3.1. Opto-Geometrical Characterization
The opto-geometrical properties of the channel waveguides and diffraction gratings constituting the micro-structured architecture on glass are illustrated in
Figure 3. The micrograph in
Figure 3a and the 3D AFM image in
Figure 3b show that, for both components of this architecture, smooth and linear patterns were obtained, and a clean interface is depicted in
Figure 3a between the bare channel waveguide and the area where the diffraction grating was imprinted (left part of the figure). The uniform diffraction effects illustrated in the inset of
Figure 3b also show that the grating was homogeneously imprinted over the whole 0.5 × 1 cm
2 mask area, where transparent stripes of 1 µm/2 µm width/pitch are present. The AFM profile in
Figure 3c illustrates a typical diffraction grating whose width/pitch closely matches that of the mask and whose uniform height of 220 nm appears to be in close agreement with that previously obtained on silicon substrates [
28]. In contrast, as illustrated in the AFM profile of
Figure 3d, the channel waveguide width is slightly greater (55 µm) than the expected value of 50 µm, and its thickness of 230 nm is also slightly greater than that previously obtained on silicon. A longer post-insolation washing would probably have enabled more closely conforming channel waveguides to be obtained. However, these small variations do not significantly alter the behavior of the device. Indeed, we previously showed that such dimensions lead to single mode and multimode waveguides in their thickness and width, respectively, and that neither thickness nor width has a critical influence on light propagation and channel waveguide/diffraction grating coupling [
28]. The AFM profiles of
Figure 3c,d also depict photo-imprinted patterns that do not present vertical edges but rather exhibit a trapezoidal profile with edge slopes of around 45°. This feature, which was also observed for channel waveguides and diffraction gratings on silicon, is attributed to diffraction effects on the edges of the transparent stripes during insolation through the mask [
32]. All these data globally demonstrate that our two-step deposition/insolation protocol initially developed on silicon substrates has been successfully extrapolated to the elaboration of a micro-structured architecture on glass with a similar quality.
3.2. Planar Waveguide/Diffraction Grating Coupling
Planar waveguide/diffraction grating coupling was preliminarily examined on the basis of theoretical considerations and optical simulation. Light propagating in a planar waveguide is described by two polarization modes, i.e., the transverse electric (TE) and transverse magnetic (TM) modes. For instance, if we consider a TE polarization (major electric field oriented parallel to the substrate plane) in the simplified case of a planar waveguide, the so-called dispersion relation for guided modes applies [
35,
36]:
where
nc,
nsub, and
nsup are the refractive indices of the waveguide core, the substrate, and the superstrate (or external medium), respectively,
d is the waveguide thickness, and
λ the wavelength. This equation can be numerically solved for a certain integer mode orders m, and when such a guided mode exists, it yields the corresponding effective index
neff. In our previous work, the waveguide effective indices were derived from Equation (1) using the thickness and refractive indices of Ti-BzAc layers determined from ellipsometric measurements [
28]. For instance, in TE mode, we derived an
neff value of 1.61 at 635 nm, which will be exploited in the following. The effective indices can then be used to assess light coupling at various wavelengths between the waveguides and diffraction gratings imprinted at their surfaces. In this configuration, light is injected into, or extracted from, the waveguide according to a finite number of diffraction angles θ
d by means of grating couplers having an appropriate period Λ. In order to achieve coupling with a guided mode of effective index n
eff, the so-called grating law must be fulfilled [
37]:
where
q is an integer that represents the diffracted coupling orders. Thus, Equation (2) enables θ
d angles to be determined where light coupling toward the guided mode occurs from a plane-wave impinging on the grating or where guided light is radiated through the grating toward the external medium. However, this equation does not take into account the grating dimensionalities (height, width, profile, and length). Thus, optical simulation is necessary to derive more reliable insights into the light coupling efficiency.
In this work, simulation was performed using the AFMM (aperiodic Fourier modal method), also known as RCWA (rigorous coupled-wave analysis) modified with PMLs (perfect matching layers). The method has been detailed in previous papers [
38,
39]. It involves a 2D simulation where electromagnetic fields and grating profiles are invariant in a transverse direction of the propagation. Hence, a planar waveguide and plane-wave excitation are used. In the experiment part, a lateral confinement waveguide is used. The method leads to the simultaneous determination of the diffraction angles θ
d and their associated diffracted coupling orders q. For practical convenience, we considered a configuration where light propagating in the core of a channel waveguide was then extracted toward the external medium through the diffraction grating. However, owing to the principle of light reciprocity, conclusions derived from this configuration can rigorously be extrapolated to the reverse case, where the diffraction grating serves to inject light into the waveguide core. The method can be used to assess gratings with a square profile by taking into account their dimensionalities. For more complicated profiles, such as the trapezoidal profiles considered in this work, a spatial discretization of the sloping part (rising and falling edges) is necessary. This part was defined by a cascade of 21 layers with a width increment of 10 nm between each layer from top to bottom of the trapezoids (Y direction in
Figure 4), and we used 163 spectral points in the vertical direction (Z direction in
Figure 4). The period number of the grating was set to 50, i.e., a grating length of 100 µm, and the electric field was calculated along a line at a height of 1.015 µm above the waveguide, to calculate the angular radiation diagram. The other main parameters used in this simulation are schematized in
Figure 4 according to previous opto-geometrical characterization, and an example of results derived from this simulation is shown in
Figure 5 for a 635 nm signal in TE mode.
Figure 5 shows a series of peaks that depict light diffraction through the grating according to a finite number of extraction angles. The main peaks are located at around 44°, 22°, and 3° with respect to the vertical direction and are associated with coupling orders of three, four, and five, respectively. These peaks exhibit a certain broadness and are positioned at angle values slightly greater than those predicted by the grating law (also illustrated in
Figure 5 for comparison purposes). This difference probably arises from the influence of the trapezoidal grating dimensionalities. In particular, the average thickness of the waveguide endowed with a grating is greater than that of a bare waveguide. A greater thickness yields a certain increase in the effective index of the actual waveguide, and this effect increases the diffracted angle, as seen in Equation (2). For simplification purposes, this feature has not been taken into account in this work. However, as discussed in the following, this simplification does not preclude drawing valuable information from the simulation. In addition, the observed peaks present different intensities that illustrate the efficiency of light coupling through the grating in relation to the extraction angle. Accordingly, the simulation illustrated in
Figure 5 shows that the most efficient light coupling at 635 nm is achieved for an extraction (or injection) angle of 44° (vs. a theoretical value of 40° according to the grating law) and a coupling order of three.
Firstly, experimental studies were performed to evaluate the extent to which diffraction coupling follows trends predicted by the simulation. In these studies, the diffraction gratings were used to inject light into the waveguide core. This configuration is the opposite of that previously simulated. Nonetheless, as previously specified, both configurations can be compared owing to the principle of light reciprocity. We firstly employed the previously described modified M-lines method to characterize a diffraction grating imprinted on a planar waveguide. In accordance with the simulation illustrated in
Figure 5, we considered light at 635 nm in the TE polarization. Three missing lines were detected for angles of around 39°, 18°, and 1° with respect to the vertical direction. As depicted in
Figure 5, these angle values are in good agreement with those deduced from the grating law, and they appear slightly weaker than the optimal coupling angle values derived from simulation. The finest missing line was observed for a diffraction angle of 39°. According to the previously mentioned waveguide effective index of 1.61 at 635 nm, the grating law allowed a coupling order of q = 3 to be determined from this angle value, which closely matches the optimal coupling order deduced from simulation. The experimental data appear, therefore, to be in rather good agreement with theoretical and simulated data, and they provide the first evidence that light coupling occurs effectively between the planar waveguide and diffraction gratings.
3.3. Light Coupling and Fiber Positioning Tolerance Assessments
In a new set of experiments, light at 635 nm was injected through the diffraction grating into the core of 55 µm width channel waveguides, and the propagated signal emerging from the polished waveguide output section was analyzed using the MATLAB software, as previously explained. These experiments were intended to provide new evidence of efficient light coupling, and we paid particular attention to the injection fiber positioning tolerance versus the grating. This tolerance is of great relevance when considering our ultimate objectives, dealing with the integration of the micro-structured architecture into a microfluidic platform devoted to fluorescence measurements in a liquid medium. Indeed, in such a configuration, positioning of the fibers enabling efficient injection of the excitation signal and efficient collect of the emission signal through diffraction gratings can be rather tricky and particularly demanding with regard to fiber position tolerance.
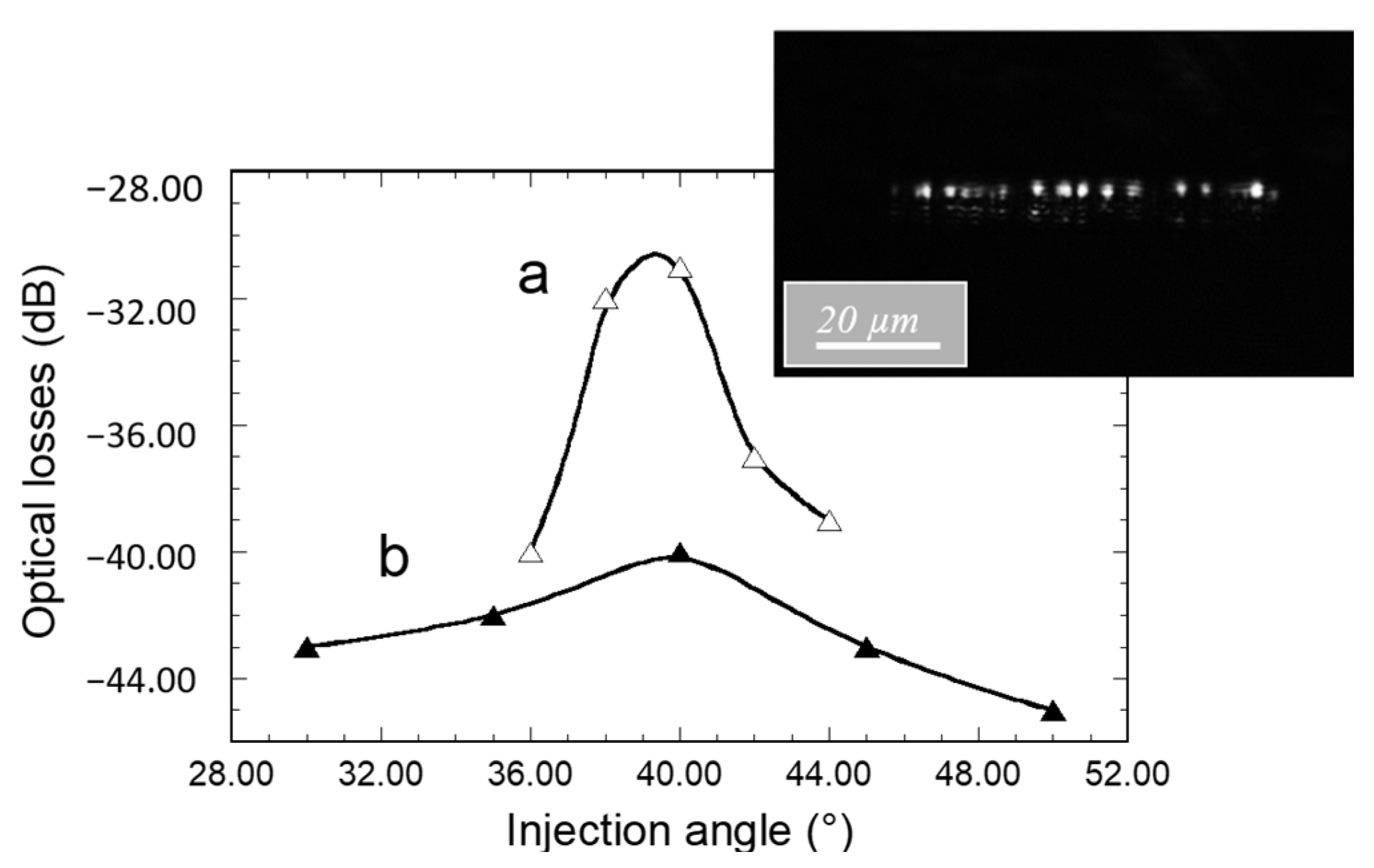
Figure 6 and
Figure 7a,b illustrate how the angular (θ
i in
Figure 2), axial (Y direction in
Figure 2), and vertical (Z direction in
Figure 2) positioning of the fiber influence the emerging signal intensity, respectively. First of all, it is important to note the correlation of experimental points in the curves of these figures, which illustrates well the high reproducibility and reliability of our measurements, i.e., the low measurement error. A typical signal emerging from the waveguide polished section is illustrated in the inset of
Figure 6, which depicts the light spots associated with many guided modes propagating in the waveguide core. On the one hand, it confirms that, due to their low thickness (230 nm), the waveguides are single mode in the vertical direction. The spot diameters are obviously larger than the waveguide core thickness, owing to light diffraction at the output facet. On the other hand, the figure illustrates a multi-mode waveguide in its width direction, i.e., several spots distributed in the waveguide width that depict the result of the interference between multiple propagated modes. These features provide new evidence that diffraction gratings effectively enable light injection into channel waveguides, and that these particular ones allow an efficient guided mode confinement in the vertical and lateral directions. The influence of the light incidence was studied for channel waveguides of length 2 and 5 mm (
Figure 6). To this end, the 4 µm core diameter injection fiber was centered on the waveguide width and positioned in contact with the diffraction grating and at a 60 µm distance from the interface between the grating and the bare waveguide. The data are given in terms of optical losses deduced from the emerging-to-injection light intensity ratio. For both studied waveguide lengths,
Figure 6 shows that the angle dependence of the extracted light intensity follows an approximately Gaussian distribution with a rather large width at mid-height around an optimal injection angle of 39–40°. This optimal value is in very close agreement with that derived from previous M-lines analyses. According to this concordance, the slightly greater angle values deduced from simulation suggest that the latter can again be optimized, for instance by taking into account the influence of the grating on the waveguide effective index. Besides, the small divergence between experimental and simulated data probably arises from the difficulty of rigorously accounting for the overall characteristics of the grating (uniformity, reproducibility, etc.). However, these divergences should be relativized when considering that, owing to the broadness of peaks illustrated in simulation data of
Figure 5, the angular dependence of diffraction coupling efficiency depicted by these peaks largely overlaps the experimentally deduced optimal coupling angles. Thus, simulation still provides a valuable guide to predicting experimental conditions. Furthermore, simulation and experimental data illustrate a tolerance of several degrees in the angular fiber positioning.
For an optimal injection angle of 39–40°,
Figure 6 depicts optical losses of 30 and 40 dB for a waveguide length of 2 and 5 mm, respectively. We firstly infer that a great part of these losses is related to the light coupling efficiency through the grating. As mentioned in the Introduction, an optimized coupling efficiency requires a grating periodicity of the order of the considered wavelength in such a way that only one coupling order is involved, as shown by Equation (2). In our case, the simulation illustrated in
Figure 5 shows that coupling through our gratings involves several orders, owing to their 2 µm periodicity. Thus, the coupling efficiency is strongly reduced and an important part of the light emerging from the injection fiber is lost by reflection at, or transmission through, the substrate. The losses illustrated in
Figure 6 also arise from propagation in the waveguide cores. Since injection losses are inferred to be identical for the waveguides illustrated in this figure, optical loss differences between both waveguide lengths essentially depict such propagation losses. Considering the data illustrated in
Figure 6 for an injection angle of 39–40°, these losses were roughly estimated to be around 3 dB/mm, i.e., around 6 dB and 15 dB for the 2 mm and 5 mm length channel waveguides, respectively. Such injection and propagation losses are obviously important, but in this work, our priority was not to optimize channel waveguide and diffraction grating performances but to propose a micro-structured architecture allowing the well-controlled and reproducible measurement of the propagated signal intensity. This is illustrated in the following for channel waveguides of 55 µm width and 5 mm length and for an injection angle of 40° with respect to the vertical direction since this angle shows a reasonable agreement between simulated and experimentally deduced values.
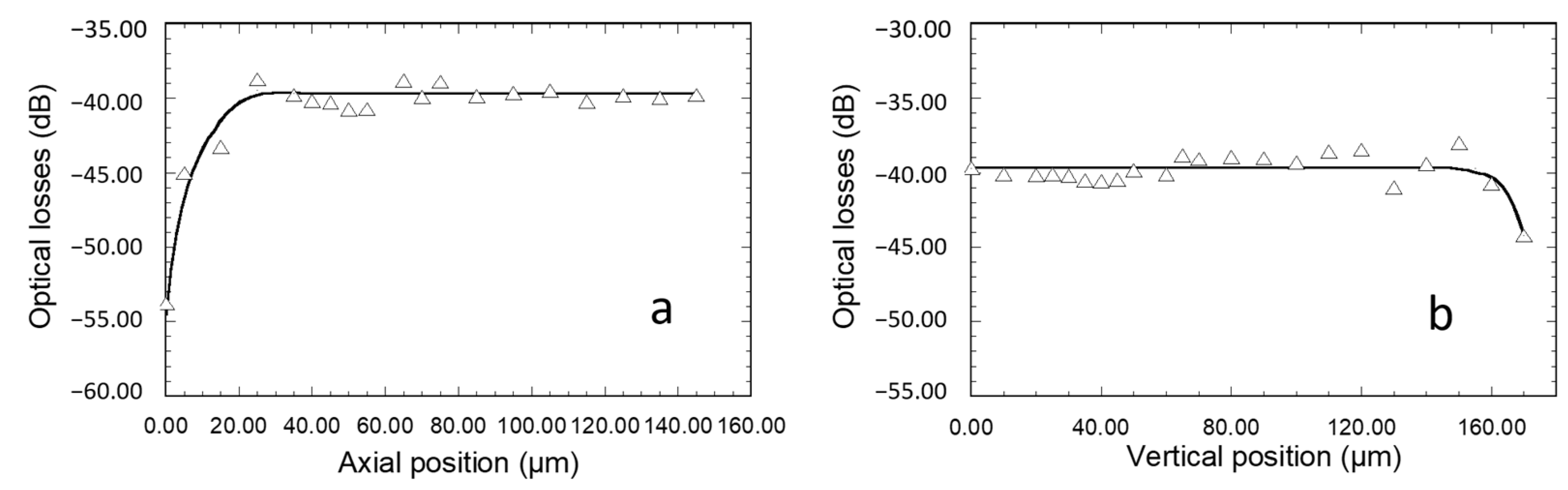
Figure 7a illustrates how the axial position of the fiber influences light emerging from the waveguide output section. The fiber, centered along the waveguide width at a few micrometers above the sample surface, was initially positioned in the vertical direction of the bare waveguide, just at the interface with the diffraction grating. No signal could be observed at the waveguide output facet, showing that light injection into the waveguide did not occur without the diffraction grating. In this case, the measured light and the corresponding optical losses of 55 dB were only related to ambient noise. Then, the fiber was progressively moved along the diffraction grating. Optical losses dropped to a value of around 45 dB after positioning the fiber in the vertical direction of the grating just at the interface with the bare waveguide, showing that light coupling had started to occur. Then, they underwent a further decrease to 40 dB when the fiber was gradually moved up to a distance of 20 µm from the interface with the bare waveguide. Finally, no loss evolution was detected with further fiber displacement up to a length of at least 120 µm, and optical losses of 40 +/−1 dB were measured. On the one hand, these data show that optimal light coupling with the channel waveguide requires a grating length of 20 µm (ten grating periods) or more. On the other hand, they show that when coupling is achieved far away from the bare channel waveguide (at least 120 µm), the eventual diffraction effects do not induce significant decoupling losses toward the external medium of the light propagating in the waveguide underneath the grating. It therefore allows an important degree of tolerance in the fiber axial positioning and the achievement of reproducible intensity measurements. A similar study was then performed to assess the tolerance in vertical positioning. The fiber was initially centered on the width of the waveguide and positioned in contact with the diffraction grating at 60 µm from the interface with the bare waveguide (in the middle of the plateau illustrated in
Figure 7a), and it was then progressively moved vertically away from the grating.
Figure 7b shows that reproducible losses of 40 +/−1 dB were measured for a fiber vertical displacement of around 150 µm, which illustrates once again an important fiber positioning tolerance allowing reproducible measurements. Then, optical losses started to increase with further vertical movement of the fiber. To summarize, the data in
Figure 6 and
Figure 7 illustrate large tolerances in the angular (several degrees) and spatial (more than 100 µm in axial and vertical directions) fiber positioning.
3.4. Fluorescence Measurements
Fluorescence measurements were performed under excitation at 450 nm to complete the assessments of light coupling through the diffraction gratings. A reference emission spectrum of a Ti-BzAc layer doped with the Rudpp fluorophore (1 mM in solution) was preliminarily collected by transmission through the glass-supported layer using a spectrophotometer and a long-pass filter with a 500 nm cutoff wavelength. As illustrated in
Figure 8, the spectrum exhibits a large emission band extending between 550 and 750 nm, with a maximum at 610 nm. This figure particularly indicates that: (i) the 450 nm wavelength is well adapted to excite the Rudpp emission in our present experimental conditions, (ii) no significant signal is detected below 550 nm, showing that the long-pass filter used enabled the excitation signal to be totally eliminated, thus allowing selective detection of the Rudpp emission, and (iii) according to the important intensity measured at 635 nm, previous assessments performed at this wavelength provided reliable information on the waveguide/diffraction grating coupling of the Rudpp emission signal.
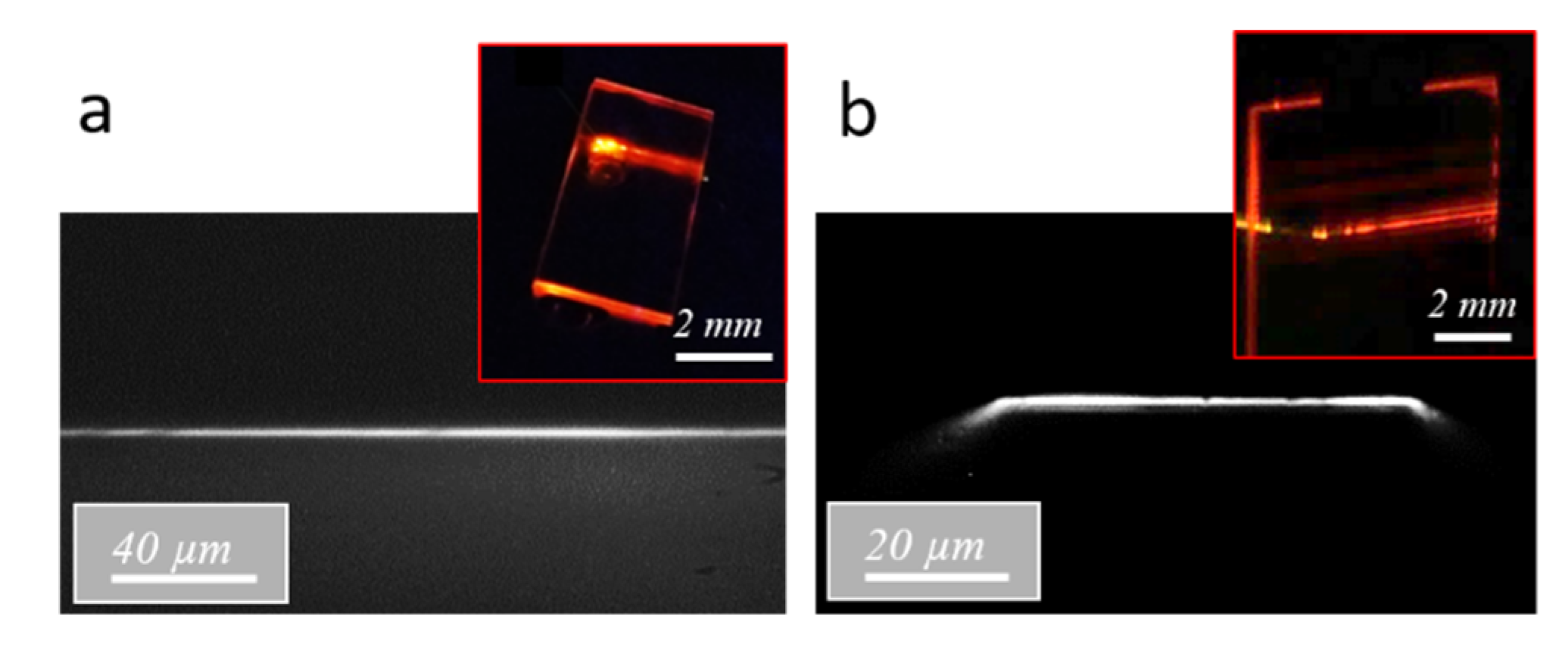
Fluorescence measurements were then performed in a guided light configuration on planar (i.e., 2D) and channel (i.e., 3D) waveguides endowed with diffraction gratings. The excitation signal was injected through the diffraction gratings in the core of the planar and channel waveguides at an incidence of 35° with respect to the vertical direction. This value, which corresponds to a coupling order of five at the 450 nm wavelength, was deduced from simulation (not illustrated here). The injection fiber was positioned in contact with the diffraction gratings at a 20 µm distance from the bare waveguides, and the emission signal was collected from the waveguide output polished facet through a 500 nm cutoff wavelength long-pass filter. As illustrated in the micrographs in
Figure 9a,b, the emerging signal was well confined within the waveguide height, similarly to the signal emerging after light injection at 635 nm through the diffraction gratings (inset of
Figure 6). For planar waveguides, no obvious lateral confinement was achieved, and the inset of
Figure 9a shows that, after propagation, significant light can be detected over a width of about 400 µm at the waveguide output. In contrast, the micrograph in
Figure 9b shows that the emission signal propagating in the channel waveguide was essentially confined in its 55 µm core width, although the inset of this figure also shows that a part of the signal seems to be radiated into the substrate underneath from either side of the waveguide.
Figure 9b also depicts a slight signal inflexion at the edges of the channel waveguide output facet, which was not clearly explained in the present state. For both kinds of waveguides excited at 450 nm, a major difference from light injection at 635 nm was that the emerging signal was not composed of separated spots but appeared in the form of a continuous horizontal line. This line shows that, in this case, light does not propagate through discrete guided modes in a coherent way. The intensity tends to an average value due to the non-coherent fluorescence signal that is isotropically emitted in the space from Rudpp sources dispersed throughout the waveguide. This observation has an important practical consequence since it supposes, in particular, that only a part of the propagating fluorescence signal is actually confined within the waveguide cores, and that another important part is coupled with the substrate and the external medium.
Our experimental set-up was not adapted to direct intensity measurements of light emerging from the waveguide output facet using a spectrophotometer, especially in the case of a planar waveguide. This measurement should be possible with a micro-structured architecture where a diffraction grating imprinted on the waveguide output would allow extraction of the emitted light, which is one of our future objectives. In the present state, the Rudpp emission intensity was derived from the previously discussed MATLAB analysis, and the output signals analyzed in this way therefore accounted for the intensity integrated over the whole spectrum illustrated in
Figure 8. However, fluorescence measurements required some adaptation of the MATLAB analysis. In previous experiments using light injection at 635 nm and propagation of guided modes, the emerging signal was analyzed from a pixel matrix of 1440 lines and 1920 columns. For fluorescence measurements, it was necessary to reduce the matrix dimensions in order to exclude the fluorescence extracted from the substrate and external medium and to selectively detect the emission signal emerging from the waveguide output. In the case of planar waveguides, this selective detection was achieved by reducing the number of pixel lines to 50, while the 1920 columns enabled the emission signal emerging from a 200 µm width section of the waveguides to be measured. For channel waveguides, the number of pixel columns was further reduced to 450 in order to also exclude fluorescence extracted from the edges of the 55 µm width waveguides. Derived fluorescence intensity measurements are illustrated in
Figure 10 for various amounts of Rudpp fluorophore in the case of planar and channel waveguides of 2 mm and 5 mm length, respectively.
Interestingly,
Figure 10 shows that both kinds of studied waveguides led to very similar trends, and in both illustrated curves the experimental points exhibit a good correlation, which once again illustrates the reproducibility and reliability of our measurements. In the planar waveguide, propagating light is expected to spread over a ~400 µm width (
Figure 9a), compared with the 55 µm width in the channel waveguide (
Figure 9b). Thus, the similar trends illustrated in
Figure 10 show that the doping-level dependence of fluorescence intensity measured in the guiding configuration is not influenced by the propagation width. The fluorescence intensity logically increases with the Rudpp amount up to a concentration in solution of around 0.5 mM, after which it plateaus, with no further intensity increase or even a slight decrease above a Rudpp threshold concentration of 1 mM. The fact that fluorescence does not continuously increase with the fluorophore amount can firstly be attributed to parasitic phenomena intrinsically occurring when the concentration of fluorophore in the host matrix exceeds a certain threshold, e.g., concentration quenching or inner filter effects that reduce the fluorescence yield [
40]. This phenomenon is also probably related to fluorescence measurements performed in the guided light configuration. Indeed, the excitation signal propagating along the waveguide cores is progressively absorbed by the Rudpp fluorophore. This means that, for a given Rudpp concentration, the excitation signal is totally absorbed after a threshold propagation length. Fluorophores located beyond this length are therefore not excited and no longer participate in the fluorescence emission, which can explain the intensity saturation depicted in
Figure 10. Thus, these new data have two important practical consequences. On the one hand, they prove that coupling at the excitation wavelength is efficiently achieved through the diffraction gratings and allows detection of the Rudpp emission in a waveguiding configuration at the output of channel waveguides, thus providing new confirmation of the potential of our micro-structured architecture. On the other hand, they indicate that the best configuration should rely on a trade-off between the doping level of Rudpp (a concentration of around 0.1 mM in this study) and the length of channel waveguides (2 to 5 mm in this study) optimized in terms of excitation and emission signal propagation.