Reversible Photo-, Thermal-, and pH-Responsive Functionalized Wood with Fluorescence Emission
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
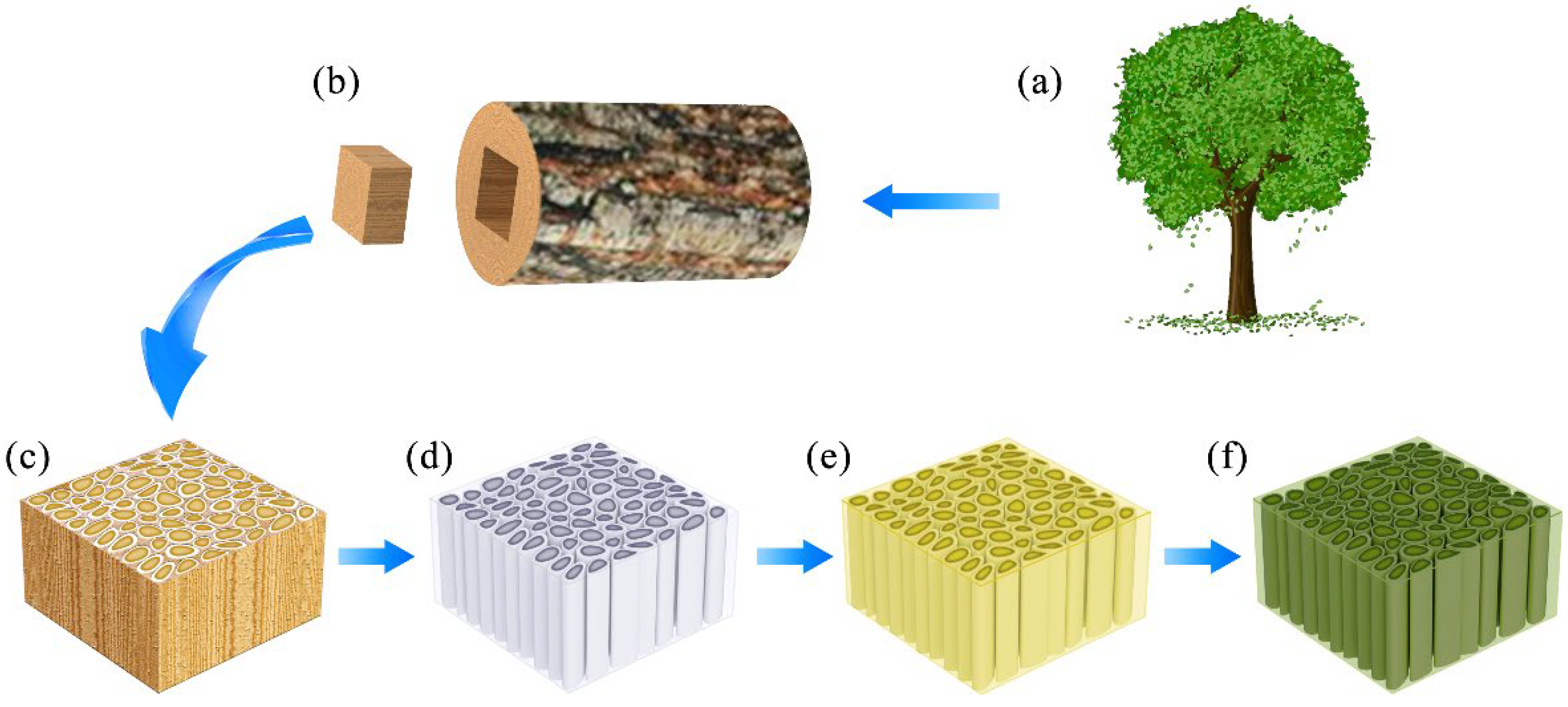
2.2. Preparation of Delignified Wood
2.3. Preparation of FUW
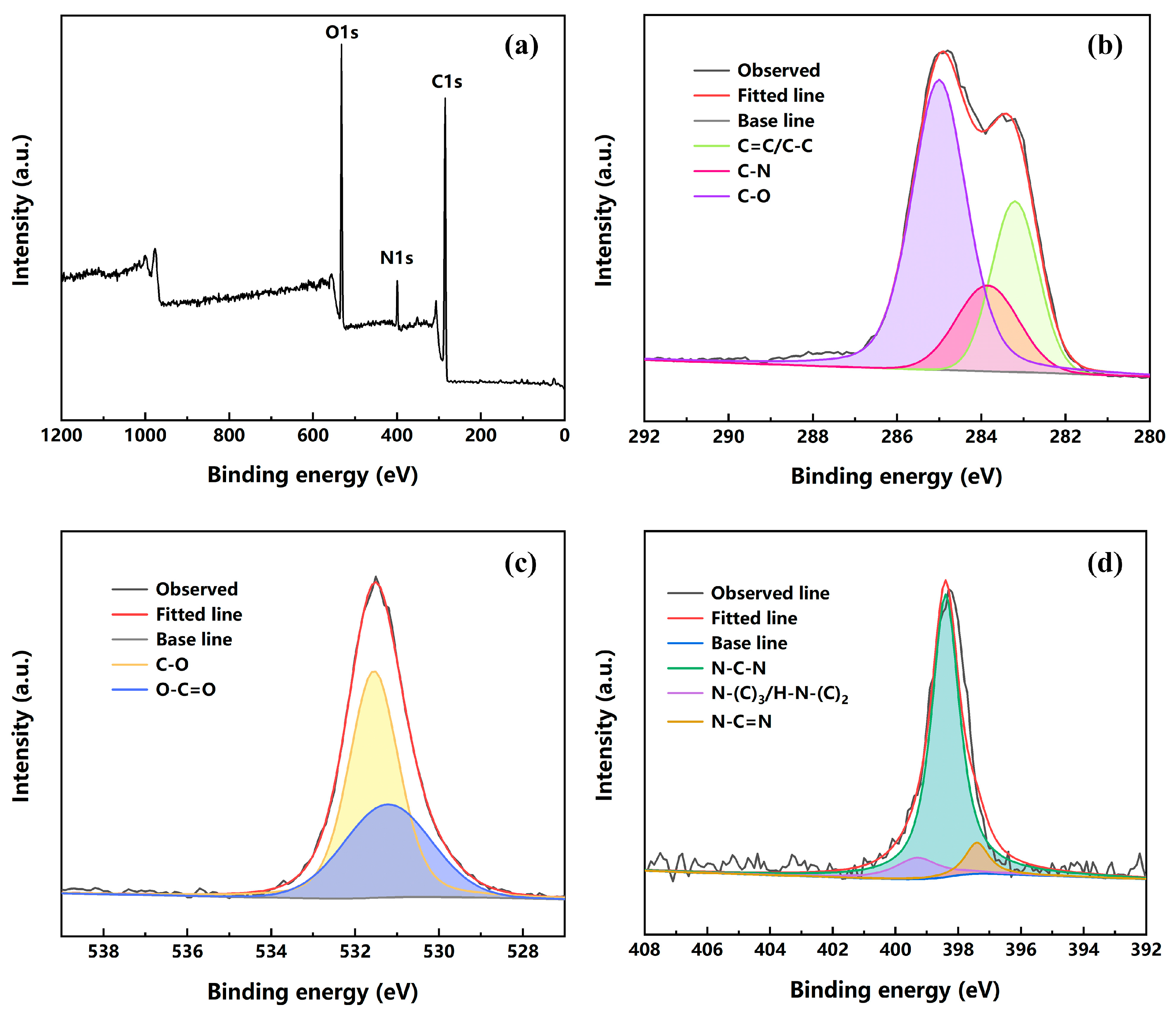
2.4. Characterization
3. Results and Discussion
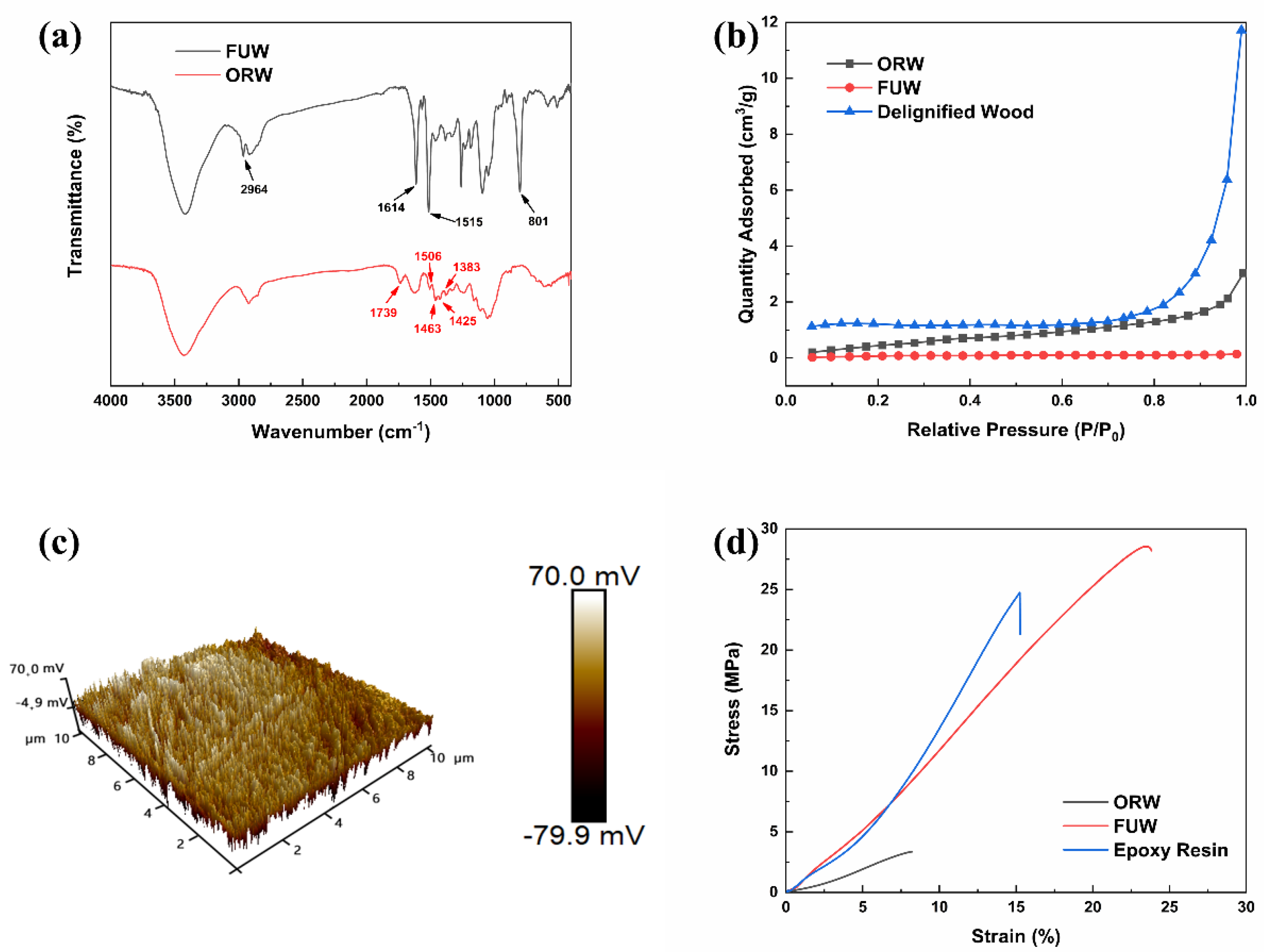
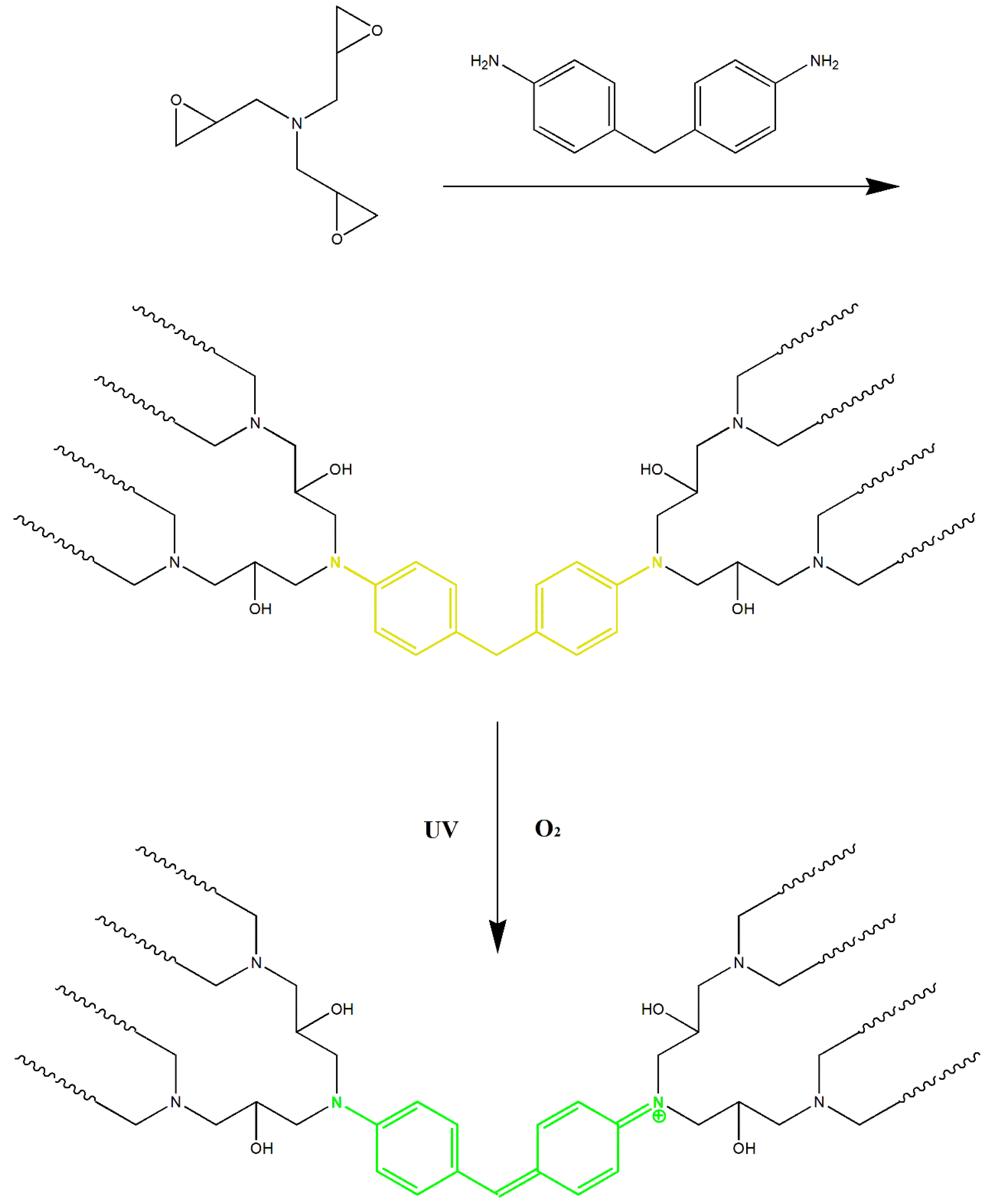
3.1. Preparation and Compatibility of FUW
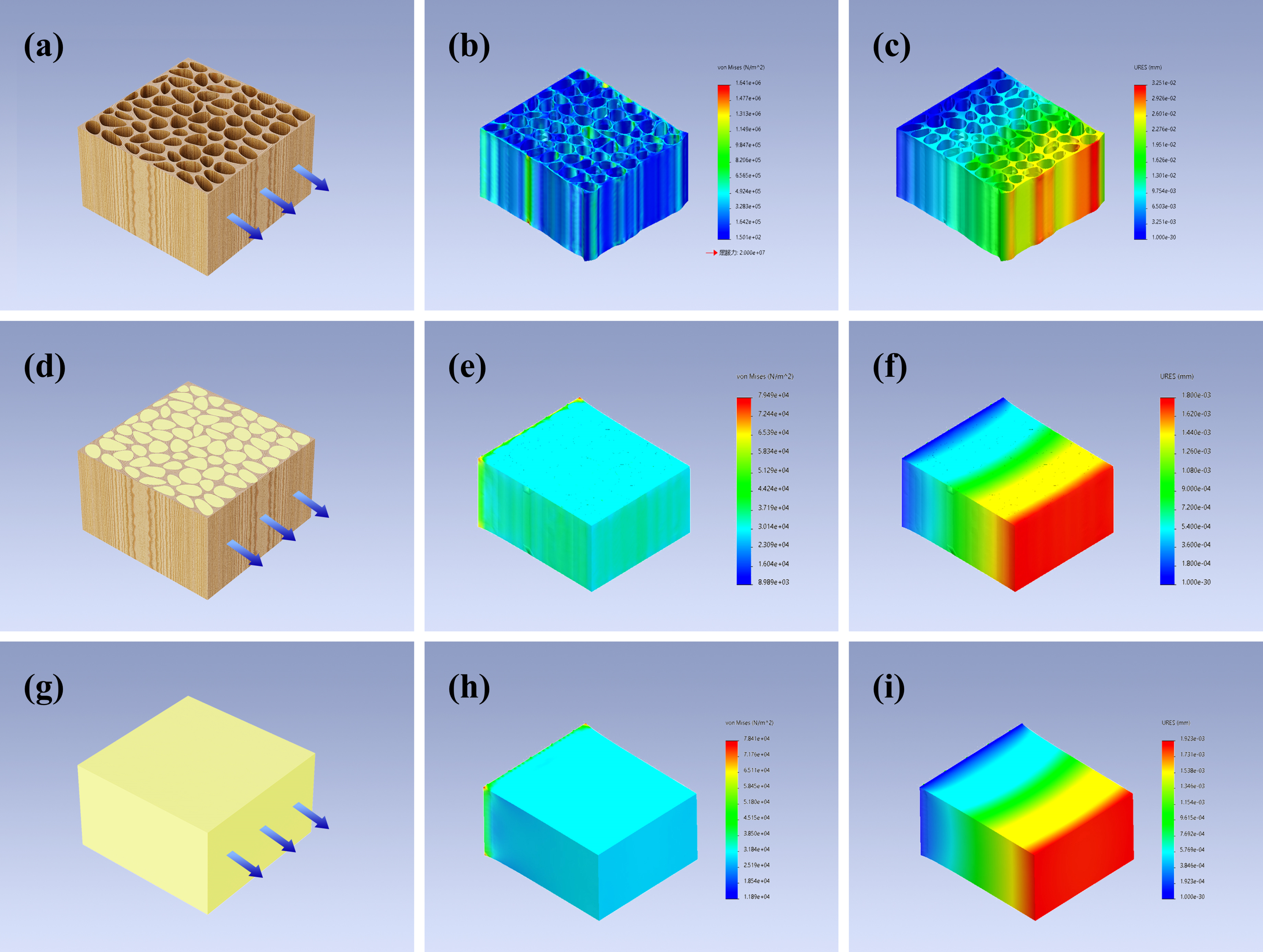
3.2. Mechanical Property and Structure of ORW and FUW
3.3. Photo-, Thermal-Response, and Fluorescence of FUW
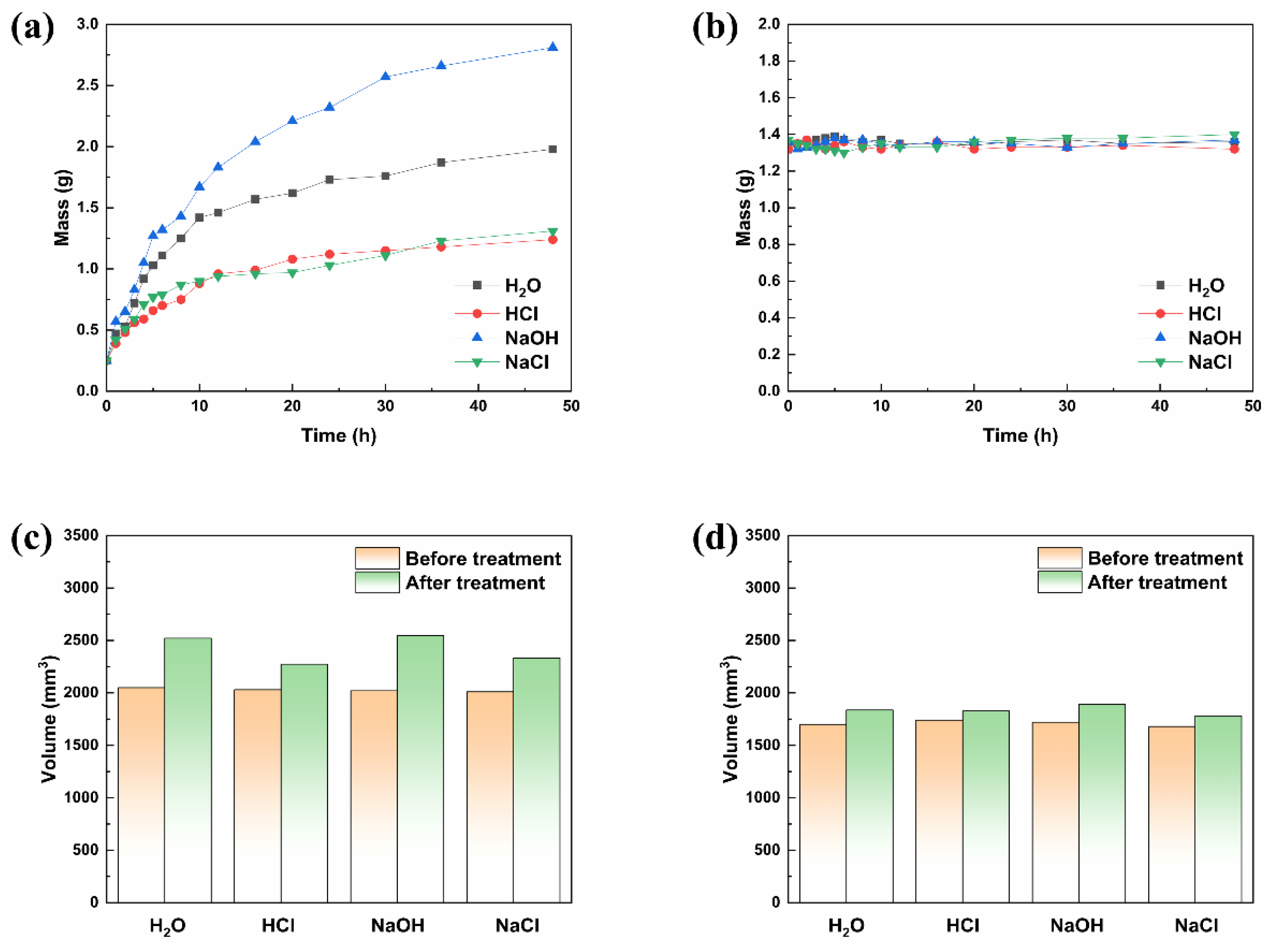
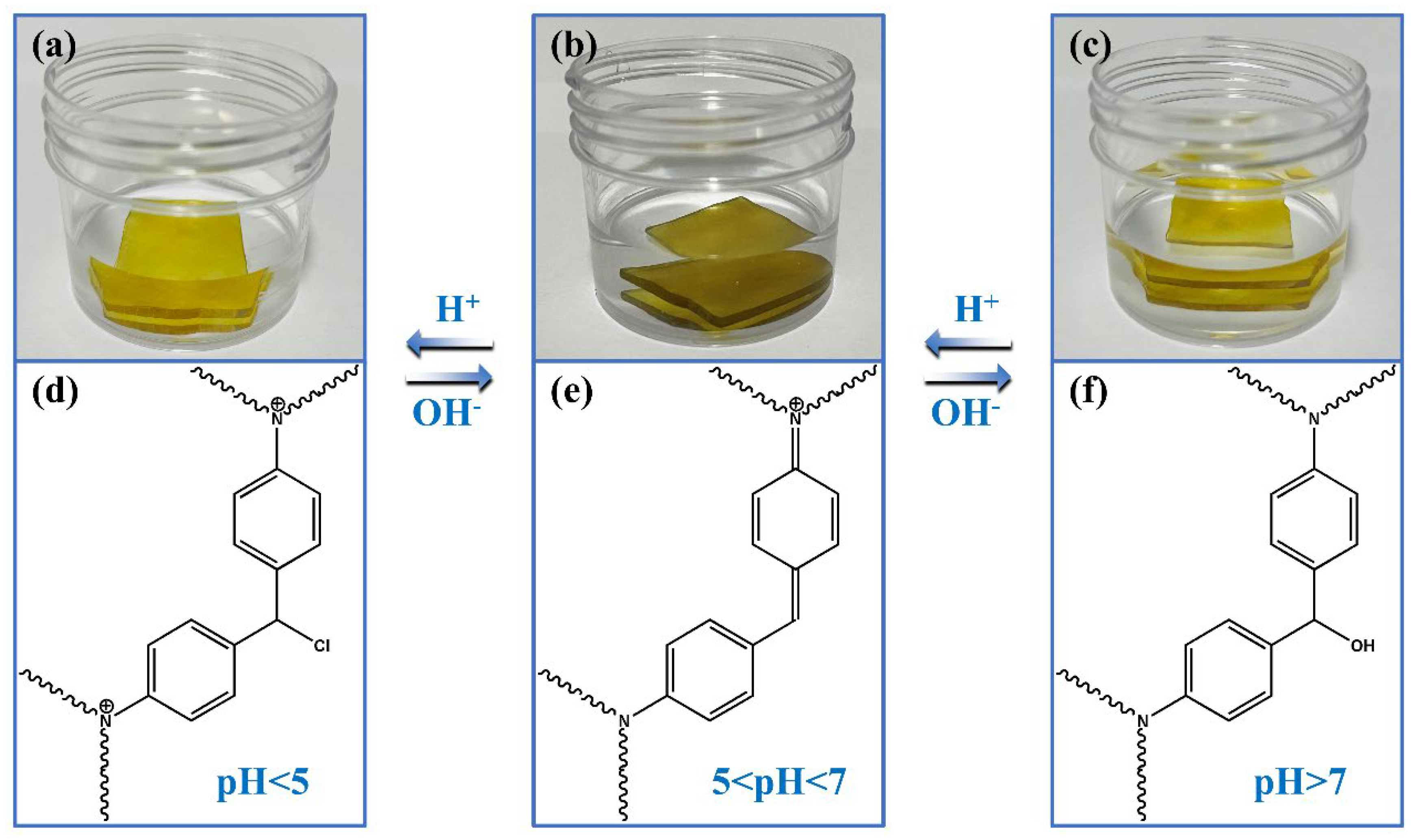
3.4. Water Resistance, Corrosion Resistance, and pH-Response of ORW and FUW
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subba Rao, A.N.; Nagarajappa, G.B.; Nair, S.; Chathoth, A.M.; Pandey, K.K. Flexible transparent wood prepared from poplar veneer and polyvinyl alcohol. Compos. Sci. Technol. 2019, 182, 107719. [Google Scholar] [CrossRef]
- Akpan, E.I.; Wetzel, B.; Friedrich, K. Eco-friendly and sustainable processing of wood-based materials. Green Chem. 2021, 23, 2198–2232. [Google Scholar] [CrossRef]
- Zhou, M.; Gu, W.; Wang, G.; Zheng, J.; Pei, C.; Fan, F.; Ji, G. Sustainable wood-based composites for microwave absorption and electromagnetic interference shielding. J. Mater. Chem. A 2020, 8, 24267–24283. [Google Scholar] [CrossRef]
- Sangregorio, A.; Muralidhara, A.; Guigo, N.; Thygesen, L.G.; Marlair, G.; Angelici, C.; Jong, E.; Sbirrazzuoli, N. Humin based resin for wood modification and property improvement. Green Chem. 2020, 22, 2786–2798. [Google Scholar] [CrossRef] [Green Version]
- Frey, M.; Schneider, L.; Masania, K.; Keplinger, T.; Burgert, I. Delignified Wood-Polymer Interpenetrating Composites Exceeding the Rule of Mixtures. ACS Appl. Mater. Interfaces 2019, 11, 35305–35311. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, H.; Li, J.; Yang, K.; Zhang, Z.; Chen, F.; Wang, B. High-Throughput Metal Trap: Sulfhydryl-Functionalized Wood Membrane Stacks for Rapid and Highly Efficient Heavy Metal Ion Removal. ACS Appl. Mater. Interfaces 2020, 12, 15002–15011. [Google Scholar] [CrossRef]
- Singha, S.; Gowda, V.; Hedenqvist, M.S. Plant Cuticle-Inspired Polyesters as Promising Green and Sustainable Polymer Materials. ACS Appl. Polym. Mater. 2021, 3, 4088–4100. [Google Scholar] [CrossRef]
- Montanari, C.; Li, Y.; Chen, H.; Yan, M.; Berglund, L.A. Transparent Wood for Thermal Energy Storage and Reversible Optical Transmittance. ACS Appl. Mater. Interfaces 2019, 11, 20465–20472. [Google Scholar] [CrossRef]
- Zhu, Z.; Fu, S.; Lavoine, N.; Lucia, L.A. Structural reconstruction strategies for the design of cellulose nanomaterials and aligned wood cellulose-based functional materials—A review. Carbohydr. Polym. 2020, 247, 116722. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, M.; Jungstedt, E.; Xu, B.; Sun, L.; Berglund, L. Optically Transparent Wood Substrate for Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 6061–6067. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Zhu, T.; Chen, Z.; Wu, Y.; Gao, Y. Transparent Wood Composites Fabricated by Impregnation of Epoxy Resin and W-Doped VO2 Nanoparticles for Application in Energy-Saving Windows. ACS Appl. Mater. Interfaces 2020, 12, 34777–34783. [Google Scholar] [CrossRef]
- Hai, L.V.; Muthoka, R.M.; Panicker, P.S.; Agumba, D.O.; Pham, H.D.; Kim, J. All-biobased transparent-wood: A new approach and its environmental-friendly packaging application. Carbohydr. Polym. 2021, 264, 118012. [Google Scholar]
- Bisht, P.; Pandey, K.K.; Barshilia, H.C. Photostable transparent wood composite functionalized with an UV-absorber. Polym. Degrad. Stab. 2021, 189, 109600. [Google Scholar] [CrossRef]
- Bi, Z.; Li, T.; Su, H.; Ni, Y.; Yan, L. Transparent Wood Film Incorporating Carbon Dots as Encapsulating Material for White Light-Emitting Diodes. ACS Sustain. Chem. Eng. 2018, 6, 9314–9323. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Howell, C.; Hunt, C.G. Functionality of Surface Mycelium Interfaces in Wood Bonding. ACS Appl. Mater. Interfaces 2020, 12, 57431–57440. [Google Scholar] [CrossRef]
- Al-Qahtani, S.; Aljuhani, E.; Felaly, R.; Alkhamis, K.; Alkabli, J.; Munshi, A.; El-Metwaly, N. Development of Photoluminescent Translucent Wood toward Photochromic Smart Window Applications. Ind. Eng. Chem. Res. 2021, 60, 8340–8350. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Chen, G.; Zhou, S.; Feng, X.; Chen, Y.; He, M.; Liu, D.; Song, T.; Qi, H. Highly Stretchable, Transparent, and Conductive Wood Fabricated by in Situ Photopolymerization with Polymerizable Deep Eutectic Solvents. ACS Appl. Mater. Interfaces 2019, 11, 14313–14321. [Google Scholar] [CrossRef]
- Moradian, M.; Islam, M.S.; Ven, T.G.M. Insoluble Regenerated Cellulose Films Made from Mildly Carboxylated Dissolving and Kraft Pulps. Ind. Eng. Chem. Res. 2021, 60, 5385–5393. [Google Scholar] [CrossRef]
- Sakakibara, K.; Moriki, Y.; Tsujii, Y. Preparation of High-Performance Polyethylene Composite Materials Reinforced with Cellulose Nanofiber: Simultaneous Nanofibrillation of Wood Pulp Fibers during Melt-Compounding Using Urea and Diblock Copolymer Dispersant. ACS Appl. Polym. Mater. 2019, 1, 178–187. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, Y.; Huang, Q.; Yang, Z.; Dong, R.; Li, L.; Tang, J.; Zhang, C.; Jiang, F. Metal Organic Framework (MOF)/Wood Derived Multi-cylinders High-Power 3D Reactor. ACS Appl. Mater. Interfaces 2021, 13, 5460–5468. [Google Scholar] [CrossRef]
- Zheng, K.; Zou, Q.; Yang, Y.; Mao, Y.; Zhang, J.; Cheng, J. The Chromogen, Structure, Inspirations, and Applications of a Photo-, pH-, thermal-, Solvent-, and Mechanical-Response Epoxy Resin. Ind. Eng. Chem. Res. 2018, 57, 13283–13290. [Google Scholar] [CrossRef]
- Zou, Q.; Ba, L.; Tan, X.; Tu, M.; Cheng, J.; Zhang, J. Tunable shape memory properties of rigid-flexible epoxy networks. J. Mater. Sci. 2016, 51, 10596–10607. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhan, X.; Luo, D.; Sun, X. Photochromic transparent wood for photo-switchable smart window applications. J. Mater. Chem. C 2019, 7, 8649–8654. [Google Scholar] [CrossRef]
- Guan, H.; Meng, J.; Cheng, Z.; Wang, X. Processing Natural Wood into a High-Performance Flexible Pressure Sensor. ACS Appl. Mater. Interfaces 2020, 12, 46357–46365. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, Y.; Xu, C.; Liu, P.; Lv, J.; Liu, Y.; Wang, Q. Removal mechanisms of aqueous Cr(VI) using apple wood biochar: A spectroscopic study. J. Hazard. Mater. 2020, 384, 121371. [Google Scholar] [CrossRef]
- Moghaddam, L.; Rencoret, J.; Maliger, V.R.; Rackemann, D.W.; Harrison, M.D.; Gutierrez, A.; Rio, J.C.; Doherty, W.O.S. Structural Characteristics of Bagasse Furfural Residue and Its Lignin Component. An NMR, Py-GC/MS, and FTIR Study. ACS Sustain. Chem. Eng. 2017, 5, 4846–4855. [Google Scholar] [CrossRef]
- Xin, J.; Li, M.; Li, R.; Wolcott, M.P.; Zhang, J. Green Epoxy Resin System Based on Lignin and Tung Oil and Its Application in Epoxy Asphalt. ACS Sustain. Chem. Eng. 2016, 4, 2754–2761. [Google Scholar] [CrossRef]
- Hernandez, E.D.; Bassett, A.W.; Sadler, J.M.; Scala, J.J.; Stanzione, J.F. Synthesis and Characterization of Bio-based Epoxy Resins Derived from Vanillyl Alcohol. ACS Sustain. Chem. Eng. 2016, 4, 4328–4339. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, J.; Cheng, J. Morphology, Structure, Miscibility, and Properties of Wholly Soy-Based Semi-interpenetrating Polymer Networks from Soy-Oil- Polyol-Based Polyurethane and Modified Soy Protein Isolate. Ind. Eng. Chem. Res. 2013, 52, 14335–14341. [Google Scholar] [CrossRef]
- Zheng, K.; Fan, X.; Mao, Y.; Lin, J.; Dai, W.; Zhang, J.; Cheng, J. The well-designed hierarchical structure of Musa basjoo for supercapacitors. Sci. Rep. 2016, 6, 20306. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Zhang, J.; Cheng, J. Miscibility, morphology, structure, and properties of porous cellulose-soy protein isolate hybrid hydrogels. J. Appl. Polym. Sci. 2016, 133, 43853. [Google Scholar] [CrossRef]
- Geng, Q.; Zhang, C.; Zheng, K.; Zhang, J.; Cheng, J.; Yang, W. Preparation and Properties of a Self-Healing, Multiresponsive Color-Change Hydrogel. Ind. Eng. Chem. Res. 2020, 59, 10689–10696. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Wu, J.; Huang, M.; Zhang, F.; Xu, J. Reversible Photo-, Thermal-, and pH-Responsive Functionalized Wood with Fluorescence Emission. Materials 2022, 15, 1229. https://doi.org/10.3390/ma15031229
Zheng K, Wu J, Huang M, Zhang F, Xu J. Reversible Photo-, Thermal-, and pH-Responsive Functionalized Wood with Fluorescence Emission. Materials. 2022; 15(3):1229. https://doi.org/10.3390/ma15031229
Chicago/Turabian StyleZheng, Kaiwen, Jiakai Wu, Munan Huang, Farao Zhang, and Junting Xu. 2022. "Reversible Photo-, Thermal-, and pH-Responsive Functionalized Wood with Fluorescence Emission" Materials 15, no. 3: 1229. https://doi.org/10.3390/ma15031229
APA StyleZheng, K., Wu, J., Huang, M., Zhang, F., & Xu, J. (2022). Reversible Photo-, Thermal-, and pH-Responsive Functionalized Wood with Fluorescence Emission. Materials, 15(3), 1229. https://doi.org/10.3390/ma15031229





