Composition of Organosilicate Coatings High-Temperature Breakdown Products and Their Distribution in the Weld
Abstract
1. Introduction
- (a)
- polyorganosiloxanes decomposition and formation of the crosslinks with the hydroxyl groups’ participation (or “siloxane structuring”) [18];
- (b)
- dehydroxylation and other structural transformations of phyllosilicates [19];
- (c)
- interaction of the polyorganosiloxanes breakdown products with the silicate and oxide components [14];
- (d)
- new crystalline phases formation, the material transition to a ceramic-like state [20].
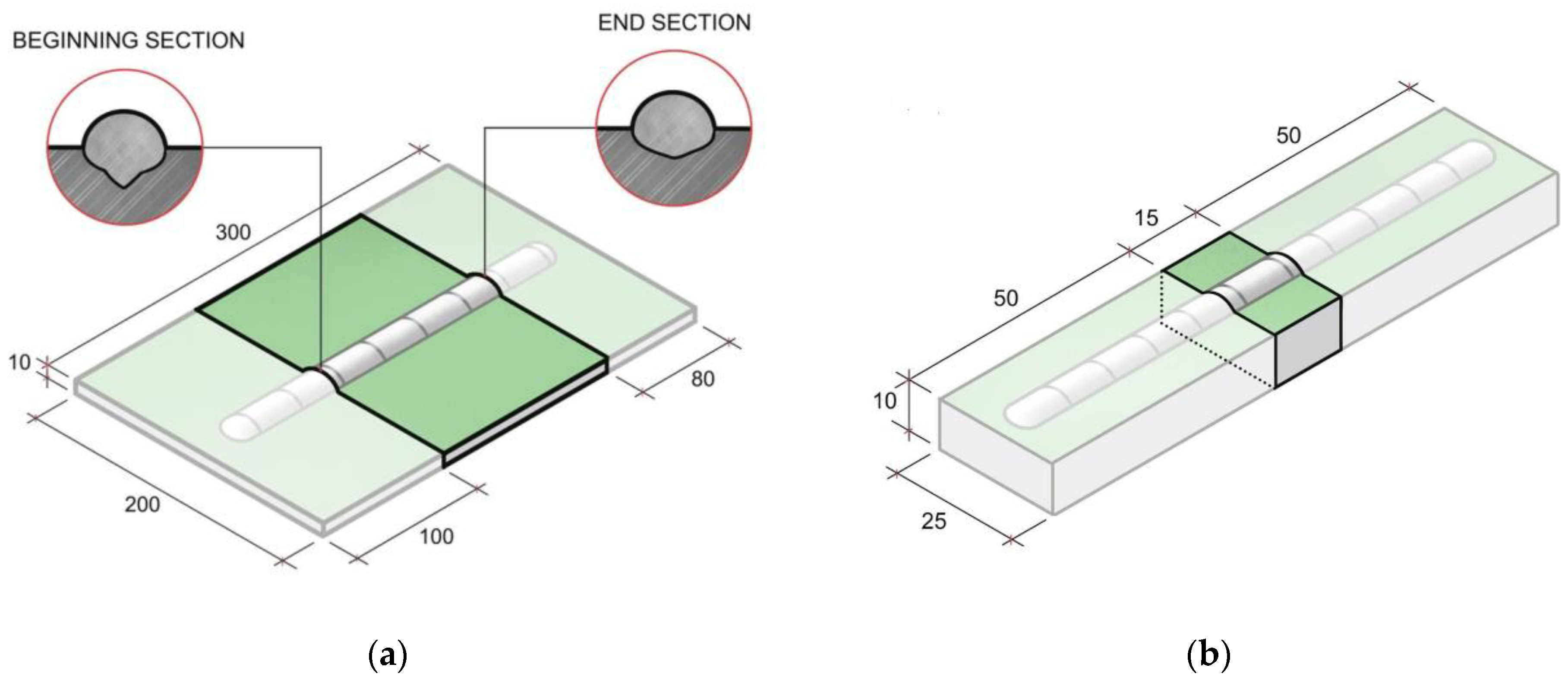
2. Materials and Methods
3. Results and Discussion
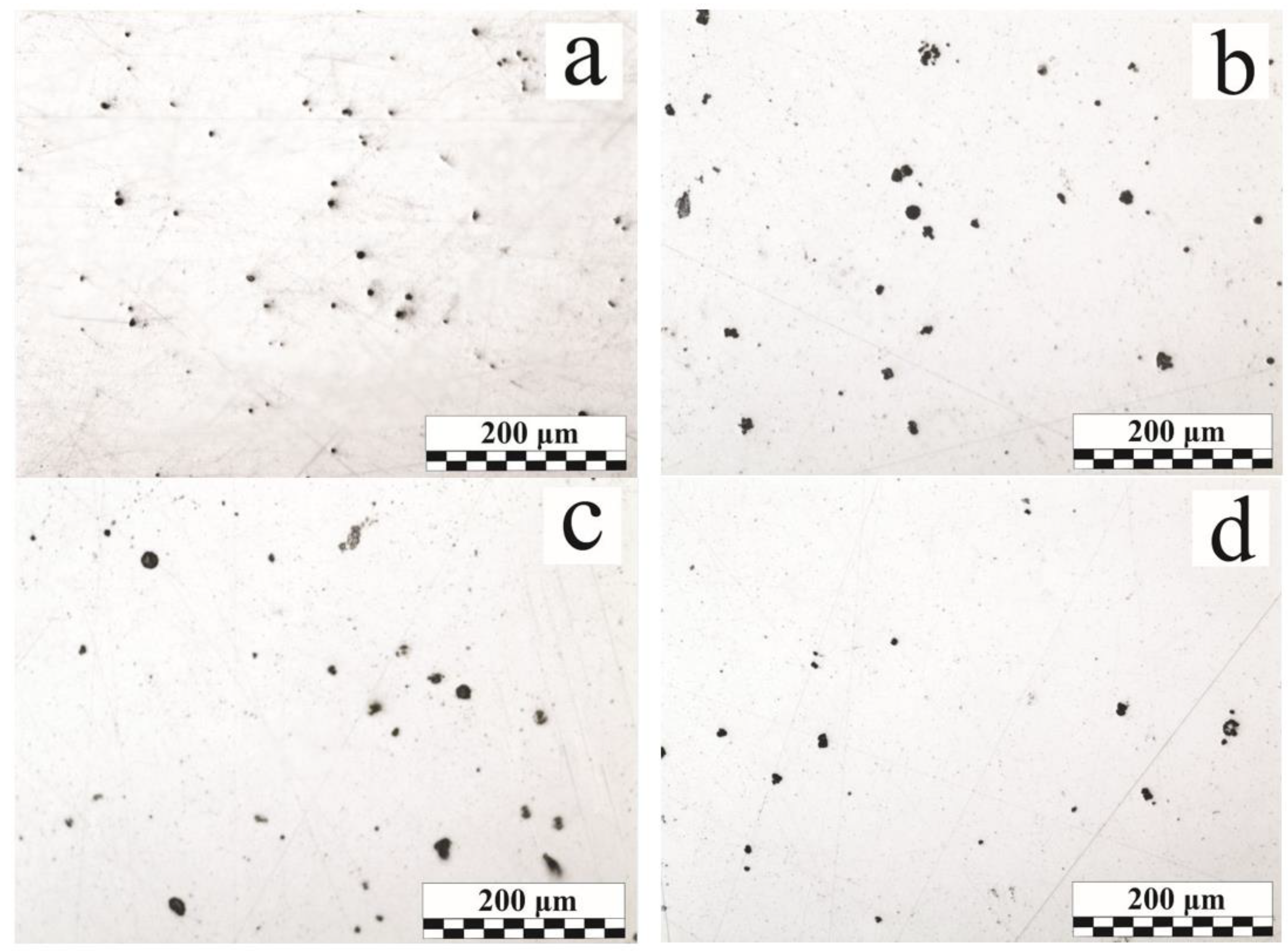
3.1. Non-Metallic Inclusions Distribution
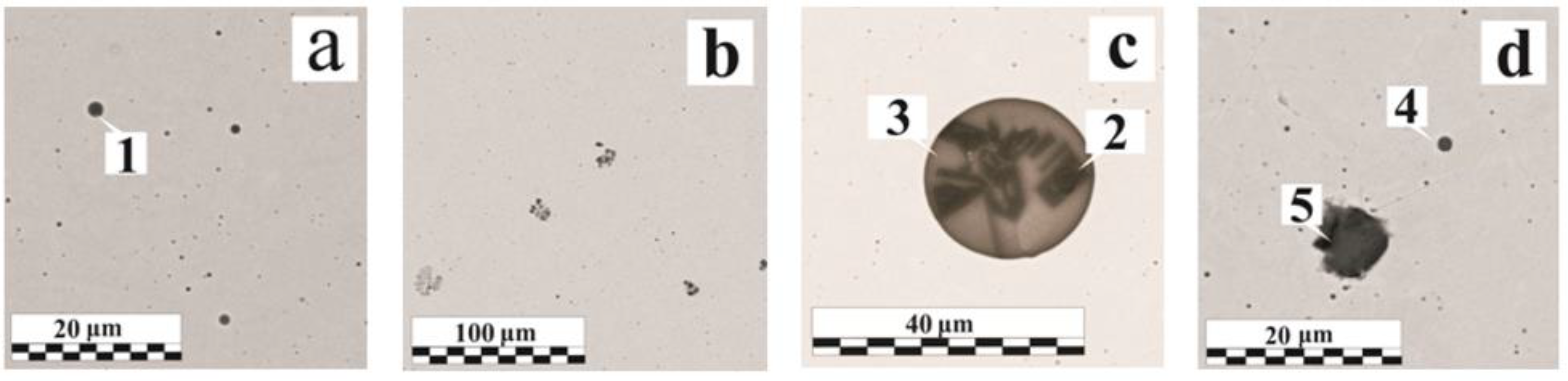
3.2. Non-Metallic Inclusions Composition and Morphology
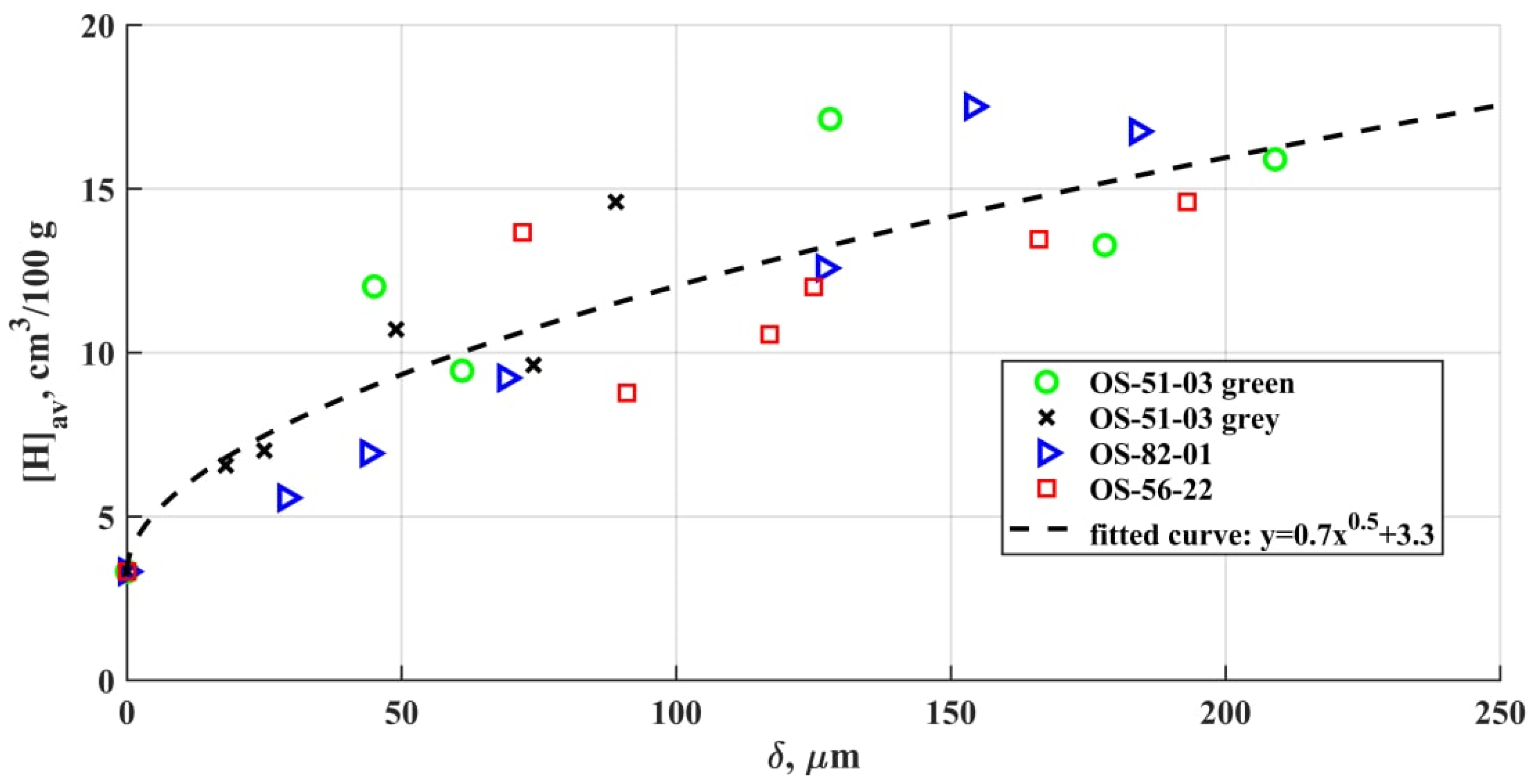
3.3. Diffusible Hydrogen Amount in the Metal
4. Conclusions
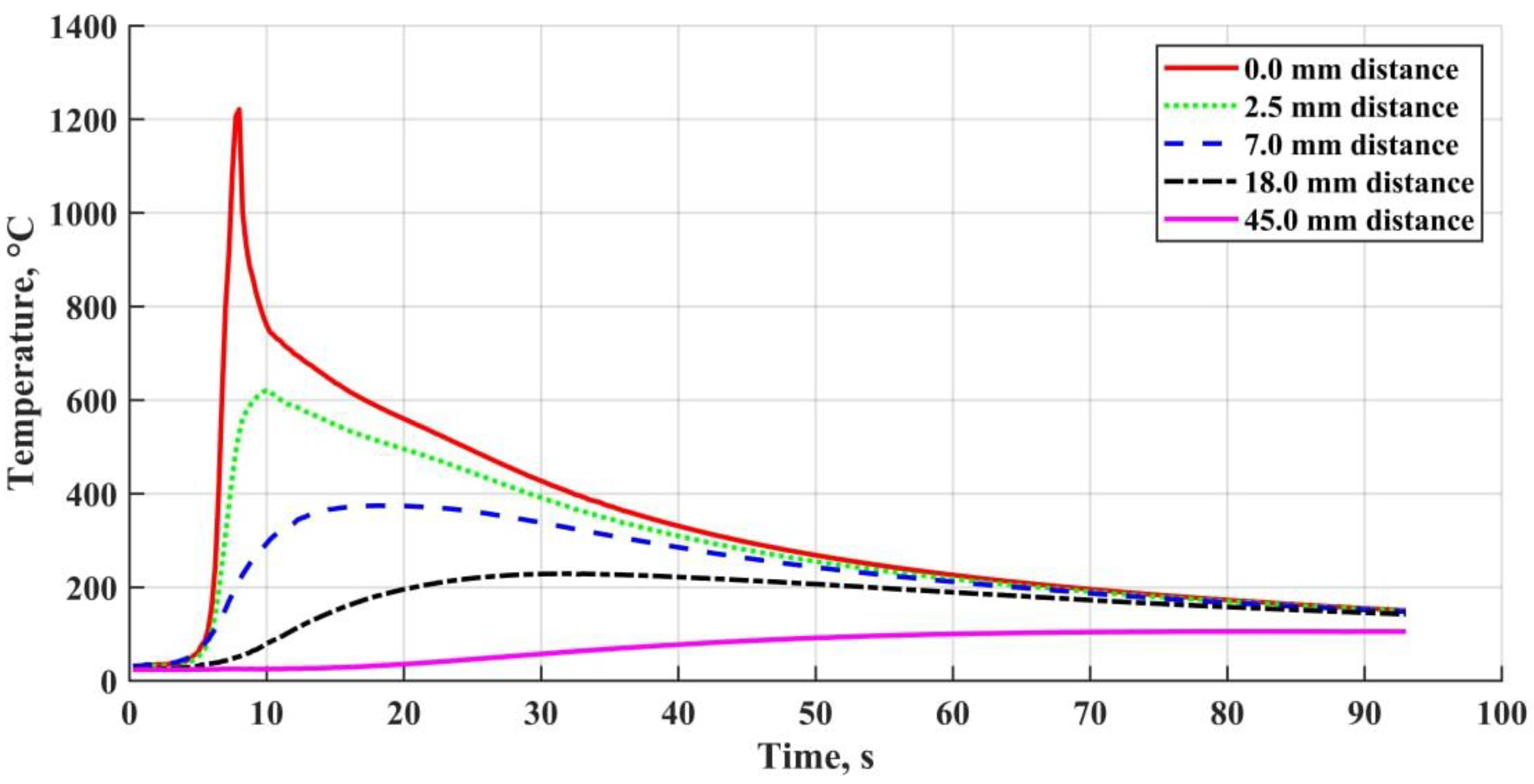
- At the heating rates of ~102 °C/s and with the temperatures up to 1200 °C, the OSCs initial structure is undergoing intense degradation. Hydrogen and oxygen, as the polymer base and hydrosilicates breakdown products, are the main elements that determine the defect formation in the weld. Hydrogen and carbon monoxide (formed as a result of a number of further reactions) are the main cause of pore formation in the metal. It was shown that the diffusible hydrogen amount rises along with the coating thickness. High metal oxidation results in the formation of nonmetallic inclusions of various morphologies and compositions, which are mostly based on the combinations of oxides: FeO, SiO2, MnO, Al2O3.
- The total porosity increases from the weld beginning to its end as a result of the accumulation of dissolved OSCs breakdown products in the liquid metal and the emergence of the large macrodefects. The total area of micropores and non-metallic inclusions in comparison to macroporosity increases slightly from the weld beginning to its end. Nonmetallic inclusions and micropores are evenly distributed over the weld cross section.
- The non-metallic inclusion size depends on the OSCs presence on the metal surface, and their composition also depends on the OSCs thickness. The inclusion size growth is provided by the intensification of the new phase precipitation at the interface or coagulation of small inclusions into larger ones. In the case of the intensification, the breakdown products of the heat-resistant phyllosilicates (Al, Mg), as well as Ca impurities, play an active role. The iron oxide inclusions mostly tend to grow due to coagulation. Inclusions of herzenite and alumina, fluorides, and phosphates are significantly rarer.
- The amount of diffusible hydrogen in the deposited metal doubles even at a 25–50 µm OSCs thickness in comparison with a bare sample. For all four brands in the range of the examined thicknesses, the amount of diffusible hydrogen is closely related to the saturated hydrogen content, which correlated with the Sieverts’ law of the square root of the hydrogen fugacity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mikhailov, M.M.; Yur’ev, S.A.; Bakhtaulova, A.S.; Yurina, V.Y. Modification of organosilicon compounds with Al2O3 nanoparticles in order to increase radiation resistance. Met. Sci. Heat Treat. 2020, 62, 81–85. [Google Scholar] [CrossRef]
- Buslaev, G.S.; Kochina, T.A.; Krasil’nikova, L.N.; Milyutina, P.A.; Shilova, O.A. Heat-resistant protective organosilicate coatings for nuclear energy. Glass Phys. Chem. 2020, 46, 357–359. [Google Scholar] [CrossRef]
- Chuppina, S.V.; Zhabrev, V.A. Organosilicate radiation-resistant deactivatable protective coatings. Prot. Met. Phys. Chem. Surf. 2013, 49, 344–347. [Google Scholar] [CrossRef]
- Kochina, T.A.; Buslaev, G.S.; Kondratenko, Y.A. Organocilicate coatings. From creation to innovations. Glass Phys. Chem. 2020, 46, 13–25. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Shevchenko, V.Y.; Shilova, O.A.; Kochina, T.A.; Barinova, L.D.; Belyi, O.V. Environmentally friendly protective coatings for transport. Her. Russ. Acad. Sci. 2019, 89, 279–286. [Google Scholar] [CrossRef]
- Kharitonov, N.P.; Krotikov, V.A.; Khudobin, Y.I.; Buslaev, G.S.; Stepanov, K.N. Organosilicate Materials, Their Properties and Application Technology [Organosilikatnyye Materialy, ikh Svoystva i Tekhnologiya Primeneniya]; Nauka: Leningrad, Russia, 1979; p. 202. [Google Scholar]
- Kharitonov, N.P.; Krotikov, V.A.; Vologdin, I.V.; Vorobiev, N.I.; Kulik, G.N.; Malyshev, V.P.; Savelyev, R.V. Application of Coatings Made of Organosilicate Material VN-30 for Corrosion Protection of Embedded Parts and Welded Joints in Prefabricated Reinforced Concrete. [Primeneniye Pokrytiy iz Organosilikatnogo Materiala VN-30 dlya Antikorrozionnoy Zashchity Zakladnykh Detaley i Svarnykh Soyedineniyv Sbornom Zhelezobetone]; Znanie: Leningrad, Russia, 1965; p. 32. [Google Scholar]
- Kharitonov, N.P.; Ostrovsky, V.V. Thermal and Thermooxidative Decomposition of Polyorganosiloxanes [Kharitonov N.P., Ostrovskiy V.V. Termicheskaya i Termookislitel’naya Destruktsiya Poliorganosiloksanov]; Nauka: Leningrad, Russia, 1982; p. 208. [Google Scholar]
- Wang, G.; Liu, X.; Mi, C.; Fan, H.; Xu, B.; Bai, X. High-temperature structural evolution and hydrolytic stability of poly(phenylborosiloxane). Pigment Resin Technol. 2018, 47, 308–314. [Google Scholar] [CrossRef]
- Dezfuli, S.M.; Sabzi, M. Deposition of ceramic nanocomposite coatings by electroplating process: A review of layer-deposition mechanisms and effective parameters on the formation of the coating. Ceram. Int. 2019, 45, 21835–21842. [Google Scholar] [CrossRef]
- Maslov, V.A. Insulating materials of high thermal stability. Russ. Electr. Eng. 2014, 85, 460–463. [Google Scholar] [CrossRef]
- Ostrovskii, V.V.; Kharitonov, N.P. Prediction of decomposition of polyorganosiloxanes at different heating rates. J. Appl. Chem. USSR 1985, 58, 2412–2414. [Google Scholar]
- Kumagai, S.; Yoshimura, N. Tracking and erosion of HTV silicone rubbers of different thickness. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 673–678. [Google Scholar] [CrossRef]
- Kumagai, S.; Yoshimura, N. Polydimethylsiloxane and alumina trihydrate system subjected to dry-band discharges or high temperature part I: Chemical structure. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 691–700. [Google Scholar] [CrossRef]
- Zavin, B.G. Organosilicate Materials [Organosilikatnyye Materialy]; Znanie: Moskow, Russia, 1986; p. 30. [Google Scholar]
- Chuppina, S.V.; Zhabrev, V.A. Chemical reactions in the course of curing of organosilicate composites and aging of organosilicate coatings. Glass Phys. Chem. 2008, 34, 82–90. [Google Scholar] [CrossRef]
- Bystritskaya, E.V.; Karpukhin, O.N.; Tsverava, V.G.; Nepovinnykh, V.I.; Rusin, M.Y. Thermal degradation of silicone sealant. Int. Polym. Sci. Technol. 2012, 39, T51–T56. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Peng, T.; You, H.; Qin, Y.; Zeng, L. Effects of muscovite matrix on photocatalytic degradation in TiO2/muscovite nanocomposites. Appl. Clay Sci. 2019, 179, 105155. [Google Scholar] [CrossRef]
- Lipowitz, J. Structure and properties of ceramic fibers prepared from organosilicon polymers. J. Inorg. Organomet. Polym. 1991, 1, 277–297. [Google Scholar] [CrossRef]
- Ptáček, P.; Opravil, T.; Šoukal, F.; Havlica, J.; Másilko, J.; Wasserbauer, J. Preparation of dehydroxylated and delaminated talc: Meta-talc. Ceram. Int. 2013, 39, 9055–9061. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, W.; Qi, L.; Yuan, L.; Huang, G.; Huang, Y.; Weng, X. The high-temperature resistance properties of polysiloxane/Al coatings with low infrared emissivity. Coatings 2018, 8, 125. [Google Scholar] [CrossRef]
- Metsik, M.S. Thermal Properties of Mica Crystals [Termicheskiye Svoystva Kristallov Slyudy]; Izd-vo Irkut. Un-Ta: Irkutsk, Russia, 1989; p. 184. [Google Scholar]
- Olson, D.L.; Dixon, R.; Liby, A.L. Welding: Theory and Practice; Elsevier Sci. Publ. B.V.: Amsterdam, The Netherlands, 1990; pp. 96–98. ISBN 9780444596383. [Google Scholar]
- Gao, W.B.; Wang, D.P.; Cheng, F.J.; Deng, C.Y.; Xu, W. Underwater wet welding for HSLA steels: Chemical composition, defects, microstructures, and mechanical properties. Acta Metall. Sin. 2015, 28, 1097–1108. [Google Scholar] [CrossRef]
- Chen, K.; Wang, D.; Hou, D.; Qu, T.; Tian, J.; Wang, H. Effect of interfacial properties on agglomeration of inclusions in molten steels. ISIJ Int. 2019, 59, 1735–1743. [Google Scholar] [CrossRef]
- Beckert, M.; Klemm, H. Methods of Metallographic Etching [Sposoby Metallograficheskogo Travleniya]; Metallurgiya: Moskow, Russia, 1988; p. 400. [Google Scholar]
- Kurushkin, D.; Mushnikov, I.; Rylkov, E.; Isupov, F.; Panchenko, O.; Golubev, I. Comparison of porosity evaluation methods using the example of dissimilar metal joints. Key Eng. Mater. 2019, 822, 129–134. [Google Scholar] [CrossRef]
- Zhabrev, L.A.; Chuppina, S.V.; Panchenko, O.V.; Repin, I.L.; Popovisch, A.A. Effect of composition and thickness of organosilicate coatings on the arc welding process. Russ. J. Appl. Chem. 2019, 92, 1274–1283. [Google Scholar] [CrossRef]
- Panchenko, O.V.; Levchenko, A.M.; Karkhin, V.A. Optimization of a Specimen Size for the Determination of the Diffusible Hydrogen Content in Metals. Appl. Mech. Mater. 2014, 698, 466–471. [Google Scholar] [CrossRef]
- Carboni, M.C.; Espinosa, D.C.R.; Tenório, J.A.S. Reduction of chromium from Al2O3-CaO-SiO2-CrOx slags by carbon dissolved in liquid iron. ISIJ Int. 2011, 51, 523–529. [Google Scholar] [CrossRef][Green Version]
- Kiviö, M.; Holappa, L. Addition of titanium oxide inclusions into liquid steel to control nonmetallic inclusions. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2012, 43, 233–240. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jo, J.O.; Lee, C.O.; Kim, D.S.; Pak, J.J. Thermodynamic relation between aluminum and titanium in liquid iron. ISIJ Int. 2008, 48, 17–22. [Google Scholar] [CrossRef][Green Version]
- Zhabrev, L.; Kurushkin, D.; Mushnikov, I.; Panchenko, O. The coatings breakdown products influence on the gas metal arc welding parameters. Coatings 2020, 10, 1061. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Ueshima, Y.; Sugiyama, M.; Mizukami, K. Influence of unstable non-equilibrium liquid iron oxide on clustering of alumina particles in steel. ISIJ Int. 2013, 53, 639–647. [Google Scholar] [CrossRef]
- Belchenko, G.I.; Gubenko, S.I. Non-Metallic Inclusions and Steel Quality [Nemetallicheskiye Vklyucheniya i Kachestvo Stali]; Tehnika: Kiev, Ukraine, 1980; p. 168. [Google Scholar]
- Kazakov, A.A.; Zhitenev, A.I. Assessment and interpretation of nonmetallic inclusions in steel. CIS Iron Steel Rev. 2018, 16, 33–38. [Google Scholar] [CrossRef]
- Roy, J.; Rai, R.N.; Saha, S.C. Effect of TiO2 enriched fluxes on the bead geometry, grain size and hardness in submerged arc welds. Int. J. Mater. Prod. Technol. 2018, 56, 313–325. [Google Scholar] [CrossRef]
- Gamutan, J.; Miki, T.; Nagasaka, T. Morphology and composition of inclusions in Si-Mn deoxidized steel at the solid-liquid equilibrium temperature. ISIJ Int. 2020, 60, 84–91. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, M.; Wang, X.; Wang, Y.; Zhao, H.; Cao, Z. Formation mechanism of SiO2-type inclusions in Si-Mn-killed steel wires containing limited aluminum content. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2015, 46, 2198–2207. [Google Scholar] [CrossRef]
- Mu, W.; Dogan, N.; Coley, K.S. Agglomeration of non-metallic inclusions at the steel/ar interface: Model application. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2017, 48, 2092–2103. [Google Scholar] [CrossRef]
- Fujiwara, H.; Tano, M.; Yamamoto, K.; Ichise, E. Solubility and activity of calcium in molten iron in equilibrium with lime and thermodynamics of calcium containing iron melts. ISIJ Int. 1995, 35, 1063–1071. [Google Scholar] [CrossRef]
- Mine, Y.; Narazaki, C.; Murakami, K.; Matsuoka, S.; Murakami, Y. Hydrogen transport in solution-treated and pre-strained austenitic stainless steels and its role in hydrogen-enhanced fatigue crack growth. Int. J. Hydrogen Energy 2009, 34, 1097–1107. [Google Scholar] [CrossRef]
- Mundra, K.; Blackburn, J.M.; DebRoy, T. Absorption and transport of hydrogen during gas metal arc welding of low alloy steel. Sci. Technol. Weld. Join. 1997, 2, 174–184. [Google Scholar] [CrossRef]
| Coating | Purpose | Organosilicon Binder | Pigments | Fillers | Polymer/(Fillers +Pigments) Ratio |
|---|---|---|---|---|---|
| OS-51-03 green | weather resistant, radiation resistant, deactivated | [(CH3)2SiO)1.0 (C6H5SiO1.5)1.25]n | TiO2 + Cr2O3 | Mg3[Si2O5]2(OH)2 + KAl2(AlSi3O10) (OH)2 | 55/45 |
| OS-51-03 grey | TiO2 + C(black) | ||||
| OS-56-22 (grey) | anti-icing | [(CH3)2SiO)1.0 (C6H5SiO1.5)1.25]n + OHSi~ [(CH3)2SiO)]n ~SiOH | ZnO + TiO2 + C(black) | Mg3[Si2O5]2(OH)2 + BaSO4 + KAl2(AlSi3O10) (OH)2 | 60/40 |
| OS-82-01 (green) | heat-resistant | {[C6H5SiO1.5][CH3SiO1.5]0.42 [(CH3)2SiO]1.33}n * | Cr2O3 | KAl2(AlSi3O10) (OH)2 | 30/70 |
| Material | C | Si | Mn | Ni | S | P | Cr | N | Cu | As | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | |||||||||||
| Substrate | 0.14–0.22 | 0.15–0.3 | 0.4–0.6 | ≤0.3 | ≤0.05 | ≤0.04 | ≤0.3 | ≤0.008 | ≤0.3 | ≤0.08 | bal. |
| ESAB OK Autrod 12.51 | 0.06–0.14 | 0.8–1.0 | 1.4–1.6 | – | ≤0.025 | ≤0.025 | – | – | – | – | bal. |
| Etchant Composition | Etching Time | Main Etched Object | Detectable Inclusions |
|---|---|---|---|
| HCl—15 mL C2H5OH—85 mL | 60 s | Cr2O3 | FeS; MnS–FeS; Al2S3–FeS; FeO; MnO –SiO2; FeO –FeS; MnO–FeO–MnS–FeS; Al2S3–FeS–Al2O3–FeO; Cr2S3–FeS–Cr2O3–FeO |
| HF—50 mL H2O—50 mL | 17 s | SiO2 | FeS; MnS–FeS; Al2S3–FeS; Cr2S3–FeS; TiS2–FeS; FeO; CaO–SiO2; MnO–FeO; FeO–SiO2; MnO–SiO2; Al2S3–FeS–Al2O3–FeO |
| C6H2(NO2)3OH—2 g NaOH—25 g H2O—75 mL | 150 s | TiO2 | partially: FeS; MnS–FeS; Al2S3–FeS; MnO–FeO; FeO–FeS; MnO–FeO–MnS–FeS; completely darken: Cr2S3–FeS; FeO–TiO2–FeS–TiS2 |
| Sample | δ, μm | Average Microdefect Diameter, μm | Relative Microdefects Content, % | Relative Porosity (Including Macropores), % | |||
|---|---|---|---|---|---|---|---|
| Beginning | End | Beginning | End | Beginning | End | ||
| No coating | 0 | 5.5 ± 1.5 | 4.9 ± 0.4 | 0.39 ± 0.23 | 0.27 ± 0.09 | 0.42 | 0.31 |
| OS-51-03 green | 95 | 5.6 ± 0.9 | 6.3 ± 0.4 | 0.55 ± 0.22 | 0.78 ± 0.31 | 0.61 | 0.69 |
| 115 | 6.0 ± 0.7 | 5.8 ± 0.6 | 0.55 ± 0.21 | 0.59 ± 0.17 | 1.09 | 1.27 | |
| 160 | 5.0 ± 0.5 | 5.8 ± 0.5 | 0.36 ± 0.08 | 0.57 ± 0.08 | 0.36 | 14.27 | |
| OS-51-03 grey | 150 | 4.2 ± 0.3 | 4.9 ± 0.6 | 0.29 ± 0.12 | 0.37 ± 0.22 | 0.63 | 8.18 |
| OS-56-22 | 185 | 4.4 ± 0.7 | 4.3 ± 1.2 | 0.48 ± 0.11 | 0.52 ± 0.15 | 0.46 | 2.64 |
| OS-82-01 | 160 | 3.6 ± 0.3 | 4.0 ± 0.8 | 0.37 ± 0.14 | 0.31 ± 0.11 | 0.54 | 1.49 |
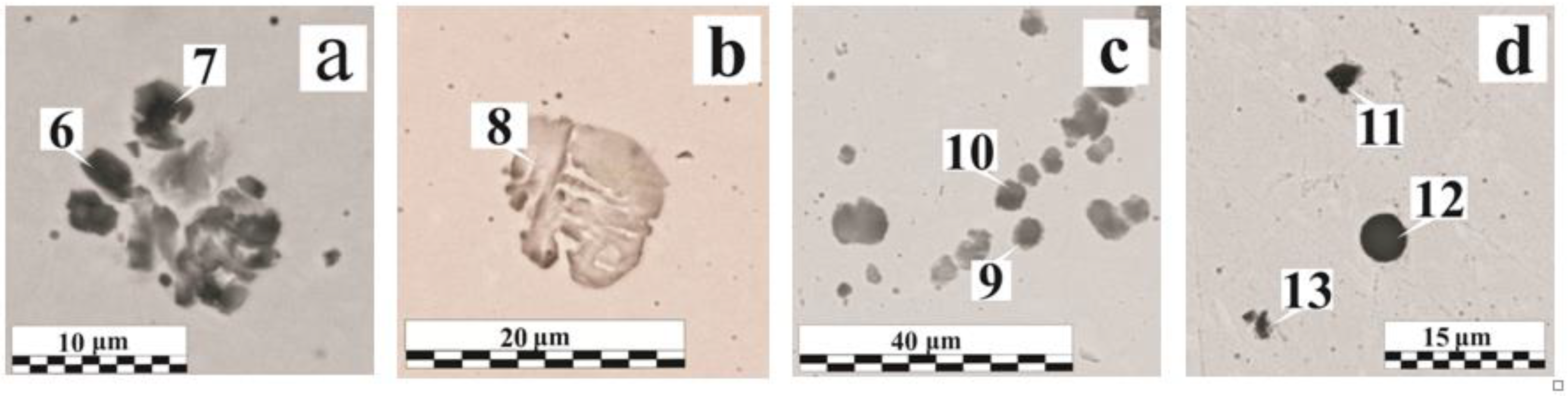
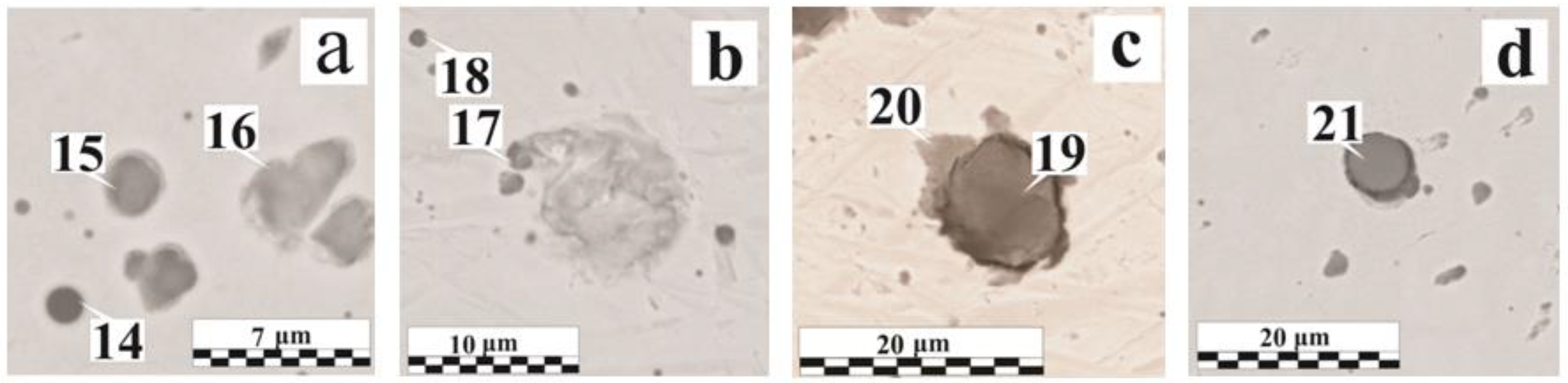
| Figure No. | Coating | δ, μm | Region No. | O | Fe | Mn | Si | Al | Ca | Cr | Mg | Ti | S | Others * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass.% | ||||||||||||||
| Figure 4a | without coating | 0 | 1 | 22.9 | 54.5 | 12.3 | 9.9 | – | – | – | – | – | 0.4 | – |
| Figure 4c | OS-51-03 green | 95 | 2 | 45.2 | 1.5 | 3.4 | 1.5 | 48.0 | – | – | – | 0.5 | – | – |
| 3 | 39.5 | 1.9 | 25.7 | 15.5 | 15.0 | 0.6 | – | 0.3 | 1.5 | – | – | |||
| Figure 4d | OS-51-03 green | 115 | 4 | 16.4 | 61.8 | 9.5 | 7.9 | 4.1 | – | – | – | – | 0.2 | – |
| 5 | 26.9 | 65.9 | 0.9 | 2.2 | – | 1.2 | 0.3 | 0.4 | – | 0.3 | P, Cu | |||
| Figure 5a | OS-51-03 green | 115 | 6 | 16.0 | 81.0 | 1.0 | 0.9 | – | 0.3 | – | – | – | – | P |
| 7 | 20.2 | 76.3 | 1.1 | 1.2 | – | 0.6 | – | – | – | – | P | |||
| Figure 5b | OS-51-03 green | 160 | 8 | 3.8 | 94.4 | 0.9 | 0.9 | – | – | – | – | – | – | – |
| Figure 5c | OS-51-03 grey | 150 | 9 | 7.0 | 88.1 | 0.8 | 2.7 | 0.3 | 0.6 | – | – | – | 0.2 | P |
| 10 | 19.5 | 63.8 | 0.6 | 15.8 | – | 0.4 | – | – | – | – | – | |||
| Figure 5d | OS-51-03 green | 160 | 11 | 23.4 | 51.9 | 0.6 | 0.4 | 23.2 | – | – | – | 0.5 | – | – |
| 12 | 46.6 | 2.6 | 4.1 | 15.4 | 9.0 | 17.4 | – | 2.1 | 2.7 | 0.2 | – | |||
| 13 | 20.0 | 54.6 | 11.7 | 5.6 | 5.0 | – | – | – | 1.1 | 2.0 | – | |||
| Figure 6a | OS-56-22 | 185 | 14 | 20.3 | 47.1 | 16.5 | 0.4 | 3.0 | – | – | – | 0.7 | 0.4 | – |
| 15 | 11.9 | 75.8 | 4.5 | 5.9 | 1.2 | 0.3 | – | – | – | – | P | |||
| 16 | 13.5 | 77.8 | 0.6 | 7.2 | – | 0.3 | – | – | – | – | P | |||
| Figure 6b | OS-82-01 | 160 | 17 | 16.8 | 58.4 | 12.3 | 9.2 | 2.1 | – | – | – | 0.3 | 0.4 | Na, Cl |
| 18 | 13.9 | 67.2 | 8.2 | 8.3 | 1.9 | – | – | – | 0.3 | 0.2 | – | |||
| Figure 6c | OS-82-01 | 160 | 19 | 29.5 | 58.5 | 0.8 | 2.1 | – | 0.3 | – | – | 0.3 | 1.4 | P |
| 20 | 10.0 | 86.9 | 0.7 | 1.2 | – | – | – | – | – | 0.2 | P | |||
| Figure 6d | OS-56-22 | 185 | 21 | 39.1 | 3.0 | 24.8 | 19.5 | 9.8 | 0.2 | – | – | 0.9 | 0.5 | F |
| Inclusion Type | Size, μm | Characteristic |
|---|---|---|
| Hercynite and Al oxides | 3–20 | Irregular triangular shape, can be separated or a part of complex inclusions |
| Fe-Mn-Si-Al (Ca or Mg) oxides | 20–25 | Usually large endogenous globular inclusions |
| Fe-Mn-Si oxides | 2–3 | Small globular inclusions, can be either separate or agglomerated into clusters |
| Fe-Si oxides | 3–7 | Relatively globular inclusions usually are presented in clusters |
| Fe oxides | 12–15 | Contain a small number of impurities, granular or globular |
| Fluorides, phosphates | 2–4 | Rare and often associated with the breakdown of heat-resistant phyllosilicates |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhabrev, L.; Kurushkin, D.; Mushnikov, I.; Shamshurin, A.; Chuppina, S.; Panchenko, O. Composition of Organosilicate Coatings High-Temperature Breakdown Products and Their Distribution in the Weld. Materials 2022, 15, 699. https://doi.org/10.3390/ma15030699
Zhabrev L, Kurushkin D, Mushnikov I, Shamshurin A, Chuppina S, Panchenko O. Composition of Organosilicate Coatings High-Temperature Breakdown Products and Their Distribution in the Weld. Materials. 2022; 15(3):699. https://doi.org/10.3390/ma15030699
Chicago/Turabian StyleZhabrev, Leonid, Dmitry Kurushkin, Igor Mushnikov, Aleksey Shamshurin, Svetlana Chuppina, and Oleg Panchenko. 2022. "Composition of Organosilicate Coatings High-Temperature Breakdown Products and Their Distribution in the Weld" Materials 15, no. 3: 699. https://doi.org/10.3390/ma15030699
APA StyleZhabrev, L., Kurushkin, D., Mushnikov, I., Shamshurin, A., Chuppina, S., & Panchenko, O. (2022). Composition of Organosilicate Coatings High-Temperature Breakdown Products and Their Distribution in the Weld. Materials, 15(3), 699. https://doi.org/10.3390/ma15030699







