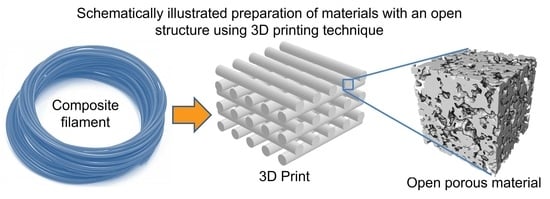
Polymer–Nickel Composite Filaments for 3D Printing of Open Porous Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation
- PLA-Ni Composite Preparation
- ABS-Ni and PVB-Ni Composites Preparation
2.3. Composite Filament Extrusion
2.4. 3D Printing
2.5. Thermal Treatment
2.6. Characterization of Materials
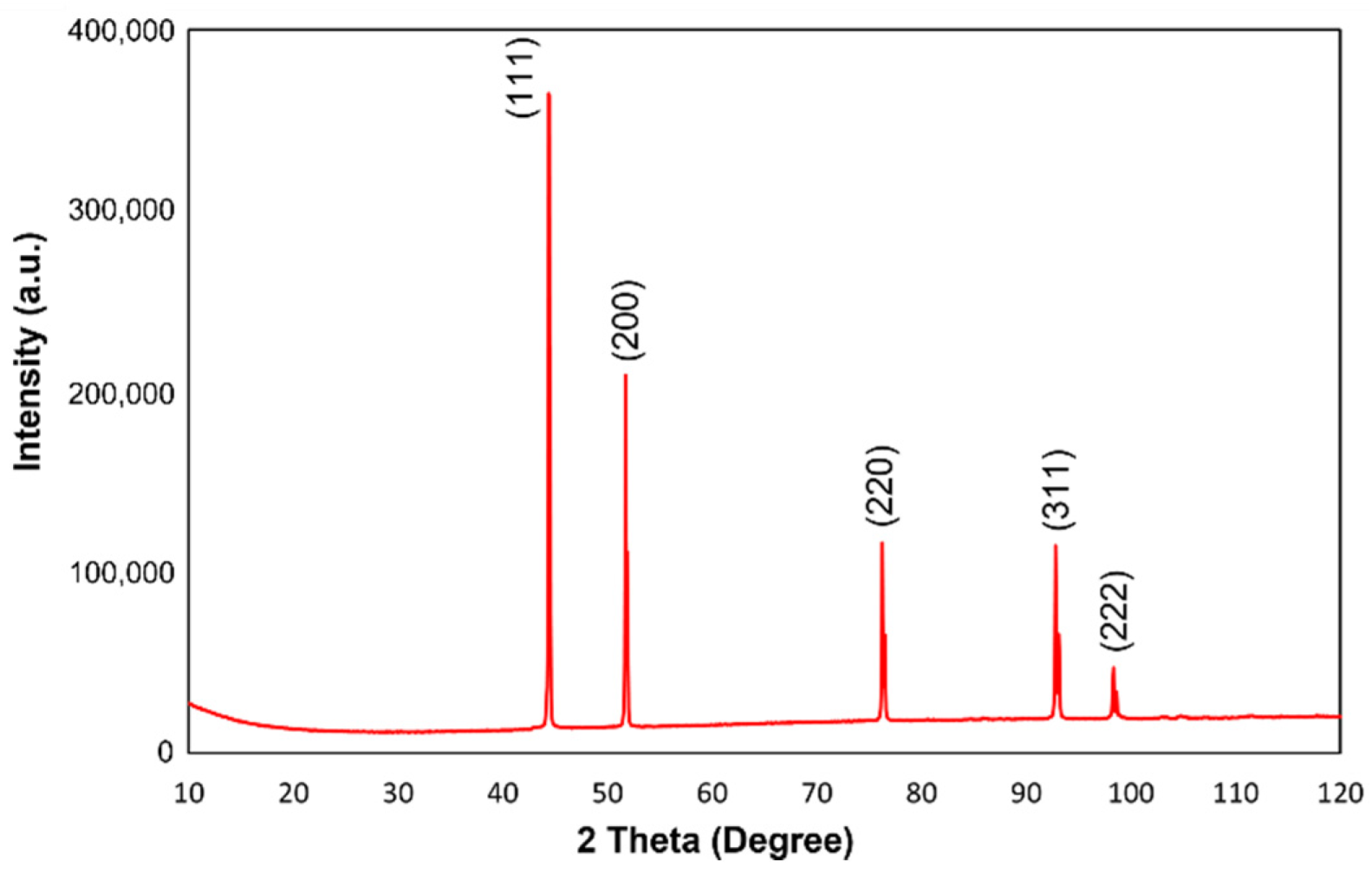
3. Results and Discussion
4. Summary and Conclusions
- Complete removal of the solvent in the drying process of composites is a crucial condition for proper filament extrusion and for obtaining a material useful in 3D printing with FDM.
- The use of acetone as a solvent in PVB and ABS based composites made it possible to carry out the process of drying and filament extrusion with an appropriate content of nickel particles.
- As the nickel content increases, the brittleness of the polymer-Ni filament increases. With a volume content of Ni above 25% for the solvent-based composite preparation method, the plasticity of the material decreases, which makes it almost impossible to be printed with FDM. For the PLA-based composite, the polymer melting method works only for nickel contents, at about 5%. At higher ones, the material is too brittle.
- It has been shown that PLA-Ni 5%, PVB-Ni 25% and ABS-Ni 25% composite filaments allow for the 3D printing of parts with a flat geometry, which after appropriate thermal treatment led to a metallic structure with an open porosity above 60%, while maintaining satisfactory mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Liu, C.J. Three-Dimensional Printing for Catalytic Applications: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1701134. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Masood, S.H.; Song, W.Q. Development of New Metal/Polymer Materials for Rapid Tooling Using Fused Deposition Modelling. Mater. Des. 2004, 25, 587–594. [Google Scholar] [CrossRef]
- Panwisawas, C.; Tang, Y.T.; Reed, R.C. Metal 3D Printing as a Disruptive Technology for Superalloys. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Azuaje, J.; Tubío, C.R.; Escalante, L.; Gómez, M.; Guitián, F.; Coelho, A.; Caamaño, O.; Gil, A.; Sotelo, E. An Efficient and Recyclable 3D Printed α-Al2O3 Catalyst for the Multicomponent Assembly of Bioactive Heterocycles. Appl. Catal. A Gen. 2017, 530, 203–210. [Google Scholar] [CrossRef]
- Stuecker, J.N.; Miller, J.E.; Ferrizz, R.E.; Mudd, J.E.; Cesarano, J. Advanced Support Structures for Enhanced Catalytic Activity. Ind. Eng. Chem. Res. 2004, 43, 51–55. [Google Scholar] [CrossRef]
- Hurt, C.; Brandt, M.; Priya, S.S.; Bhatelia, T.; Patel, J.; Selvakannan, P.R.; Bhargava, S. Combining Additive Manufacturing and Catalysis: A Review. Catal. Sci. Technol. 2017, 7, 3421–3439. [Google Scholar] [CrossRef]
- Zhu, C.; Qi, Z.; Beck, V.A.; Luneau, M.; Lattimer, J.; Chen, W.; Worsley, M.A.; Ye, J.; Duoss, E.B.; Spadaccini, C.M.; et al. Toward Digitally Controlled Catalyst Architectures: Hierarchical Nanoporous Gold via 3D Printing. Sci. Adv. 2018, 4, eaas9459. [Google Scholar] [CrossRef] [Green Version]
- Hurt, C.W. Investigation of Additively Manufactured Substrates in Heterogeneous Catalysis. Master’s Thesis, RMIT University, Melbourne, VIC, Australia, 2017. [Google Scholar]
- Ludwig, T.; von Seckendorff, J.; Troll, C.; Fischer, R.; Tonigold, M.; Rieger, B.; Hinrichsen, O. Additive Manufacturing of Al2O3-Based Carriers for Heterogeneous Catalysis. Chem. Ing. Tech. 2018, 90, 703–707. [Google Scholar] [CrossRef]
- Tubío, C.R.; Azuaje, J.; Escalante, L.; Coelho, A.; Guitián, F.; Sotelo, E.; Gil, A. 3D Printing of a Heterogeneous Copper-Based Catalyst. J. Catal. 2016, 334, 110–115. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Sitek, R.; Buhagiar, J.; Kurzydłowski, K.J.; Świeszkowski, W. Laser and Electron Beam Additive Manufacturing Methods of Fabricating Titanium Bone Implants. Appl. Sci. 2017, 7, 657. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Adeoye, A.O.M. 3D Printing of Bone Scaffolds with Hybrid Biomaterials. Compos. Part B Eng. 2019, 158, 428–436. [Google Scholar] [CrossRef]
- Do, A.V.; Smith, R.; Acri, T.M.; Geary, S.M.; Salem, A.K. 3D Printing Technologies for 3D Scaffold Engineering. In Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Thorston, UK, 2018; pp. 203–234. ISBN 9780081009796-6. [Google Scholar]
- Lahtinen, E.; Hänninen, M.M.; Kinnunen, K.; Tuononen, H.M.; Väisänen, A.; Rissanen, K.; Haukka, M. Porous 3D Printed Scavenger Filters for Selective Recovery of Precious Metals from Electronic Waste. Adv. Sustain. Syst. 2018, 2, 1800048. [Google Scholar] [CrossRef]
- Low, Z.X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D Printing of Separation Membranes and Comparison to Related Unconventional Fabrication Techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef] [Green Version]
- Kalsoom, U.; Nesterenko, P.N.; Paull, B. Current and Future Impact of 3D Printing on the Separation Sciences. TrAC-Trends Anal. Chem. 2018, 105, 492–502. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, T.; Li, X.; Hu, J.; Li, T.; Lin, X.; Huang, W. 3D Printing Well Organized Porous Iron-Nickel/Polyaniline Nanocages Multiscale Supercapacitor. J. Alloys Compd. 2018, 760, 78–83. [Google Scholar] [CrossRef]
- Azuaje, J.; Rama, A.; Mallo-Abreu, A.; Boado, M.G.; Majellaro, M.; Tubío, C.R.; Prieto, R.; García-Mera, X.; Guitián, F.; Sotelo, E.; et al. Catalytic Performance of a Metal-Free Graphene Oxide-Al2O3 Composite Assembled by 3D Printing. J. Eur. Ceram. Soc. 2021, 41, 1399–1406. [Google Scholar] [CrossRef]
- Lawson, S.; Li, X.; Thakkar, H.; Rownaghi, A.A.; Rezaei, F. Recent Advances in 3D Printing of Structured Materials for Adsorption and Catalysis Applications. Chem. Rev. 2021, 6246–6291. [Google Scholar] [CrossRef]
- Browne, M.P.; Redondo, E.; Pumera, M. 3D Printing for Electrochemical Energy Applications. Chem. Rev. 2020, 120, 2783–2810. [Google Scholar] [CrossRef]
- Tauster, S.J. Strong Metal-Support Interactions. Acc. Chem. Res. 1987, 20, 389–394. [Google Scholar] [CrossRef]
- Czelej, K.; Cwieka, K.; Colmenares, J.C.; Kurzydlowski, K.J.; Xu, Y.J. Toward a Comprehensive Understanding of Enhanced Photocatalytic Activity of the Bimetallic PdAu/TiO2 Catalyst for Selective Oxidation of Methanol to Methyl Formate. ACS Appl. Mater. Interfaces 2017, 9, 31825–31833. [Google Scholar] [CrossRef] [PubMed]
- Middelkoop, V.; Vamvakeros, A.; de Wit, D.; Jacques, S.D.M.; Danaci, S.; Jacquot, C.; de Vos, Y.; Matras, D.; Price, S.W.T.; Beale, A.M. 3D Printed Ni/Al2O3 Based Catalysts for CO2 Methanation-a Comparative and Operando XRD-CT Study. J. CO2 Util. 2019, 33, 478–487. [Google Scholar] [CrossRef]
- Castles, F.; Isakov, D.; Lui, A.; Lei, Q.; Dancer, C.E.J.; Wang, Y.; Janurudin, J.M.; Speller, S.C.; Grovenor, C.R.M.; Grant, P.S. Microwave Dielectric Characterisation of 3D-Printed BaTiO3/ABS Polymer Composites. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrado Perez, A.R.; Roberson, D.A.; Wicker, R.B. Fracture Surface Analysis of 3D-Printed Tensile Specimens of Novel ABS-Based Materials. J. Fail. Anal. Prev. 2014, 14, 343–353. [Google Scholar] [CrossRef]
- Khatri, B.; Lappe, K.; Noetzel, D.; Pursche, K.; Hanemann, T. A 3D-Printable Polymer-Metal Soft-Magnetic Functional Composite-Development and Characterization. Materials 2018, 11, 189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-Printed Magnetic Fe3O4/MBG/PCL Composite Scaffolds with Multifunctionality of Bone Regeneration, Local Anticancer Drug Delivery and Hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D Printable Graphene Composite. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Stenzel, O.; Pecho, O.; Holzer, L.; Neumann, M.; Schmidt, V. Predicting Effective Conductivities Based on Geometric Microstructure Characteristics. AIChE J. 2016, 62, 1834–1843. [Google Scholar] [CrossRef]
- Gaiselmann, G.; Neumann, M.; Holzer, L.; Hocker, T.; Prestat, M.R.; Schmidt, V. Stochastic 3D Modeling of La0.6Sr0.4CoO3-δ Cathodes Based on Structural Segmentation of FIB-SEM Images. Comput. Mater. Sci. 2013, 67, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Gaiselmann, G.; Neumann, M.; Schmidt, V.; Pecho, O.; Hocker, T.; Holzer, L. Quantitative Relationships between Microstructure and Effective Transport Properties Based on Virtual Materials Testing. AIChE J. 2014, 60, 1983–1999. [Google Scholar] [CrossRef]
- Neumann, M.; Staněk, J.; Pecho, O.M.; Holzer, L.; Beneš, V.; Schmidt, V. Stochastic 3D Modeling of Complex Three-Phase Microstructures in SOFC-Electrodes with Completely Connected Phases. Comput. Mater. Sci. 2016, 118, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Haj Ibrahim, S.; Neumann, M.; Klingner, F.; Schmidt, V.; Wejrzanowski, T. Analysis of the 3D Microstructure of Tape-Cast Open-Porous Materials via a Combination of Experiments and Modeling. Mater. Des. 2017, 133, 216–223. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J.; Zhou, J.; et al. Dry Reforming of Methane on a Highly-Active Ni-CeO2 Catalyst: Effects of Metal-Support Interactions on C−H Bond Breaking. Angew. Chem. 2016, 128, 7581–7585. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lu, M. Role of CeO2 in Ni/CeO2-Al2O3 Catalysts for Carbon Dioxide Reforming of Methane. Appl. Catal. B Environ. 1998, 19, 267–277. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Haj Ibrahim, S.; Cwieka, K.; Loeffler, M.; Milewski, J.; Zschech, E.; Lee, C.G. Multi-Modal Porous Microstructure for High Temperature Fuel Cell Application. J. Power Sources 2018, 373, 85–94. [Google Scholar] [CrossRef]
- Nötzel, D.; Hanemann, T. New Feedstock System for Fused Filament Fabrication of Sintered Alumina Parts. Materials 2020, 13, 4461. [Google Scholar] [CrossRef] [PubMed]
- Podsiadły, B.; Skalski, A.; Słoma, M. Conductive ABS/Ni Composite Filaments for Fused Deposition Modeling of Structural Electronics. Proc. Adv. Intell. Syst. Comput. Springer Verl. 2020, 1044, 62–70. [Google Scholar]
- Lei, Z.; Chen, Z.; Zhou, Y.; Liu, Y.; Xu, J.; Wang, D.; Shen, Y.; Feng, W.; Zhang, Z.; Chen, H. Novel Electrically Conductive Composite Filaments Based on Ag/Saturated Polyester/Polyvinyl Butyral for 3D-Printing Circuits. Compos. Sci. Technol. 2019, 180, 44–50. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, Z.; Peng, H.; Shen, Y.; Feng, W.; Liu, Y.; Zhang, Z.; Chen, Y. Fabrication of Highly Electrical Conductive Composite Filaments for 3D-Printing Circuits. J. Mater. Sci. 2018, 53, 14495–14505. [Google Scholar] [CrossRef]
- Valino, A.D.; Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D Printing of Thermoplastic Polymer Composites and Nanocomposites. Prog. Polym. Sci. 2019, 98, 101162. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive Manufacturing of Metallic and Ceramic Components by the Material Extrusion of Highly-Filled Polymers: A Review and Future Perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiee, M.; Farahani, R.D.; Therriault, D. Multi-Material 3D and 4D Printing: A Survey. Adv. Sci. 2020, 7, 1902307. [Google Scholar] [CrossRef] [PubMed]
- Wejrzanowski, T.; Cwieka, K.; Skibinski, J.; Brynk, T.; Haj Ibrahim, S.; Milewski, J.; Xing, W. Metallic Foam Supported Electrodes for Molten Carbonate Fuel Cells. Mater. Des. 2020, 193, 108864. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Cwieka, K.; Skibinski, J.; Lysik, A.; Ibrahim, S.H.; Milewski, J.; Xing, W.; Lee, C.G. Microstructure Driven Design of Porous Electrodes for Molten Carbonate Fuel Cell Application: Recent Progress. Int. J. Hydrogen Energy 2020, 45, 25719–25732. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Kurzydlowski, K.J. Stereology of Grains in Nano-Crystals. Solid State Phenomena; Trans Tech Publications Ltd.: Bach, Switzerland, 2003; Volume 94, pp. 221–228. [Google Scholar]
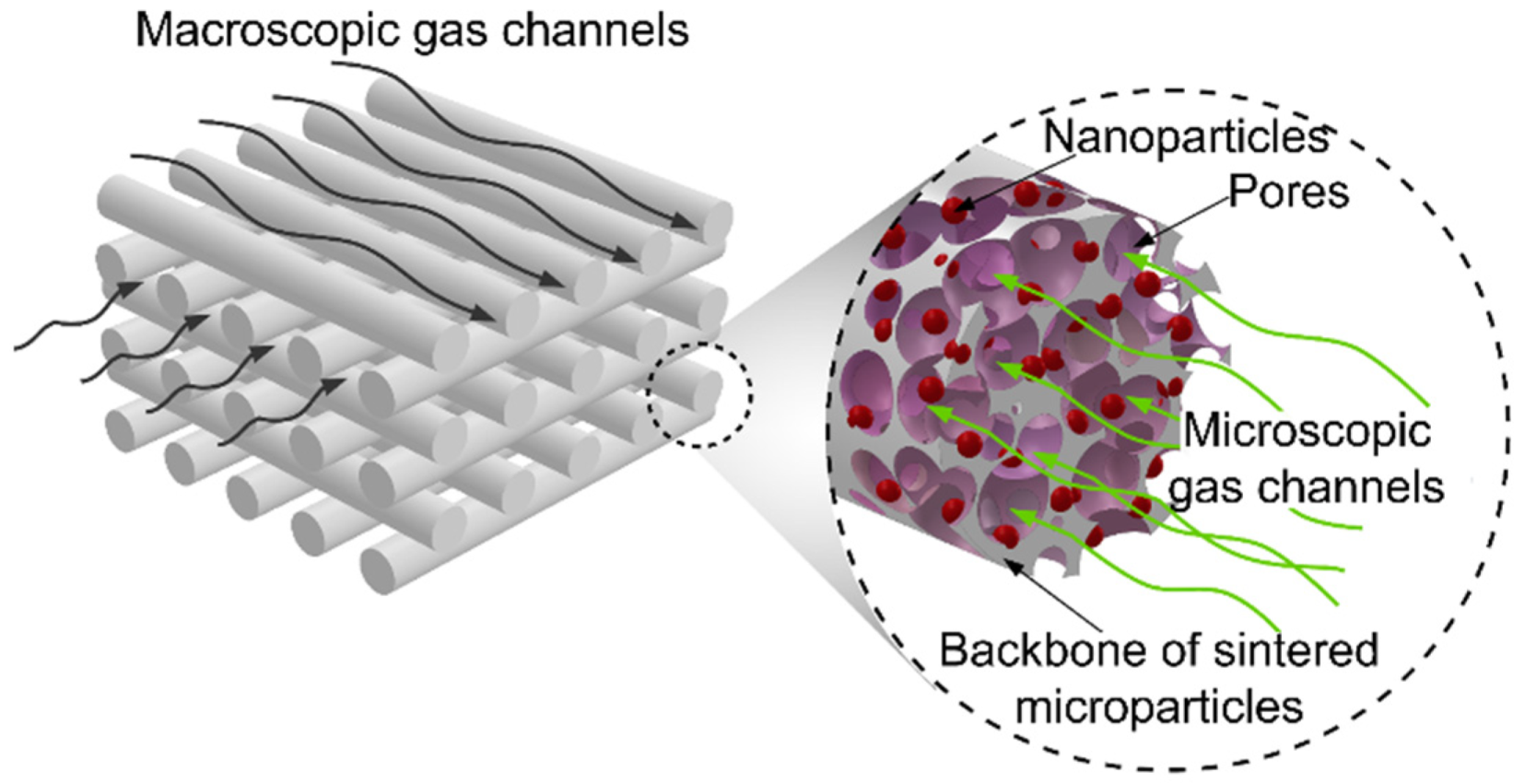
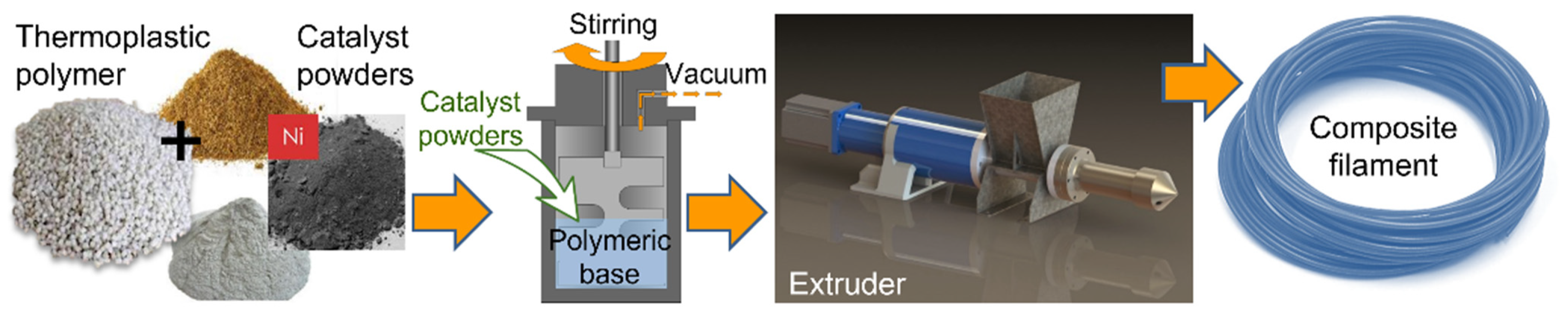
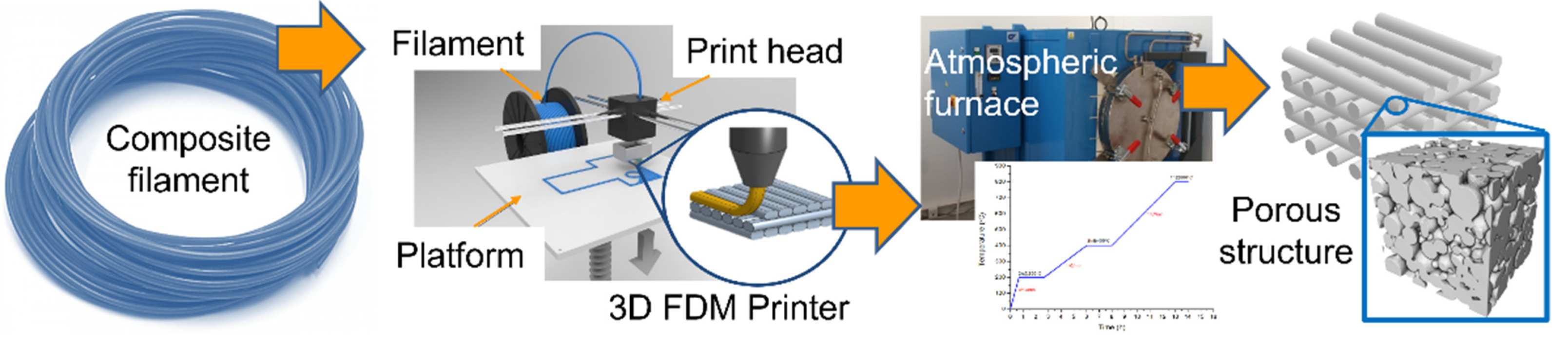

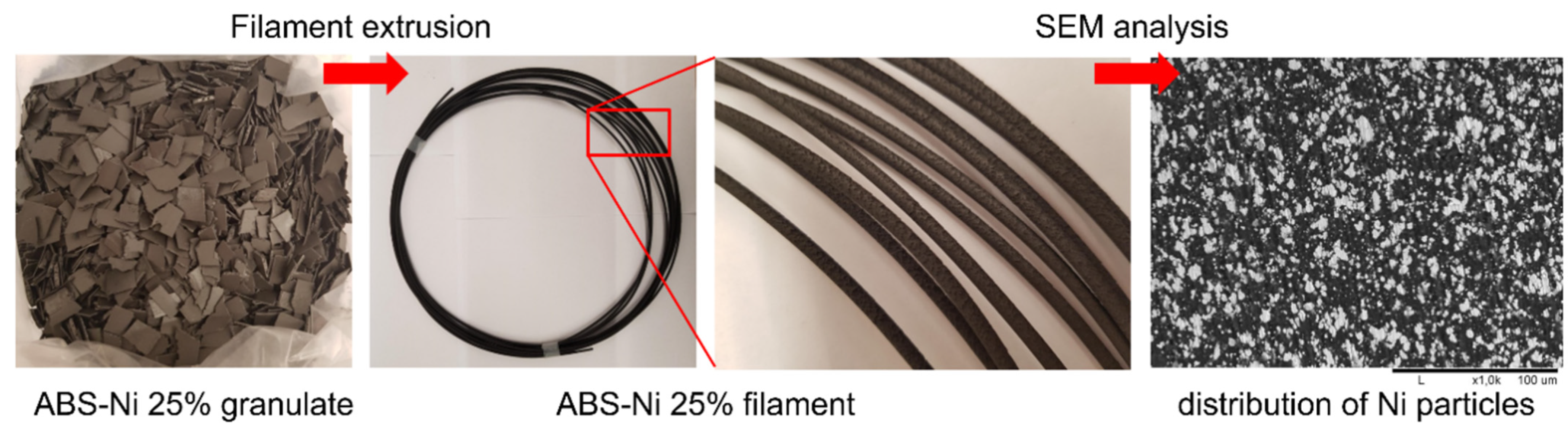
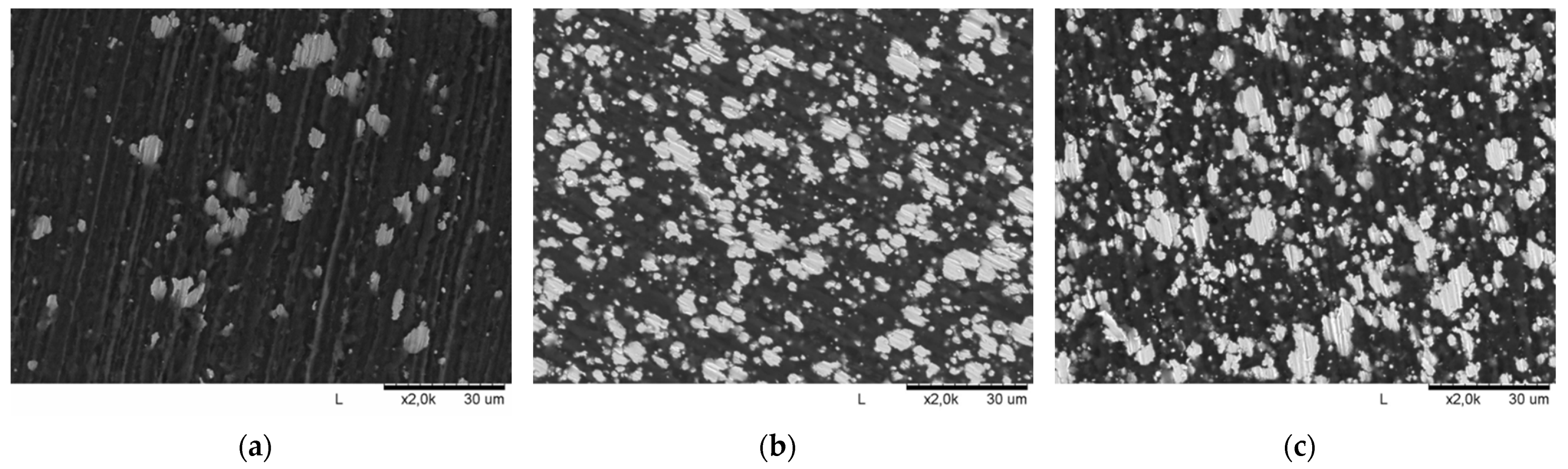
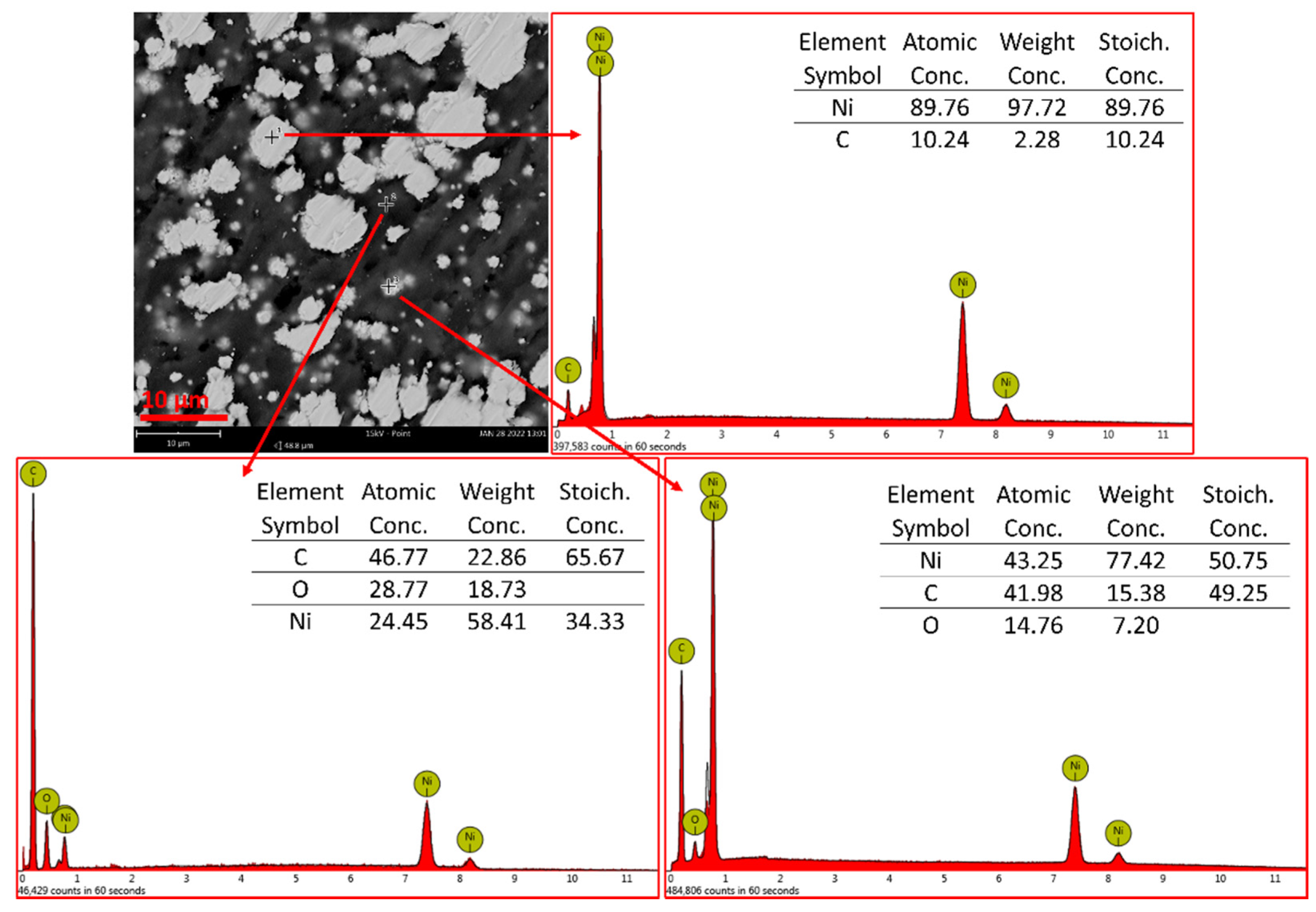
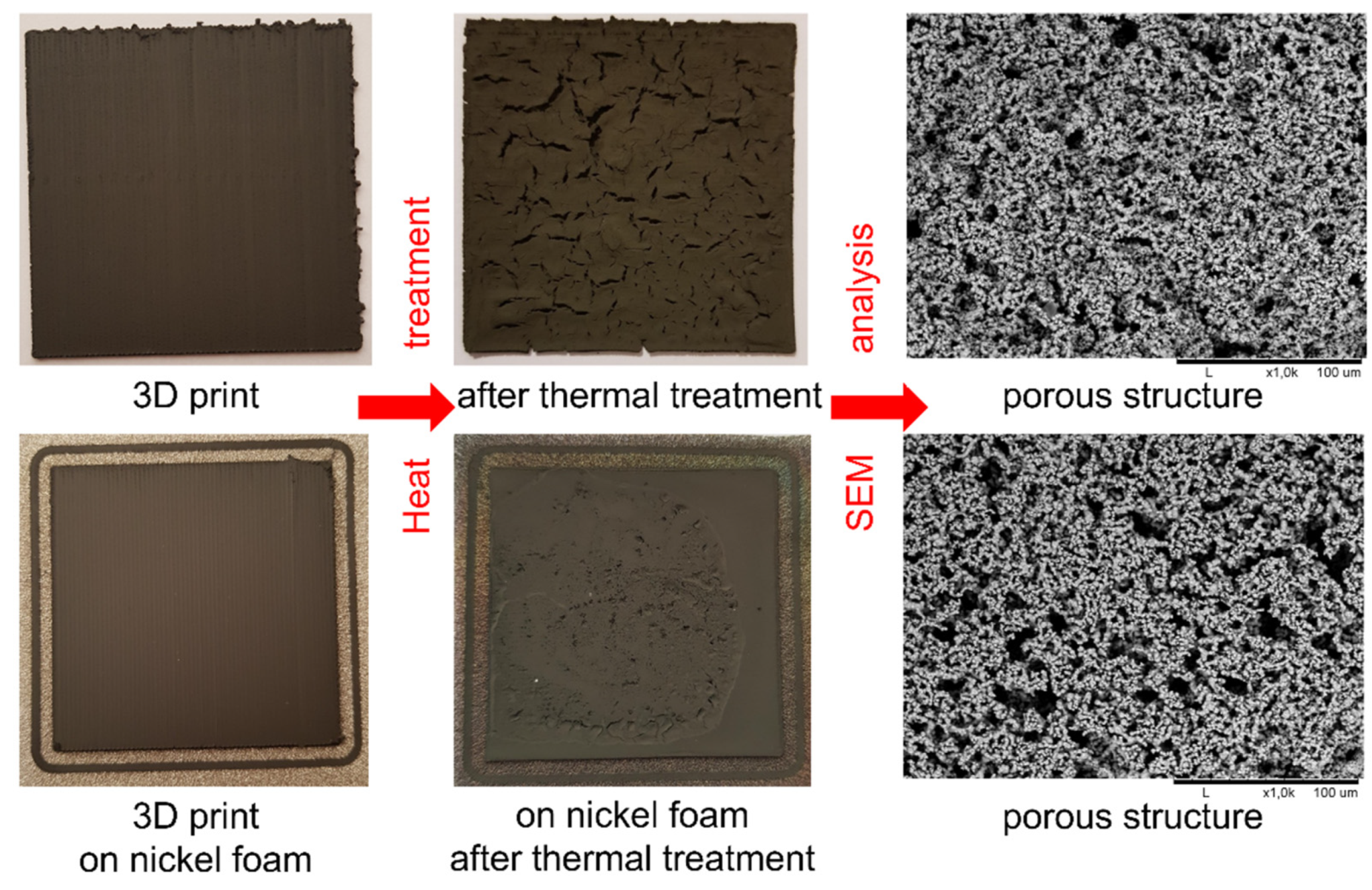


| Polymer Used | Ni Volume Fraction in a Composite (vol%) | Polymer Amount (g) |
|---|---|---|
| PLA | 5 | 264.7 |
| 10 | 125.4 | |
| 20 | 55.7 | |
| PVB | 10 | 111.31 |
| 15 | 70.04 | |
| 20 | 49.58 | |
| 25 | 37.09 | |
| 50 | 12.37 | |
| ABS | 10 | 105.19 |
| 20 | 46.82 | |
| 25 | 35.03 |
| PLA-Ni 5% | PVB-Ni 25% | ABS-Ni 25% | |
|---|---|---|---|
| Heaters | Set temperature (℃) | ||
| 4 | 170 | 170 | 220 |
| 3 | 185 | 195 | 230 |
| 2 | 190 | 200 | 230 |
| 1 | 180 | 200 | 240 |
| Average extrusion speed (rpm) | |||
| ~4.0 | ~3.0 | ~3.5 | |
| Item | PLA-Ni 5% | PVB-Ni 25% | ABS-Ni 25% |
|---|---|---|---|
| Printing temperature | 220 °C | 230 °C | 250 °C |
| Printing bed temperature | 65 °C | 85 °C | 85 °C |
| Printing speed | 15 mm/s | ||
| Single layer height | 0.4 mm | ||
| Line width | 0.4 mm | ||
| Print dimensions | 5 cm × 5 cm × 0.12 cm | ||
| Infill Line Directions | 90 degrees | ||
| Ni Powder vol% in Composite | PLA-Ni | PVB-Ni | ABS-Ni |
|---|---|---|---|
| 50% | - | unsuitable for extrusion (89 wt% of Ni) | - |
| 25% | - | + (73 wt% of Ni) | + (74 wt% of Ni) |
| 20% | unsuitable for extrusion (64 wt% of Ni) | + (67 wt% of Ni) | + (68 wt% of Ni) |
| 15% | - | + (59 wt% of Ni) | - |
| 10% | filament too brittle, it breaks (44 wt% of Ni) | + (47 wt% of Ni) | + (49 wt% of Ni) |
| 5% | + (27% wt% of Ni) | - | - |
| Average Parameter | Ni Foam | Thermally Treated Ni Foam | PLA-Ni 5% on Ni Foam | PVB-Ni 25% | ABS-Ni 25% | ||
|---|---|---|---|---|---|---|---|
| - | on Ni Foam | - | on Ni Foam | ||||
| Total porosity (%) | 90.9 | 86.1 | 86.6 | 60.7 | 71.8 | 65.7 | 76.3 |
| Open porosity (%) | 90.8 | 85.5 | 84.1 | 55.2 | 68.3 | 59.8 | 71.6 |
| Closed porosity (%) | 0.1 | 0.6 | 2.4 | 5.4 | 3.5 | 5.9 | 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackiewicz, E.; Wejrzanowski, T.; Adamczyk-Cieślak, B.; Oliver, G.J. Polymer–Nickel Composite Filaments for 3D Printing of Open Porous Materials. Materials 2022, 15, 1360. https://doi.org/10.3390/ma15041360
Mackiewicz E, Wejrzanowski T, Adamczyk-Cieślak B, Oliver GJ. Polymer–Nickel Composite Filaments for 3D Printing of Open Porous Materials. Materials. 2022; 15(4):1360. https://doi.org/10.3390/ma15041360
Chicago/Turabian StyleMackiewicz, Ewelina, Tomasz Wejrzanowski, Bogusława Adamczyk-Cieślak, and Graeme J. Oliver. 2022. "Polymer–Nickel Composite Filaments for 3D Printing of Open Porous Materials" Materials 15, no. 4: 1360. https://doi.org/10.3390/ma15041360
APA StyleMackiewicz, E., Wejrzanowski, T., Adamczyk-Cieślak, B., & Oliver, G. J. (2022). Polymer–Nickel Composite Filaments for 3D Printing of Open Porous Materials. Materials, 15(4), 1360. https://doi.org/10.3390/ma15041360