J. 2001, 34, 184–188. [Google Scholar] [CrossRef]
- Richhawal, A.P.; Shetty, V.K.; Singh, S.S.; Jadhav, N.L. Short term effect of intracanal calcium hydroxide on the compressive strength of root dentin of mature human permanent teeth. IOSR J. 2019, 18, 36–39. [Google Scholar]
- Wigler, R.; Dvir, R.; Weisman, A.; Matalon, S.; Kfir, A. Efficacy of XP-endo finisher files in the removal of calcium hydroxide paste from artificial standardized grooves in the apical third of oval root canals. Int. Endod. J. 2017, 50, 700–705. [Google Scholar] [CrossRef]
- Evans, M.; Davies, J.K.; Sundqvist, G.; Figdor, D. Mechanisms involved in the resistance of enterococcus faecalis to calcium hydroxide. Aust. Endod. J. 2002, 35, 221–228. [Google Scholar] [CrossRef]
- Rôças, I.N.; Siqueira, J.F.; Santos, K.R.N. Association of enterococcus faecalis with different forms of periradicular diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef]
- Tennert, C.; Fuhrmann, M.; Wittmer, A.; Karygianni, L.; Altenburger, M.J.; Pelz, K.; Hellwig, E.; Al-Ahmad, A. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J. Endod. 2014, 40, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Little, J.W. Complementary and alternative medicine: Impact on dentistry. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Aumeeruddy, M.Z.; Zengin, G.; Mahomoodally, M.F. A Review of the Traditional and Modern Uses of Salvadora persica L. (Miswak): Toothbrush tree of Prophet Muhammad. J. Ethnopharmacol. 2018, 213, 409–444. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Alsareii, S.A. A review of the therapeutic effects of using Miswak (Salvadora persica) on oral health. Saudi Med. J. 2015, 36, 530–543. [Google Scholar] [CrossRef]
- Balto, H.; Al-Howiriny, T.; Al-Somily, A.; Siddiqui, Y.; Al-Sowygh, Z.; Halawany, H.; Al-Hadlaq, S. Screening for the antimicrobial activity of Salvadora persica extracts against Enterococcus faecalis and Candida albicans. Int. J. Phytomed 2013, 5, 486–492. [Google Scholar]
- WHO. Consensus statement on oral hygiene. Int. Dent. J. 2000, 50, 139. [Google Scholar]
- Paliwal, S.; Chauhan, R.; Siddiqui, A.A.; Paliwal, S.; Sharma, J. Evaluation of antifungal activity of Salvadora persica Linn. leaves. Indian J. Nat. Prod. Resour. 2007, 6, 372–374. [Google Scholar]
- Mervat, E.-H.; Ali, H.M.; Ashmawy, N.A.; Salem, M.Z.M. Chemical Composition and Bioactivity of Salvadora persica Extracts against Some Potato Bacterial Pathogens. BioResources 2017, 12, 1835–1849. [Google Scholar]
- Akhtar, J.; Siddique, K.; Bi, S.; Mujeeb, M. A Review on Phytochemical and Pharmacological Investigations of Miswak (Salvadora persica Linn). J. Pharm. Bioallied Sci. 2011, 3, 113–117. [Google Scholar]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.K.; Gustafsson, A.; Pütsep, K. Benzyl Isothiocyanate, a Major component from the roots of Salvadora persica is highly active against gram-negative bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar]
- Khatak, M.; Khatak, S.; Siddqui, A.; Vasudeva, N.; Aggarwal, A.; Aggarwal, P. Salvadora persica. Pharmacogn. Rev. 2010, 8, 209–214. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; AL Sahli, A.A.A.; Alaraidh, I.A.; Al-Homaidan, A.A.; Mostafa, E.M.; EL-Gaaly, G.A. Assessment of antioxidant activities in roots of miswak (Salvadora persica) plants grown at two different locations in Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.T. Benzylamides from Salvadora persica. Arch. Pharm. Res. 2006, 29, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabawi, N.; Al Sheikh Abdal, A.; Taha, M. The antimicrobial activity of Salvadora persica solution (Miswak-Siwak) as root canal irrigant (a comparative study). Univ. Sharjah J. Pure Appl. Sci. 2007, 4, 69–91. [Google Scholar]
- Balto, H.; Ghandourah, B.; Al-Sulaiman, H. The efficacy of Salvadora persica Extract in the elimination of the intracanal smear layer: A SEM study. Saudi Dent. J. 2012, 24, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sabawi, N.; Al-Naimi, A.; Yahya, E. An in Vitro and in Vivo antibacterial effect of different plant extracts on Enterococcus faecalis as intracanal medicament. J. Int. Oral Health 2020, 12, 362–369. [Google Scholar] [CrossRef]
- Zancan, R.F.; Vivan, R.R.; Milanda Lopes, M.R.; Weckwerth, P.H.; de Andrade, F.B.; Ponce, J.B.; Duarte, M.A.H. Antimicrobial activity and physicochemical properties of calcium hydroxide pastes used as intracanal medication. J. Endod. 2016, 42, 1822–1828. [Google Scholar] [CrossRef]
- Kalamegam, G.; Sait, K.H.W.; Ahmed, F.; Kadam, R.; Pushparaj, P.N.; Anfinan, N.; Rasool, M.; Jamal, M.S.; Abu-Elmagd, M.; Al-Qahtani, M. Human wharton’s jelly stem cell (HWJSC) extracts inhibit ovarian cancer cell lines OVCAR3 and SKOV3 in vitro by inducing cell cycle arrest and apoptosis. Front. Oncol. 2018, 8, 592. [Google Scholar] [CrossRef] [Green Version]
- Dahl, J.E.; Frangou-Polyzois, M.J.; Polyzois, G.L. In vitro biocompatibility of denture relining materials. Gerodontology 2006, 23, 17–22. [Google Scholar] [CrossRef]
- Labban, N.; Yassen, G.; Windsor, L.; Platt, J. The direct cytotoxic effects of medicaments used in endodontic regeneration on human dental pulp cells. Dent. Traumatol. 2014, 30, 429–434. [Google Scholar] [CrossRef]
- Al-Khatib, Z.Z.; Baum, R.H.; Morse, D.R.; Yesilsoy, C.; Bhambhani, S.; Furst, M.L. The antimicrobial effect of various endodontic sealers. Oral Surg. Oral Med. Oral Pathol. 1990, 70, 784–790. [Google Scholar] [CrossRef]
- Madhubala, M.M.; Srinivasan, N.; Ahamed, S. Comparative evaluation of propolis and triantibiotic mixture as an intracanal medicament against Enterococcus faecalis. J. Endod. 2011, 37, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Pourhashemi, S.J.; Sadegh, M.; Salehi, Y.; Fard, M.J.K. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent. 2015, 43, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, M.; Orstavik, D. In vitro infection and disinfection of dentinal tubules. J. Dent. Res. 1987, 66, 1375–1379. [Google Scholar] [CrossRef]
- Sundqvist, G.; Figdor, D.; Persson, S.; Sjögren, U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 86–93. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Aguiar, A.S.; Guerreiro-Tanomaru, J.M.; Faria, G.; Leonardo, R.T.; Tanomaru-Filho, M. Antimicrobial Activity and PH of calcium hydroxide and zinc oxide nanoparticles intracanal medication and association with chlorhexidine. J. Contemp. Dent. Pract. 2015, 16, 624–629. [Google Scholar]
- Palanikumar, L.; Ramasamy, S.N.; Balachandran, C. Size-dependent antimicrobial response of zinc oxide nanoparticles. IET Nanobiotechnology 2014, 8, 111–117. [Google Scholar] [CrossRef]
- Simsek, N.; Bulut, E.T.; Ahmetoğlu, F.; Alan, H. Determination of trace elements in rat organs implanted with endodontic repair materials by ICP-MS. J. Mater. Sci. Mater. Med. 2016, 27, 46. [Google Scholar] [CrossRef]
- Halawany, H.S. A Review on Miswak (Salvadora persica) and its effect on various aspects of oral health. Saudi Dent. J. 2012, 24, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, E.; Al-Harbi, K. Phytochemical screening and antibacterial activity of crude aqueous and ethanol extracts of Salvadora persica L. Stem (Miswak) from Saudi Arabia. J. Phytopharm. 2015, 4, 243–247. [Google Scholar] [CrossRef]
- Al-Ayed, M.; Asaad, A.; Qureshi, M.; Attia, H.; AlMarrani, A. Antibacterial activity of Salvadora persica L. (Miswak) extracts against multidrug resistant bacterial clinical isolates. Evid.-Based Complement. Altern. Med. 2016, 13, 79–83. [Google Scholar]
- Al-bayati, F.A.; Sulaiman, K.D. In vitro antimicrobial activity of salvadora persica l. extracts against some isolated oral pathogens in Iraq. Turkish J. Biol. 2008, 32, 57–62. [Google Scholar]
- Plant Solvent Extraction Method Using Ethanol: 3 Steps—Cole-Parmer. Available online: https://www.coleparmer.com/tech-article/solvent-extraction-method-ethanol (accessed on 22 January 2022).
- Zhang, W.J.; Björn, L.O. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia 2009, 80, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Skarupova, D.; Vostalova, J.; Svobodova, A.R. Ultraviolet a protective potential of plant extracts and phytochemicals. Biomed. Pap. 2020, 164, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Al-Nimer, M.S.M.; Wahbee, Z. Ultraviolet light assisted extraction of flavonoids and allantoin from aqueous and alcoholic extracts of Symphytum officinale. J. Intercult. Ethnopharmacol. 2017, 6, 280–283. [Google Scholar] [CrossRef] [Green Version]
- Nadir, B.; Khalid, A.M. Effect of miswak extract on healthy human dentin. Saudi Dent. J. 1999, 11, 46–52. [Google Scholar]
- ISO—ISO 10993-1:2018—Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. Available online: https://www.iso.org/standard/68936.html (accessed on 29 June 2021).
- Tabatabaei, F.; Moezizadeh, M.; Javand, F. Effects of extracts of Salvadora persica on proliferation and viability of human dental pulp stem cells. J. Conserv. Dent. 2015, 18, 315–320. [Google Scholar] [PubMed] [Green Version]
- Balto, H.; Al-Manei, K.; Bin-Mohareb, T.; Shakoor, Z.; Al-Hadlaq, S. Cytotoxic effect of Salvadora persica extracts on human gingival fibroblast cells. Saudi Med. J. 2014, 35, 810–815. [Google Scholar]
- Rajabalian, S.; Mohammadi, M.; Mozaffari, B. Cytotoxicity evaluation of Persica mouthwash on cultured human and mouse cell lines in the presence and absence of fetal calf serum. Indian J. Dent. Res. 2009, 20, 169–173. [Google Scholar] [PubMed]
- Darmani, H.; Nusayr, T.; Al-Hiyasat, A.S. Effects of extracts of miswak and derum on proliferation of Balb/C 3T3 fibroblasts and viability of cariogenic bacteria. Int. J. Dent. Hyg. 2006, 4, 62–66. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, A.I.; Yap, A.S. Contact inhibition (of proliferation) redux. Curr. Opin. Cell Biol. 2012, 24, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N. Exploiting Molecular Methods to Explore Endodontic Infections: Part 2—Redefining the endodontic microbiota. J. Endod. 2005, 31, 488–498. [Google Scholar] [CrossRef]
- Siqueira, J.J.; Rôças, I. Clinical implications and microbiology of bacterial persistance after treatment procedures. J. Endodonotics 2008, 34, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Karibian, D.; Cavaillon, J.M.; Haeffner-Cavaillon, N. Structural and functional analyses of bacterial lipopolysaccharides. Microbes Infect. 2002, 4, 915–926. [Google Scholar] [CrossRef]
- Basrani, B.; Tjäderhane, L.; Santos, J.M.; Pascon, E.; Grad, H.; Lawrence, H.P.; Friedman, S. Efficacy of Chlorhexidine- and Calcium Hydroxide-Containing Medicaments against Enterococcus faecalis in Vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 618–624. [Google Scholar] [CrossRef]
- Barbosa, C.A.M.; Gonçalves, R.B.; Siqueira, J.F.; De Uzeda, M. Evaluation of the antibacterial activities of calcium hydroxide, chlorhexidine, and camphorated paramonochlorophenol as intracanal medicament. A clinical and laboratory study. J. Endod. 1997, 23, 297–300. [Google Scholar] [CrossRef]
- Balto, H.; Al-Sanie, I.; Al-Beshri, S.; Aldrees, A. Effectiveness of Salvadora persica extracts against common oral pathogens. Saudi Dent. J. 2017, 29, 1–6. [Google Scholar] [CrossRef]
- Gupta, D.; Kamat, S.; Hugar, S.; Nanjannawar, G.; Kulkarni, R. A comparative evaluation of the antibacterial efficacy of Thymus Vulgaris, Salvadora persica, Acacia Nilotica, Calendula Arvensis, and 5% sodium hypochlorite against Enterococcus faecalis: An in-vitro study. J. Conserv. Dent. 2020, 23, 97–101. [Google Scholar] [CrossRef]
- Siqueira, J.; José, F. Treatment of Endodontic Infections; Quintessence: Chicago, IL, USA, 2011. [Google Scholar]
- Distel, J.W.; Hatton, J.F.; Gillespie, M.J. Biofilm formation in medicated root canals. J. Endod. 2002, 28, 689–693. [Google Scholar] [CrossRef]
- Hartke, A.; Lemarinier, S.; Pichereau, V.; Auffray, Y. Survival of Enterococcus faecalis in seawater microcosms is limited in the presence of bacterivorous zooflagellates. Curr. Microbiol. 2002, 44, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, L.; Valera, M.; Oliveira, L.; Carvalho, C.; Camargo, C.; Jorge, A. Effect of zingiber officinale and propolis on microorganisms and endotoxins in root canals. J. Appl. Oral Sci. 2013, 21, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, D.; Haapasalo, M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent. Traumatol. 1990, 6, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Stojicic, S.; Shen, Y.; Haapasalo, M. Effect of the source of biofilm bacteria, level of biofilm maturation, and type of disinfecting agent on the susceptibility of biofilm bacteria to antibacterial agents. J. Endod. 2013, 39, 473–477. [Google Scholar] [CrossRef]
- Siqueira, J.F.J.; De Uzeda, M.; Fonseca, M. A Scanning electron microscopic evaluation of in vitro dentinal tubules penetration by selected anaerobic bacteria. J. Endod. 1996, 22, 308–310. [Google Scholar] [CrossRef]
- Pereira, T.C.; da Silva Munhoz Vasconcelos, L.R.; Graeff, M.S.Z.; Ribeiro, M.C.M.; Duarte, M.A.H.; de Andrade, F.B. Intratubular decontamination ability and physicochemical properties of calcium hydroxide pastes. Clin. Oral Investig. 2019, 23, 1253–1262. [Google Scholar] [CrossRef]
- Hayashi, T.; Makino, K.; Ohnishi, M.; Kurokawa, K.; Ishii, K.; Yokoyama, K.; Han, C.G.; Ohtsubo, E.; Nakayama, K.; Murata, T.; et al. Complete genome sequence of enterohemorrhagic Eschelichia Coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001, 8, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Swimberghe, R.C.D.; Coenye, T.; De Moor, R.J.G.; Meire, M.A. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2019, 52, 604–628. [Google Scholar] [CrossRef] [Green Version]


| Study Group | Group Discription |
|---|---|
| Group 1 | 94 mg of S. persica extract in 4 mL of normal saline (n = 5) |
| Group 2 | 94 mg of Metapaste in 4 mL of normal saline (n = 5) |
| Group 3 | 4 mL of normal saline (n = 5) |
| Time Points | 0 h | 2 h | 4 h | 1 Day | 3 Days | 7 Days |
|---|---|---|---|---|---|---|
| S.persica extract | 3.79 ± 0.36 c | 3.79 ± 0.38 c | 3.83 ± 0.40 c | 3.91 ± 0.37 c | 3.93 ± 0.37 c | 3.75 ± 0.39 c |
| Metapaste | 12.42 ± 0.11 a | 12.42 ± 0.09 a | 12.39 ± 0.08 a | 12.51 ± 0.03 a | 12.48 ± 0.13 a | 12.40 ± 0.21 a |
| Positive control | 5.40 ± 0.07 b * | 6.04 ± 0.41 b | 6.24 ± 0.50 b | 5.89 ± 0.32 b | 6.04 ± 0.30 b | 6.13 ± 0.40 b |
| Bacterial Type | E. faecalis | E. coli | S. epidermids | S. mutans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time period (Days) | 1 | 3 | 7 | 1 | 3 | 7 | 1 | 3 | 7 | 1 | 3 | 7 |
| S. Persica extract (± SD) | 13.3 ± 1.2 a | 15 ± 1.7 a | 13.3 ± 1.2 a | 14.3 ± 0.6 a | 15.3 ± 1.5 | 15.3 ± 1.2 | 16.6 ± 0.6 | 17.3 ± 1.2 | 16.3 ± 1.2 | 15 ± 2.6 a | 15 ± 1 a | 14.6 ± 1.5 a |
| Metapaste (± SD) | 0 | 0 | 0 | 0 | 16 ± 2 | 13.6 ± 0.6 | 18 ± 2 | 17.6 ± 0.6 | 16.6 ± 2.3 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdeltawab, S.S.; Abu Haimed, T.S.; Bahammam, H.A.; Arab, W.T.; Abou Neel, E.A.; Bahammam, L.A. Biocompatibility and Antibacterial Action of Salvadora persica Extract as Intracanal Medication (In Vitro and Ex Vivo Experiment). Materials 2022, 15, 1373. https://doi.org/10.3390/ma15041373
Abdeltawab SS, Abu Haimed TS, Bahammam HA, Arab WT, Abou Neel EA, Bahammam LA. Biocompatibility and Antibacterial Action of Salvadora persica Extract as Intracanal Medication (In Vitro and Ex Vivo Experiment). Materials. 2022; 15(4):1373. https://doi.org/10.3390/ma15041373
Chicago/Turabian StyleAbdeltawab, Samah Samir, Tariq S. Abu Haimed, Hammam Ahmed Bahammam, Wafaa Talat Arab, Ensanya A. Abou Neel, and Laila Ahmed Bahammam. 2022. "Biocompatibility and Antibacterial Action of Salvadora persica Extract as Intracanal Medication (In Vitro and Ex Vivo Experiment)" Materials 15, no. 4: 1373. https://doi.org/10.3390/ma15041373
APA StyleAbdeltawab, S. S., Abu Haimed, T. S., Bahammam, H. A., Arab, W. T., Abou Neel, E. A., & Bahammam, L. A. (2022). Biocompatibility and Antibacterial Action of Salvadora persica Extract as Intracanal Medication (In Vitro and Ex Vivo Experiment). Materials, 15(4), 1373. https://doi.org/10.3390/ma15041373






