Magnesium-Based Alloys Used in Orthopedic Surgery
Abstract
1. Introduction
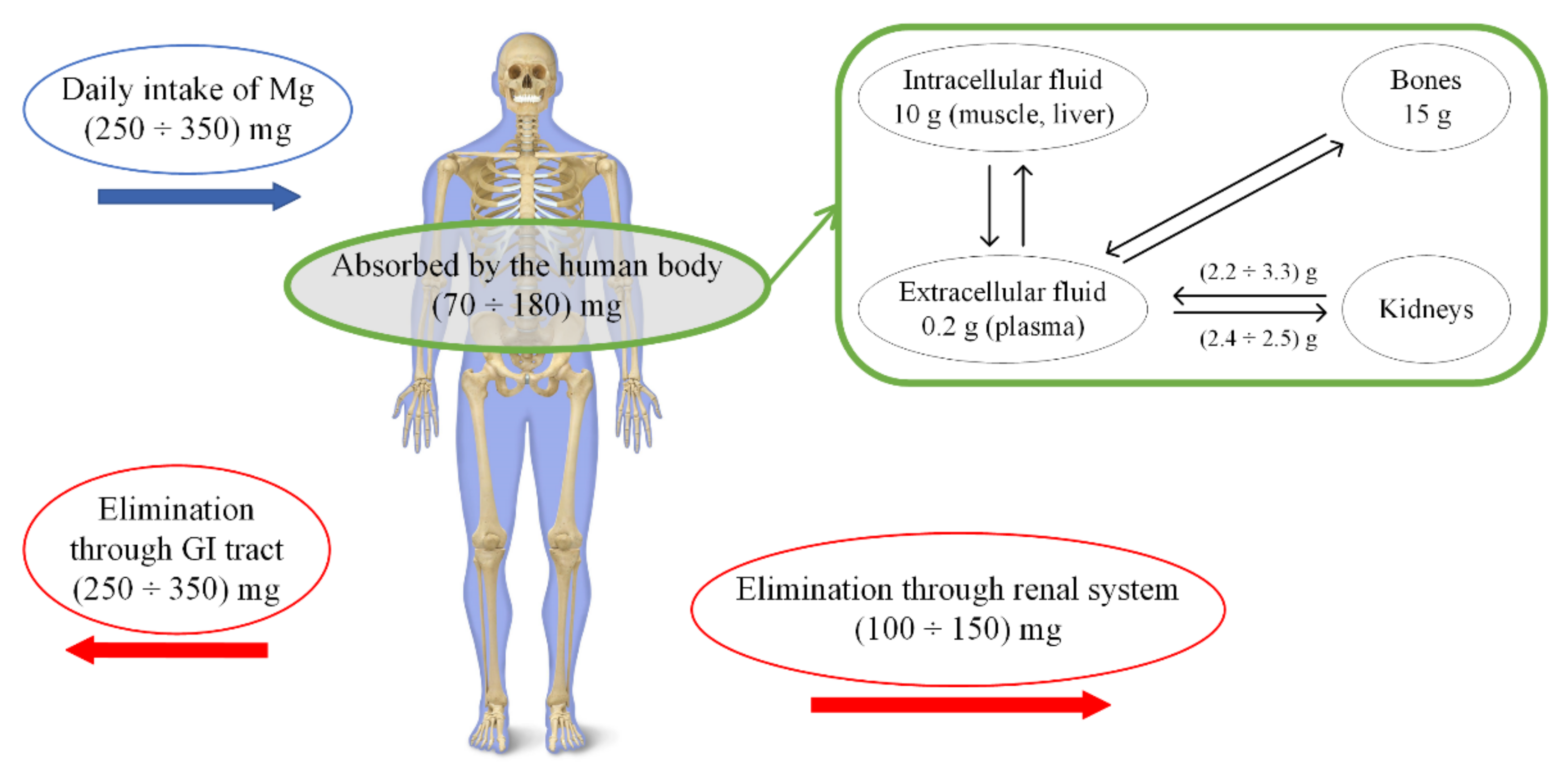
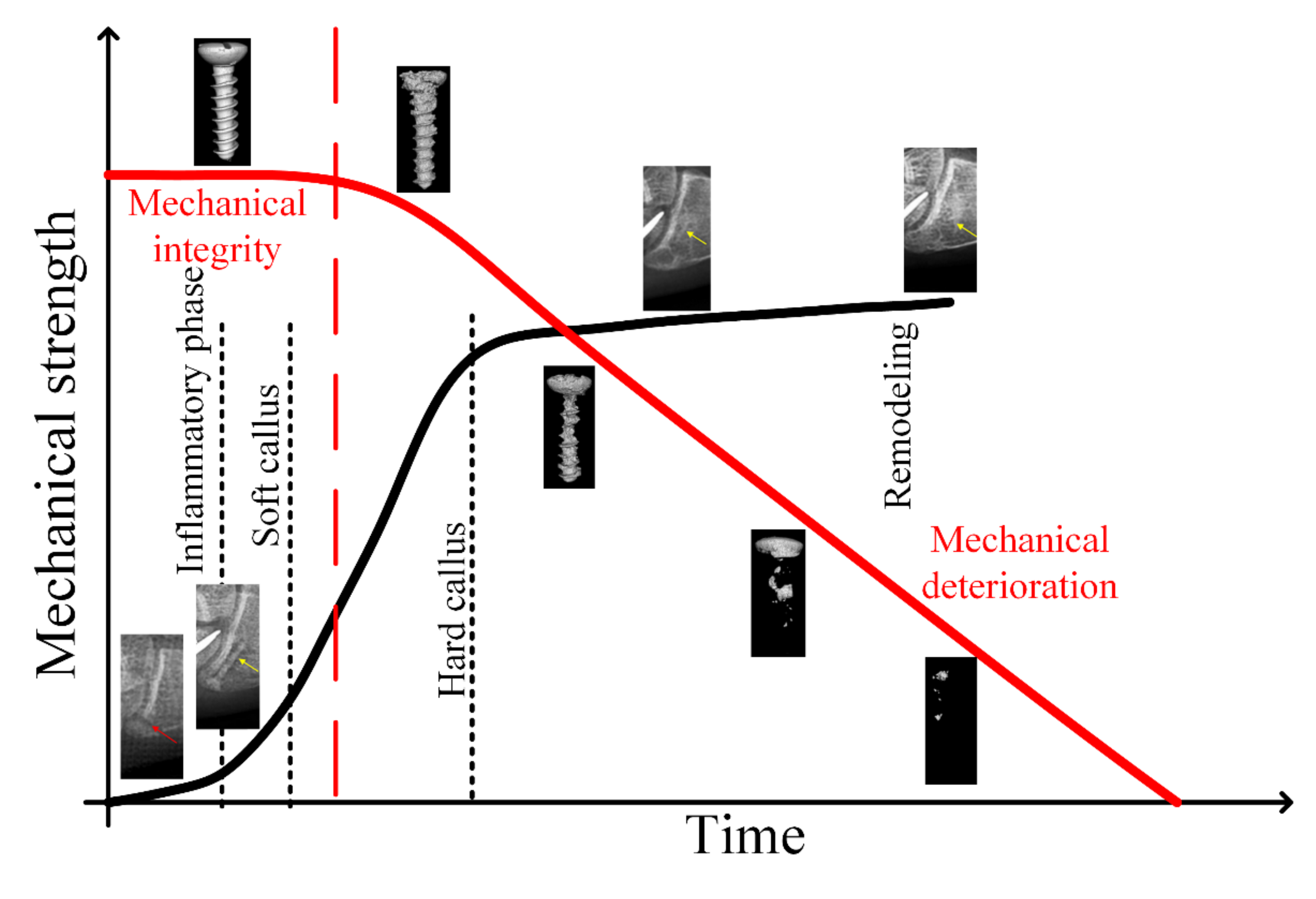
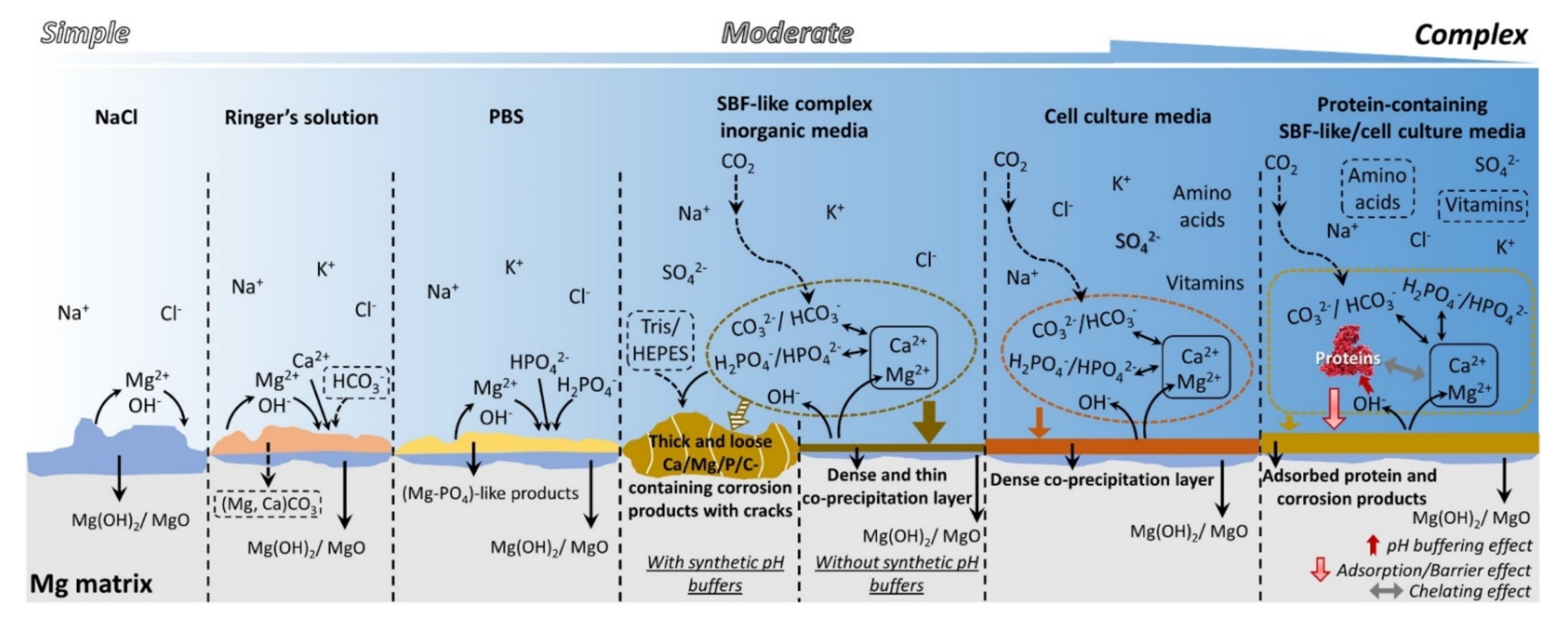
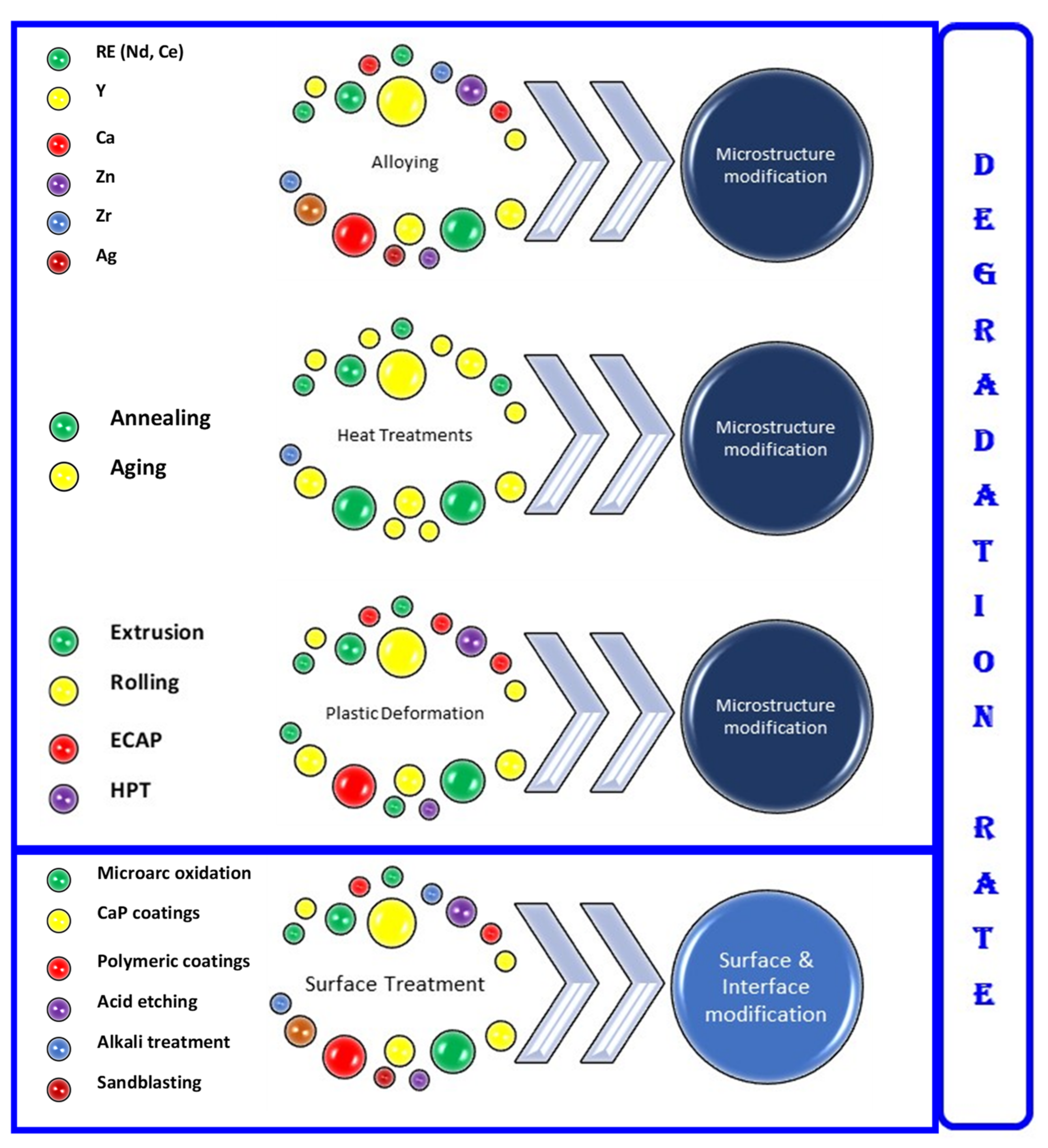
2. Attributes of Mg Alloys for Temporary Orthopedic Implants
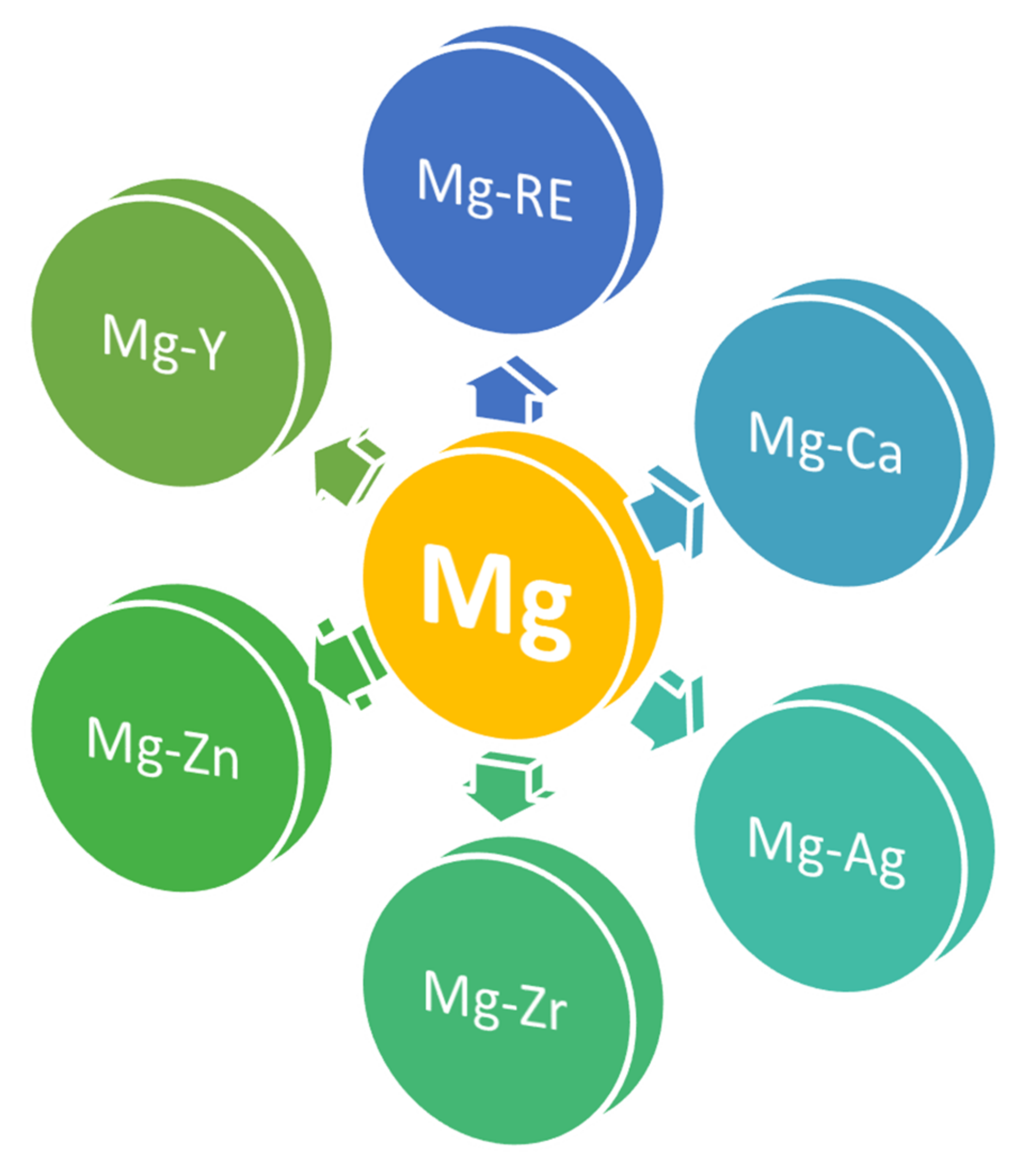
3. Current Status of Mg Alloys for Orthopedic Applications
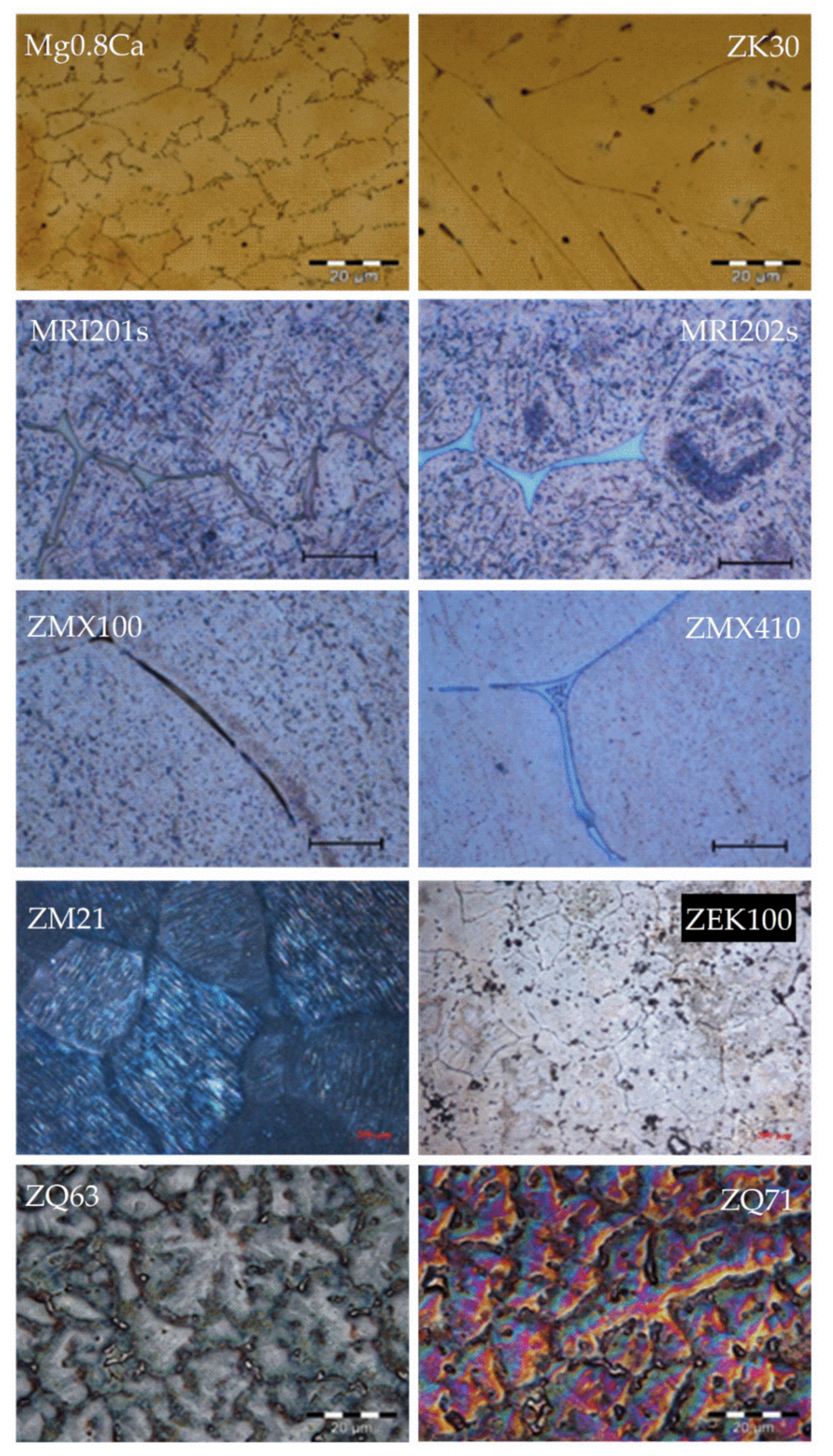
3.1. Mg–RE-Based Alloys
3.2. Mg–Zn-Based Alloys
3.3. Mg–Ca-Based Alloys
3.4. Mg–Zr-Based Alloys
3.5. Mg–Sr-Based Alloys
3.6. Mg–Ag-Based Alloys
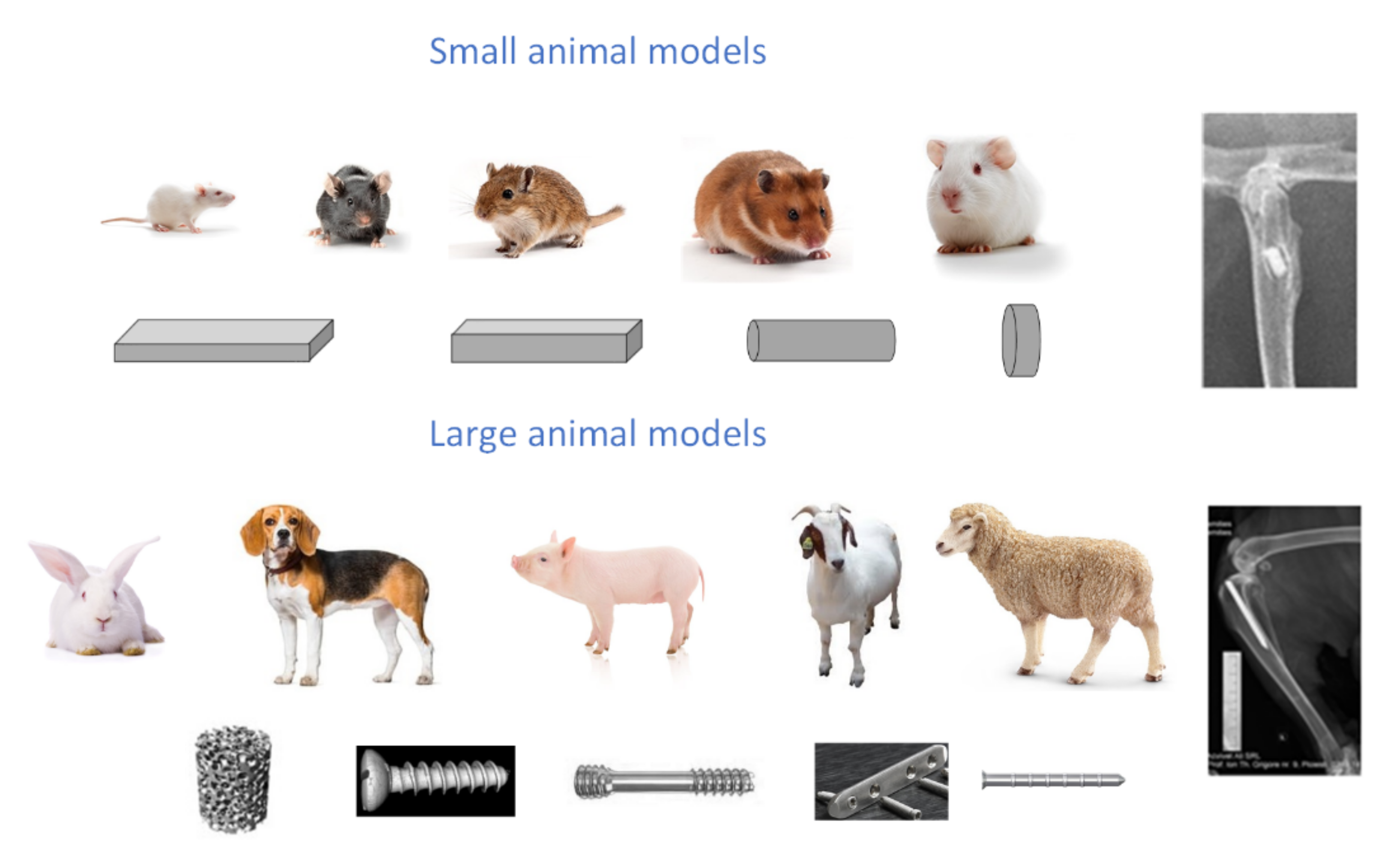
4. Animal Testing
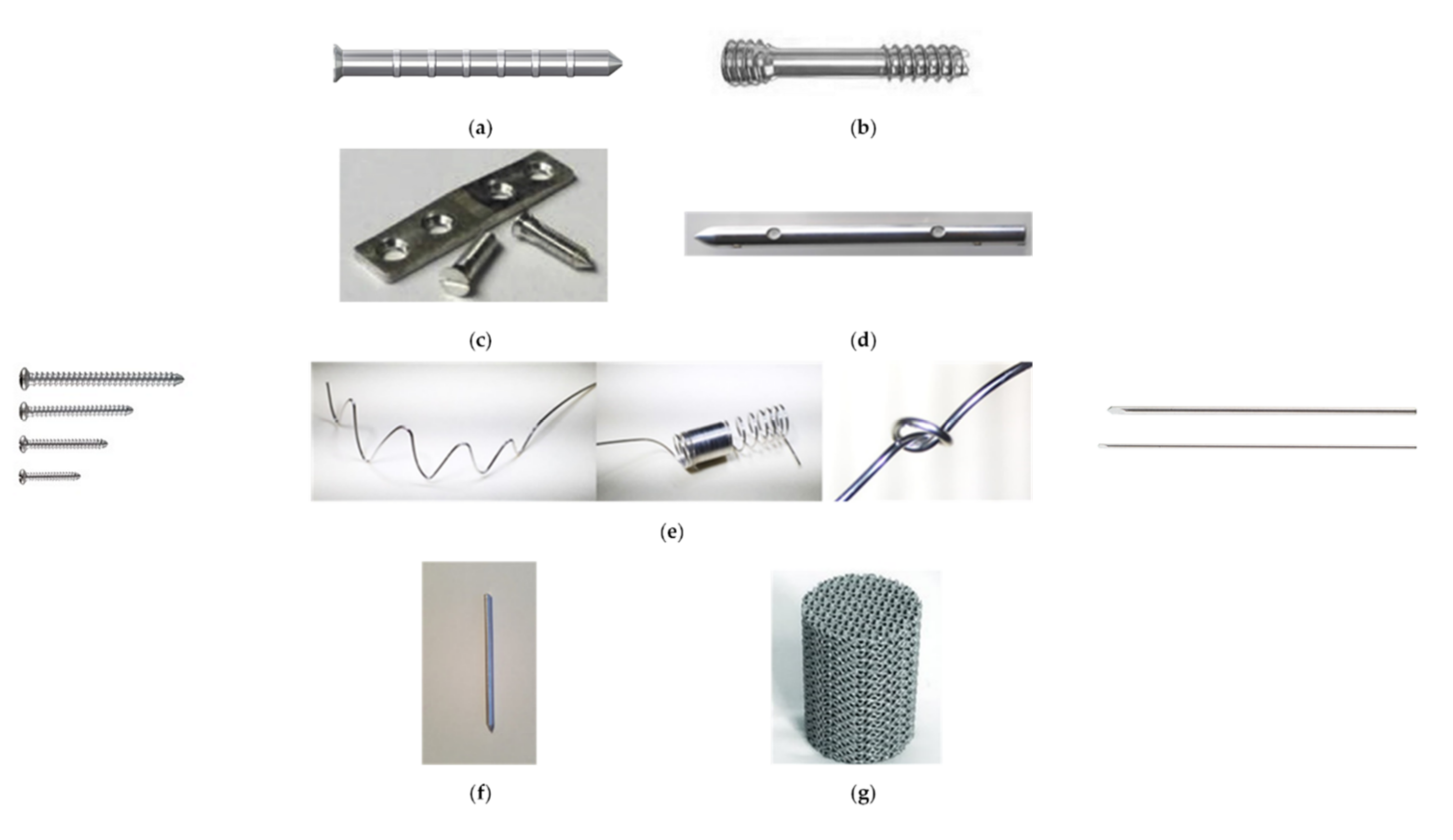
5. Clinical Translation of Mg-Based Materials for Temporary Implants’ Manufacture
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kulinets, I. Biomaterials and Their Applications in Medicine. In Regulatory Affairs for Biomaterials and Medical Devices; Woodhead Publishing: Sawston, UK, 2014; pp. 1–10. ISBN 978-0-85709-542-8. [Google Scholar]
- Williams, D. The Williams Dictionary of Biomaterials; Liverpool University Press: Liverpool, UK, 1999; ISBN 978-0-85323-734-1. [Google Scholar]
- Antoniac, I.V.; Albu, M.G.; Antoniac, A.; Rusu, L.C.; Ghica, M.V. Collagen–Bioceramic Smart Composites. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 301–324. ISBN 978-3-319-12460-5. [Google Scholar]
- Laurencin, C.T.; Badon, M.A. Regenerative Engineering in the Field of Orthopedic Surgery. In Biologics in Orthopaedic Surgery; Elsevier: St. Louis, MO, USA, 2019; pp. 201–213. ISBN 978-0-323-55140-3. [Google Scholar]
- Vespa, J.; Medina, L.; Armstrong, D.M. Demographic Turning Points for the United States: Population Projections for 2020 to 2060; U.S. Census Bureau: Suitland, MD, USA, 2020; p. 15. [Google Scholar]
- Tian, L.; Tang, N.; Ngai, T.; Wu, C.; Ruan, Y.; Huang, L.; Qin, L. Hybrid Fracture Fixation Systems Developed for Orthopaedic Applications: A General Review. J. Orthop. Transl. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, E.D.; Jun, E.J.; Yoo, H.S.; Lee, J.W. Analysis of Trends and Prospects Regarding Stents for Human Blood Vessels. Biomater. Res. 2018, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Rigatelli, G.; Carraro, U.; Barbiero, M.; Zanchetta, M.; Pedon, L.; Dimopoulos, K.; Rigatelli, G.; Maiolino, P.; Cobelli, F.; Riccardi, R.; et al. New Advances in Dynamic Cardiomyoplasty: Doppler Flow Wire Shows Improved Cardiac Assistance in Demand Protocol. ASAIO J. 2002, 48, 119–123. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- González, J.E.G.; Mirza-Rosca, J.C. Study of the Corrosion Behavior of Titanium and Some of Its Alloys for Biomedical and Dental Implant Applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Nica, M.; Cretu, B.; Ene, D.; Antoniac, I.; Gheorghita, D.; Ene, R. Failure Analysis of Retrieved Osteosynthesis Implants. Materials 2020, 13, 1201. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Stoia, D.I.; Ghiban, B.; Tecu, C.; Miculescu, F.; Vigaru, C.; Saceleanu, V. Failure analysis of a humeral shaft locking compression plate—Surface investigation and simulation by finite element method. Materials 2019, 12, 1128. [Google Scholar] [CrossRef]
- Jin, W.; Chu, P.K. Orthopedic Implants. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–439. ISBN 978-0-12-805144-3. [Google Scholar]
- Mozumder, M.S.; Mairpady, A.; Mourad, A.-H.I. Polymeric Nanobiocomposites for Biomedical Applications. J. Biomed. Mater. Res. 2017, 105, 1241–1259. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Purnama, A.; Hermawan, H.; Couet, J.; Mantovani, D. Assessing the Biocompatibility of Degradable Metallic Materials: State-of-the-Art and Focus on the Potential of Genetic Regulation. Acta Biomater. 2010, 6, 1800–1807. [Google Scholar] [CrossRef]
- Waizy, H.; Diekmann, J.; Weizbauer, A.; Reifenrath, J.; Bartsch, I.; Neubert, V.; Schavan, R.; Windhagen, H. In Vivo Study of a Biodegradable Orthopedic Screw (MgYREZr-Alloy) in a Rabbit Model for up to 12 Months. J. Biomater. Appl. 2014, 28, 667–675. [Google Scholar] [CrossRef]
- Rahim, M.I.; Eifler, R.; Rais, B.; Mueller, P.P. Alkalization Is Responsible for Antibacterial Effects of Corroding Magnesium. J. Biomed. Mater. Res. 2015, 103, 3526–3532. [Google Scholar] [CrossRef]
- Naujokat, H.; Seitz, J.-M.; Açil, Y.; Damm, T.; Möller, I.; Gülses, A.; Wiltfang, J. Osteosynthesis of a Cranio-Osteoplasty with a Biodegradable Magnesium Plate System in Miniature Pigs. Acta Biomater. 2017, 62, 434–445. [Google Scholar] [CrossRef]
- Aktan, C.; Ertan, M.B.; Turan, A.; Kose, O. Fixation of Small Osteochondral Fragments in a Comminuted Distal Humerus Fracture with Magnesium Bioabsorbable Screws: A Case Report. Cureus 2018, 10, e3752. [Google Scholar] [CrossRef]
- Gigante, A.; Setaro, N.; Rotini, M.; Finzi, S.S.; Marinelli, M. Intercondylar Eminence Fracture Treated by Resorbable Magnesium Screws Osteosynthesis: A Case Series. Injury 2018, 49, S48–S53. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, L.; Yuan, G.; Dai, K.; Pei, J.; Hao, Y. Dual Modulation of Bone Formation and Resorption with Zoledronic Acid-Loaded Biodegradable Magnesium Alloy Implants Improves Osteoporotic Fracture Healing: An in Vitro and in Vivo Study. Acta Biomater. 2018, 65, 486–500. [Google Scholar] [CrossRef]
- Lam, W.-H.; Tso, C.-Y.; Tang, N.; Cheung, W.-H.; Qin, L.; Wong, R.M.-Y. Biodegradable Magnesium Screws in Elbow Fracture Fixation: Clinical Case Series. J. Orthop. Trauma Rehabil. 2021. [Google Scholar] [CrossRef]
- Holweg, P.; Berger, L.; Cihova, M.; Donohue, N.; Clement, B.; Schwarze, U.; Sommer, N.G.; Hohenberger, G.; van den Beucken, J.J.J.P.; Seibert, F.; et al. A Lean Magnesium–Zinc–Calcium Alloy ZX00 Used for Bone Fracture Stabilization in a Large Growing-Animal Model. Acta Biomater. 2020, 113, 646–659. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.-T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In Vivo Study of Magnesium Plate and Screw Degradation and Bone Fracture Healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef]
- Könneker, S.; Krockenberger, K.; Pieh, C.; von Falck, C.; Brandewiede, B.; Vogt, P.M.; Kirschner, M.H.; Ziegler, A. Comparison of Scaphoid Fracture Osteosynthesis by Magnesium-Based Headless Herbert Screws with Titanium Herbert Screws: Protocol for the Randomized Controlled SCAMAG Clinical Trial. BMC Musculoskelet. Disord. 2019, 20, 357. [Google Scholar] [CrossRef]
- Acar, B.; Unal, M.; Turan, A.; Kose, O. Isolated Lateral Malleolar Fracture Treated with a Bioabsorbable Magnesium Compression Screw. Cureus 2018, 10, e2539. [Google Scholar] [CrossRef]
- Chow, D.H.K.; Wang, J.; Wan, P.; Zheng, L.; Ong, M.T.Y.; Huang, L.; Tong, W.; Tan, L.; Yang, K.; Qin, L. Biodegradable Magnesium Pins Enhanced the Healing of Transverse Patellar Fracture in Rabbits. Bioact. Mater. 2021, 6, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- El-Rahman, S. Neuropathology of Aluminum Toxicity in Rats (Glutamate and GABA Impairment). Pharmacol. Res. 2003, 47, 189–194. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Sankara Narayanan, T.S.N. Corrosion Behavior of Commercially Pure Mg and ZM21 Mg Alloy in Ringer’s Solution—Long Term Evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef]
- Kose, O. Magnesium (MgYREZr) Bioabsorbable Screws in Orthopedic Surgery. Available online: https://military-medicine.com/article/3830-magnesium-mgyrezr-bioabsorbable-screws-in-orthopedic-surgery.html (accessed on 6 November 2021).
- Hermawan, H. Biodegradable Metals; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-31169-7. [Google Scholar]
- Zhang, H.; Shang, S.-L.; Wang, Y.; Chen, L.-Q.; Liu, Z.-K. Thermodynamic Properties of Laves Phases in the Mg–Al–Ca System at Finite Temperature from First-Principles. Intermetallics 2012, 22, 17–23. [Google Scholar] [CrossRef]
- Friedrich, H.E.; Mordike, B.L. Magnesium Technology: Metallurgy, Design Data, Applications; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2006; ISBN 978-3-540-20599-9. [Google Scholar]
- Antoniac, I.V.; Filipescu, M.; Barbaro, K.; Bonciu, A.; Birjega, R.; Cotrut, C.M.; Galvano, E.; Fosca, M.; Fadeeva, I.V.; Vadalà, G.; et al. Iron Ion-Doped Tricalcium Phosphate Coatings Improve the Properties of Biodegradable Magnesium Alloys for Biomedical Implant Application. Adv. Mater. Interfaces 2020, 7, 2000531. [Google Scholar] [CrossRef]
- Istrate, B.; Rau, J.V.; Munteanu, C.; Antoniac, I.V.; Saceleanu, V. Properties and in Vitro Assessment of ZrO2-Based Coatings Obtained by Atmospheric Plasma Jet Spraying on Biodegradable Mg-Ca and Mg-Ca-Zr Alloys. Ceram. Int. 2020, 46, 15897–15906. [Google Scholar] [CrossRef]
- Gu, D.; Hagedorn, Y.-C.; Meiners, W.; Meng, G.; Batista, R.J.S.; Wissenbach, K.; Poprawe, R. Densification Behavior, Microstructure Evolution, and Wear Performance of Selective Laser Melting Processed Commercially Pure Titanium. Acta Mater. 2012, 60, 3849–3860. [Google Scholar] [CrossRef]
- Allavikutty, R.; Gupta, P.; Santra, T.S.; Rengaswamy, J. Additive Manufacturing of Mg Alloys for Biomedical Applications: Current Status and Challenges. Curr. Opin. Biomed. Eng. 2021, 18, 100276. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.-U.; Mol, J.M.C.; Weinans, H.; et al. Additively Manufactured Biodegradable Porous Magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, C.; Shen, L.; Zhao, Z.; Peng, S.; Shuai, C. In-Situ Deposition of Apatite Layer to Protect Mg-Based Composite Fabricated via Laser Additive Manufacturing. J. Magnes. Alloys, 2021; in press. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg Bone Implant: Features, Developments and Perspectives. Mater. Design 2020, 185, 108259. [Google Scholar] [CrossRef]
- Lévesque, J.; Hermawan, H.; Dubé, D.; Mantovani, D. Design of a pseudo-physiological test bench specific to the development of biodegradable metallic biomaterials. Acta Biomater. 2008, 4, 284–295. [Google Scholar] [CrossRef]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Adam, R.; Gheorghiu, N.; Orban, H.; Antoniac, I.; Barbilian, A. Evolution toward New Bioabsorbable Material for Osteosynthesis Implants Used in Foot and Ankle Surgery. Arch. Balk. Med. Union 2016, 51, 190–198. [Google Scholar]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current Status and Outlook on the Clinical Translation of Biodegradable Metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and Metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current Status on Clinical Applications of Magnesium-Based Orthopaedic Implants: A Review from Clinical Translational Perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Abed, E.; Moreau, R. Importance of Melastatin-like Transient Receptor Potential 7 and Magnesium in the Stimulation of Osteoblast Proliferation and Migration by Platelet-Derived Growth Factor. Am. J. Physiol.-Cell Physiol. 2009, 297, C360–C368. [Google Scholar] [CrossRef] [PubMed]
- He, L.Y.; Zhang, X.M.; Liu, B.; Tian, Y.; Ma, W.H. Effect of Magnesium Ion on Human Osteoblast Activity. Braz. J. Med. Biol. Res. 2016, 49, e5257. [Google Scholar] [CrossRef]
- Sun, Y.; Helmholz, H.; Willumeit-Römer, R. Preclinical in Vivo Research of Magnesium-Based Implants for Fracture Treatment: A Systematic Review of Animal Model Selection and Study Design. J. Magnes. Alloys 2021, 9, 351–361. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Cama, G.; Komlev, V.S.; Ravaglioli, A. Bioactive Materials for Bone Tissue Engineering. BioMed Res. Int. 2016, 2016, 3741428. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for Modifying Current Cytotoxicity Testing Standards for Biodegradable Magnesium-Based Materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium Ion Stimulation of Bone Marrow Stromal Cells Enhances Osteogenic Activity, Simulating the Effect of Magnesium Alloy Degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y. Research on an Mg–Zn Alloy as a Degradable Biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, J.; Yu, H.; Chen, C. Degradable Magnesium-Based Alloys for Biomedical Applications: The Role of Critical Alloying Elements. J. Biomater. Appl. 2019, 33, 1348–1372. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Ulrich Kainer, K.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Hamad, K. Highly-Ductile Magnesium Alloys: Atomistic-Flow Mechanisms and Alloy Designing. Materials 2019, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Sekar, P.; Narendranath, S.; Desai, V. Recent progress in in vivo studies and clinical applications of magnesium based biodegradable implants—A review. J. Magnes. Alloys 2021, 9, 1147–1163. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized Bone Grafting Fixed by Biodegradable Magnesium Screw for Treating Osteonecrosis of the Femoral Head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef]
- Ghasali, E.; Bordbar-Khiabani, A.; Alizadeh, M.; Mozafari, M.; Niazmand, M.; Kazemzadeh, H.; Ebadzadeh, T. Corrosion Behavior and In-Vitro Bioactivity of Porous Mg/Al2O3 and Mg/Si3N4 Metal Matrix Composites Fabricated Using Microwave Sintering Process. Mater. Chem. Phys. 2019, 225, 331–339. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Lupescu, S.; Antoniac, V.I.; Sindilar, E. Structural Characterization of Mg-0.5Ca-XY Biodegradable Alloys. KEM 2018, 782, 129–135. [Google Scholar] [CrossRef]
- Dobatkin, S.V.; Rokhlin, L.L.; Lukyanova, E.A.; Murashkin, M.Y.; Dobatkina, T.V.; Tabachkova, N.Y. Structure and Mechanical Properties of the Mg-Y-Gd-Zr Alloy after High Pressure Torsion. Mater. Sci. Eng. A 2016, 667, 217–223. [Google Scholar] [CrossRef]
- Aghion, E.; Levy, G. The Effect of Ca on the in Vitro Corrosion Performance of Biodegradable Mg–Nd–Y–Zr Alloy. J. Mater. Sci. 2010, 45, 3096–3101. [Google Scholar] [CrossRef]
- Chen, H.; Kang, S.B.; Yu, H.; Cho, J.; Kim, H.W.; Min, G. Effect of Heat Treatment on Microstructure and Mechanical Properties of Twin Roll Cast and Sequential Warm Rolled ZK60 Alloy Sheets. J. Alloys Compd. 2009, 476, 324–328. [Google Scholar] [CrossRef]
- Kang, Y.H.; Huang, Z.H.; Wang, S.C.; Yan, H.; Chen, R.S.; Huang, J.C. Effect of Pre-Deformation on Microstructure and Mechanical Properties of WE43 Magnesium Alloy II: Aging at 250 and 300 °C. J. Magnes. Alloys 2020, 8, 103–110. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Shoeib, M.A. Corrosion Studies and Microstructure of Mg−Zn−Ca Alloys for Biomedical Applications. Surf. Interfaces 2019, 14, 108–116. [Google Scholar] [CrossRef]
- Tie, D.; Liu, H.; Guan, R.; Holt-Torres, P.; Liu, Y.; Wang, Y.; Hort, N. In Vivo Assessment of Biodegradable Magnesium Alloy Ureteral Stents in a Pig Model. Acta Biomater. 2020, 116, 415–425. [Google Scholar] [CrossRef]
- Duley, P.; Sanyal, S.; Bandyopadhyay, T.K.; Mandal, S. Homogenization-Induced Age-Hardening Behavior and Room Temperature Mechanical Properties of Mg-4Zn-0.5Ca-0.16Mn (Wt%) Alloy. Mater. Design 2019, 164, 107554. [Google Scholar] [CrossRef]
- Li, Y.; Wen, C.; Mushahary, D.; Sravanthi, R.; Harishankar, N.; Pande, G.; Hodgson, P. Mg–Zr–Sr Alloys as Biodegradable Implant Materials. Acta Biomater. 2012, 8, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Fu, P.; Ding, W. Effects of Extrusion and Heat Treatment on the Mechanical Properties and Biocorrosion Behaviors of a Mg–Nd–Zn–Zr Alloy. J. Mech. Behav. Biomed. Mater. 2012, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Lamaka, S.V.; Lu, X.; Zheludkevich, M.L. Selecting medium for corrosion testing of bioabsorbable magnesium and other metals—A critical review. Corros. Sci. 2020, 171, 108722. [Google Scholar] [CrossRef]
- Liu, C.; Xin, Y.; Tian, X.; Chu, P.K. Degradation Susceptibility of Surgical Magnesium Alloy in Artificial Biological Fluid Containing Albumin. J. Mater. Res. 2007, 22, 1806–1814. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Electrodeposition of Ca–P Coatings on Biodegradable Mg Alloy: In Vitro Biomineralization Behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef]
- Adam, R.; Orban, H.; Dragomir, L.; Milea, C.; Antoniac, I.; Barbilian, A. Investigation of Biodegradation Behavior of an Mg-1Ca Alloy during In Vivo Testing. KEM 2017, 752, 87–92. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Jin, W.; Wu, G.; Feng, H.; Wang, W.; Zhang, X.; Chu, P.K. Improvement of Corrosion Resistance and Biocompatibility of Rare-Earth WE43 Magnesium Alloy by Neodymium Self-Ion Implantation. Corros. Sci. 2015, 94, 142–155. [Google Scholar] [CrossRef]
- Liu, D.; Yang, D.; Li, X.; Hu, S. Mechanical Properties, Corrosion Resistance and Biocompatibilities of Degradable Mg-RE Alloys: A Review. J. Mater. Res. Technol. 2019, 8, 1538–1549. [Google Scholar] [CrossRef]
- Trivedi, P.; Nune, K.C.; Misra, R.D.K. Degradation behaviour of magnesium-rare earth biomedical alloys. Mater.Technol. 2016, 31, 1–6. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In Vitro Corrosion and Biocompatibility of Binary Magnesium Alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, F.; Cotrut, C.; Ficai, A.; Rau, J.V.; Grosu, E.; Antoniac, A.; Tecu, C.; Cristescu, I. Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating. Materials 2020, 13, 263. [Google Scholar] [CrossRef]
- Lotfabadi, A.F.; Bakhsheshi-Rad, H.R.; Idris, M.H.; Hamzah, E.; Kasiri-Asgarani, M. The Role of Solution Heat Treatment on Corrosion and Mechanical Behaviour of Mg–Zn Biodegradable Alloys. Can. Metall. Q. 2016, 55, 53–64. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, Z.; Tang, C.; Li, W.; You, C.; Chen, M. The mechanical and corrosion resistance of Mg-Zn-Ca-Ag alloys: The influence of Ag content3. J. Mater. Res. Technol. 2020, 9, 10863–10875. [Google Scholar] [CrossRef]
- Kavyani, M.; Ebrahimi, G.R.; Ezatpour, H.R.; Jahazi, M. Microstructure Refinement, Mechanical and Biocorrosion Properties of Mg–Zn–Ca–Mn Alloy Improved by a New Severe Plastic Deformation Process. J. Magnes. Alloys, 2021; in press. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation and Characterization of a New Biomedical Mg–Zn–Ca Alloy. Mater. Design 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Qiao, X.G.; Xu, S.W.; Wu, K.; Kamado, S.; Kojima, Y. In-Situ Quasicrystal-Reinforced Magnesium Matrix Composite Processed by Equal Channel Angular Extrusion (ECAE). J. Mater. Sci. 2005, 40, 2587–2590. [Google Scholar] [CrossRef]
- Kawamura, Y.; Hayashi, K.; Inoue, A.; Masumoto, T. Rapidly Solidified Powder Metallurgy Mg97Zn1Y2 Alloys with Excellent Tensile Yield Strength above 600 MPa. Mater. Trans. 2001, 42, 1172–1176. [Google Scholar] [CrossRef]
- Wu, L.; Cui, C.; Wu, R.; Li, J.; Zhan, H.; Zhang, M. Effects of Ce-Rich RE Additions and Heat Treatment on the Microstructure and Tensile Properties of Mg–Li–Al–Zn-Based Alloy. Mater. Sci. Eng. A 2011, 528, 2174–2179. [Google Scholar] [CrossRef]
- Qian, M.; Das, A. Grain Refinement of Magnesium Alloys by Zirconium: Formation of Equiaxed Grains. Scr. Mater. 2006, 54, 881–886. [Google Scholar] [CrossRef]
- Huan, Z.G.; Leeflang, M.A.; Zhou, J.; Fratila-Apachitei, L.E.; Duszczyk, J. In Vitro Degradation Behavior and Cytocompatibility of Mg–Zn–Zr Alloys. J. Mater. Sci. Mater. Med. 2010, 21, 2623–2635. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Wang, Y.; Zeng, X.; Ding, W. Effect of Nd and Y Addition on Microstructure and Mechanical Properties of As-Cast Mg–Zn–Zr Alloy. J. Alloys Compd. 2007, 427, 115–123. [Google Scholar] [CrossRef]
- Nanda, I.P.; Hassim, M.H.; Idris, M.H.; Jahare, M.H.; Abdulmalik, S.S.; Arafat, A. Mechanical and Degradation Properties of Zinc Adopted Magnesium Alloys for Biomedical Application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 602, 012094. [Google Scholar] [CrossRef]
- Telang, V.S.; Pemmada, R.; Thomas, V.; Ramakrishna, S.; Tandon, P.; Nanda, H.S. Harnessing Additive Manufacturing for Magnesium-Based Metallic Bioimplants: Recent Advances and Future Perspectives. Curr. Opin. Biomed. Eng. 2021, 17, 100264. [Google Scholar] [CrossRef]
- Lesz, S.; Hrapkowicz, B.; Karolus, M.; Gołombek, K. Characteristics of the Mg-Zn-Ca-Gd Alloy after Mechanical Alloying. Materials 2021, 14, 226. [Google Scholar] [CrossRef]
- Zhang, E.; He, W.; Du, H.; Yang, K. Microstructure, Mechanical Properties and Corrosion Properties of Mg–Zn–Y Alloys with Low Zn Content. Mater. Sci. Eng. A 2008, 488, 102–111. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, N.; Zheng, Y.F.; Ruan, L. In Vitro Degradation Performance and Biological Response of a Mg–Zn–Zr Alloy. Mater. Sci. Eng. B 2011, 176, 1778–1784. [Google Scholar] [CrossRef]
- Bita, A.I.; Antoniac, A.; Cotrut, C.; Vasile, E.; Ciuca, I.; Niculescu, M.; Antoniac, I. In Vitro Degradation and Corrosion Evaluation of Mg-Ca Alloys for Biomedical Applications. J. Optoelectron. Adv. Mater. 2016, 18, 394–398. [Google Scholar]
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-Ceramic Coated Mg-Ca Alloys for Biomedical Implant Applications. Mater. Sci. Eng. C 2016, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Rad, H.R.B.; Idris, M.H.; Kadir, M.R.A.; Farahany, S. Microstructure Analysis and Corrosion Behavior of Biodegradable Mg–Ca Implant Alloys. Mater. Design 2012, 33, 88–97. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Filipescu, M.; Cotrut, C.; Fosca, M.; Nistor, L.C.; Birjega, R.; Dinescu, M. Hydroxyapatite Coatings on Mg-Ca Alloy Prepared by Pulsed Laser Deposition: Properties and Corrosion Resistance in Simulated Body Fluid. Ceram. Int. 2018, 44, 16678–16687. [Google Scholar] [CrossRef]
- Seong, J.W.; Kim, W.J. Development of Biodegradable Mg–Ca Alloy Sheets with Enhanced Strength and Corrosion Properties through the Refinement and Uniform Dispersion of the Mg2Ca Phase by High-Ratio Differential Speed Rolling. Acta Biomater. 2015, 11, 531–542. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The Development of Binary Mg–Ca Alloys for Use as Biodegradable Materials within Bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Levi, G.; Avraham, S.; Zilberov, A.; Bamberger, M. Solidification, Solution Treatment and Age Hardening of a Mg–1.6wt.% Ca–3.2wt.% Zn Alloy. Acta Mater. 2006, 54, 523–530. [Google Scholar] [CrossRef]
- Berglund, I.S.; Brar, H.S.; Dolgova, N.; Acharya, A.P.; Keselowsky, B.G.; Sarntinoranont, M.; Manuel, M.V. Synthesis and Characterization of Mg-Ca-Sr Alloys for Biodegradable Orthopedic Implant Applications. J. Biomed. Mater. Res. 2012, 100B, 1524–1534. [Google Scholar] [CrossRef]
- Fernandes, D.; Resende, C.; Cavalcanti, J.; Liu, D.; Elias, C. Biocompatibility of Bioabsorbable Mg–Ca Alloys with Rare Earth Elements Addition. J. Mater. Sci. Mater. Med. 2019, 30, 134. [Google Scholar] [CrossRef]
- Mohamed, A.; El-Aziz, A.M.; Breitinger, H.-G. Study of the Degradation Behavior and the Biocompatibility of Mg–0.8Ca Alloy for Orthopedic Implant Applications. J. Magnes. Alloys 2019, 7, 249–257. [Google Scholar] [CrossRef]
- Bita, A.-I.; Stan, G.E.; Niculescu, M.; Ciuca, I.; Vasile, E.; Antoniac, I. Adhesion Evaluation of Different Bioceramic Coatings on Mg–Ca Alloys for Biomedical Applications. J. Adhes. Sci. Technol. 2016, 30, 1968–1983. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, B.; Zhao, R.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhu, X.; Iulian, A.V.; Zhang, X. Evaluation on the Corrosion Resistance, Antibacterial Property and Osteogenic Activity of Biodegradable Mg-Ca and Mg-Ca-Zn-Ag Alloys. J. Magnes. Alloys, 2021; in press. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Zhao, Y.; Chen, M. Corrosion Degradation Behavior of Mg–Ca Alloy with High Ca Content in SBF. Trans. Nonferr. Metals Soc. China 2015, 25, 3339–3347. [Google Scholar] [CrossRef]
- Mareci, D.; Bolat, G.; Izquierdo, J.; Crimu, C.; Munteanu, C.; Antoniac, I.; Souto, R.M. Electrochemical Characteristics of Bioresorbable Binary MgCa Alloys in Ringer’s Solution: Revealing the Impact of Local PH Distributions during in-Vitro Dissolution. Mater. Sci. Eng. C 2016, 60, 402–410. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, J.; Wen, C.; Zhang, D.; Li, Y. Mechanical Properties, in Vitro Corrosion and Biocompatibility of Newly Developed Biodegradable Mg-Zr-Sr-Ho Alloys for Biomedical Applications. Sci. Rep. 2016, 6, 31990. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; An, J.; Luo, D.-M.; Hu, W.-Y.; Li, Y.C.; Hodgson, P.; Wen, C.E. Microstructures and Mechanical Properties of as Cast Mg–Zr–Ca Alloys for Biomedical Applications. Mater. Technol. 2012, 27, 52–54. [Google Scholar] [CrossRef][Green Version]
- Zhao, C.; Pan, F.; Zhang, L.; Pan, H.; Song, K.; Tang, A. Microstructure, Mechanical Properties, Bio-Corrosion Properties and Cytotoxicity of as-Extruded Mg-Sr Alloys. Mater. Sci. Eng. C 2017, 70, 1081–1088. [Google Scholar] [CrossRef]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In Vitro and in Vivo Studies on a Mg–Sr Binary Alloy System Developed as a New Kind of Biodegradable Metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef]
- Tie, D.; Feyerabend, F.; Müller, W.-D.; Schade, R.; Liefeith, K.; Kainer, K.; Willumeit, R. Antibacterial Biodegradable Mg-Ag Alloys. Eur. Cells Mater. 2013, 25, 284–298. [Google Scholar] [CrossRef]
- Bohlen, J.; Meyer, S.; Wiese, B.; Luthringer-Feyerabend, B.J.C.; Willumeit-Römer, R.; Letzig, D. Alloying and Processing Effects on the Microstructure, Mechanical Properties, and Degradation Behavior of Extruded Magnesium Alloys Containing Calcium, Cerium, or Silver. Materials 2020, 13, 391. [Google Scholar] [CrossRef]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W.; et al. Revolutionizing Biodegradable Metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Jähn, K.; Saito, H.; Taipaleenmäki, H.; Gasser, A.; Hort, N.; Feyerabend, F.; Schlüter, H.; Rueger, J.M.; Lehmann, W.; Willumeit-Römer, R.; et al. Intramedullary Mg2Ag Nails Augment Callus Formation during Fracture Healing in Mice. Acta Biomater. 2016, 36, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Staras, A.; Voicu, S.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.; Pandele, A.; Croitoru, S.; Miculescu, F.; et al. Characterization and In Vitro and In Vivo Assessment of a Novel Cellulose Acetate-Coated Mg-Based Alloy for Orthopedic Applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In Vivo Evaluation of Biodegradable Magnesium Alloy Bone Implant in the First 6 Months Implantation. J. Biomed. Mater. Res. Part A 2009, 90, 882–893. [Google Scholar] [CrossRef]
- Chou, D.-T.; Hong, D.; Oksuz, S.; Schweizer, R.; Roy, A.; Lee, B.; Shridhar, P.; Gorantla, V.; Kumta, P.N. Corrosion and Bone Healing of Mg-Y-Zn-Zr-Ca Alloy Implants: Comparative in Vivo Study in a Non-Immobilized Rat Femoral Fracture Model. J. Biomater. Appl. 2019, 33, 1178–1194. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Noorani, S.; Costello, B.J.; Sfeir, C. Fracture Healing Using Degradable Magnesium Fixation Plates and Screws. J. Oral Maxillofac. Surg. 2015, 73, 295–305. [Google Scholar] [CrossRef]
- Cheng, M.; Wahafu, T.; Jiang, G.; Liu, W.; Qiao, Y.; Peng, X.; Cheng, T.; Zhang, X.; He, G.; Liu, X. A Novel Open-Porous Magnesium Scaffold with Controllable Microstructures and Properties for Bone Regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yang, Z.Y.; Tan, L.L.; Li, H.; Zhang, Y.Z. An Animal Experimental Study of Porous Magnesium Scaffold Degradation and Osteogenesis. Braz. J. Med. Biol. Res. 2014, 47, 715–720. [Google Scholar] [CrossRef]
- Antoniac, I.; Adam, R.; Biță, A.; Miculescu, M.; Trante, O.; Petrescu, I.M.; Pogărășteanu, M. Comparative Assessment of In Vitro and In Vivo Biodegradation of Mg-1Ca Magnesium Alloys for Orthopedic Applications. Materials 2020, 14, 84. [Google Scholar] [CrossRef]
- Marukawa, E.; Tamai, M.; Takahashi, Y.; Hatakeyama, I.; Sato, M.; Higuchi, Y.; Kakidachi, H.; Taniguchi, H.; Sakamoto, T.; Honda, J.; et al. Comparison of Magnesium Alloys and Poly-l-Lactide Screws as Degradable Implants in a Canine Fracture Model: Comparison of Mg Alloys and PLLA Screws in Canine Fracture Model. J. Biomed. Mater. Res. 2016, 104, 1282–1289. [Google Scholar] [CrossRef]
- Kong, X.; Wang, L.; Li, G.; Qu, X.; Niu, J.; Tang, T.; Dai, K.; Yuan, G.; Hao, Y. Mg-Based Bone Implants Show Promising Osteoinductivity and Controllable Degradation: A Long-Term Study in a Goat Femoral Condyle Fracture Model. Mater. Sci. Eng. C 2018, 86, 42–47. [Google Scholar] [CrossRef]
- Imwinkelried, T.; Beck, S.; Iizuka, T.; Schaller, B. Effect of a Plasmaelectrolytic Coating on the Strength Retention of in Vivo and in Vitro Degraded Magnesium Implants. Acta Biomater. 2013, 9, 8643–8649. [Google Scholar] [CrossRef]
- Schaller, B. In Vivo Degradation of a New Concept of Magnesium-Based Rivet-Screws in the Minipig Mandibular Bone. Materials Sci. Eng. C 2016, 8, 247–254. [Google Scholar] [CrossRef]
- Bita, A.-I.; Antoniac, I.; Ciuca, I. Potential Use of Mg-Ca Alloys for Orthopedic Applications. U.P.B. Sci. Bull. Ser. B 2016, 78, 173–184. [Google Scholar]
- Choo, J.T.; Lai, S.H.S.; Tang, C.Q.Y.; Thevendran, G. Magnesium-Based Bioabsorbable Screw Fixation for Hallux Valgus Surgery—A Suitable Alternative to Metallic Implants. Foot Ankle Surg. 2019, 25, 727–732. [Google Scholar] [CrossRef]
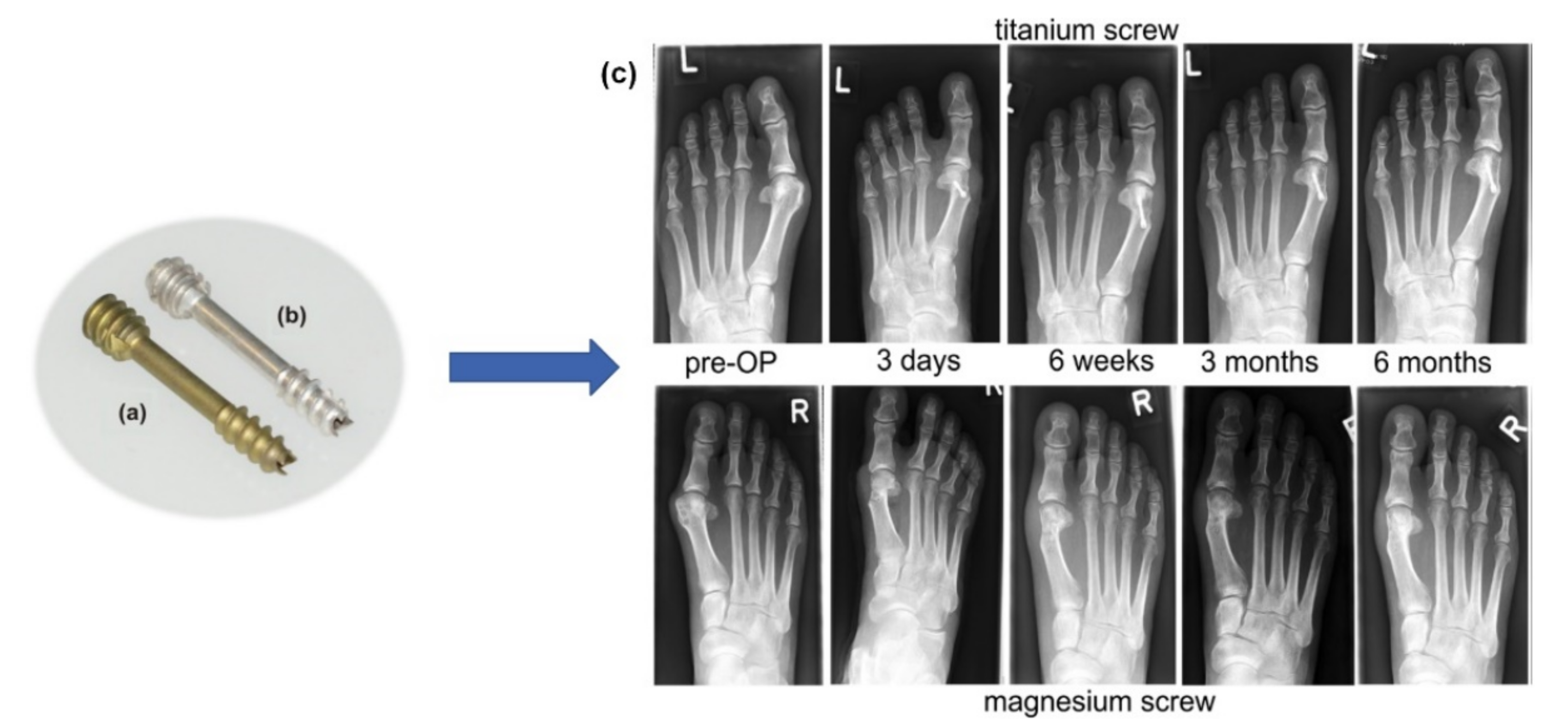
- Plaass, C.; von Falck, C.; Ettinger, S.; Sonnow, L.; Calderone, F.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Waizy, H.; Daniilidis, K.; et al. Bioabsorbable Magnesium versus Standard Titanium Compression Screws for Fixation of Distal Metatarsal Osteotomies—3 Year Results of a Randomized Clinical Trial. J. Orthop. Sci. 2018, 23, 321–327. [Google Scholar] [CrossRef]
- Klauser, H. Internal Fixation of Three-Dimensional Distal Metatarsal I Osteotomies in the Treatment of Hallux Valgus Deformities Using Biodegradable Magnesium Screws in Comparison to Titanium Screws. Foot Ankle Surg. 2019, 25, 398–405. [Google Scholar] [CrossRef]
- Acar, B.; Kose, O.; Turan, A.; Unal, M.; Kati, Y.A.; Guler, F. Comparison of Bioabsorbable Magnesium versus Titanium Screw Fixation for Modified Distal Chevron Osteotomy in Hallux Valgus. BioMed Res. Int. 2018, 2018, 5242806. [Google Scholar] [CrossRef]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable Magnesium-Based Screw Clinically Equivalent to Titanium Screw in Hallux Valgus Surgery: Short Term Results of the First Prospective, Randomized, Controlled Clinical Pilot Study. BioMed Eng. OnLine 2013, 12, 62. [Google Scholar] [CrossRef]
- May, H.; Alper Kati, Y.; Gumussuyu, G.; Yunus Emre, T.; Unal, M.; Kose, O. Bioabsorbable Magnesium Screw versus Conventional Titanium Screw Fixation for Medial Malleolar Fractures. J. Orthop. Traumatol. 2020, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Acar, B.; Kose, O.; Unal, M.; Turan, A.; Kati, Y.A.; Guler, F. Comparison of Magnesium versus Titanium Screw Fixation for Biplane Chevron Medial Malleolar Osteotomy in the Treatment of Osteochondral Lesions of the Talus. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Turan, A.; Unal, M.; Acar, B.; Guler, F. Fixation of Medial Malleolar Fractures with Magnesium Bioabsorbable Headless Compression Screws: Short-Term Clinical and Radiological Outcomes in Eleven Patients. Arch. Orthop. Trauma Surg. 2018, 138, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, H.; Ziegler, A.; Lauer, G.; Franke, A. Osteosynthesis of the Mandibular Condyle With Magnesium-Based Biodegradable Headless Compression Screws Show Good Clinical Results During a 1-Year Follow-Up Period. J. Oral Maxillofac. Surg. 2021, 79, 637–643. [Google Scholar] [CrossRef]
- Leonhardt, H.; Franke, A.; McLeod, N.M.H.; Lauer, G.; Nowak, A. Fixation of Fractures of the Condylar Head of the Mandible with a New Magnesium-Alloy Biodegradable Cannulated Headless Bone Screw. Br. J. Oral Maxillofac. Surg. 2017, 55, 623–625. [Google Scholar] [CrossRef]
- Biber, R.; Pauser, J.; Geßlein, M.; Bail, H.J. Magnesium-Based Absorbable Metal Screws for Intra-Articular Fracture Fixation. Case Rep. Orthop. 2016, 2016, 9673174. [Google Scholar] [CrossRef]
- Grieve, P.; O’Carroll, S.; Albastaki, O. Six cas de série de patients de Magnezix®. Une vis métallique absorbable pour la fixation de la fracture du carpe et des fusions entre les carpes. Hand Surg. Rehabil. 2017, 36, 488–489. [Google Scholar] [CrossRef]
- Meier, R.; Panzica, M. First results with a resorbable MgYREZr compression screw in unstable scaphoid fractures show extensive bone cysts. Handchir. Mikrochir. Plast. Chir. 2017, 49, 37–41. [Google Scholar] [CrossRef]
- Turan, A.; Kati, Y.A.; Acar, B.; Kose, O. Magnesium Bioabsorbable Screw Fixation of Radial Styloid Fractures: Case Report. Jnl. Wrist Surg. 2020, 09, 150–155. [Google Scholar] [CrossRef]
- Wichelhaus, A.; Emmerich, J.; Mittlmeier, T. A Case of Implant Failure in Partial Wrist Fusion Applying Magnesium-Based Headless Bone Screws. Case Rep. Orthop. 2016, 2016, 7049130. [Google Scholar] [CrossRef]
- Deguchi, T.; Takano-Yamamoto, T.; Kanomi, R.; Hartsfield, J.K.; Roberts, W.E.; Garetto, L.P. The Use of Small Titanium Screws for Orthodontic Anchorage. J. Dent. Res. 2003, 82, 377–381. [Google Scholar] [CrossRef]
- Shiwaku, K.; Suzuki, T.; Matsumura, T.; Takashima, H.; Otsubo, H.; Yamashita, T. Bioabsorbable Interference Screws Can Be Used with Less Tunnel Widening in Anatomic Rectangular Tunnel Anterior Cruciate Ligament Reconstruction with a Bone–Patellar-Tendon–Bone Graft. Knee 2020, 27, 1293–1299. [Google Scholar] [CrossRef]
- Pins–Syntellix AG. Available online: https://www.syntellix.de/en/products/advantages-and-application/pins.html (accessed on 7 December 2021).
- Krämer, M.; Schilling, M.; Eifler, R.; Hering, B.; Reifenrath, J.; Besdo, S.; Windhagen, H.; Willbold, E.; Weizbauer, A. Corrosion Behavior, Biocompatibility and Biomechanical Stability of a Prototype Magnesium-Based Biodegradable Intramedullary Nailing System. Mater. Sci. Eng. C 2016, 59, 129–135. [Google Scholar] [CrossRef]
- Resoloy®: A Magnesium Alloy for Absorbable Devices-Fort Wayne Metals. Available online: https://www.fwmetals.com/services/r-d/rd-update/previous-rd-updates/resoloy-a-magnesium-alloy-for-absorbable-devices/ (accessed on 16 October 2021).
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Moharamzadeh, K.; Boccaccini, A.R.; Tayebi, L. Porous Magnesium-Based Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2017, 71, 1253–1266. [Google Scholar] [CrossRef]
- All–Syntellix AG. Available online: https://www.syntellix.de/en/products/product-overview/all.html (accessed on 16 October 2021).
- Yu, X.; Zhao, D.; Huang, S.; Wang, B.; Zhang, X.; Wang, W.; Wei, X. Biodegradable Magnesium Screws and Vascularized Iliac Grafting for Displaced Femoral Neck Fracture in Young Adults. BMC Musculoskelet. Disord. 2015, 16, 329. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Z.; Wang, M.; Huang, W.; Ke, J.; Zhao, D.; Yin, Q.; Zhang, Y. Treatment of Trauma-Induced Femoral Head Necrosis with Biodegradable Pure Mg Screw-Fixed Pedicle Iliac Bone Flap. J. Orthop. Transl. 2019, 17, 133–137. [Google Scholar] [CrossRef]
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H.; et al. Long-Term Clinical Study and Multiscale Analysis of in Vivo Biodegradation Mechanism of Mg Alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef]
- Kozakiewicz, M. Are Magnesium Screws Proper for Mandibular Condyle Head Osteosynthesis? Materials 2020, 13, 2641. [Google Scholar] [CrossRef]
- Kania, A.; Nowosielski, R.; Gawlas-Mucha, A.; Babilas, R. Mechanical and Corrosion Properties of Mg-Based Alloys with Gd Addition. Materials 2019, 12, 1775. [Google Scholar] [CrossRef]
- Rahman, M.; Dutta, N.K.; Roy Choudhury, N. Magnesium Alloys With Tunable Interfaces as Bone Implant Materials. Front. Bioeng. Biotechnol. 2020, 8, 564. [Google Scholar] [CrossRef]
- Huang, S.; Wang, B.; Zhang, X.; Lu, F.; Wang, Z.; Tian, S.; Li, D.; Yang, J.; Cao, F.; Cheng, L.; et al. High-Purity Weight-Bearing Magnesium Screw: Translational Application in the Healing of Femoral Neck Fracture. Biomaterials 2020, 238, 119829. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, H. A Facile Method to Prepare a Superhydrophobic Magnesium Alloy Surface. Materials 2020, 13, 4007. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Antoniac, A.; Vasile, E.; Tecu, C.; Fosca, M.; Yankova, V.G.; Rau, J.V. In Vitro Characterization of Novel Nanostructured Collagen-Hydroxyapatite Composite Scaffolds Doped with Magnesium with Improved Biodegradation Rate for Hard Tissue Regeneration. Bioact. Mater. 2021, 6, 3383–3395. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Sheng, Y.; Huang, L.; Chow, D.H.-K.; Chau, W.H.; Tang, N.; Ngai, T.; Wu, C.; Lu, J.; Qin, L. An Innovative Mg/Ti Hybrid Fixation System Developed for Fracture Fixation and Healing Enhancement at Load-Bearing Skeletal Site. Biomaterials 2018, 180, 173–183. [Google Scholar] [CrossRef] [PubMed]

| Fracture Type | Implant Type | Mg-Based Alloy | Animal Model/Human | Ref. |
|---|---|---|---|---|
| Skull fracture | Four-hole plates (1 mm thickness, 22 mm in length) and cortical bone screws (2 mm in diameter, 5 mm in length) | WE43/Mg-Y-RE-Zr (Syntellix AG) | Miniature pig | [20] |
| Comminuted distal humerus fracture | 2.7 mm diameter screws | Mg-Y-RE-Zr (MAGNEZIX®, Syntellix AG) | Human male 50 years old (2018) | [21] |
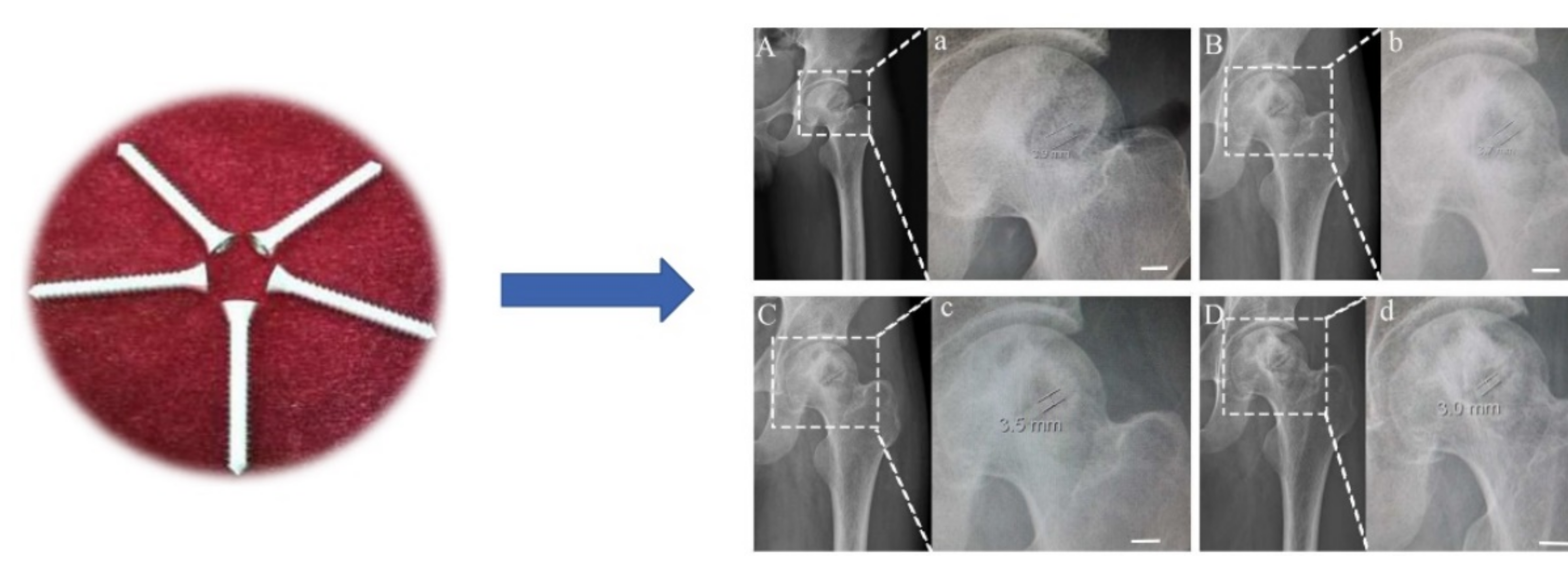
| Tibial spine fracture | Magnesium screws | Mg-Y-RE-Zr (MAGNEZIX®, Syntellix AG) | Human female 20 years old (2018) | [22] |
| Femoral fracture in osteoporotic bone | Intramedullary nail | Mg-Nd-Zn-Zr (JDBM) integrated with the anti-catabolic drug zoledronic acid (ZA) | Rat | [23] |
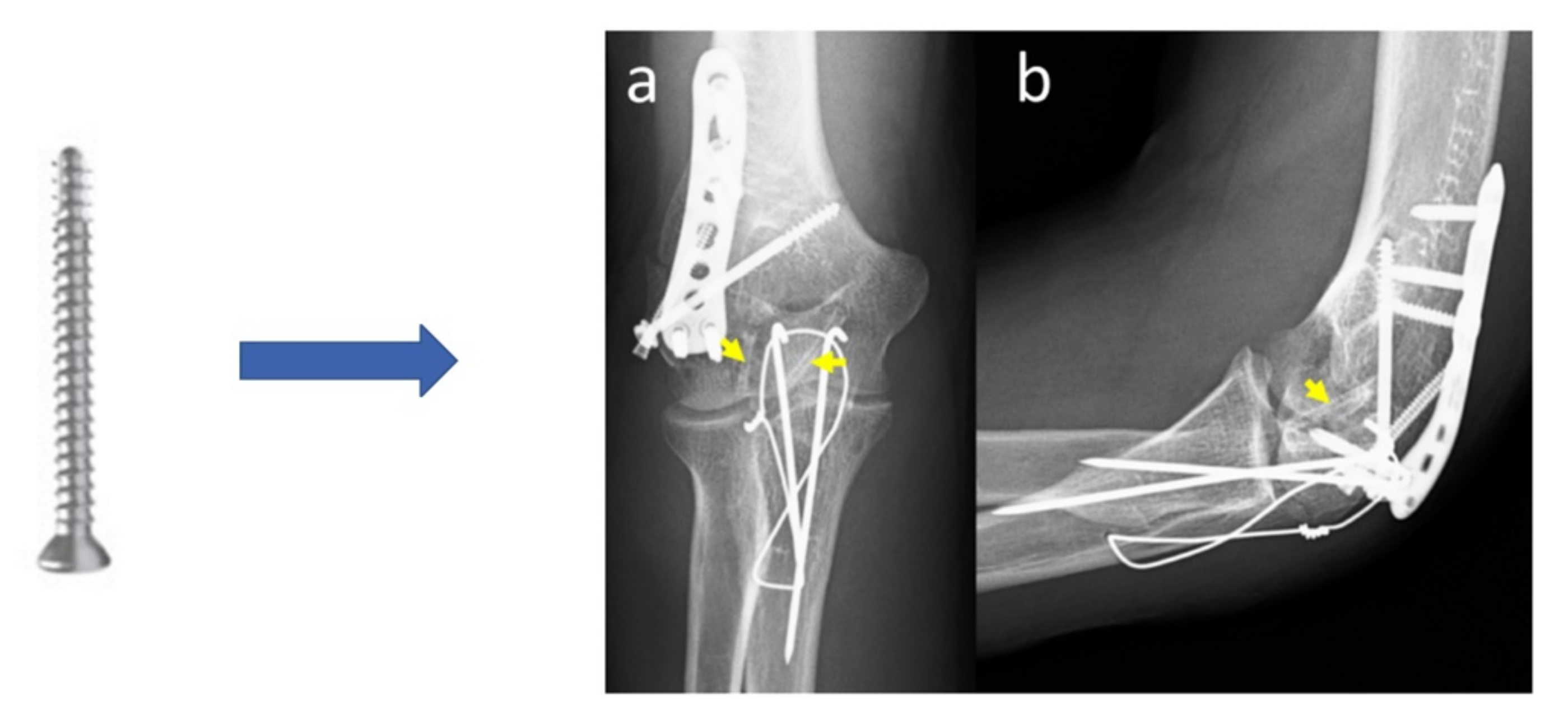
| Elbow fracture | 2.7 mm diameter screws | Mg-Y-RE-Zr (MAGNEZIX®, Syntellix AG) | Human female 48 years old (2020) | [24] |
| Osteotomized bone in sheep | screw | Mg-Zn-Ca (ZX00) | Sheep | [25] |
| Ulna fracture | Plates with an area of 20 mm × 4.5 mm, thickness of 1–1.5 mm, screws 7 mm in length, | Pure Mg (99.9%) | White rabbit | [26] |
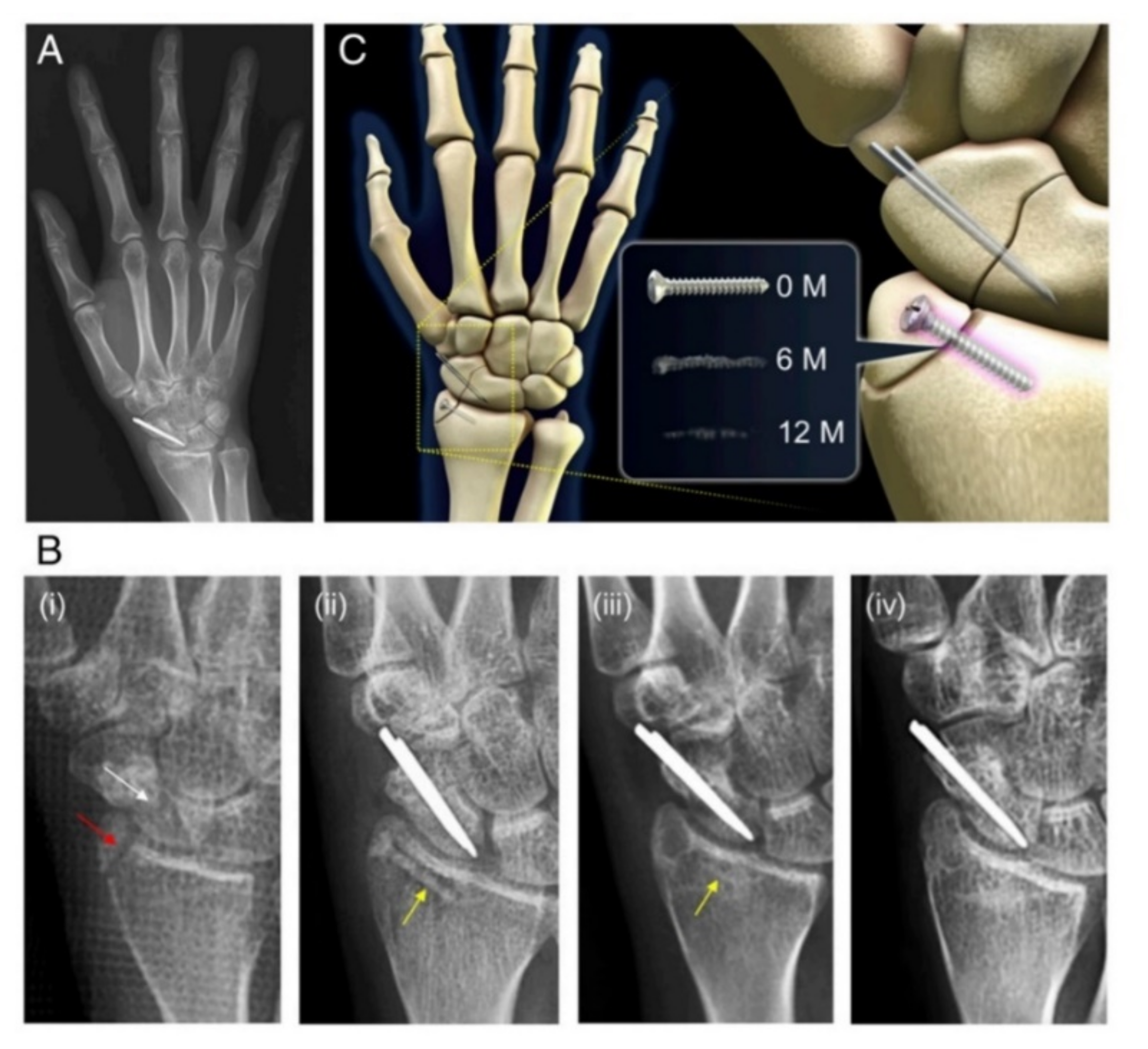
| Scaphoid fracture | Magnesium-based headless Herbert screw | Mg-Y-RE-Zr/WE43 | Human (190 patients) | [27] |
| Isolated lateral malleolar fracture | Cannulated headless compression screws | Mg-Y-RE-Zr | Human female 19 years old (2018) | [28] |
| Patella fracture | Fixation pin with a diameter of 1 mm | Pure Mg (99.9%) | New Zealand white rabbits | [29] |
| Alloying Element | Physiological and Toxicological Characteristics |
|---|---|
| Magnesium (Mg) | The normal blood quantity of Mg ranges between 0.73 and 1.06 mmol/L. It has a benefic effect on metabolism, cells proliferation, and protein synthesis. It regulates the activity of about 350 different proteins, and it stabilizes the DNA and RNA. It has a long-term influence on cellular reactions. |
| Calcium (Ca) | The normal blood quantity of Ca ranges between 0.919 and 0.993 mg/L. It is the most abundant mineral material in the human body and is deposed in bones. The skeletal, renal, and intestinal homeostases regulate the Ca quantity. |
| Zinc (Zn) | The normal blood quantity of Zn is between 12.4 and 17.4 μmol/L. It is a trace element that is essential for the immune system. It is considered an enzymatic agent for bones and cartilages. High concentrations can exhibit neurotoxic effects. |
| Manganese (Mn) | The normal blood quantity of Mn must be lower than 0.8 μg/L. It is an essential trace element. Mn plays an important role in the metabolic circuit of lipids, amino acids, and carbohydrates. It has an influence on the immune system, bone growth, and blood coagulation. At high concentrations, it can exhibit neurotoxic effects. |
| Rare earth (RE) | A lot of rare earths exhibit anticancer properties. |
| Strontium (Sr) | The normal blood quantity of Sr should be equal to 0.17 mg (total). Notably, 99% of total Sr is located in bones, and it proves a metabolic effect on bone and stimulates new bone formation. Sr in high doses results in hypocalcemia or skeletal unwanted effects. |
| Zirconium (Zr) | The normal blood quantity of Zr must be lower than 0.250 mg (total). Zr shows low ionic toxicity and good biocompatibility. Zr accumulates in the bone and nervous system. |
| Impurity | Physiological and Toxicological Characteristics |
|---|---|
| Nickel (Ni) | The normal blood quantity of Ni ranges between 0.05 and 0.23 μg/L. It manifests allergenic properties, and it can induce metal sensitization. It has a strong carcinogenic and genotoxic effect. |
| Beryllium (Be) | The toxic dose is considered higher than 2 μg/m3. It has carcinogenic potential and induces metal sensitization. |
| Iron (Fe) | The normal blood quantity of Fe is between 5 and 17.6 g/L. It is regulated and deposited through human body metabolism. |
| Copper (Cu) | The normal blood quantity of Cu ranges between 74 and 131 μmol/L. |
| Magnesium Alloy | Element (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASTM | Al | Zn | Mn | Re a | Zr | Y | Ca | Ag | Nd | Cu | Mg |
| AZ31A | 2.4 | 0.50 | 0.15 | - | - | - | - | - | - | - | bal |
| AZ31B | 2.4 | 0.50 | 0.05 | - | - | - | - | - | - | - | bal |
| AZ61A | 5.5 | 0.50 | 0.15 | - | - | - | - | - | - | - | bal |
| AZ80A | 7.8 | 0.20 | 0.12 | - | - | - | - | - | - | - | bal |
| MC1 | - | - | - | - | - | - | 0.80 | - | - | - | bal |
| ZK60 | - | 8.00 | - | - | - | 1.50 | - | - | - | - | bal |
| ZM21 | - | 2.00 | 1.16 | - | - | 0.16 | - | - | - | - | bal |
| EW10 | - | - | - | - | 0.50 | 0.50 | - | - | 1.20 | - | bal |
| ZEK 100 | - | 1.00 | - | 0.12 | 0.20 | 0.17 | - | - | - | - | bal |
| ZMX410 | - | 4.30 | 0.62 | - | - | - | 0.30 | - | - | - | bal |
| ZE41 | - | 4.20 | - | 1.30 | 0.40 | - | - | - | - | - | bal |
| ZC71A | - | 6.0 | 0.5 | - | - | - | - | - | - | 1.00 | bal |
| WE54A | - | - | - | 1.50 | - | 4.75 | - | - | - | - | bal |
| WE43A | - | - | - | 2.40 | - | 3.70 | - | - | - | - | bal |
| ZQ71 | - | 7.2 | - | - | 1.30 | 0.20 | - | 1.50 | - | - | bal |
| ZQ63 | - | 6.4 | - | - | 1.00 | 0.16 | - | 2.50 | - | - | bal |
| MRI 201s | - | 0.3 | - | - | 0.60 | 2.10 | - | - | 3.20 | - | bal |
| MRI 202s | - | 0.3 | - | - | 0.40 | 0.21 | - | - | 3.10 | - | bal |
| ZMX 410 | - | 4.3 | 0.62 | - | - | - | 0.30 | - | - | - | bal |
| ZMX 100 | - | 1.3 | 0.51 | - | 0.03 | - | 0.38 | - | - | - | bal |
| Alloy Composition (wt.%) | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) | Hardness | Ref. |
|---|---|---|---|---|---|
| Mg-4.71Y-4.58Gd-0.31Zr. | 255 | 330 | 15 | 1200 Hv | [66] |
| EW10 (Mg-1.2Nd-0.5Y-0.5Zr) | 77 ± 4 | 175 ± 11 | 12 ± 3 | - | [67] |
| EW10 + 0.4Ca | 74 ± 5 | 135 ± 11 | 5 ± 1 | - | [67] |
| ZK60 (Mg-8Zn-1.5Y) | 390 | 445 | 8.3 | 69 Hv | [68] |
| WE43 (Mg-4.38Y-2.72Nd-1.1Gd-0.56Zr) | 145 ± 16 | 204 ± 6 | 6.9 ± 0.5 | 85 Hv | [69] |
| Mg- 5Zn | 68 ± 1.5 | 185 ± 5 | 9.2 ± 0.5 | - | [57] |
| Mg-4Zn-0.2Ca | 58.1 ± 1 | 255 ± 5 | 17.5 ± 1 | - | [70] |
| Mg–Zn -Mn | 78 ± 2 | 175 ± 3 | 12 | 68 Hv | [71] |
| Mg- 4Zn-0.5Ca-0.16Mn | 175 | 180 | 0.2 | 70 Hv | [72] |
| Mg-2Zr-2Sr | 80 | 290 | 15 | - | [73] |
| JDBM (Mg-3.13Nd-0.16Zn-0.41Zr) | 189 ± 2 | 243 ± 3 | 21 ± 0.9 | - | [74] |
| Animal Model | Age/Weight | Fracture Site | Mg-Based Implant Type | Implant Type | Follow-Up | Ref. |
|---|---|---|---|---|---|---|
| Mouse | 10 weeks | femur | Mg-2Ag | Intramedullary nail | 133 days | [121] |
| Mouse | 3 months | femur | Mg-2Sr | Rods | 30 days | [117] |
| Albino rat | 8 weeks | femur | Mg-1Ca-0.2Mn-0.6Zr | Intramedullary bar | 180 days | [122] |
| Rat | - | femur cortical bone | Mg-1Zn-0.8Mn | Rod implant | 182 days | [123] |
| Sprague-Dawley (SD) rat | 250 g | femur | Mg-4Y-2Zn-1Zr-0.6Ca (WZ42) | Intramedullary pin and wire | 98 days | [124] |
| Sprague-Dawley (SD) rat | 9 months | femur | Mg-3Nd-0.2Zn-0.4Zr (JDBM) | Intramedullary pin | 84 days | [23] |
| Sprague-Dawley (SD) rat | 8 weeks/220 g | femur | Mg-0.8Ca Mg-0.8Ca-5Zn-1.5Ag Mg-0.8Ca-5Zn-2.5Ag | Cylindrical samples | 28 days | [111] |
| Dunkin Hartley guinea pig | 658 g | femur | WE43 | Rods | 126 days | [125] |
| New Zealand White (NZW) rabbit | 19 weeks | ulna | 99.9% Mg | Plate and screw | 28 days | [126] |
| New Zealand White (NZW) rabbit | 6 months/2.5 kg | femur | Mg-5Zr Mg-1Zr-2Sr Mg-2Zr-5Sr | Cylindrical samples | 90 days | [73] |
| New Zealand White (NZW) rabbit | 3.87 kg | lateral epicondyle femur | High purity Mg | Porous scaffold | 112 days | [127] |
| New Zealand White (NZW) rabbit | 6 months | right femoral condyle | High purity Mg | 4-hole cylindrical scaffold | 84 days | [128] |
| Oryctolagus Cuniculus rabbit | 3.5 kg | femur | Mg-1Ca | Parallelepiped samples | 42 days | [129] |
| Beagle dog | 1 year-10 kg | tibia | WE43 | Screw | 84 days | [130] |
| Goat | - | femur | JDBM | Screw | 548 days | [131] |
| Sheep | 1 months | tibia | Mg-0.45Zn-0.45Ca (ZX00) | Screw | 84 days | [25] |
| Mini pig | 14 months, 50 kg | frontal bone | WE43 | Osteosynthesis plates and screws | 210 days | [20] |
| Miniature mini pig | 30–36 months, 53 kg | frontal bone | WE43 | Plate with plasma electrolytic coating | 168 days | [132] |
| Mini pig | 53 kg | mandibular bone | modified WE43 | Based rivet screws | 168 days | [133] |
| Clinical Needs | Patients | Clinical Outcomes | Follow-Up | Complications | Ref. |
|---|---|---|---|---|---|
| Hallux valgus | 24 | Similar functional outcomes with titanium screw used as control group | 12 months | None | [135] |
| 13 | Similar functional outcomes with titanium screw used as control group | 3 years | None | [136] | |
| 100 | Similar functional outcomes with titanium screw used as control group | 12.2 weeks | Soft tissue irritation, delayed wound healing, screw fracture | [137] | |
| 16 | Excellent | 17.6 months | Prolonged swelling | [138] | |
| 13 | Both groups (Mg and Ti screws) were similar regarding the functional outcomes | 6 months | None | [139] | |
| Malleolar fracture and osteotomy | 23 | Similar functional outcomes with titanium screw used as control group | 1 year | None | [140] |
| 12 | Similar to control titanium screw group | 1 year | Pain and irritation | [141] | |
| 11 | Excellent | 17 months | None | [142] | |
| Mandible fracture | 6 | Improvement in mouth opening, left and right laterotrusion and protrusion distance | 1 year | - | [143] |
| 5 | Excellent with good occlusion | 3 months | One screw fracture, revised with Mg screw | [144] | |
| Humeral fracture (elbow) | 1 | Excellent | 4 months | None | [21] |
| 1 | Excellent | 24 months | None | [145] | |
| Carpus | 6 | Good results | 6–18 months | None | [146] |
| 5 | Excellent | 24 months | Extensive resorption cysts in the case of 3 patients | [147] | |
| Knee intercondylar tibial eminence fracture | 3 | Excellent with new bone formation observed at the end of the follow-up time | 12 months | None | [22] |
| Distal radius fractures | 2 | Excellent | 27 months | None | [148] |
| 1 | Poor | 6 weeks | Revision following loosening and backing out of the screw, osteolysis and pain | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniac, I.; Miculescu, M.; Mănescu, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. https://doi.org/10.3390/ma15031148
Antoniac I, Miculescu M, Mănescu V, Stere A, Quan PH, Păltânea G, Robu A, Earar K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials. 2022; 15(3):1148. https://doi.org/10.3390/ma15031148
Chicago/Turabian StyleAntoniac, Iulian, Marian Miculescu, Veronica Mănescu (Păltânea), Alexandru Stere, Pham Hong Quan, Gheorghe Păltânea, Alina Robu, and Kamel Earar. 2022. "Magnesium-Based Alloys Used in Orthopedic Surgery" Materials 15, no. 3: 1148. https://doi.org/10.3390/ma15031148
APA StyleAntoniac, I., Miculescu, M., Mănescu, V., Stere, A., Quan, P. H., Păltânea, G., Robu, A., & Earar, K. (2022). Magnesium-Based Alloys Used in Orthopedic Surgery. Materials, 15(3), 1148. https://doi.org/10.3390/ma15031148






.png)



