TEOS Nanocomposites for the Consolidation of Carbonate Stone: The Effect of Nano-HAp and Nano-SiO2 Modifiers
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Stone Samples
2.3. Preparation of Sols
2.4. Drying Environments
- (a)
- Fifteen millilitres of the formulations were placed inside cylindrical flasks (3.5 cm diameter) with pinholes (needle-size) in the cap (xerogels);
- (b)
- Ten millilitres of the formulations were mixed with 10 g of the CaCO3 powder and placed inside cylindrical flasks (calcite blends);
- (c)
- The formulations were applied to sound and aged limestone samples () by two different procedures: capillary suction and brushing. Specifications are given in Section 2.6.1.
2.5. Characterisation of the Formulations
2.6. Efficacy
2.6.1. Treated Stone Samples
2.6.2. Consolidating Effect
2.7. Colour Variation
3. Results and Discussion
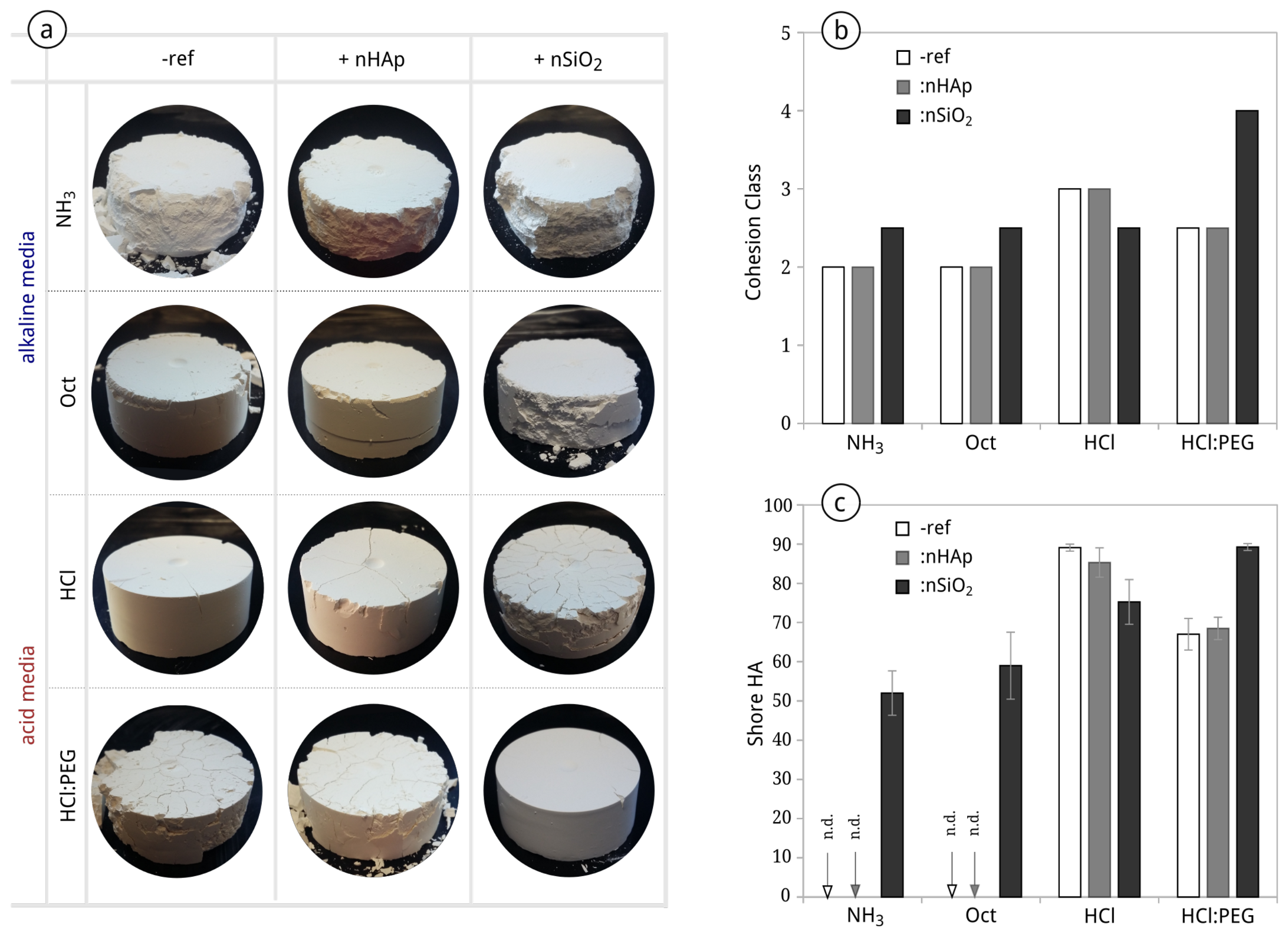
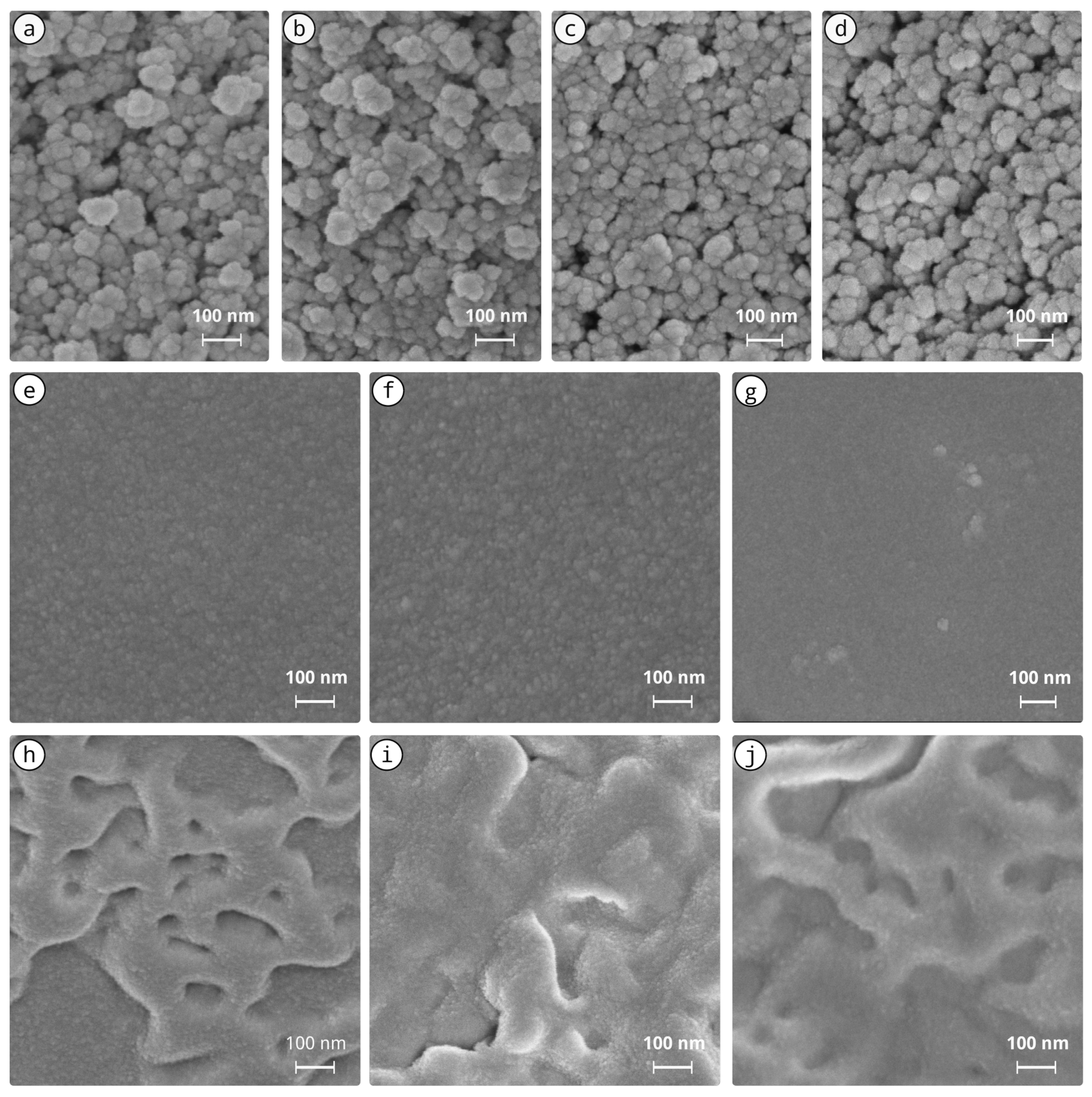
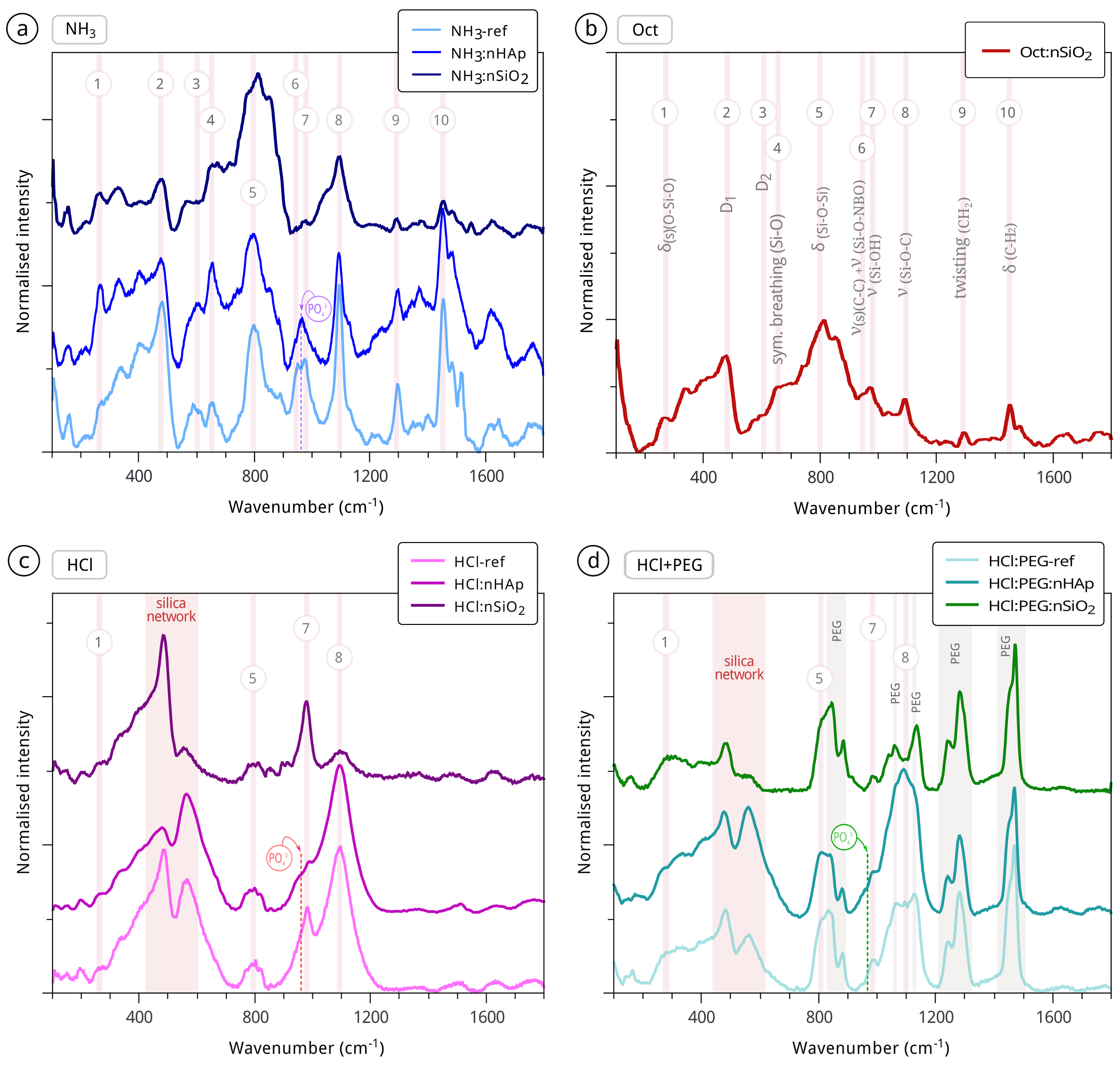
3.1. Sols and Xerogels
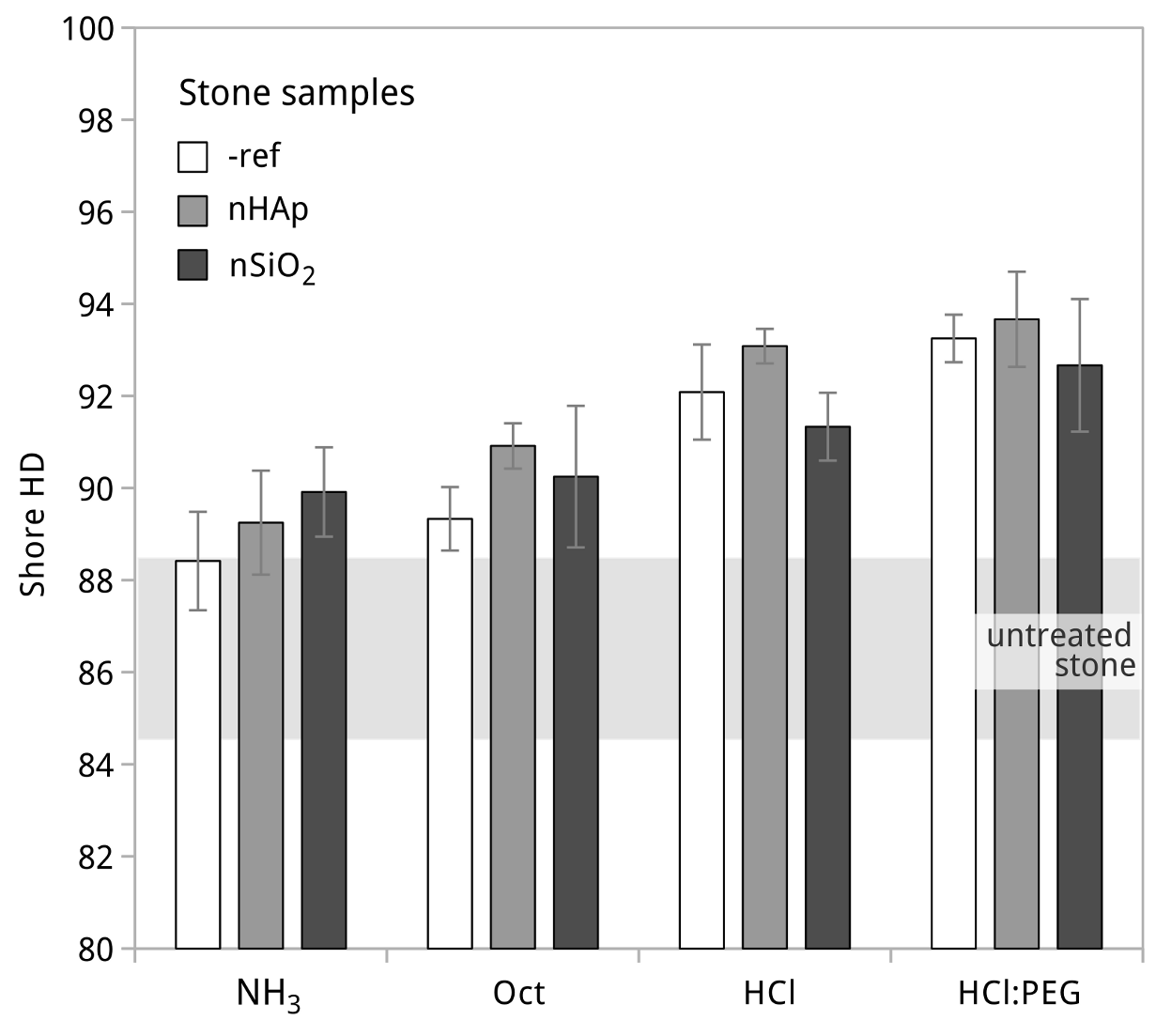
3.2. Potential Initial Efficacy and Compatibility Issues
3.3. Treatment Trials to Reduce the Risk of Incompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A. Ançã Stone Samples

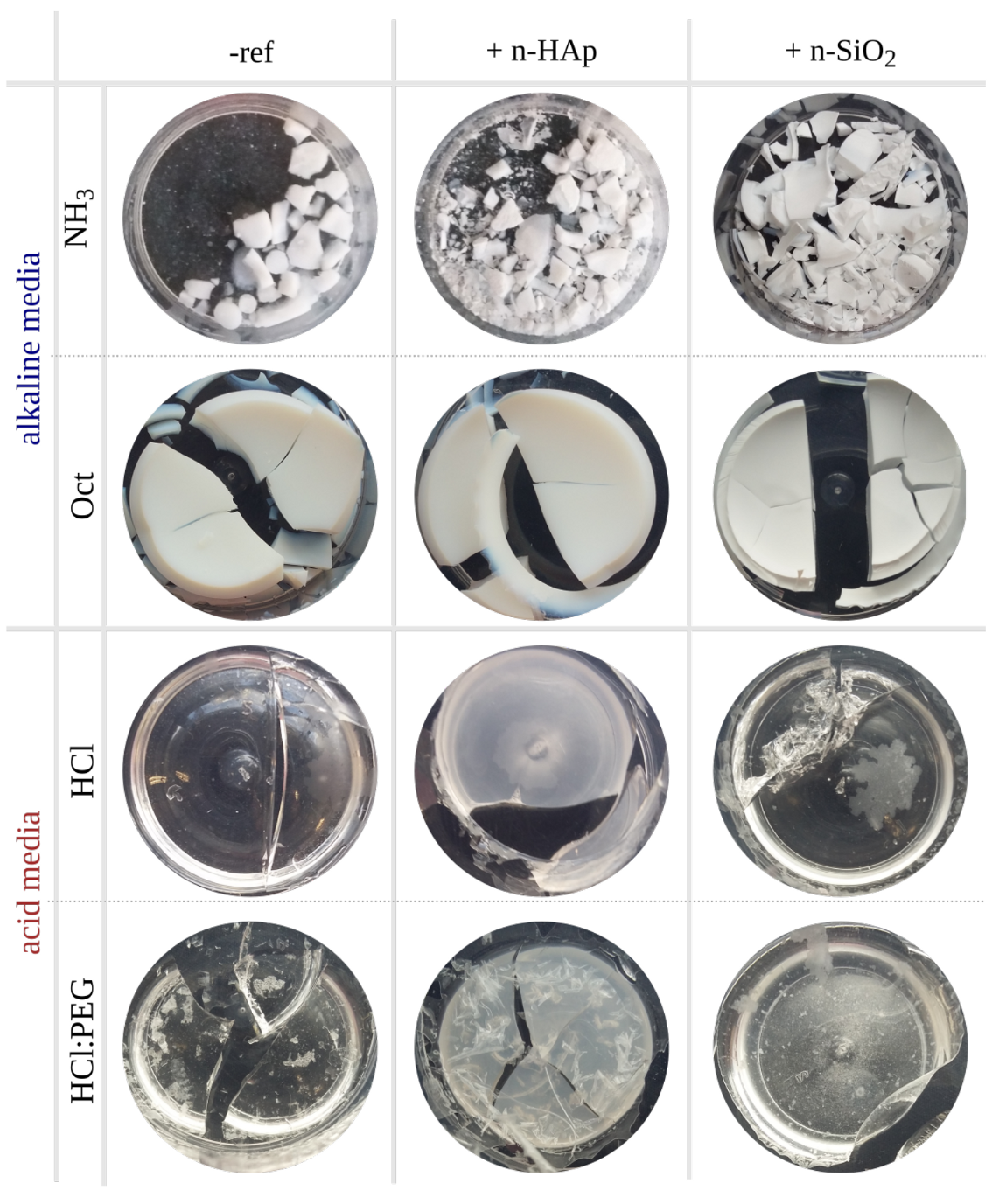
Appendix B. Calcite Blends
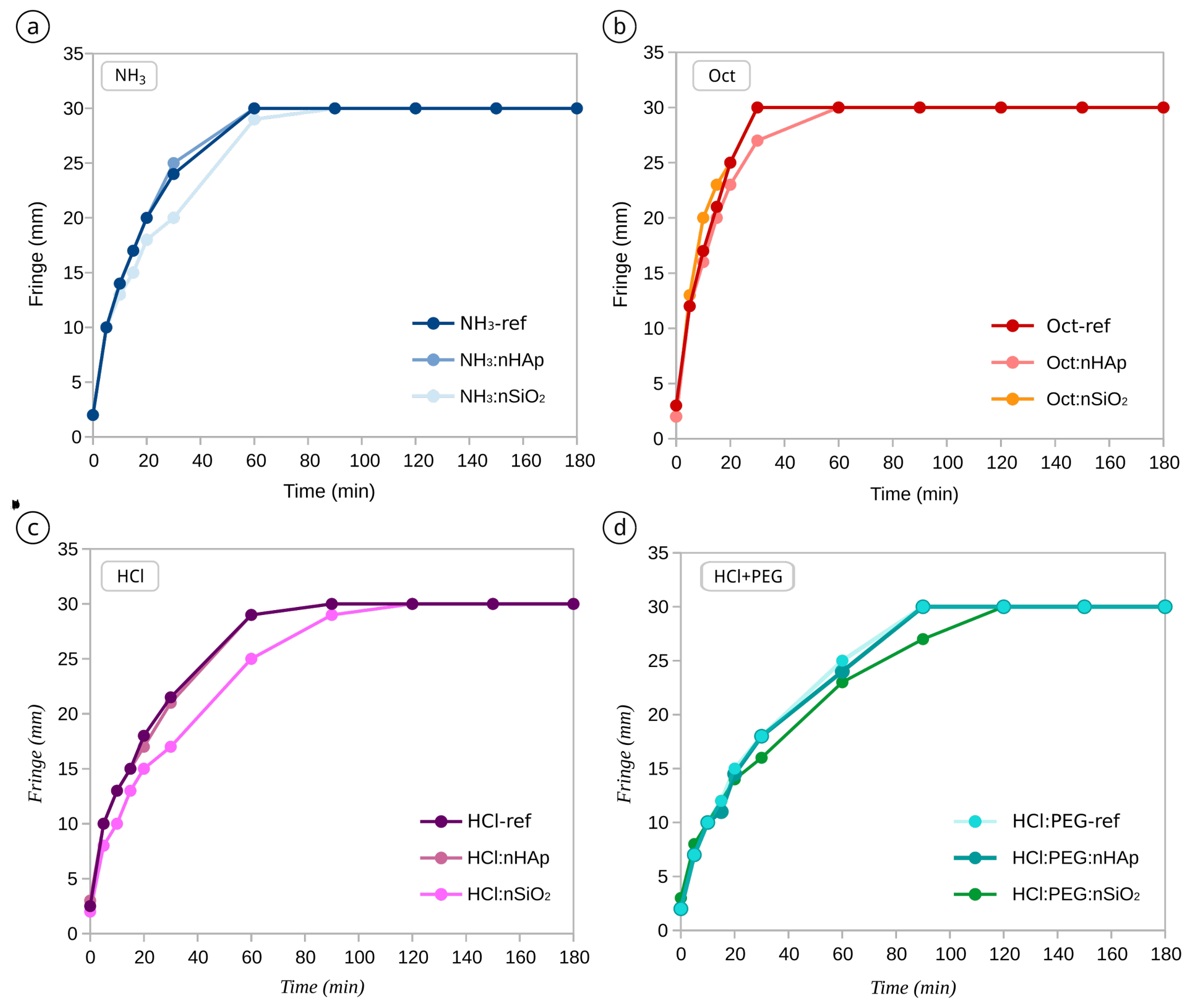
Appendix C. Capillary Suction
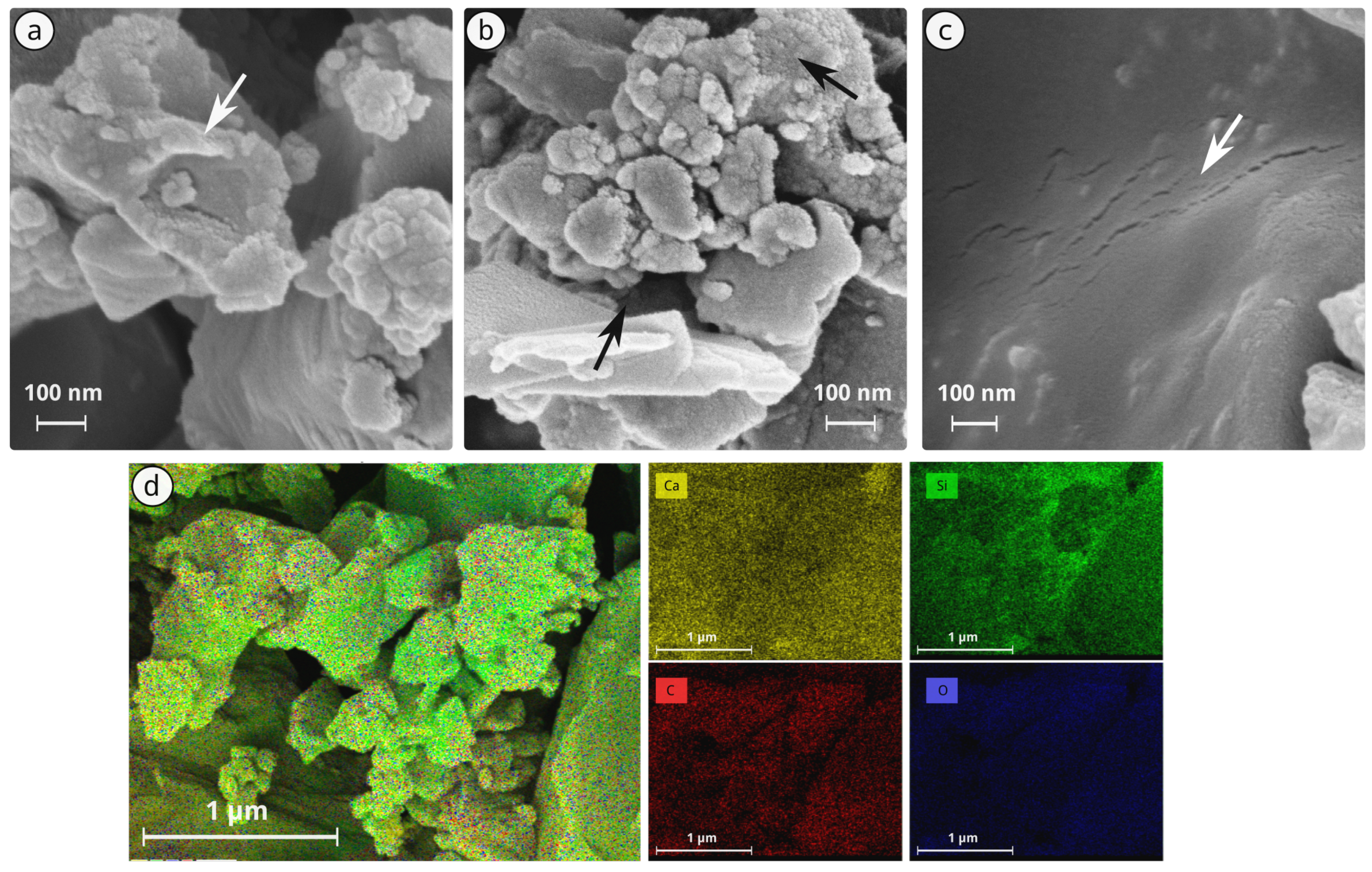
Appendix D. SEM after Stone Treatment (Supplementary)
References
- Delgado Rodrigues, J.; Ferreira Pinto, A.P. Stone consolidation by biomineralisation. Contribution for a new conceptual and practical approach to consolidate soft decayed limestones. J. Cult. Herit. 2019, 39, 82–92. [Google Scholar] [CrossRef]
- Bianco, L. Geochemistry, mineralogy and textural properties of the lower globigerina limestone used in the built heritage. Minerals 2021, 11, 740. [Google Scholar] [CrossRef]
- Rescic, S.; Fratini, F.; Tiano, P. On-site evaluation of the ‘mechanical’ properties of Maastricht limestone and their relationship with the physical characteristics. Geol. Soc. Spec. Publ. 2010, 331, 203–208. [Google Scholar] [CrossRef]
- Salvi, A.; Menendez, B. Experimental Determination of Salt Content in Artificial Weathered Samples of Sedimentary Stones. In Proceedings of the SWBSS 2021: Fifth International Conference on Salt Weathering of Buildings and Stone Sculptures, Delft, The Netherlands, 22–24 September 2021; pp. 89–98. [Google Scholar]
- Ferreira Pinto, A.P.; Delgado Rodrigues, J. Impacts of consolidation procedures on colour and absorption kinetics of carbonate stones. Stud. Conserv. 2014, 59, 79–90. [Google Scholar] [CrossRef]
- Delgado Rodrigues, J. Consolidation of decayed stones. A delicate problem with few practical solutions. Hist. Constr. 2001, 3–14. [Google Scholar]
- Possenti, E.; Colombo, C.; Conti, C.; Marinoni, N.; Merlini, M.; Negrotti, R.; Realini, M.; Gatta, G.D. Consolidation of building materials with a phosphate-based treatment: Effects on the microstructure and on the 3D pore network. Mater. Charact. 2019, 154, 315–324. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Jroundi, F.; Pascolini, C.; Rodriguez-Navarro, C.; Piñar-Larrubia, G.; Rodriguez-Gallego, M.; González-Muñoz, M.T. Consolidation of quarry calcarenite by calcium carbonate precipitation induced by bacteria activated among the microbiota inhabiting the stone. Int. Biodeterior. Biodegrad. 2008, 62, 352–363. [Google Scholar] [CrossRef]
- Ševčík, R.; Viani, A.; Mancini, L.; Appavou, M.S.; Machová, D. Investigation of nano-microstructural changes in Maastricht limestone after treatment with nanolime suspension. Appl. Phys. A: Mater. Sci. Process. 2020, 126, 367. [Google Scholar] [CrossRef]
- Wheeler, G.; Goins, E.S. Alkoxysilanes and the Consolidation of Stone; Getty Publications: Los Angeles, CA USA, 2005. [Google Scholar]
- Scherer, G.W.; Wheeler, G.S. Silicate consolidants for stone. Key Eng. Mater. 2009, 391, 1–25. [Google Scholar] [CrossRef]
- Delgado Rodrigues, J.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Rodriguez-Navarro, C.; Piñar, G.; Carrillo-Rosúa, F.; Rodriguez-Gallego, M.; Gonzalez-Muñoz, M. Consolidation of degraded ornamental porous limestone stone by calcium carbonate precipitation induced by the microbiota inhabiting the stone. Chemosphere 2007, 68, 1929–1936. [Google Scholar] [CrossRef] [Green Version]
- Pintus, A.; Aragoni, M.C.; Carcangiu, G.; Giacopetti, L.; Isaia, F.; Lippolis, V.; Maiore, L.; Meloni, P.; Arca, M. Density functional theory modelling of protective agents for carbonate stones: A case study of oxalate and oxamate inorganic salts. New J. Chem. 2018, 42, 11593–11600. [Google Scholar] [CrossRef]
- Taglieri, G.; Otero, J.; Daniele, V.; Gioia, G.; Macera, L.; Starinieri, V.; Charola, A.E. The biocalcarenite stone of Agrigento (Italy): Preliminary investigations of compatible nanolime treatments. J. Cult. Herit. 2018, 30, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Erşan, Y.Ç.; Wang, J.; Fraeye, D.; Boon, N.; De Belie, N. Surface Consolidation of Maastricht Limestone by Means of Bacillus Sphaericus under Varying Treatment Conditions. J. Mater. Civ. Eng. 2020, 32, 04020342. [Google Scholar] [CrossRef]
- Badreddine, D.; Beck, K.; Brunetaud, X.; Chaaba, A.; Al-Mukhtar, M. Nanolime consolidation of the main building stone of the archaeological site of Volubilis (Morocco). J. Cult. Herit. 2020, 43, 98–107. [Google Scholar] [CrossRef]
- Verganelaki, A.; Kapridaki, C.; Maravelaki-Kalaitzaki, P. Modified Tetraethoxysilane with Nanocalcium Oxalate in One-Pot Synthesis for Protection of Building Materials. Ind. Eng. Chem. Res. 2015, 54, 7195–7206. [Google Scholar] [CrossRef]
- Gheno, G.; Badetti, E.; Brunelli, A.; Ganzerla, R.; Marcomini, A. Consolidation of Vicenza, Arenaria and Istria stones: A comparison between nano-based products and acrylate derivatives. J. Cult. Herit. 2018, 32, 44–52. [Google Scholar] [CrossRef]
- Chelazzi, D.; Camerini, R.; Giorgi, R.; Baglioni, P. Nanomaterials for the consolidation of stone artifacts. Adv. Mater. Conserv. Stone 2018, 151–173. [Google Scholar] [CrossRef]
- Zornoza-Indart, A.; Lopez-Arce, P. Silica nanoparticles (SiO2): Influence of relative humidity in stone consolidation. J. Cult. Herit. 2016, 18, 258–270. [Google Scholar] [CrossRef]
- Verganelaki, A.; Kilikoglou, V.; Karatasios, I.; Maravelaki-Kalaitzaki, P. A biomimetic approach to strengthen and protect construction materials with a novel calcium-oxalate–silica nanocomposite. Constr. Build. Mater. 2014, 62, 8–17. [Google Scholar] [CrossRef]
- Ruggiero, L.; Fidanza, M.R.; Iorio, M.; Tortora, L.; Caneva, G.; Ricci, M.A.; Sodo, A. Synthesis and Characterization of TEOS Coating Added with Innovative Antifouling Silica Nanocontainers and TiO2 Nanoparticles. Front. Mater. 2020, 7, 185. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A Mater. Sci. Process. 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N.; Savva, A.; Panayiotou, C. Superhydrophobic polymer-particle composite films produced using various particle sizes. Surf. Interface Anal. 2012, 44, 870–875. [Google Scholar] [CrossRef]
- Ban, M.; Mascha, E.; Weber, J.; Rohatsch, A.; Rodrigues, J.D. Efficiency and compatibility of selected alkoxysilanes on porous carbonate and silicate stones. Materials 2019, 12, 156. [Google Scholar] [CrossRef] [Green Version]
- Mosquera, M.J.; Bejarano, M.; De la Rosa-Fox, N.; Esquivias, L. Producing crack-free colloid-polymer hybrid gels by tailoring porosity. Langmuir 2003, 19, 951–957. [Google Scholar] [CrossRef]
- Mosquera, M.J.; De Los Santos, D.M.; Montes, A. Producing new stone consolidants for the conservation of monumental stones. Mater. Res. Soc. Symp. Proc. 2005, 852, 81–87. [Google Scholar] [CrossRef]
- Salazar-Hernández, C.; Alquiza, M.J.P.; Salgado, P.; Cervantes, J. TEOS-colloidal silica-PDMS-OH hybrid formulation used for stone consolidation. Appl. Organomet. Chem. 2010, 24, 481–488. [Google Scholar] [CrossRef]
- Kim, E.K.; Won, J.; Do, J.Y.; Kim, S.D.; Kang, Y.S. Effects of silica nanoparticle and GPTMS addition on TEOS-based stone consolidants. J. Cult. Herit. 2009, 10, 214–221. [Google Scholar] [CrossRef]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol–gel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 1: Effectiveness and compatibility in comparison with ethyl silicate. Constr. Build. Mater. 2016, 102, 918–930. [Google Scholar] [CrossRef]
- Celik, S.E.; Gulen, J.; Viles, H.A. Evaluating the effectiveness of DAP as a consolidant on Turkish building stones. Constr. Build. Mater. 2020, 262, 120765. [Google Scholar] [CrossRef]
- Pesce, C.; Moretto, L.M.; Orsega, E.F.; Pesce, G.L.; Corradi, M.; Weber, J. Effectiveness and compatibility of a novel sustainable method for stone consolidation based on di-ammonium phosphate and calcium-based nanomaterials. Materials 2019, 12, 3025. [Google Scholar] [CrossRef] [Green Version]
- Capitelli, F.; Dida, B.; Ventura, G.D.; Baldassarre, F.; Capelli, D.; Senesi, G.S.; Mele, A.; Siliqi, D. Functional Nano-Hydroxyapatite for Applications in Conservation of Stony Monuments of Cultural Heritage. Proceedings 2021, 62, 11. [Google Scholar] [CrossRef]
- Maravelaki, P.; Verganelaki, A. A hybrid consolidant of nano-hydroxyapatite and silica inspired from patinas for stone conservation. In Advanced Materials for the Conservation of Stone; Springer: Cham, Switzerland, 2018; pp. 79–95. [Google Scholar] [CrossRef]
- Sassoni, E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials 2018, 11, 557. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.; Sena da Fonseca, B.; Ferreira Pinto, A.P.; Piçarra, S.; Montemor, M.F. Synthesis and application of hydroxyapatite nanorods for improving properties of stone consolidants. Ceram. Int. 2022. forthcoming. [Google Scholar]
- Facio, D.; Ordoñez, J.; Gil, M.; Carrascosa, L.; Mosquera, M. New Consolidant-Hydrophobic Treatment by Combining SiO2 Composite and Fluorinated Alkoxysilane: Application on Decayed Biocalcareous Stone from an 18th Century Cathedral. Coatings 2018, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Barberena-Fernández, A.M.; Blanco-Varela, M.T.; Carmona-Quiroga, P.M. Use of nanosilica- or nanolime-additioned TEOS to consolidate cementitious materials in heritage structures: Physical and mechanical properties of mortars. Cem. Concr. Compos. 2019, 95, 271–276. [Google Scholar] [CrossRef]
- Ferreira Pinto, A.P.; Delgado Rodrigues, J. Stone consolidation: The role of treatment procedures. J. Cult. Herit. 2008, 9, 38–53. [Google Scholar] [CrossRef]
- Sena da Fonseca, B.; Piçarra, S.; Ferreira Pinto, A.P.; Montemor, M.F. Polyethylene glycol oligomers as siloxane modificators in consolidation of carbonate stones. Pure Appl. Chem. 2016, 88, 1117–1128. [Google Scholar] [CrossRef]
- Sena da Fonseca, B.; Ferreira Pinto, A.P.; Rodrigues, A.; Piçarra, S.; Montemor, M.F. The role of properties on the decay susceptibility and conservation issues of soft limestones: Contribution of Anç a. J. Build. Eng. 2021, 44, 102997. [Google Scholar] [CrossRef]
- Sena Da Fonseca, B.; Piçarra, S.; Ferreira Pinto, A.P.; Ferreira, M.J.; Montemor, M.F. TEOS-based consolidants for carbonate stones: The role of N1-(3-trimethoxysilylpropyl)diethylenetriamine. New J. Chem. 2017, 41, 2458–2467. [Google Scholar] [CrossRef]
- Rodrigues, A.; Sena da Fonseca, B.; Ferreira Pinto, A.P.; Piçarra, S.; Montemor, M.F. Tailoring alkoxysilanes with poly(ethylene glycol) as potential consolidants for carbonate stones. Constr. Build. Mater. 2021, 289, 123048. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, X.; Du, A.; Wu, S.; Shen, J.; Zhou, B. Timing of polyethylene glycol addition for the control of SiO2 sol structure and sol–gel coating properties. J. Coat. Technol. Res. 2017, 14, 447–454. [Google Scholar] [CrossRef]
- Rodrigues, A.; Sena da Fonseca, B.; Ferreira Pinto, A.P.; Piçarra, S.; Montemor, M.F. Exploring alkaline routes for production of TEOS-based consolidants for carbonate stones using amine catalysts. New J. Chem. 2021, 45, 3833–3847. [Google Scholar] [CrossRef]
- Sena da Fonseca, B.; Piçarra, S.; Ferreira Pinto, A.P.; Montemor, M.D.F. Development of formulations based on TEOS-dicarboxylic acids for consolidation of carbonate stones. New J. Chem. 2016, 40, 7493–7503. [Google Scholar] [CrossRef]
- Sena da Fonseca, B.; Ferreira Pinto, A.P.; Piçarra, S.; Montemor, M.F. Alkoxysilane-based sols for consolidation of carbonate stones: Proposal of methodology to support the design and development of new consolidants. J. Cult. Herit. 2020, 43, 51–63. [Google Scholar] [CrossRef]
- Ferreira Pinto, A.P.; Delgado Rodrigues, J. Study of consolidation effects in carbonate stones by means of drilling resistance. In Proceedings of the 6th International Symposium on the Conservation of Monuments in the Mediterranean Basin, Lisbon, Portugal, 7–10 April 2004. [Google Scholar]
- Graziani, G.; Sassoni, E.; Franzoni, E. Consolidation of porous carbonate stones by an innovative phosphate treatment: Mechanical strengthening and physical-microstructural compatibility in comparison with TEOS-based treatments. Herit. Sci. 2015, 3, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Ziegenbalg, G. Stone Conservation for Refurbishment of Buildings (Project Final Report); Technical Report; IBZ-SALZCHEMIE GmbH & Co. KG: Halsbruecke, Germany, 31 August 2011. [Google Scholar]
- Wacker. Wacker, Silres BS OH 100: Safety Data Sheet (Version 2.6—Date of Last Alteration: 601 11/16/2019); Technical Report; Wacker: Munich, Germany, 2019. [Google Scholar]
- Illescas, J.F.; Mosquera, M.J. Producing surfactant-synthesized nanomaterials in situ on a building substrate, without volatile organic compounds. ACS Appl. Mater. Interfaces 2012, 4, 4259–4269. [Google Scholar] [CrossRef]
- Pinho, L.; Mosquera, M.J. Titania-silica nanocomposite photocatalysts with application in stone self-cleaning. J. Phys. Chem. C 2011, 115, 22851–22862. [Google Scholar] [CrossRef]
- Ravaine, D.; Seminel, A.; Charbouillot, Y.; Vincens, M. A new family of organically modified silicates prepared from gels. J. Non-Cryst. Solids 1986, 82, 210–219. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Balachandran, C.; Muñoz, J.F.; Arnold, T. Characterization of alkali silica reaction gels using Raman spectroscopy. Cem. Concr. Res. 2017, 92, 66–74. [Google Scholar] [CrossRef]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of silica nanoparticles—polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- De Ferri, L.; Lorenzi, A.; Lottici, P.P. OctTES/TEOS system for hybrid coatings: Real-time monitoring of the hydrolysis and condensation by Raman spectroscopy. J. Raman Spectrosc. 2016, 47, 699–705. [Google Scholar] [CrossRef]
- Ramanauskaite, L.; Snitka, V. The synthesis of controlled shape nanoplasmonic silver-silica structures by combining sol–gel technique and direct silver reduction. Nanoscale Res. Lett. 2015, 10, 133. [Google Scholar] [CrossRef] [Green Version]
- Capeletti, L.B.; Baibich, I.M.; Butler, I.S.; Dos Santos, J.H. Infrared and Raman spectroscopic characterization of some organic substituted hybrid silicas. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 619–625. [Google Scholar] [CrossRef]
- De Oliveira, A.A.; Ciminelli, V.; Dantas, M.S.; Mansur, H.S.; Pereira, M.M. Acid character control of bioactive glass/polyvinyl alcohol hybrid foams produced by sol–gel. J. Sol-Gel Sci. Technol. 2008, 47, 335–346. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Zhan, Y.; Zheng, C.; Wang, G. A simple route to hydroxyapatite nanofibers. Mater. Lett. 2002, 56, 496–501. [Google Scholar] [CrossRef]
- Hernández Ortiz, G.M.; Parra, R.; Fanovich, M.A. Comparative hydrothermal synthesis of hydroxyapatite by using cetyltrimethylammonium bromide and hexamethylenetetramine as additives. Ceram. Int. 2018, 44, 3658–3663. [Google Scholar] [CrossRef]
- Alessi, A.; Agnello, S.; Buscarino, G.; Gelardi, F.M. Raman and IR investigation of silica nanoparticles structure. J. Non-Cryst. Solids 2013, 362, 20–24. [Google Scholar] [CrossRef]
- Jung, H.; Gupta, R.; Oh, E.; Kim, Y.; Whang, C. Vibrational spectroscopic studies of sol–gel derived physical and chemical bonded ORMOSILs. J. Non-Cryst. Solids 2005, 351, 372–379. [Google Scholar] [CrossRef]
- Murru, A.; Fort, R. Diammonium hydrogen phosphate (DAP) as a consolidant in carbonate stones: Impact of application methods on effectiveness. J. Cult. Herit. 2020, 42, 45–55. [Google Scholar] [CrossRef]
- Berto, T.; Godts, S.; Clercq, H.D. The Effects of Commercial Ethyl Silicate Based Consolidation Products on Limestone. In Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone: Investigation Methods Commercially, Glasgow, Scotland, 6–10 September 2016; pp. 271–279. [Google Scholar]
- Delgado Rodrigues, J.; Ferreira Pinto, A.P.; Rodrigues da Costa, D. Tracing of decay profiles and evaluation of stone treatments by means of microdrilling techniques. J. Cult. Herit. 2002, 3, 117–125. [Google Scholar] [CrossRef]



| TEOS | EtOH | Catalyst | H2O | PEG Added | Designation | Modifier (1:0.001) | Designation | |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.6 | HCl | down to pH’ = 3.2 | 2.1 | — | HCl-ref | nano-HAp | HCl:nHAp |
| nano-SiO2 | HCl:nSiO2 | |||||||
| 0.11 | HCl:PEG-ref | nano-HAp | HCl:PEG:nHAp | |||||
| nano-SiO2 | HCl:PEG:nSiO2 | |||||||
| Oct | 0.002 | 1.5 | — | Oct-ref | nano-HAp | Oct:nHAp | ||
| Oct-ref | nano-SiO2 | Oct:nSiO2 | ||||||
| NH3 | 0.008 | — | NH3-ref | nano-HAp | NH3:nHAp | |||
| NH3-ref | nano-SiO2 | NH3:nSiO2 | ||||||
| Sols Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Viscosity (mPa·s) | Gelling Time (d) | Density (g/mL) | |||||||
| -ref | nHAp | nSiO2 | -ref | nHAp | nSiO2 | -ref | nHAp | nSiO2 | |
| NH3 | 1.1 | 1.0 | 1.1 | 13d | 13d | 13d | 0.83 | 0.84 | 0.84 |
| Oct | 0.9 | 0.8 | 0.9 | 6d | 6d | 6d | 0.84 | 0.84 | 0.85 |
| HCl | 1.7 | 1.7 | 1.6 | 14d | 14d | 14d | 0.83 | 0.84 | 0.85 |
| HCl:PEG | 1.9 | 1.8 | 2.0 | 13d | 13d | 13d | 0.87 | 0.86 | 0.86 |
| Dry Residue (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Xerogels | Calcite Blends | Stone Samples | |||||||
| -ref | nHAp | nSiO2 | -ref | nHAp | nSiO2 | -ref | nHAp | nSiO2 | |
| NH3 | 2% | 2% | 4% | 3% | 3% | 6% | 1% | 1% | 2% |
| Oct | 12% | 12% | 7% | 11% | 11% | 10% | 2% | 2% | 2% |
| HCl | 14% | 14% | 16% | 12% | 12% | 14% | 15% | 13% | 18% |
| HCl:PEG | 19% | 19% | 19% | 19% | 20% | 19% | 25% | 20% | 20% |
| Units: kg/m2 | Absorbed Product | Dry Residue | ||||
|---|---|---|---|---|---|---|
| -ref | nHAp | nSiO2 | -ref | nHAp | nSiO2 | |
| NH3 | 7.48 | 7.50 | 8.16 | 0.06 | 0.08 | 0.15 |
| Oct | 9.21 | 8.56 | 10.17 | 0.15 | 0.18 | 0.14 |
| HCl | 8.66 | 8.66 | 8.35 | 1.14 | 1.05 | 1.37 |
| HCl:PEG | 7.31 | 7.87 | 8.38 | 1.65 | 1.42 | 1.48 |
| Total Colour Variation (*) | |||||||
|---|---|---|---|---|---|---|---|
| Alkaline | Acid | ||||||
| ref | nHAp | nSiO2 | ref | nHAp | nSiO2 | ||
| blackNH3 | 1.6 | 5.1 | 1.1 | HCl | 8.4 | 4.8 | 8.4 |
| Oct | 3.4 | 0.6 | 0.4 | HCl:PEG | 11.5 | 11.7 | 16.0 |
| Dry Residue (%) | (kg/m2) | |||
|---|---|---|---|---|
| SS Capillary | SS Brushing | AS Brushing | All Cases | |
| HCl:nHAp | 13% | 17% | 17% | |
| HCl:nSiO2 | 18% | 18% | 18% | |
| SS Capillary Abs. | SS Brushing | AS Brushing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DR↑ | Depth | HD | * | DR↑ | Depth | HD | * | DR↑ | Depth | HD | * | |
| HCl:nHAp | 62% | 30 | 93.1 | 4.8 | 22% | 30 | 87.9 | 1.3 | 55% | 30 | 90.7 | 2.0 |
| HCl:nSiO2 | 73% | 30 | 91.3 | 8.4 | 15% | 15 | 89.4 | 2.4 | 37% | 20 | 90.4 | 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.; Sena da Fonseca, B.; Ferreira Pinto, A.P.; Piçarra, S.; Montemor, M.d.F. TEOS Nanocomposites for the Consolidation of Carbonate Stone: The Effect of Nano-HAp and Nano-SiO2 Modifiers. Materials 2022, 15, 981. https://doi.org/10.3390/ma15030981
Rodrigues A, Sena da Fonseca B, Ferreira Pinto AP, Piçarra S, Montemor MdF. TEOS Nanocomposites for the Consolidation of Carbonate Stone: The Effect of Nano-HAp and Nano-SiO2 Modifiers. Materials. 2022; 15(3):981. https://doi.org/10.3390/ma15030981
Chicago/Turabian StyleRodrigues, Alexandra, Bruno Sena da Fonseca, Ana Paula Ferreira Pinto, Susana Piçarra, and Maria de Fátima Montemor. 2022. "TEOS Nanocomposites for the Consolidation of Carbonate Stone: The Effect of Nano-HAp and Nano-SiO2 Modifiers" Materials 15, no. 3: 981. https://doi.org/10.3390/ma15030981
APA StyleRodrigues, A., Sena da Fonseca, B., Ferreira Pinto, A. P., Piçarra, S., & Montemor, M. d. F. (2022). TEOS Nanocomposites for the Consolidation of Carbonate Stone: The Effect of Nano-HAp and Nano-SiO2 Modifiers. Materials, 15(3), 981. https://doi.org/10.3390/ma15030981









