Experimental Study on Carbonation Durability of Kaolin Strengthened with Slag Portland Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Sample Preparation
2.2. Experimental Scheme
2.2.1. Carbonation Test (CDT)
2.2.2. Uniaxial Compressive Strength Test (CUST)
2.2.3. XRD and SEM + EDS Tests
3. Results
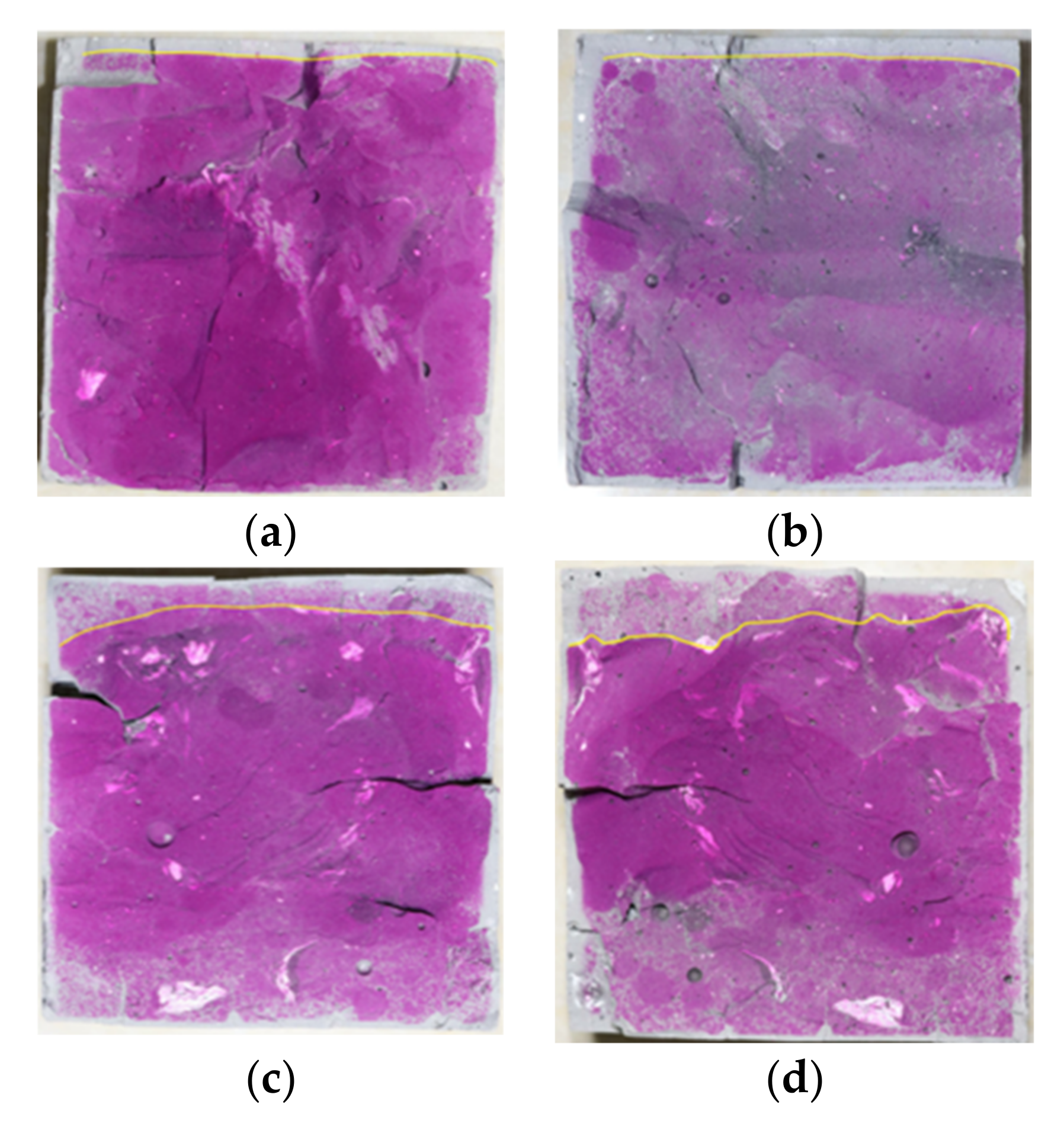
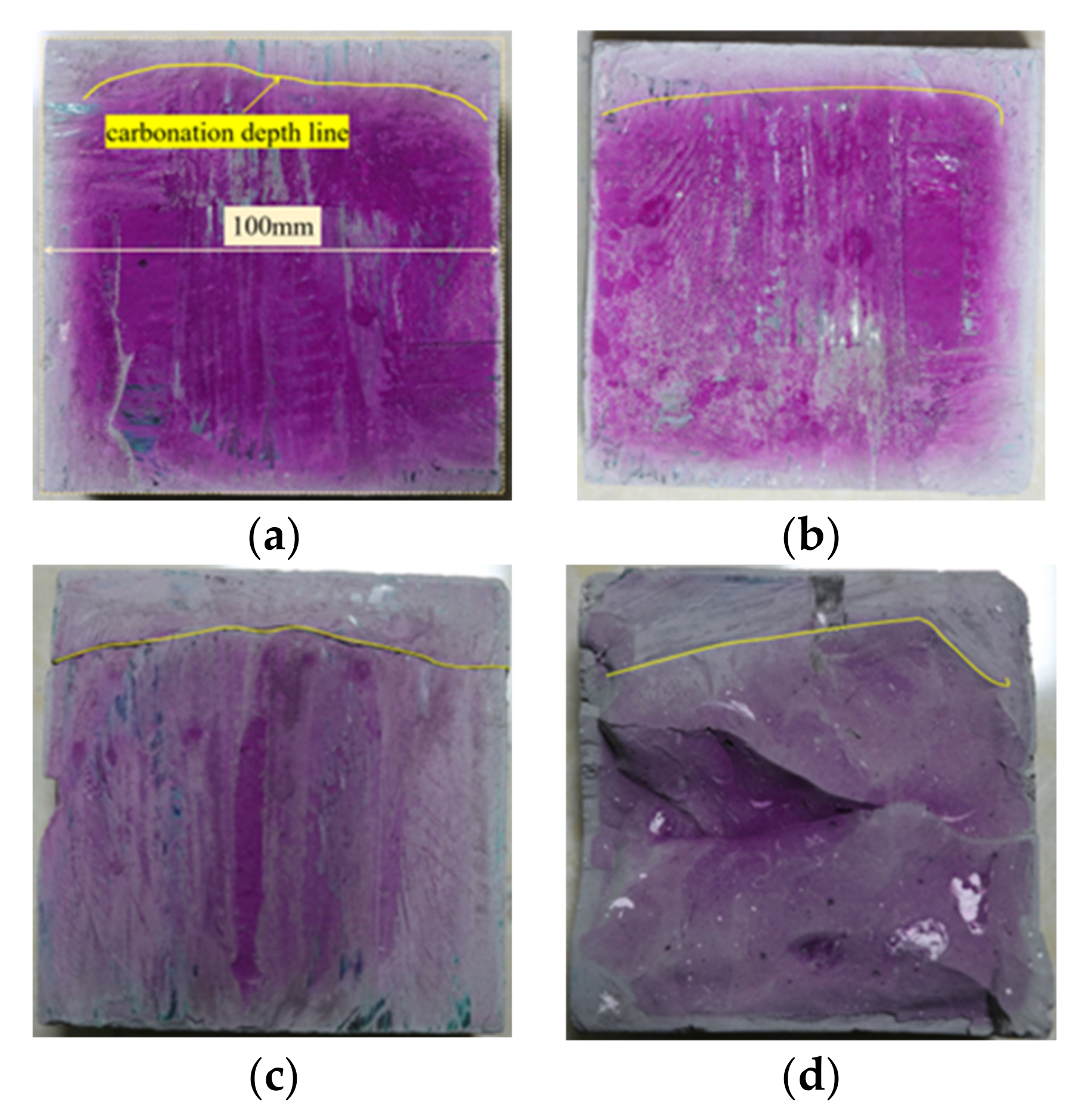
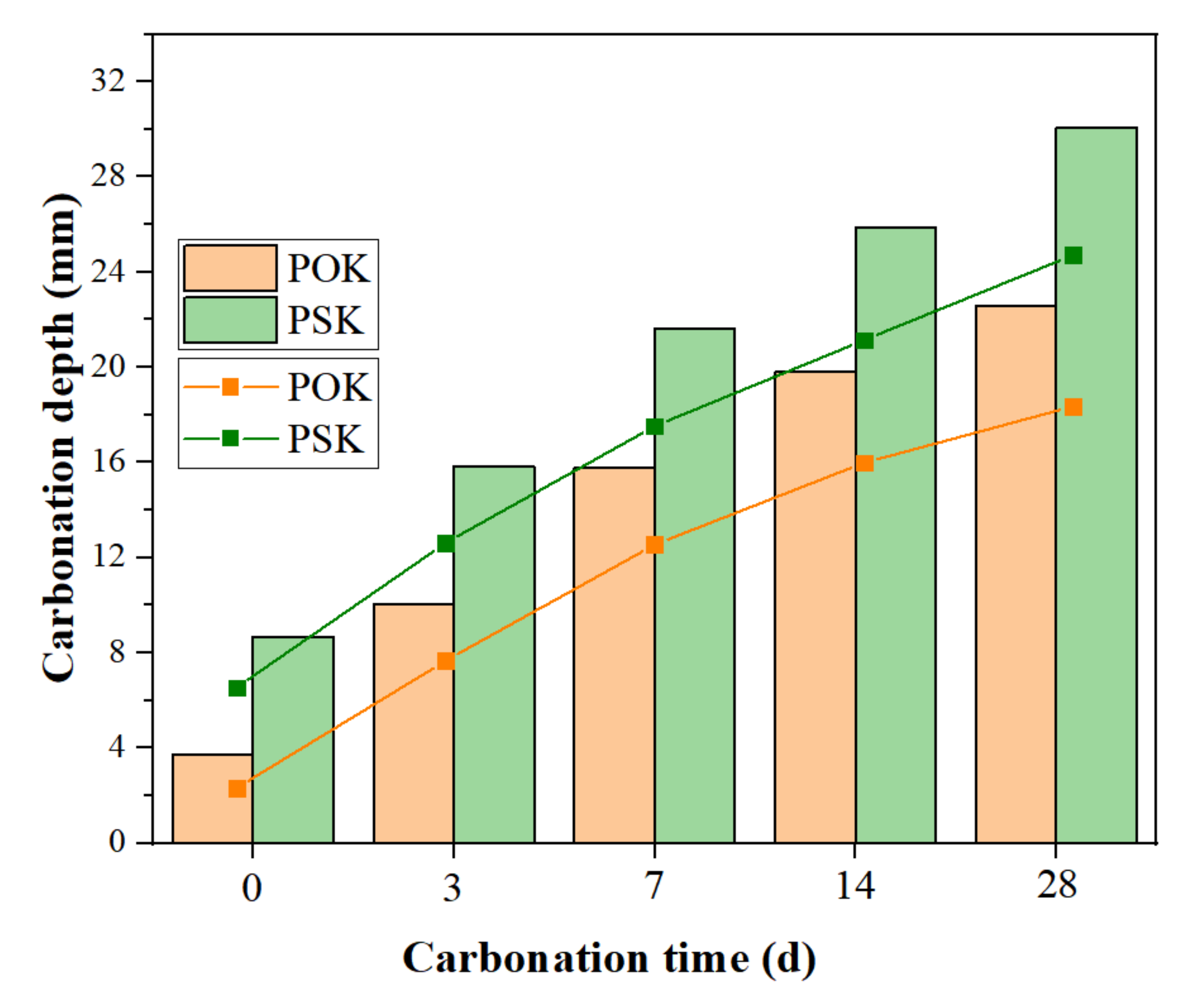
3.1. Analysis of Carbonation Depth Test (CDT) Results
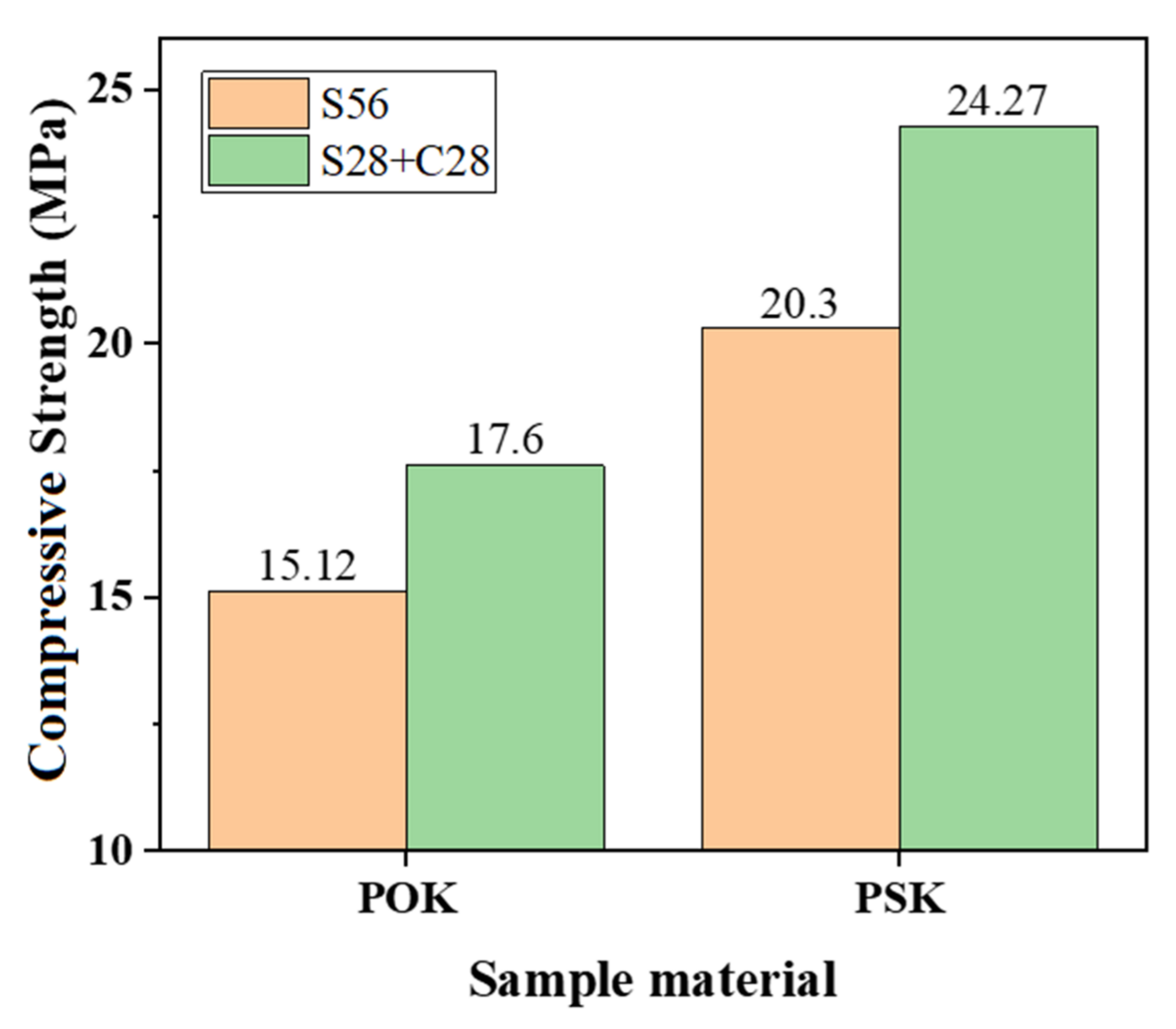
3.2. Analysis of CUST Results
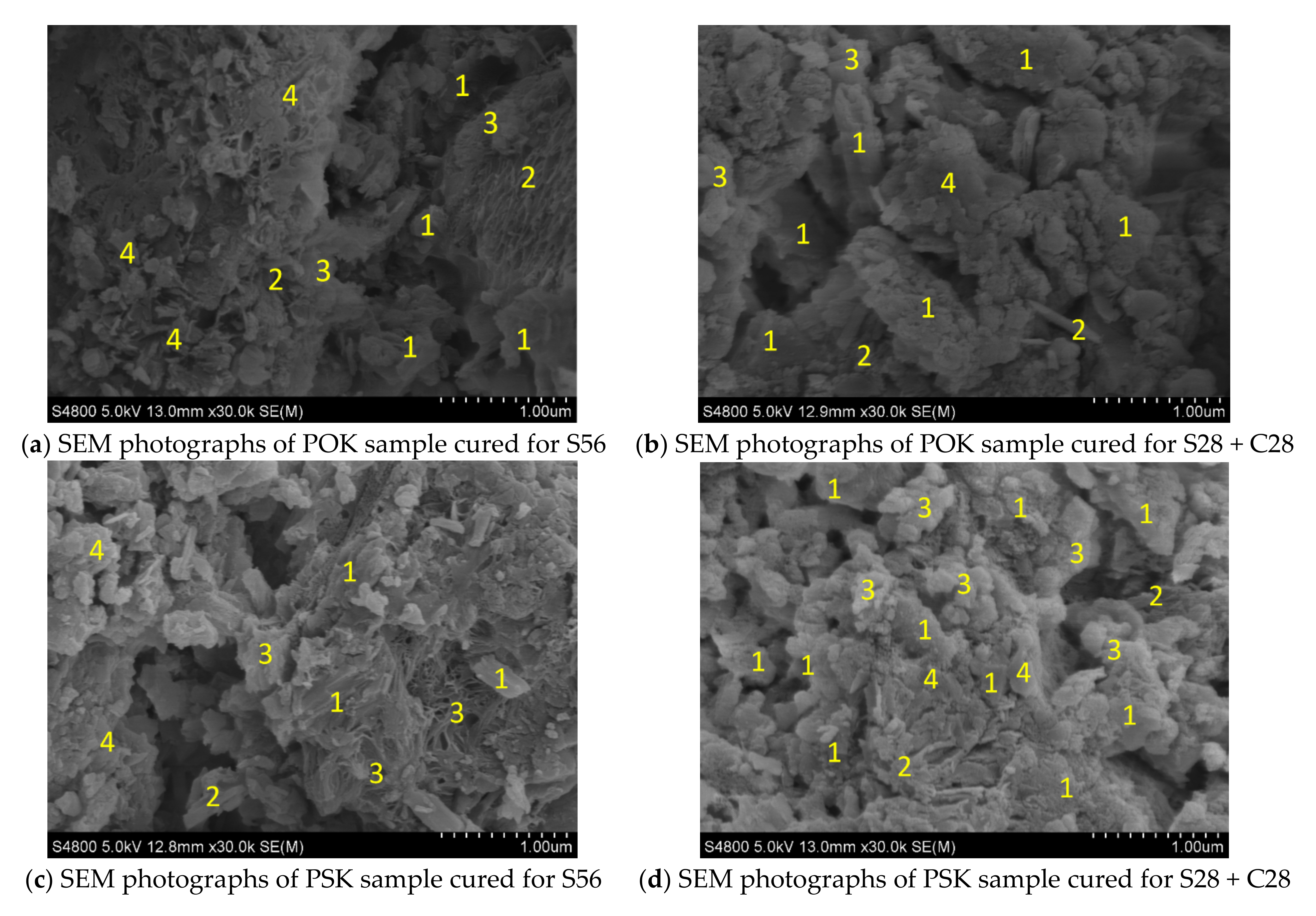
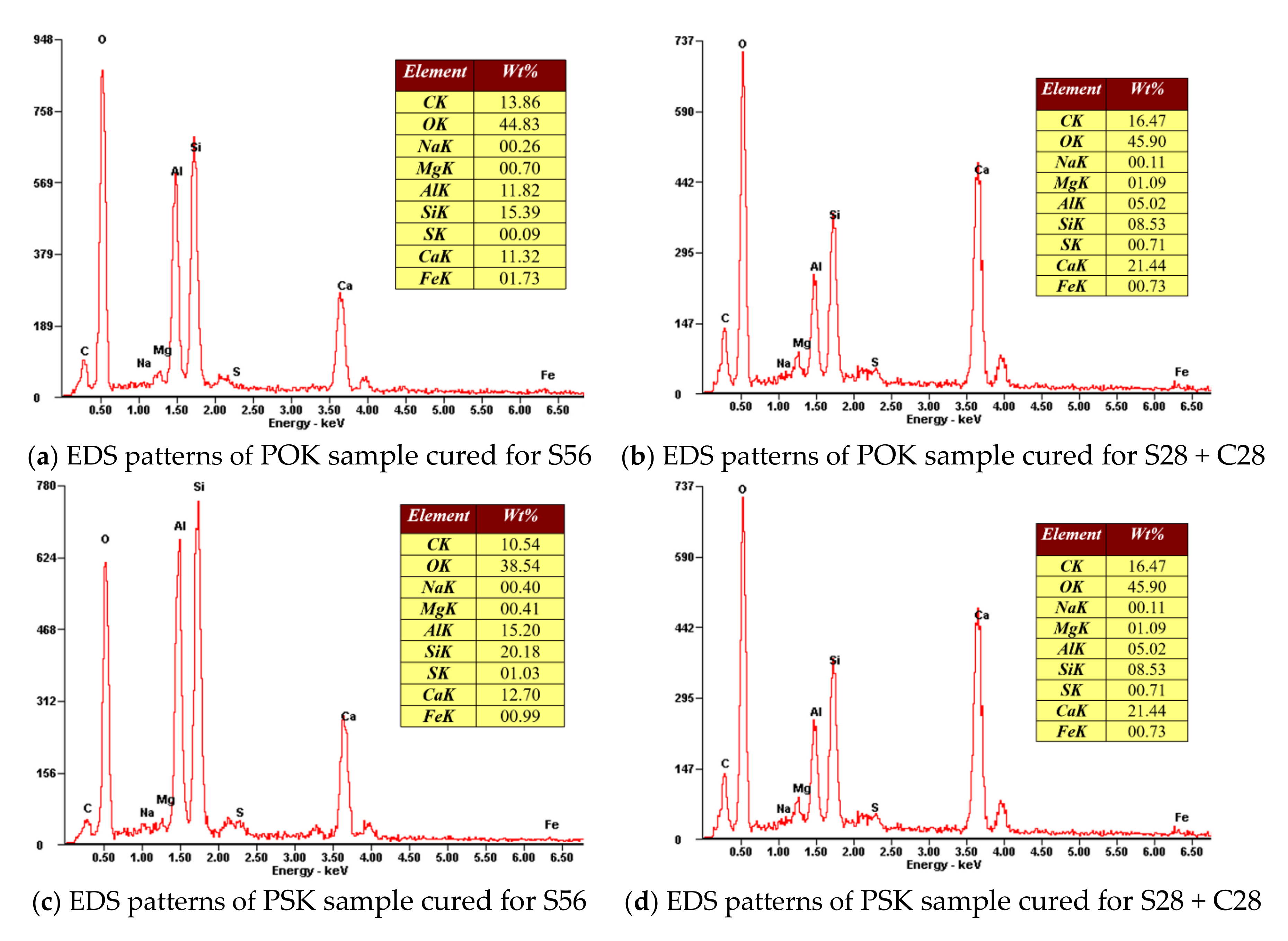
3.3. Analysis of SEM and EDS Data
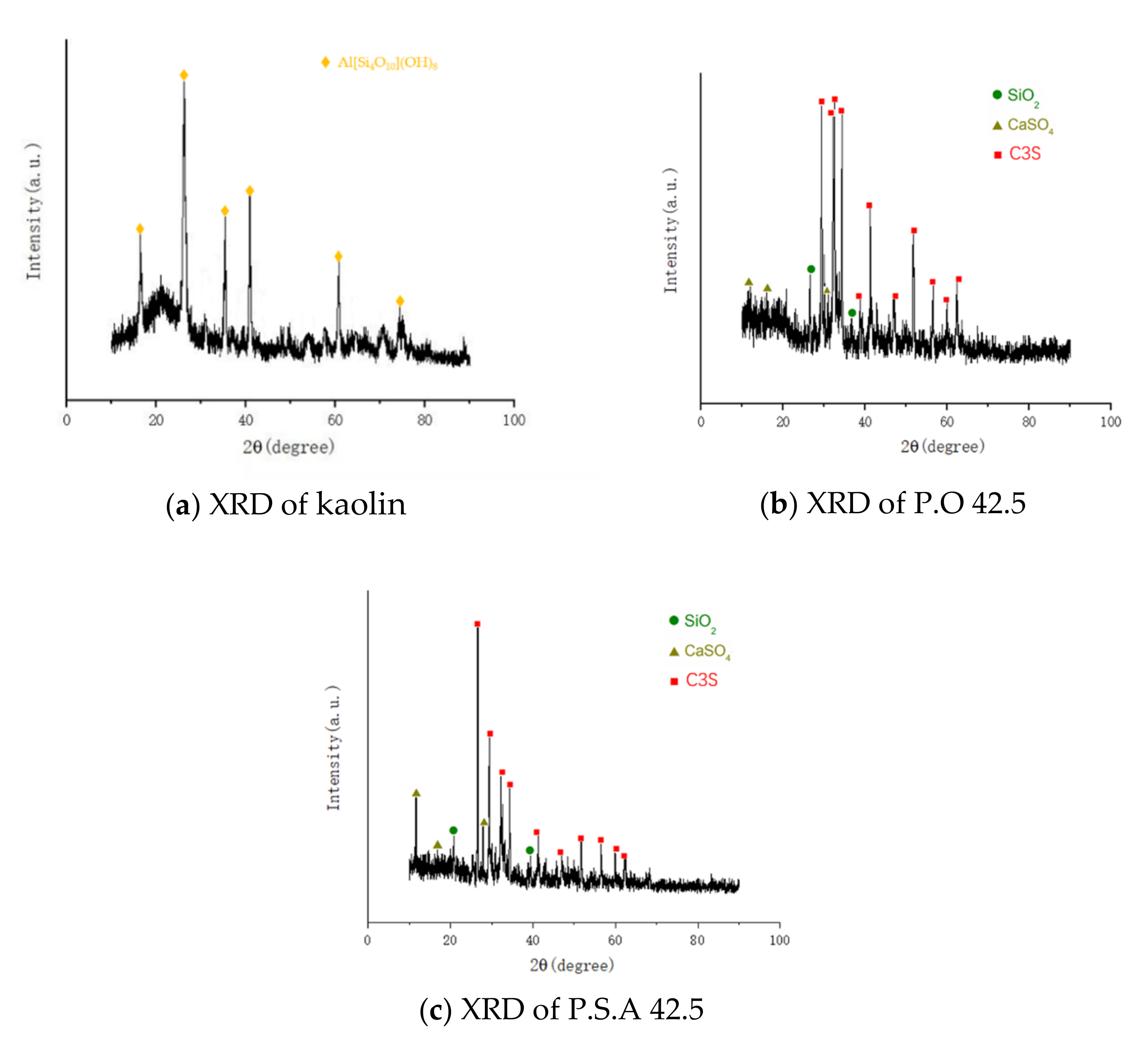
3.4. Analysis of XRD Test Results
4. Discussion and Suggestions
5. Conclusions
- (1)
- The content of OH− was reduced during hydration and carbonation of the PSK samples. The active substances contained in PSK samples can react with the CH produced during cement hydration to reduce the content of OH−. In the S28 + C28 curing environment, OH− in the PSK samples reacts with CO2. Both processes greatly reduce the content of OH−.
- (2)
- The POK and PSK samples were carbonized in the S28 + C28 environment. The carbonation depth in the PSK sample at each curing time was deeper than that of the POK sample, and the color of the phenolphthalein reagent on PSK was significantly lighter than in the case of the POK sample. This shows that the carbonation durability of slag Portland cement is weaker than that of ordinary Portland cement.
- (3)
- The strength of the PSK sample after carbonation was higher than that without carbonation. The strength of the PSK sample after carbonation was higher than that of the POK sample after carbonation. This shows that the carbonation of slag Portland cement-reinforced soil improves its strength.
- (4)
- After comprehensive analysis of SEM, EDS and XRD, the elements and products of POK and PSK samples before and after hydration and carbonation are basically the same. The main products after hydration and carbonation are CaCO3, CaSO4, SiO2, Aft, AFm, CH, C-S-H, C2S, etc. The increase of crystals content such as calcium carbonate and the decrease of calcium hydroxide content are the main factors for the improvement of PSK sample strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanjuán, M.Á.; Argiz, C.; Mora, P.; Zaragoza, A. Carbon dioxide uptake in the roadmap 2050 of the Spanish cement industry. Energies 2020, 13, 3452. [Google Scholar] [CrossRef]
- Kang, G.; Tsuchida, T.; Kim, Y. Strength and stiffness of cement-treated marine dredged clay at various curing stages. Constr. Build. Mater. 2017, 132, 71–84. [Google Scholar] [CrossRef]
- Gyeong-o, K.; Takashi, T.; Athapaththu, A.M.R.G. Engineering behavior of cement-treated marine dredged clay during early and later stages of curing. Eng. Geol. 2016, 209, 163–174. [Google Scholar]
- Pu, S.; Zhu, Z.; Song, W.; Wan, Y.; Wang, H.; Song, S.; Zhang, J. Mechanical and microscopic properties of cement stabilized silt. KSCE J. Civ. Eng. 2020, 24, 2333–2344. [Google Scholar] [CrossRef]
- Kim, A.R.; Chang, I.; Cho, G.C.; Shim, S.H. Strength and dynamic properties of cement-mixed korean marine clays. KSCE J. Civ. Eng. 2018, 22, 1150–1161. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Pan, Y.; Jia, L.; Yi, J.T.; Hu, J.; Wu, C. Strength evaluation of marine clay stabilized by cementitious binder. Mar. Georesources Geotechnol. 2020, 38, 730–743. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, M.Y.; Li, S.L.; Wei, X.J.; Do, T.N. The pore pressure model of cement soil under cyclic loading. Mater. Res. Innov. 2015, 19 (Suppl. S8), 409–415. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, L.; Tang, Q.; Zhang, Y.; Yang, N.; Lu, C. Evaluation of carbonation effects on cement-solidified contaminated soil used in road subgrade. Adv. Mater. Sci. Eng. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Liu, W.; Lin, S.; Li, Y.; Long, W.; Dong, Z.; Tang, L. Slag blended cement paste carbonation under different CO2 concentrations: Controls on mineralogy and morphology of products. Materials 2020, 13, 3404. [Google Scholar] [CrossRef]
- Luo, Z.T.; Wang, Y.; Yang, G.J.; Ye, J.Y.; Zhang, W.S.; Liu, Z.C.; Mu, Y.D. Effect of curing temperature on carbonation behavior of steel slag compacts. Constr. Build. Mater. 2021, 291, 123369. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Estévez, E.; Argiz, C.; Barrio, D.D. Effect of curing time on granulated blast-furnace slag cement mortars carbonation. Cem. Concr. Compos. 2018, 90, 257–265. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C.; Sanjuán, M.Á. Updating carbon storage capacity of spanish cements. Sustainability 2018, 10, 4806. [Google Scholar] [CrossRef] [Green Version]
- Sanjuán, M.Á.; Estévez, E.; Argiz, C. Carbon dioxide absorption by blast-furnace slag mortars in function of the curing intensity. Energies 2019, 12, 2346. [Google Scholar] [CrossRef] [Green Version]
- Sanjuán, M.Á.; Piñeiro, A.; Rodríguez, O. Ground granulated blast furnace slag efficiency coefficient (k value) in concrete. Applications and limits. Mater. Construcción 2011, 61, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Pu, X.C.; Gan, C.C.; He, G.; Bai, G.; Wu, L.X.; Chen, M.Z. Study on durability of Alkali Slag concrete. Concrete 1991, 5, 13–20 + 46. (In Chinese) [Google Scholar]
- Wu, Y.; Shi, K.; Yu, J.; Han, T.; Li, D. Research on strength degradation of soil solidified by steel slag powder and cement in seawater erosion. J. Mater. Civ. Eng. 2020, 32, 4020181. [Google Scholar] [CrossRef]
- Monsif, M.Y.; Liu, J.; Gurpersaud, N. Microstructural analyses of cement-based binders in stabilizing Champlain Sea clay. Geotechnol. Geol. Eng. 2021, 39, 4963–4981. [Google Scholar] [CrossRef]
- Allahverdi, A.; Maleki, A.; Mahinroosta, M. Chemical activation of slag-blended Portland cement. J. Build. Eng. 2018, 18, 76–83. [Google Scholar] [CrossRef]
- Ge, L.; Wang, C.C.; Hung, C.W.; Liao, W.C.; Zhao, H. Assessment of strength development of slag cement stabilized kaolinite. Constr. Build. Mater. 2018, 184, 492–501. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, A.; Deng, Y.; Cui, Y.; Yu, Z.; Yu, C. Strength performance of cement/slag-based stabilized soft clays. Constr. Build. Mater. 2019, 211, 909–918. [Google Scholar] [CrossRef]
- Saafan, M.A.; Etman, Z.A.; Ellakany, D.M. Microstructure and durability of ground granulated blast furnace slag cement Mortars. Iran. J. Sci. Technol. Trans. Civ. Eng. 2020, 45, 1457–1465. [Google Scholar] [CrossRef]
- GB 175-2007; Common Portland Cement. State Administration for Market Regulation and Standardization Administration of the People’s Republic of China: Beijing, China, 2007.
- JGJ/T 233-2011; Specification for Mix Proportion Design of Cement Soil. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2011.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2009.
- Behfarnia, K.; Rostami, M. An assessment on parameters affecting the carbonation of alkali-activated slag concrete. J. Clean. Prod. 2017, 157, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.F.; Han, J.D.; Liu, W.Q.; Wang, S.G.; Han, P. Study on carbonation process of slag cement mortar with large dosage. Bull. Chin. Ceram. Soc. 2015, 34, 591–596. [Google Scholar]
- Shi, Z.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental studies and thermodynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Shi, Z.G.; Shi, C.J.; Wan, S.; Li, N.; Zhang, Z.H. Effect of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to carbonation. Cem. Concr. Res. 2001, 31, 1277–1283. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Vázquez, T. Carbonation process of alkali-activated slag mortars. J. Mater. Sci. 2006, 41, 3071–3082. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasit, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Cambridge, UK, 2014; pp. 319–329. [Google Scholar]
- Lange, L.C.; Hills, C.D.; Poole, A.B. The effect of accelerated carbonation on the properties of cement-solidified waste forms. Waste Manag. 1996, 16, 757–763. [Google Scholar] [CrossRef]
- Lange, L.C.; Hills, C.D.; Poole, A.B. Effect of carbonation on properties of blended and non-blended cement solidified waste forms. J. Hazard. Mater. 1997, 52, 193–212. [Google Scholar] [CrossRef]
- Bernal, S.A. Effect of the activator dose on the compressive strength and accelerated carbonation resistance of alkali silicate-activated slag/metakaolin blended materials. Constr. Build. Mater. 2015, 98, 217–226. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. A reaction engineering approach to the problem of concrete carbonation. Aiche J. 1989, 35, 1639–1650. [Google Scholar] [CrossRef]
- Li, S.F. Image Analysis of Cement Microstructure. Master’s Thesis, University of Jinan, Jinan, China, 2004. [Google Scholar]
- Du, Y.B.; Ge, Y. Effect of metakaolin, glass powder and limestone filler on microstructure and properties of cement paste. J. Build. Mater. 2021. [Google Scholar]
- Li, N.; Farzadnia, N.; Shi, C.J. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Rivera, R.A.; Sanjuán, M.Á.; Martín, D.A.; Costafreda, J.L. Performance of ground granulated blast-furnace slag and coal fly ash ternary portland cements exposed to natural carbonation. Materials 2021, 14, 3239. [Google Scholar] [CrossRef]
- Groves, G.W.; Brough, A.; Richardson, I.G.; Dobson, C.M. Progressive changes in the structure of hardened C3S cement pastes due to carbonation. J. Am. Ceram. Soc. 1991, 74, 2891–2896. [Google Scholar] [CrossRef]
- Zou, R.Z.; Chen, X.T. Kinetic study on carbon dioxide decomposition reaction of ettringite. J. Hebei Inst. Light Chem. Technol. 1991, 1, 42–48. (In Chinese) [Google Scholar]
- Grounds, T.; Midgley, H.G.; Novell, D.V. Carbonation of ettringite by atmospheric carbon dioxide. Thermochim. Acta 1988, 135, 347–352. [Google Scholar] [CrossRef]
- Hadj-sadok, A.; Kenai, S.; Courard, L.; Darimont, A. Microstructure and durability of mortars modified with medium active blast furnace slag. Constr. Build. Mater. 2011, 25, 1018–1025. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of carbonation on alkali-activated slag paste. J. Am. Ceram. Soc. 2006, 89, 3211–3221. [Google Scholar] [CrossRef]

| Material Type | CaO (%) | SiO2 (%) | Al2O3 (%) | SO3 (%) | Fe2O3 (%) | MgO (%) | NaO2 (%) | K2O (%) | Cl (%) | LOI (%) | Specific Surface Area (m2/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaolin | 0.55 | 57.03 | 30.32 | 0.07 | 1.42 | 0.49 | / | / | / | / | / |
| P.S.A 42.5 | 51.81 | 26.48 | 10.09 | 2.54 | 2.58 | 3.96 | / | / | / | 1.33 | 326 |
| P.O 42.5 | 56.23 | 25.09 | 6.02 | 2.13 | 3.87 | 2.38 | 0.46 | 0.59 | 0.05 | 2.55 | 341 |
| Sample Type | Sample Material | Curing Sequence (Time and Sequence) | Test Sequence | |||||
|---|---|---|---|---|---|---|---|---|
| a. Standard Curing | b. Carbonation Curing | a. Phase I Test | b. Phase II Test | |||||
| Time | Quantity | Time | Quantity | Test Type | Test Type | |||
| Sample 1 | P.O 42.5 + Kaolin (POK) | 28 | 12 | 3 | 3 | CDT | / | |
| 7 | 3 | |||||||
| 14 | 3 | |||||||
| 28 | 3 | |||||||
| P.S.A 42.5 + Kaolin (PSK) | 28 | 12 | 3 | 3 | ||||
| 7 | 3 | |||||||
| 14 | 3 | |||||||
| 28 | 3 | |||||||
| Sample 2 | P.O 42.5 + Kaolin (POK) | 56 | 3 | / | / | CUST | XRD | SEM |
| 28 | 3 | 28 | 3 | XRD | SEM | |||
| P.S.A 42.5 + Kaolin (PSK) | 56 | 3 | / | / | XRD | SEM | ||
| 28 | 3 | 28 | 3 | XRD | SEM | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ma, Y.; Wang, F.; Shi, Z.; You, H.; Tian, Y.; Liu, Y.; Hu, Z.; Song, H.; Wang, D.; et al. Experimental Study on Carbonation Durability of Kaolin Strengthened with Slag Portland Cement. Materials 2022, 15, 1240. https://doi.org/10.3390/ma15031240
Wang Q, Ma Y, Wang F, Shi Z, You H, Tian Y, Liu Y, Hu Z, Song H, Wang D, et al. Experimental Study on Carbonation Durability of Kaolin Strengthened with Slag Portland Cement. Materials. 2022; 15(3):1240. https://doi.org/10.3390/ma15031240
Chicago/Turabian StyleWang, Qingbiao, Yiming Ma, Fuqiang Wang, Zhenyue Shi, Hongyue You, Yuanyuan Tian, Yunfei Liu, Zhongjing Hu, Hongxu Song, Dong Wang, and et al. 2022. "Experimental Study on Carbonation Durability of Kaolin Strengthened with Slag Portland Cement" Materials 15, no. 3: 1240. https://doi.org/10.3390/ma15031240
APA StyleWang, Q., Ma, Y., Wang, F., Shi, Z., You, H., Tian, Y., Liu, Y., Hu, Z., Song, H., Wang, D., Sun, Y., Yang, R., & Sun, H. (2022). Experimental Study on Carbonation Durability of Kaolin Strengthened with Slag Portland Cement. Materials, 15(3), 1240. https://doi.org/10.3390/ma15031240








