Preparation of Allyl Alcohol Oligomers Using Dipicolinate Oxovanadium(IV) Coordination Compound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
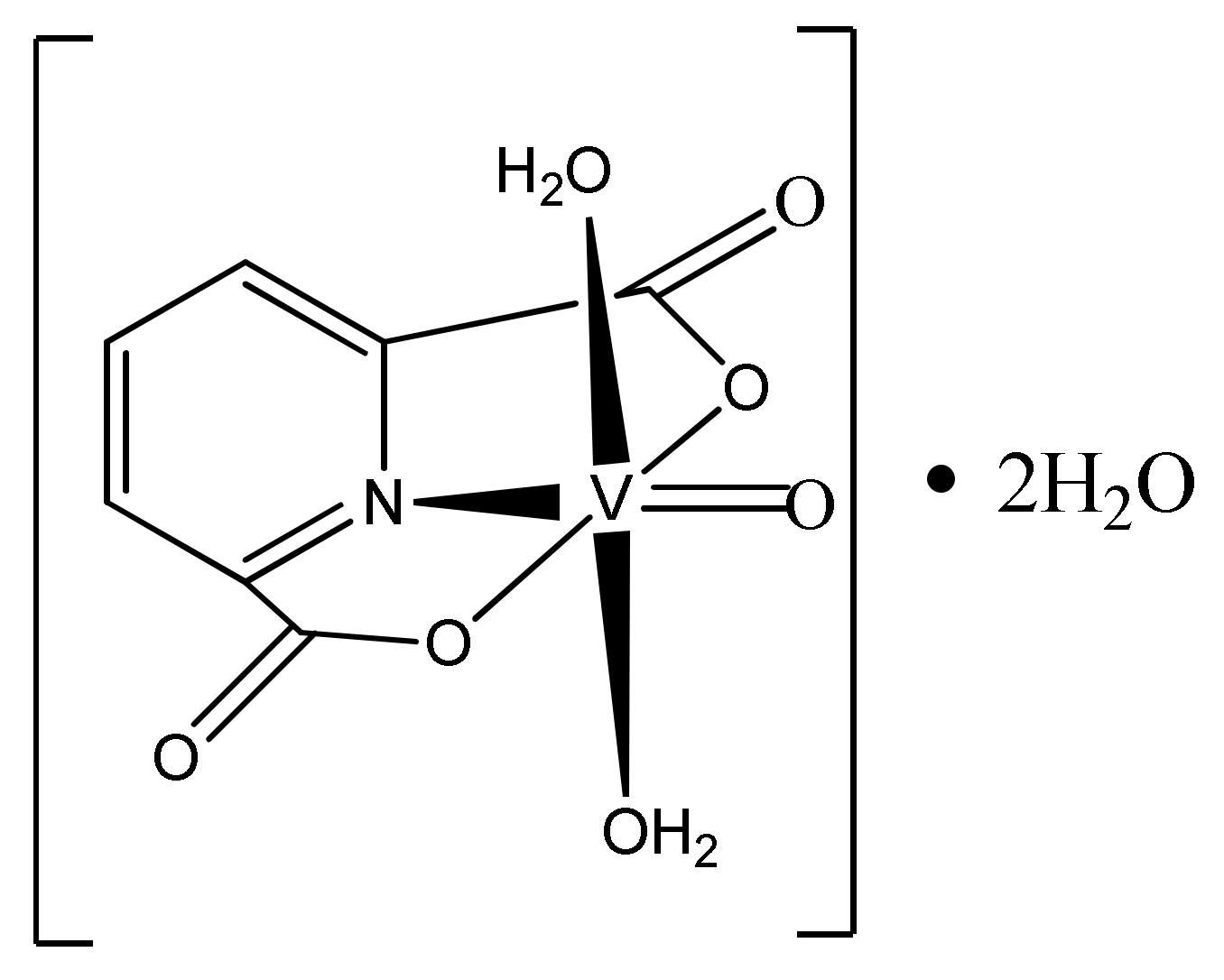
2.2. Dipicolinate Oxovanadium(IV) Complex Synthesis
2.3. Elemental Analysis of the Oxovanadium(IV) Complex Compound with Pyridine-2,6-Dicarboxylate Anion
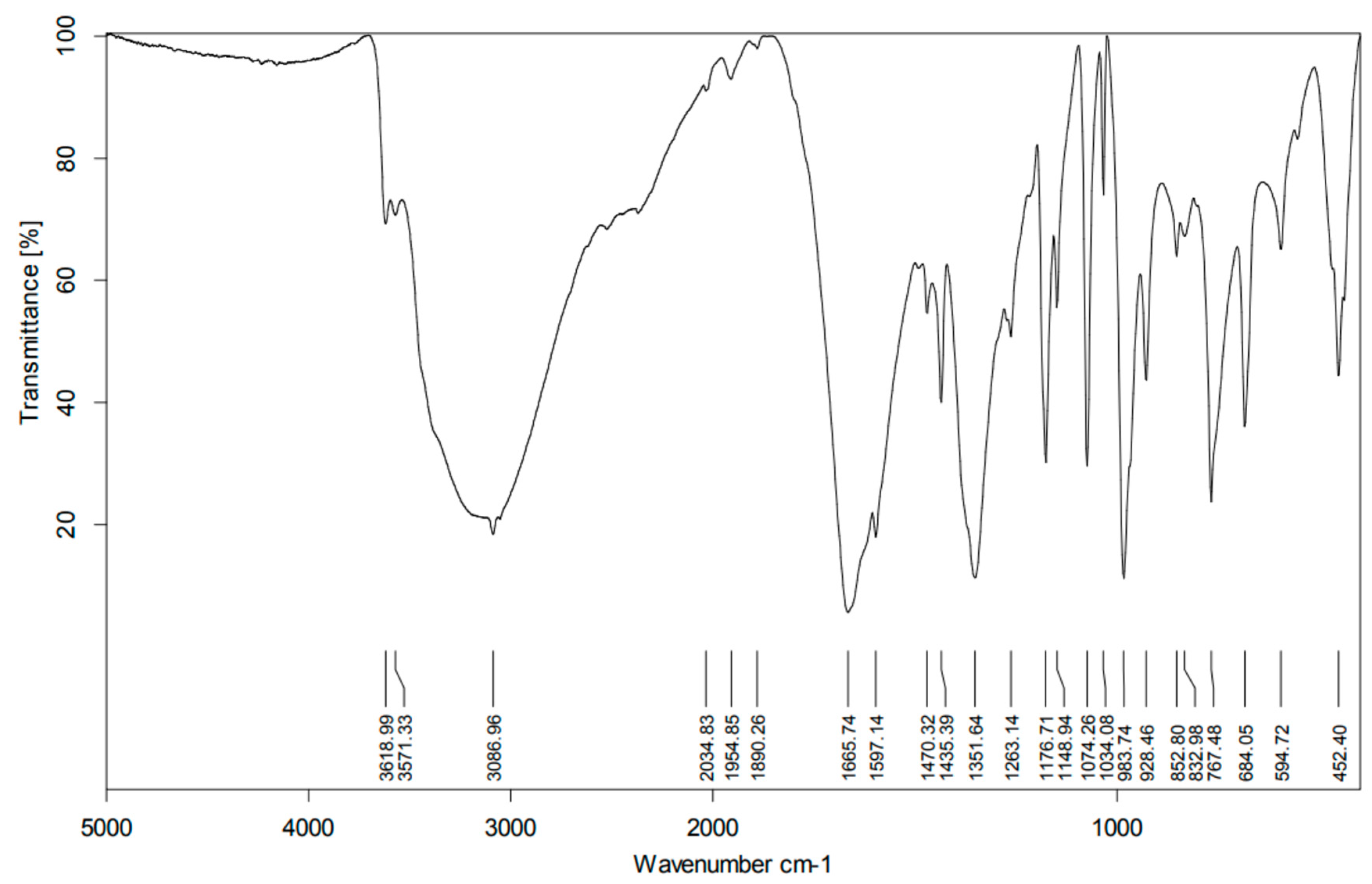
2.4. Infrared (IR) Spectra
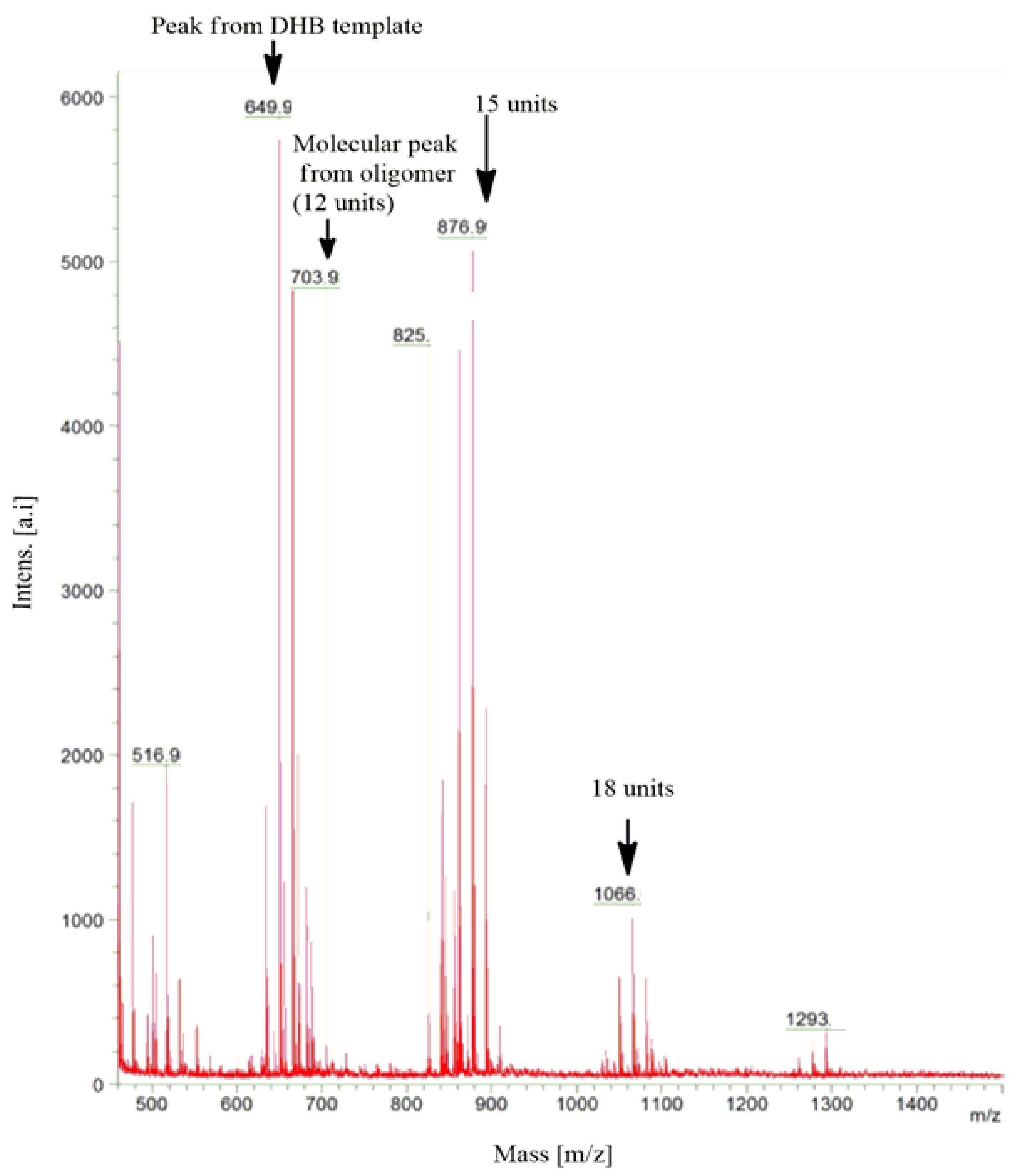
2.5. Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF-MS) Spectra
2.6. Nuclear Magnetic Resonance (NMR) Spectra
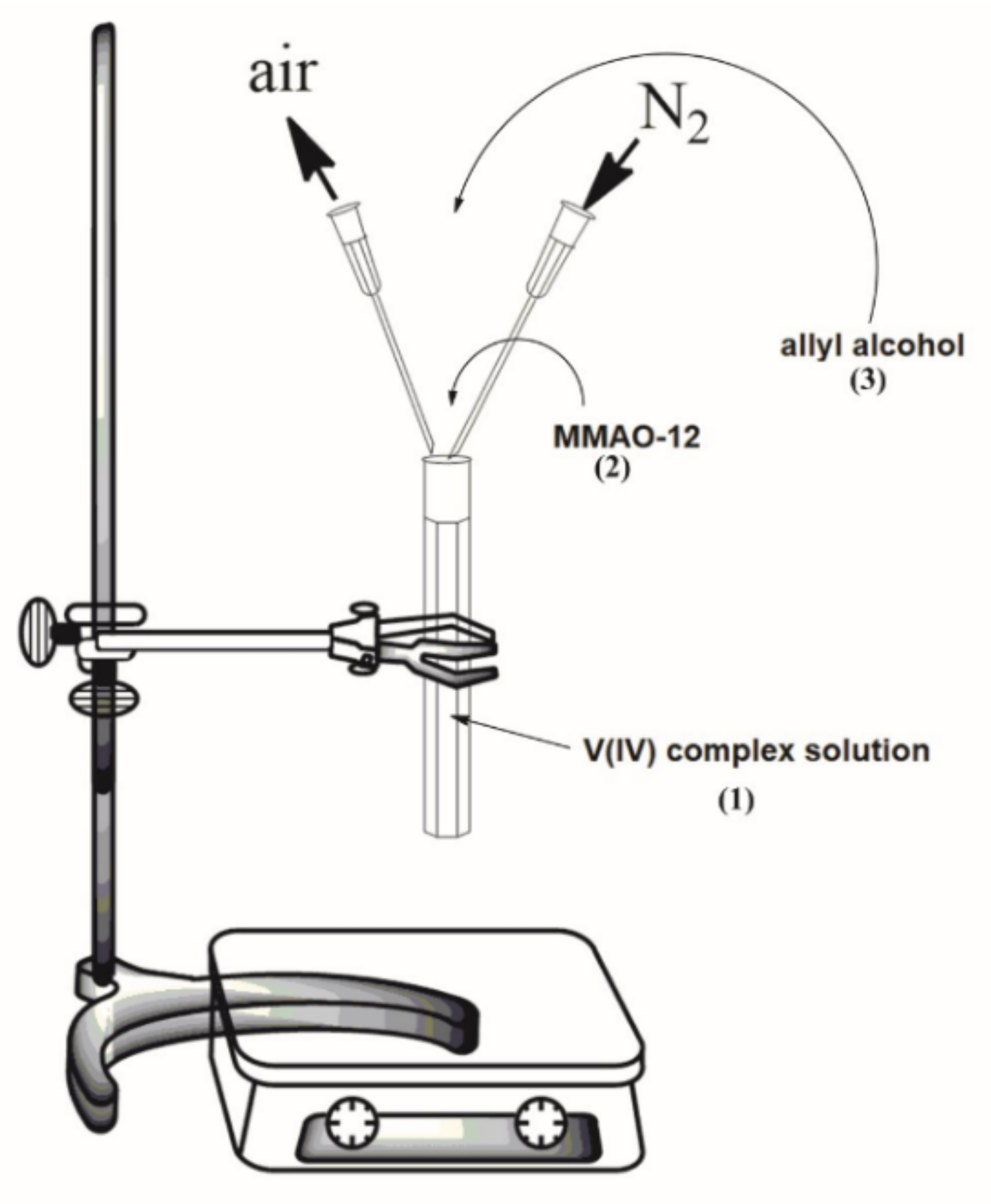
2.7. The Oligomerization Process
3. Results and Discussion
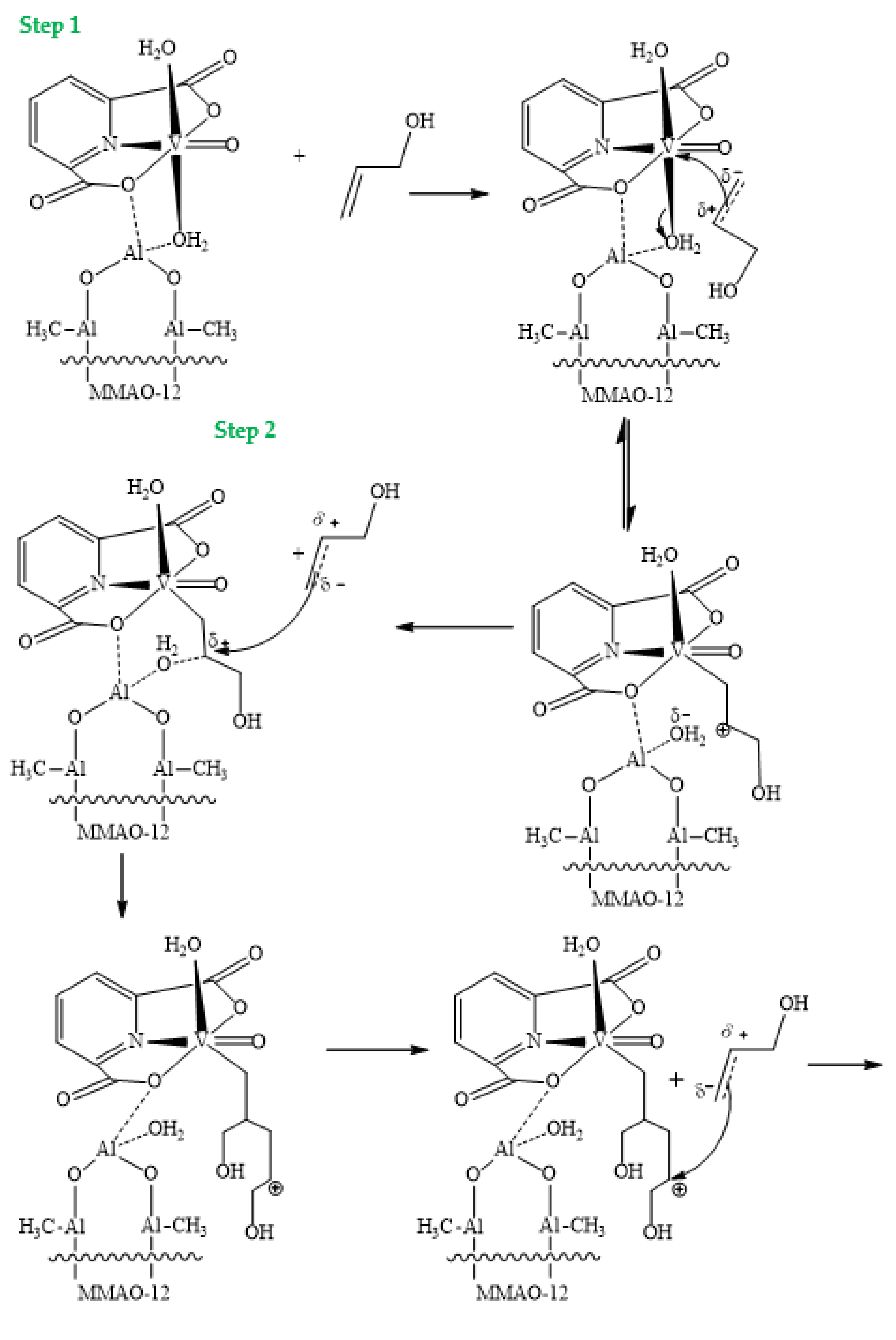
4. The Proposed Mechanism of the Oligomerization Reaction of 2-Propen-1-ol Catalyzed by [VO(dipic)(H2O)2] 2 H2O + MMAO-12
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Verma, A.; Budiyal, L.; Sanjay, M.R.; Siengchin, S. Processing and characterization analysis of pyrolyzed oil rubber (from waste tires)-epoxy polymer blend composite for lightweight structures and coatings applications. Polym. Eng. Sci. 2019, 59, 2041–2051. [Google Scholar] [CrossRef]
- Baricevic, A.; Pezer, A.; Rukavina, M.J.; Serdar, M.; Stirmer, N. Effect of polymer fibers recycled from waste tires on properties of wet-sprayed concrete. Constr. Build. Mater. 2018, 176, 135–144. [Google Scholar] [CrossRef]
- Rezić, I.; Haramina, T.; Rezić, T. Metal nanoparticles and carbon nanotubes—Perfect antimicrobial nano-fillers in polymer-based food packaging materials. In Food Packaging: Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 7, pp. 497–532. [Google Scholar]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rößler, F.; Günther, K.; Lasagni, A.F. In-volume structuring of a bilayered polymer foil using direct laser interference patterning. Appl. Surf. 2018, 440, 1166–1171. [Google Scholar] [CrossRef]
- Passlack, U.; Simon, N.; Buche, V.; Harendt, C.; Stieglitz, T.; Burghartz, J.N. Investigation of long-term stability of hybrid systems-in-foil (HySiF) for biomedical applications. In Proceedings of the 2020 IEEE 8th Electronics System-Integration Technology Conference (ESTC), Tønsberg, Norway, 15–18 September 2020; pp. 1–6. [Google Scholar]
- Firozjaii, A.M.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2020, 6, 115–122. [Google Scholar] [CrossRef]
- Zhou, W.; Xin, C.; Chen, S.; Yu, Q.; Wang, K. Polymer-enhanced foam flooding for improving heavy oil recovery in thin reservoirs. Energy Fuels 2020, 34, 4116–4128. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Al-Obaidi, A.; Kunke, A.; Kräusel, V. Hot single-point incremental forming of glass-fiber-reinforced polymer (PA6GF47) supported by hot air. J. Manuf. Process 2019, 43, 17–25. [Google Scholar] [CrossRef]
- Xu, J.; Merlier, F.; Avalle, B.; Vieillard, V.; Debré, P.; Haupt, K.; Tse Sum Bui, B. Molecularly imprinted polymer nanoparticles as potential synthetic antibodies for immunoprotection against HIV. ACS Appl. Mater. Interfaces 2019, 11, 9824–9831. [Google Scholar] [CrossRef] [PubMed]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef] [Green Version]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, B.R.; Genjo, T.; Bekier, M., II; Cox, S.J.; Stoddard, A.K.; Ivanova, M.; Yasuhara, K.; Fierke, C.A.; Wang, Y.; Ramamoorthy, A. Alzheimer’s amyloid-beta intermediates generated using polymer-nanodiscs. Commun. Chem. 2018, 54, 12883–12886. [Google Scholar] [CrossRef]
- Carradori, D.; Balducci, C.; Re, F.; Brambilla, D.; Le Droumaguet, B.; Flores, O.; Gaudin, A.; Mura, S.; Forloni, G.; Ordoñez-Gutierrez, L.; et al. Antibody-functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s disease-like transgenic mouse model. Nanomedicine 2018, 14, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-E.; Lim, S.; Pang, Y.-L.; Ong, H.-C.; Lee, K.-T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energ. Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Putra, M.D.; Irawan, C.; Udiantoro; Ristianingsih, Y.; Nata, I.F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Clean. Prod. 2018, 195, 1249–1258. [Google Scholar] [CrossRef]
- Shen, Z.; Cao, M.; Zhang, Z.; Pu, J.; Zhong, C.; Li, J.; Ma, H.; Li, F.; Zhu, J.; Pan, F.; et al. Efficient Ni2Co4P3 nanowires catalysts enhance ultrahigh–loading lithium–sulfur conversion in a microreactor–like battery. Adv. Funct. Mater. 2020, 30, 1906661. [Google Scholar] [CrossRef]
- Gawdzik, B.; Kamizela, A.; Szyszkowska, A. Lactones with a fragrance properties. Chemist 2015, 69, 346–349. [Google Scholar]
- Kamizela, A.; Gawdzik, B.; Urbaniak, M.; Lechowicz, Ł.; Białońska, A.; Gonciarz, W.; Chmiela, M. Synthesis, characterization, cytotoxicity, and antibacterial properties of trans-γ-Halo-δ-lactones. ChemistryOpen 2018, 7, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Drzeżdżon, J.; Piotrowska-Kirschling, A.; Malinowski, J.; Kloska, A.; Gawdzik, B.; Chmurzyński, L.; Jacewicz, D. Antimicrobial, cytotoxic, and antioxidant activities and physicochemical characteristics of chromium(III) complexes with picolinate, dipicolinate, oxalate, 2, 2′-bipyridine, and 4, 4′-dimethoxy-2, 2′-bipyridine as ligands in aqueous solutions. J. Mol. Liq. 2019, 282, 441–447. [Google Scholar] [CrossRef]
- Gawdzik, B.; Iwanek, W. Synthesis, structure, and stereochemistry of the bora derivatives of 1-[(2-hydroxy-1-naphthyl) methyl] proline. Tetrahedron Asymmetry 2005, 16, 2019–2023. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Sikorski, A.; Chmurzyński, L.; Jacewicz, D. New type of highly active chromium(III) catalysts containing both organic cations and anions designed for polymerization of beta-olefin derivatives. Sci.Rep. 2018, 8, 2315. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef]
- Vitorino, M.J.; Devic, T.; Tromp, M.; Férey, G.; Visseaux, M. Lanthanide Metal-Organic Frameworks as Ziegler–Natta Catalysts for the Selective Polymerization of Isoprene. Chem. Phys. 2009, 210, 1923–1932. [Google Scholar] [CrossRef]
- Kayda, A.S.; Rumyantsev, A.V.; Zubkevich, S.V.; Zhizhko, P.A.; Takazova, R.U.; Tuskaev, V.A.; Gagieva, S.C.; Buzin, M.I.; Shatokhin, S.S.; Nikiforova, G.G.; et al. Vanadium(V) imido chlorides and n-propoxides—Towards a rational design of vanadium imido precatalysts for ethylene polymerization. J. Organomet. Chem. 2021, 934, 121665. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ghosh, S.; Nandi, A.K. X-ray crystal structure of [VO(DPA)(H2O) 2]· 2H2O (DPA = Dipicolinate dianion). Transit. Met. Chem. 1999, 24, 183–185. [Google Scholar] [CrossRef]
- Rončević, S.; Nemet, I.; Ferri, T.Z.; Matković-Čalogović, D. Characterization of nZVI nanoparticles functionalized by EDTA and dipicolinic acid: A comparative study of metal ion removal from aqueous solutions. RSC Adv. 2019, 9, 31043–31051. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, P.; Ghosh, S.; Mak, T.C. A new route for the synthesis of bis(pyridine dicarboxylato) bis(triphenylphosphine) complexes of ruthenium(II) and X-ray structural characterisation of the biologically active trans-[Ru(PPh3)2(L1H)2](L1H2= pyridine 2, 3-dicarboxylic acid). Polyhedron 2001, 20, 975–980. [Google Scholar] [CrossRef]
- Wu, J.-Q.; Li, Y.-S. Well-defined vanadium complexes as the catalysts for olefin polymerization. Coord. Chem. Rev. 2011, 255, 2303–2314. [Google Scholar] [CrossRef]
- Buglyó, P.; Crans, D.C.; Nagy, E.M.; Lindo, R.L.; Yang, L.; Smee, J.J.; Jin, W.; Chi, L.-H.; Godzala, M.E.; Willsky, G.R. Aqueous chemistry of the vanadiumIII (VIII) and the VIII− dipicolinate systems and a comparison of the effect of three oxidation states of vanadium compounds on diabetic hyperglycemia in rats. Inorg. Chem. 2005, 44, 5416–5427. [Google Scholar] [CrossRef]
- Yue, Z.; Xiaoda, Y.; Kui, W. Permeation of vanadium (III, IV, V)-dipicolinate complexes across MDCK cell monolayer and comparison with Caco-2 cells. Chin. Sci. Bull. 2005, 50, 1854–1859. [Google Scholar] [CrossRef]
- Pranczk, J.; Jacewicz, D.; Wyrzykowski, D.; Wojtczak, A.; Tesmar, A.; Chmurzynski, L. Crystal structure, antioxidant properties and characteristics in aqueous solutions of the oxidovanadium(IV) complex [VO(IDA)phen] · 2H2O. Eur. J. Inorg. 2015, 2015, 3343–3349. [Google Scholar] [CrossRef]
- Guan, T.S.; Hee, N.C.; Lai, T.F.; Khoon, L.E.; Mansor, S.M.; Balraj, P.; Chu, T.L.; Yamin, B.M.; Ng, S.W. Oxovanadium(IV) dipicolinate: Structure nucleolytic and anticancer property. Mod. Appl. Sci 2008, 2, 117. [Google Scholar] [CrossRef] [Green Version]
- Pobłocki, K.; Drzeżdżon, J.; Kostrzewa, T.; Jacewicz, D. Coordination complexes as a new generation photosensitizer for photodynamic anticancer therapy. Int. J. Moc. Sci 2021, 22, 8052. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal-complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Gawdzik, B.; Drzeżdżon, J.; Siarhei, T.; Sikorski, A.; Malankowska, A.; Kowalczyk, P.; Jacewicz, D. Catalytic activity of new oxovanadium (IV) microclusters with 2-phenylpyridine in olefin oligomerization. Materials 2021, 14, 7670. [Google Scholar] [CrossRef]
- Malinowski, J.; Jacewicz, D.; Gawdzik, B.; Drzeżdżon, J. New chromium (III)-based catalysts for ethylene oligomerization. Sci. Rep. 2020, 10, 16578. [Google Scholar] [CrossRef] [PubMed]
- Bersted, B.H.; Belford, R.L.; Paul, I.C. Crystal and molecular structure of orthorhombic vanadyl(IV) pyridine-2, 6-dicarboxylate tetrahydrate. Inorg. Chem. 1968, 7, 1557–1562. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Pawlak, M.; Matyka, N.; Sikorski, A.; Gawdzik, B.; Jacewicz, D. Relationship between Antioxidant Activity and Ligand Basicity in the Dipicolinate Series of Oxovanadium(IV) and Dioxovanadium(V) Complexes. Int. J. Mol. Sci. 2021, 22, 9886. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, W.; Smee, J.J.; Baruah, B.; Willsky, G.R.; Crans, D.C. Anti-diabetic effects of vanadium (III, IV, V)–chlorodipicolinate complexes in streptozotocin-induced diabetic rats. Biometals 2009, 22, 895–905. [Google Scholar] [CrossRef]
- Iio, K.; Kobayashi, K.; Matsunaga, M. Radical polymerization of allyl alcohol and allyl acetate. Polym. Adv. Technol. 2007, 18, 953–958. [Google Scholar] [CrossRef]
- Sawada, H.; Tanba, K.I.; Oue, M.; Kawase, T.; Mitani, M.; Minoshima, Y.; Nakajima, H.; Nishida, M.; Moriya, Y. Synthesis and properties of novel fluoroalkylated allyl alcohol oligomers. Polymer 1994, 35, 4028–4030. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Chmurzyński, L.; Jacewicz, D. Geometric isomerism effect on catalytic activities of bis(oxalato) diaquochromates(III) for 2-chloroallyl alcohol oligomerization. J. Chem. Sci. 2018, 130, 116. [Google Scholar] [CrossRef] [Green Version]
- Cossee, P. Ziegler-Natta catalysis I: Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J. Catal. 1964, 3, 80–88. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G.; Cavallo, L. Do new century catalysts unravel the mechanism of stereocontrol of old Ziegler-Natta catalysts? Acc. Chem. Res. 2004, 37, 231–241. [Google Scholar] [CrossRef]
- Allegra, G. Discussion on the mechanism of polymerization of α-olefins with Ziegler-Natta catalysts Macromol. Chem. Phys. 1971, 145, 235–246. [Google Scholar] [CrossRef]

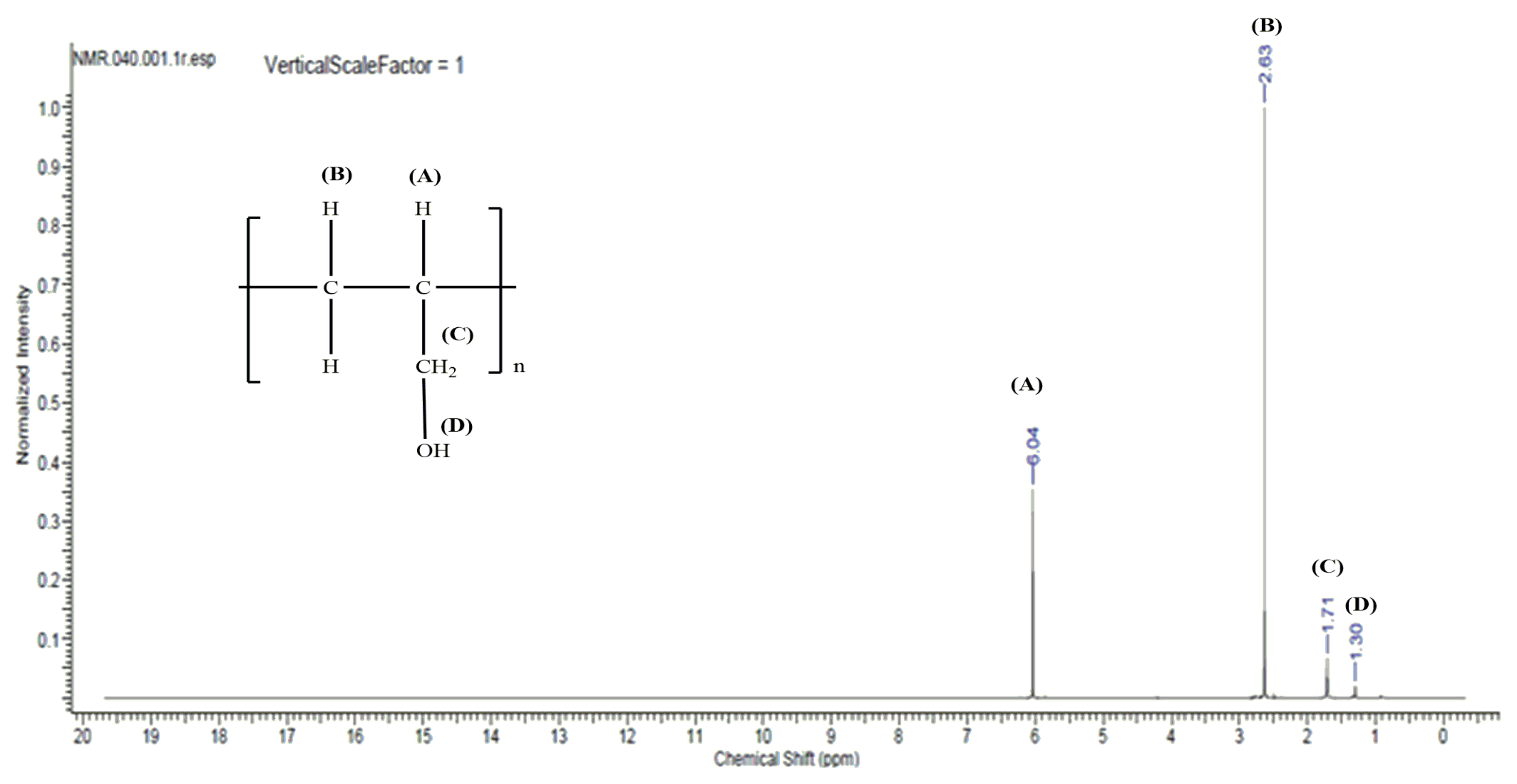


| Complex Compound | Percentage [%] | |||||
|---|---|---|---|---|---|---|
| %C | %H | %N | ||||
| AE | T | AE | T | AE | T | |
| [VO(dipic)(H2O)2] 2 H2O | 27.64 | 27.46 | 3.60 | 4.25 | 4.70 | 4.58 |
| Wavenumber [cm−1] | Type of Vibration with Function Group |
|---|---|
| 3571 | v(OH) |
| 1665 | v(COO) of dipic |
| 1352 | v(COO) of dipic |
| 983 | V=O stretching frequency |
| 452 | stretching vibration of the V-N |
| Wavenumber [cm−1] | Type of Vibration | Function Group |
|---|---|---|
| 3425 | stretching vibrations | −OH |
| 2992 | stretching vibrations | −CH |
| 1651 | stretching vibrations | C=C |
| 1438 | bending vibrations | −CH2 |
| Peak Value | Assigned Hydrogen Atoms |
|---|---|
| 6.04 | CH2=CH- (oligomer) |
| 2.63 | CH2=CH- (monomer) |
| 1.71 | HO-CH2- (oligomer) |
| 1.30 | -OH (oligomer) |
| Peak Value | Assigned Carbons Atoms |
|---|---|
| 70.79 | HO-CH2-CH-CH2- (oligomer) |
| 70.76–70.31 | HO-CH2-CH-CH2- (oligomer) |
| 37.64 | -CH2-OH (oligomer) |
| Catalyst Efficiency | Catalytic Activity [g∙mmol−1∙bar−1∙h−1] |
|---|---|
| Very low | <1 |
| Low | 1–10 |
| Moderate | 10–100 |
| High | 100–1000 |
| Very high | >1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pobłocki, K.; Jacewicz, D.; Walczak, J.; Gawdzik, B.; Kramkowski, K.; Drzeżdżon, J.; Kowalczyk, P. Preparation of Allyl Alcohol Oligomers Using Dipicolinate Oxovanadium(IV) Coordination Compound. Materials 2022, 15, 695. https://doi.org/10.3390/ma15030695
Pobłocki K, Jacewicz D, Walczak J, Gawdzik B, Kramkowski K, Drzeżdżon J, Kowalczyk P. Preparation of Allyl Alcohol Oligomers Using Dipicolinate Oxovanadium(IV) Coordination Compound. Materials. 2022; 15(3):695. https://doi.org/10.3390/ma15030695
Chicago/Turabian StylePobłocki, Kacper, Dagmara Jacewicz, Juliusz Walczak, Barbara Gawdzik, Karol Kramkowski, Joanna Drzeżdżon, and Paweł Kowalczyk. 2022. "Preparation of Allyl Alcohol Oligomers Using Dipicolinate Oxovanadium(IV) Coordination Compound" Materials 15, no. 3: 695. https://doi.org/10.3390/ma15030695
APA StylePobłocki, K., Jacewicz, D., Walczak, J., Gawdzik, B., Kramkowski, K., Drzeżdżon, J., & Kowalczyk, P. (2022). Preparation of Allyl Alcohol Oligomers Using Dipicolinate Oxovanadium(IV) Coordination Compound. Materials, 15(3), 695. https://doi.org/10.3390/ma15030695






