Cobalt Minimisation in Violet Co3P2O8 Pigment
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inorganic Crystal Structure Database (ICSD Web); Fachinformationszentrum (FIZ): Karlsruhe, Germany, 2021.
- DCMA. Classification and Chemical Description of the Mixed Metal Oxide Inorganic Coloured Pigments, 2nd ed.; Metal Oxides and Ceramics Colors Subcommittee, Dry Color Manufacturer’s Ass.: Washington, DC, USA, 1982.
- Sarver, J.F. Figure. 2309. System CoO-Co(PO3)2. Trans. Brit. Ceram. Soc. 1966, 65, 196. [Google Scholar]
- Tena, M.A.; Mendoza, R.; Trobajo, C.; García, J.R.; García-Granda, S. Ceramic pigments from CoxNi3-xP2O8 (0 ≤ x ≤ 3) solid solutions. Ceram. Int. 2021, 47, 29888–29899. [Google Scholar] [CrossRef]
- Roczniki, J.B. Figure 272. System MgO-P2O5. Chemistry 1958, 32, 19. [Google Scholar]
- Thejus, P.K.; Koley, B.; Nishanth, K.G. An intense purple chromophore based on Co2+ in distorted tetrahedral coordination. Dyes Pigm. 2018, 158, 267–276. [Google Scholar] [CrossRef]
- Nord, A.G.; Stefanidis, T. Structure Refinements of Co3(PO4)2. A Note on the Reliability of Powder Diffraction Studies. Acta Chem. Scand. 1983, A37, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Junghwa, H.; Seungwan, S.; Seungtae, H. Strontium magnesium phosphate, Sr2 + xMg3-xP 4O15 (x ~ 0.36), from laboratory X-ray powder data. Acta Cryst. 2011, C67, i1–i3. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. (September 2018-ILL-JRC). Fullprof.2k Computer Program, version 6.50 France; 2018. Available online: http://www.ill.eu/sites/fullprof/ (accessed on 16 January 2022).
- Chapon, L.; Rodriguez-Carvajal, J. FPStudio Computer Program, version 2.0; Rutherford Appleton Laboratory: Oxfordshire, UK; Institut Laue Langevin: Grenoble, France, 2008. [Google Scholar]
- Nord, A.G.; Stefanidis, T. The cation distribution in two (Co, Mg)3(PO4)2 solid solutions. Z. Fur Krist. 1980, 153, 141–149. [Google Scholar] [CrossRef]
- Nord, A.G.; Stefanidis, T. The cation distribution between five- and six-coordinated sites in some (Mg, Me)3(PO4)2) solid solutions. Mat. Res. Bull. 1980, 15, 1183–1191. [Google Scholar] [CrossRef]
- Aaddane, A.; Kacimi, M.; Ziyad, M. Oxidative dehydrogenation of ethane and propane over magnesium–cobalt phosphates CoxMg3−x(PO4)2. Catal. Lett. 2001, 73, 47–53. [Google Scholar] [CrossRef]
- Forés, A.; Llusar, M.; Badenes, J.A.; Calbo, J.; Tena, M.A.; Monrós, G. Cobalt minimisation in willemite (CoxZn2−xSiO4) ceramic pigments. Green Chem. 2000, 2, 93–100. [Google Scholar] [CrossRef]
- Commission Internationale del’Eclairage. Recommendations on Uniform Color Spaces, Color Difference Equations, Phychometrics Color Terms. 1978. In Supplement No. 2 of CIE Publication No. 15 (E1-1.31); Bureau Central de la CIE: Paris, France, 1971. [Google Scholar]
- West, A.R. Solid State Chemistry and Its Applications; John Wiley & Sons: Chichester, UK, 1984. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier Science B. V.: Amsterdam, The Netherlands, 1977; pp. 507–511. [Google Scholar]
- Jesionowski, T.; Ciesielczyk, F. Inorganic, Hybrid and Functional Pigments. In Encyclopedia of Color Science and Technology; Shamey, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Llusar, M.; Forés, A.; Badenes, J.A.; Tena, M.A.; Monrós, G. Colour analysis of some cobalt-based blue pigments. J. Eur. Ceram. Soc. 2001, 21, 1121–1130. [Google Scholar] [CrossRef]
- Molinari, C.; Conte, S.; Zanelli, C.; Ardit, M.; Cruciani, G.; Dondi, M. Ceramic pigments and dyes beyond the inkjet revolution: From technological requeriments to constraints in colorant design. Ceram. Int. 2020, 46, 21839–21872. [Google Scholar] [CrossRef]
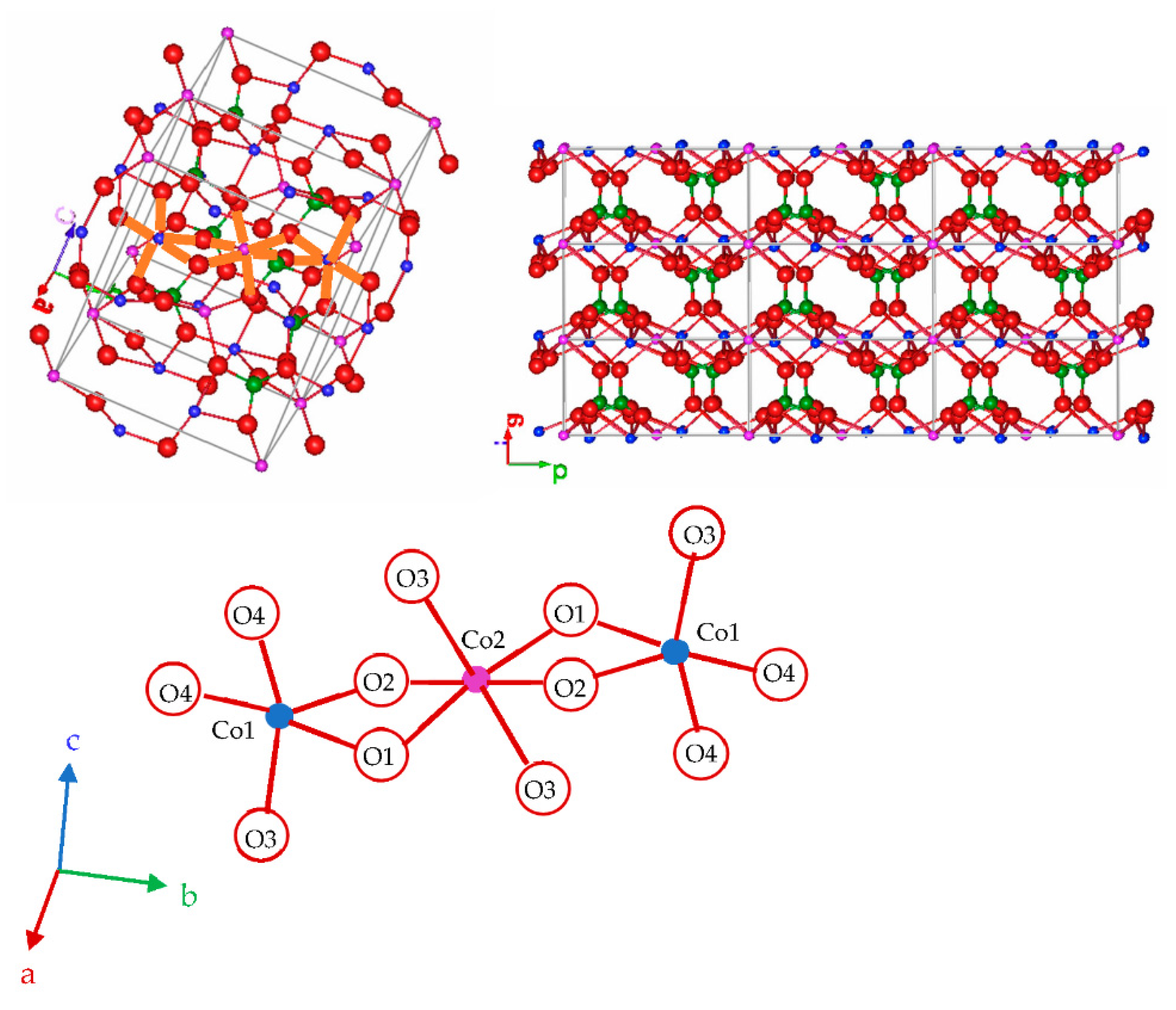
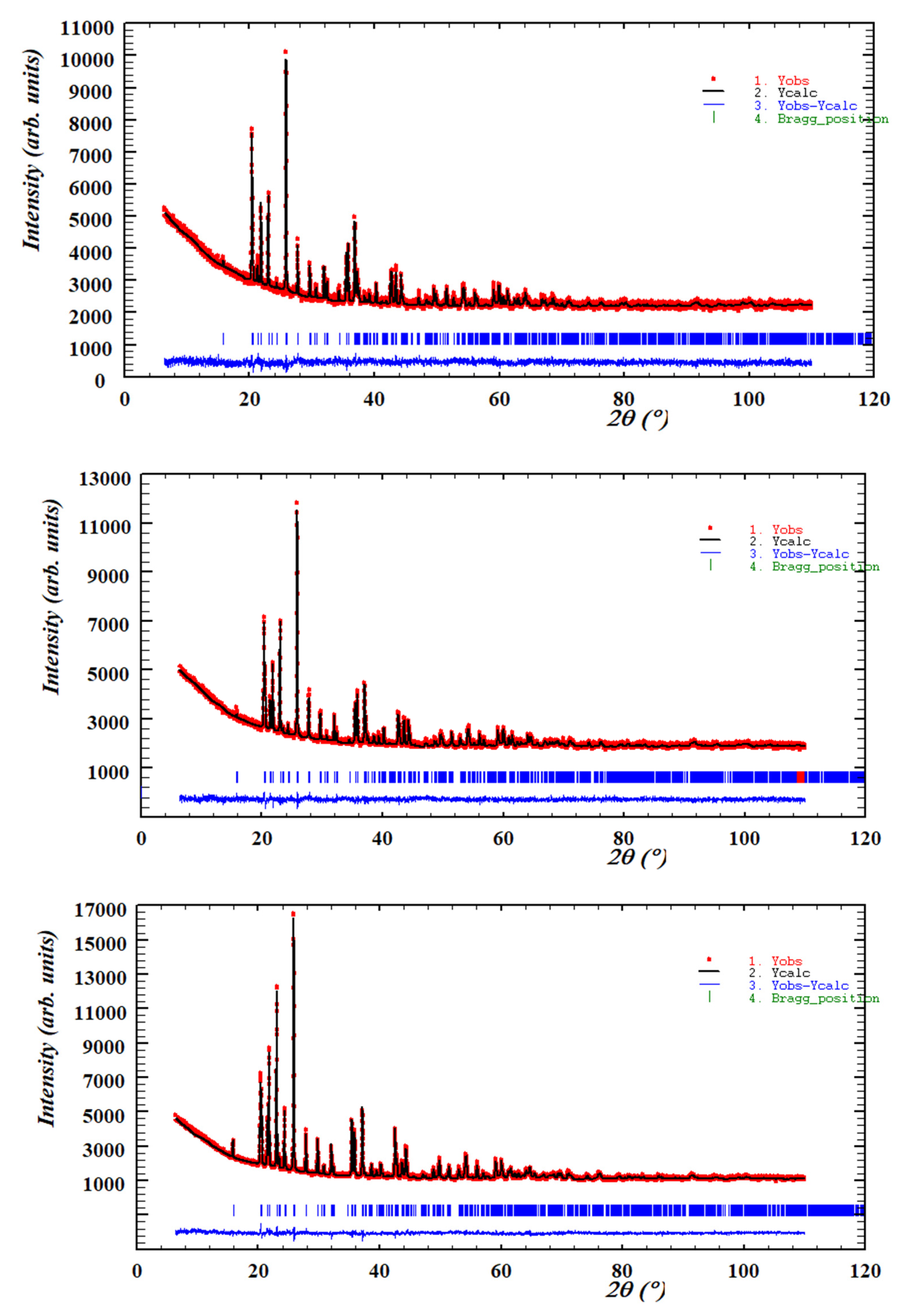

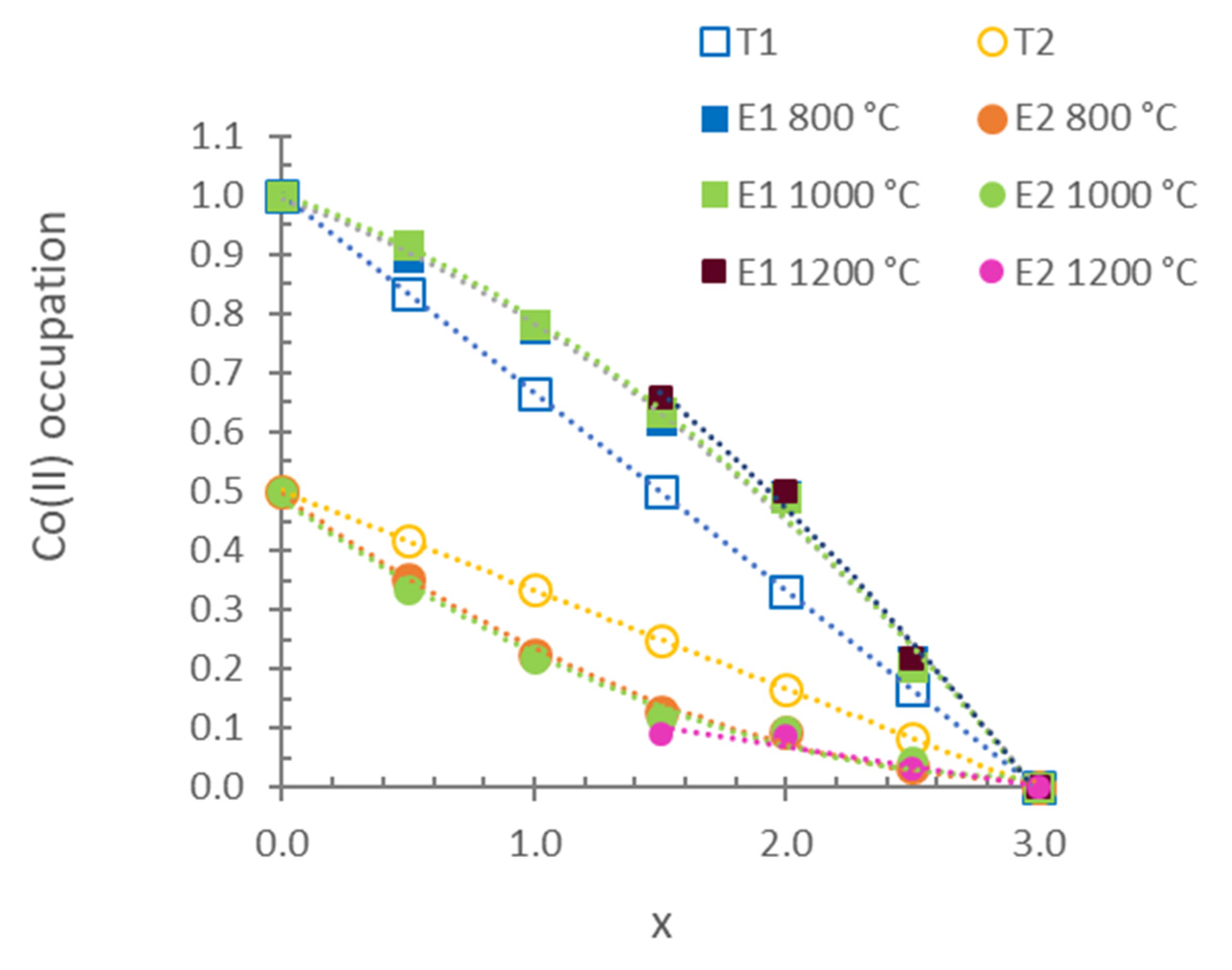
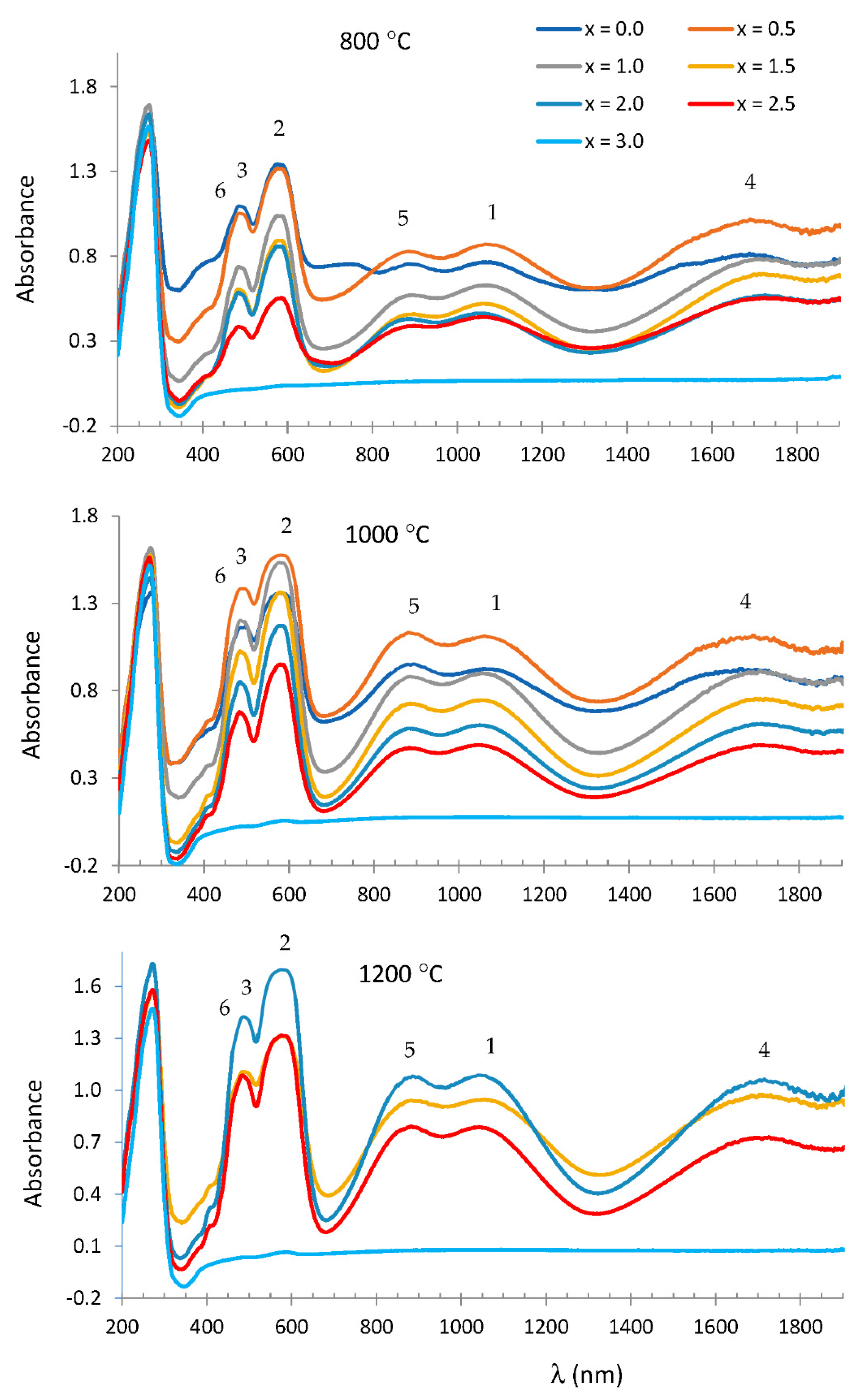

| X | 800 °C | 1000 °C | 1200 °C |
|---|---|---|---|
| 0.0 | C(s) | C(s) | |
| 0.5 | C(s) | C(s) | |
| 1.0 | C(s) | C(s) | |
| 1.5 | C(s) | C(s) | C(s) |
| 2.0 | C(s), M(vw) | C(s) | C(s) |
| 2.5 | C(s), M(vw) | C(s) | C(s) |
| 3.0 | C(s), M(w) | C(s) | C(s) |
| T (°C) | x | a (Å) | b (Å) | c (Å) | β (°) | V (Å3) |
|---|---|---|---|---|---|---|
| 800 | 0.0 | 5.0629(1) | 8.3618(2) | 8.7888(2) | 121.003(1) | 318.92(1) |
| 800 | 0.5 | 5.0655(1) | 8.3377(2) | 8.7938(2) | 120.882(1) | 318.75(1) |
| 800 | 1.0 | 5.0691(1) | 8.3131(1) | 8.7999(2) | 120.840(1) | 318.40(1) |
| 800 | 1.5 | 5.07242(9) | 8.2887(1) | 8.8076(1) | 120.8274(9) | 318.018(9) |
| 800 | 2.0 | 5.07424(8) | 8.2691(1) | 8.8169(1) | 120.8303(8) | 317.675(8) |
| 800 | 2.5 | 5.0734(1) | 8.2505(2) | 8.8237(2) | 120.854(1) | 317.07(1) |
| 800 | 3.0 | 5.07529(9) | 8.2370(1) | 8.8316(2) | 120.862(1) | 316.93(1) |
| 1000 | 0.0 | 5.06288(8) | 8.3631(1) | 8.7894(1) | 120.9897(8) | 319.027(8) |
| 1000 | 0.5 | 5.06491(9) | 8.3386(1) | 8.7933(2) | 120.8665(9) | 318.78(1) |
| 1000 | 1.0 | 5.0677(1) | 8.3143(2) | 8.7981(2) | 120.820(1) | 318.35(1) |
| 1000 | 1.5 | 5.07022(8) | 8.2914(1) | 8.8056(1) | 120.7983(8) | 317.975(8) |
| 1000 | 2.0 | 5.07251(6) | 8.2704(1) | 8.8146(1) | 120.813(7) | 317.588(7) |
| 1000 | 2.5 | 5.07328(5) | 8.25084(9) | 8.82223(9) | 120.8395(5) | 317.073(6) |
| 1000 | 3.0 | 5.07521(4) | 8.23586(8) | 8.83144(7) | 120.8663(5) | 316.860(5) |
| 1200 | 1.5 | 5.07299(7) | 8.2876(1) | 8.8068(1) | 120.8024(8) | 318.034(7) |
| 1200 | 2.0 | 5.07408(4) | 8.26660(7) | 8.81422(8) | 120.8155(5) | 317.520(5) |
| 1200 | 2.5 | 5.07550(3) | 8.24883(5) | 8.82347(5) | 120.8519(4) | 317.139(3) |
| 1200 | 3.0 | 5.07675(2) | 8.23098(4) | 8.83270(4) | 120.8969(3) | 316.712(3) |
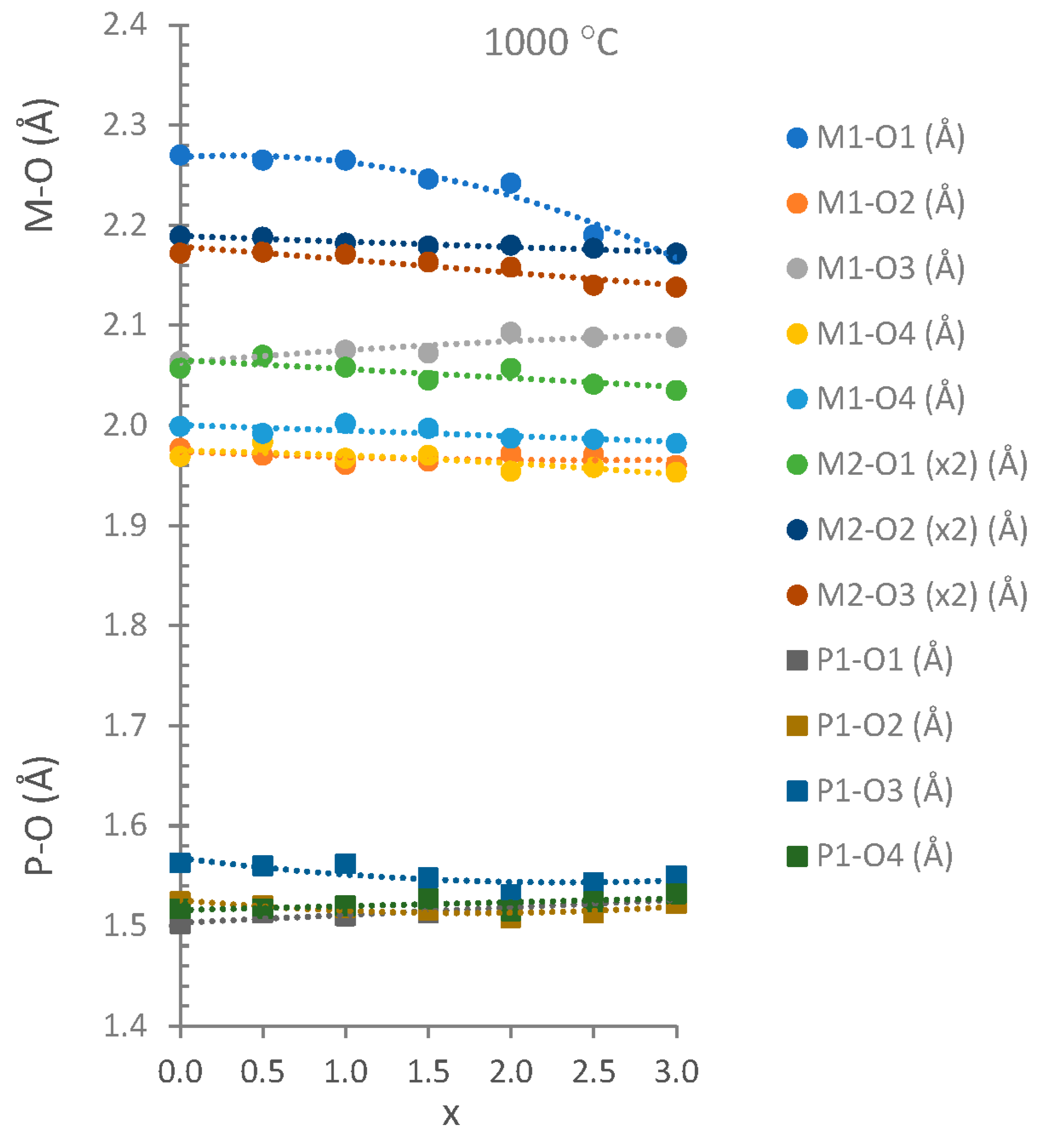
| T (°C) | X | M1-O1 (Å) | M1-O2 (Å) | M1-O3 (Å) | M1-O4 (Å) | M1-O4 (Å) | M2-O1 (x2) (Å) | M2-O2 (x2) (Å) | M2-O3 (x2) (Å) |
|---|---|---|---|---|---|---|---|---|---|
| 800 | 0.0 | 2.274(6) | 1.973(5) | 2.065(7) | 1.960(5) | 1.996(5) | 2.052(8) | 2.195(4) | 2.174(4) |
| 800 | 0.5 | 2.273(6) | 1.970(5) | 2.076(7) | 1.985(5) | 1.992(5) | 2.089(7) | 2.184(4) | 2.173(4) |
| 800 | 1.0 | 2.253(5) | 1.969(5) | 2.070(7) | 1.969(4) | 1.999(4) | 2.059(7) | 2.183(4) | 2.164(4) |
| 800 | 1.5 | 2.246(5) | 1.972(4) | 2.074(6) | 1.961(4) | 1.995(4) | 2.054(6) | 2.185(3) | 2.155(4) |
| 800 | 2.0 | 2.242(5) | 1.979(4) | 2.091(6) | 1.941(4) | 1.985(4) | 2.061(6) | 2.188(3) | 2.153(4) |
| 800 | 2.5 | 2.228(5) | 1.967(4) | 2.102(4) | 1.946(4) | 1.991(4) | 2.050(5) | 2.187(3) | 2.150(3) |
| 800 | 3.0 | 2.226(5) | 1.950(5) | 2.098(6) | 1.941(4) | 1.980(4) | 2.058(5) | 2.182(3) | 2.153(3) |
| 1000 | 0.0 | 2.270(6) | 1.977(5) | 2.064(7) | 1.969(5) | 1.999(4) | 2.057(7) | 2.189(4) | 2.172(4) |
| 1000 | 0.5 | 2.265(6) | 1.970(5) | 2.069(7) | 1.983(4) | 1.992(4) | 2.070(7) | 2.188(4) | 2.173(4) |
| 1000 | 1.0 | 2.265(6) | 1.961(5) | 2.075(7) | 1.967(5) | 2.002(5) | 2.058(7) | 2.182(4) | 2.171(4) |
| 1000 | 1.5 | 2.246(5) | 1.964(4) | 2.072(6) | 1.970(4) | 1.997(4) | 2.045(6) | 2.179(3) | 2.163(4) |
| 1000 | 2.0 | 2.242(5) | 1.972(4) | 2.093(6) | 1.954(4) | 1.987(4) | 2.057(6) | 2.180(3) | 2.158(4) |
| 1000 | 2.5 | 2.190(4) | 1.971(4) | 2.088(5) | 1.958(3) | 1.986(4) | 2.041(4) | 2.177(3) | 2.140(3) |
| 1000 | 3.0 | 2.171(3) | 1.960(3) | 2.088(4) | 1.953(3) | 1.982(3) | 2.035(3) | 2.172(2) | 2.138(2) |
| 1200 | 1.5 | 2.229(6) | 1.979(5) | 2.078(7) | 1.950(5) | 1.983(5) | 2.036(8) | 2.201(4) | 2.157(4) |
| 1200 | 2.0 | 2.231(5) | 1.956(4) | 2.067(6) | 1.965(4) | 2.009(4) | 2.037(6) | 2.190(3) | 2.171(4) |
| 1200 | 2.5 | 2.203(4) | 1.968(4) | 2.085(5) | 1.961(3) | 2.000(4) | 2.042(4) | 2.162(3) | 2.151(3) |
| 1200 | 3.0 | 2.166(3) | 1.956(3) | 2.081(4) | 1.965(3) | 1.988(3) | 2.033(3) | 2.172(2) | 2.138(2) |
| T (°C) | X | P1-O1 (Å) | P1-O2 (Å) | P1-O3 (Å) | P1-O4 (Å) |
|---|---|---|---|---|---|
| 800 | 0.0 | 1.944(9) | 1.527(5) | 1.561(5) | 1.512(5) |
| 800 | 0.5 | 1.521(9) | 1.520(5) | 1.566(5) | 1.517(5) |
| 800 | 1.0 | 1.512(9) | 1.526(5) | 1.550(5) | 1.527(5) |
| 800 | 1.5 | 1.516(8) | 1.521(4) | 1.546(4) | 1.519(4) |
| 800 | 2.0 | 1.518(7) | 1.508(4) | 1.537(4) | 1.521(4) |
| 800 | 2.5 | 1.516(7) | 1.507(4) | 1.534(3) | 1.515(4) |
| 800 | 3.0 | 1.524(6) | 1.520(4) | 1.544(3) | 1.527(4) |
| 1000 | 0.0 | 1.502(9) | 1.524(5) | 1.563(5) | 1.517(5) |
| 1000 | 0.5 | 1.513(9) | 1.520(5) | 1.560(5) | 1.517(5) |
| 1000 | 1.0 | 1.510(9) | 1.518(5) | 1.562(5) | 1.520(5) |
| 1000 | 1.5 | 1.513(7) | 1.516(4) | 1.548(4) | 1.527(4) |
| 1000 | 2.0 | 1.514(7) | 1.508(4) | 1.531(4) | 1.515(4) |
| 1000 | 2.5 | 1.519(5) | 1.513(3) | 1.543(3) | 1.524(3) |
| 1000 | 3.0 | 1.532(4) | 1.522(3) | 1.550(3) | 1.532(3) |
| 1200 | 1.5 | 1.516(9) | 1.526(5) | 1.522(5) | 1.520(5) |
| 1200 | 2.0 | 1.520(7) | 1.483(4) | 1.549(4) | 1.526(4) |
| 1200 | 2.5 | 1.505(5) | 1.511(3) | 1.537(3) | 1.548(3) |
| 1200 | 3.0 | 1.530(4) | 1.516(3) | 1.549(3) | 1.527(3) |
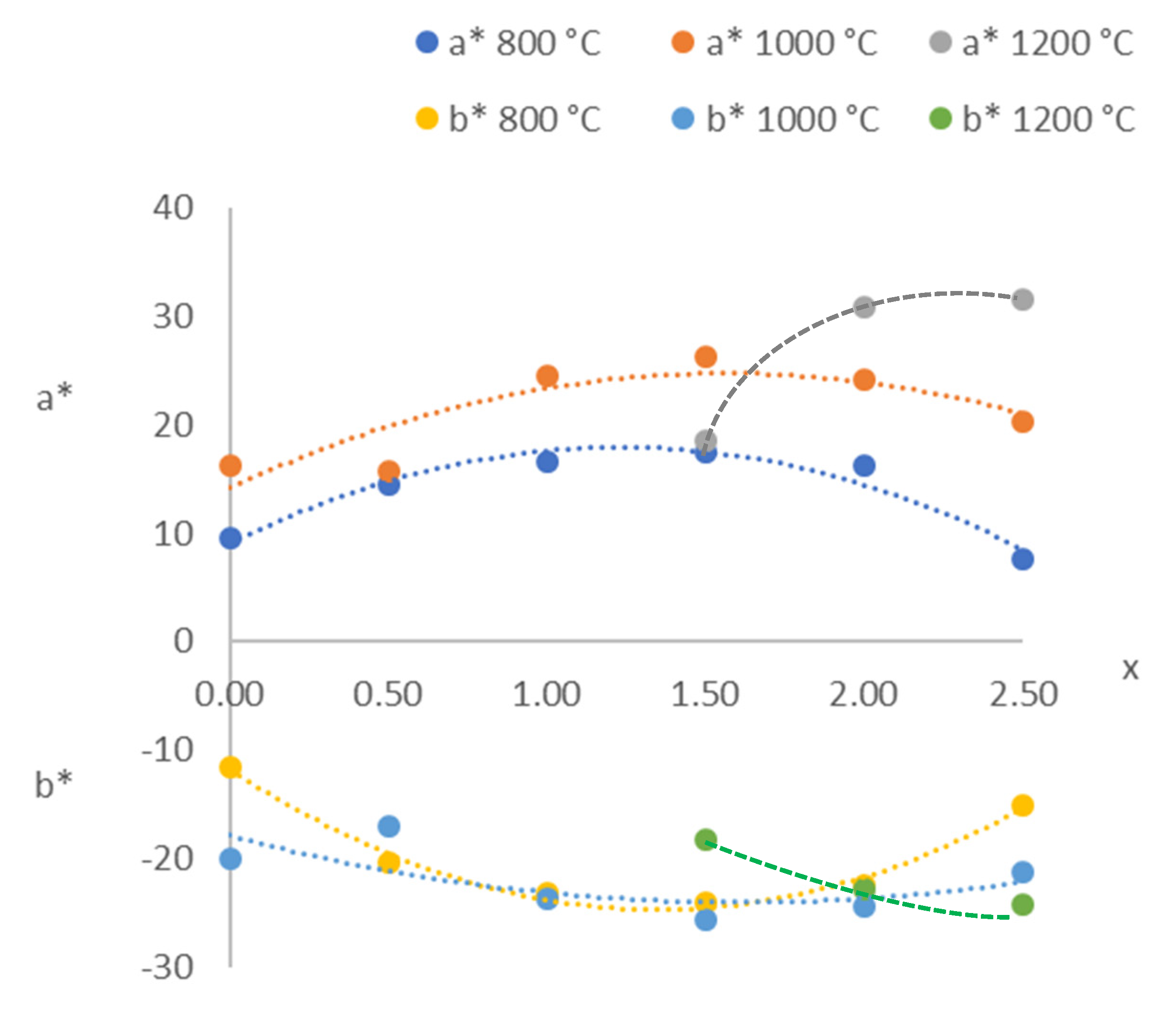
| x. | Raw Material | 300 °C | 600 °C | 800 °C | 1000 °C | 1200 °C | |
|---|---|---|---|---|---|---|---|
| 0.0 | L* | 51.36 | 32.62 | 35.23 | 36.73 | 31.07 | |
| a* | 7.72 | 8.62 | 7.64 | 9.52 | 16.22 | ||
| b* | –24.72 | –11.72 | –10.64 | −11.50 | −20.05 | ||
| Colour | Violet | Purple | Dark violet | Dark violet | Purple | ||
| 0.5 | L* | 49.71 | 31.21 | 34.54 | 37.07 | 29.30 | |
| a* | 6.00 | 7.88 | 10.72 | 14.57 | 15.72 | ||
| b* | −30.36 | –18.94 | −22.32 | −20.45 | −16.98 | ||
| Colour | Violet | Purple | Purple | Purple | Purple | ||
| 1.0 | L* | 37.31 | 44.69 | 51.37 | 52.43 | 35.18 | |
| a* | 7.78 | 4.06 | 12.73 | 16.58 | 24.48 | ||
| b* | –29.41 | −27.07 | −25.60 | −23.25 | −23.78 | ||
| Colour | Violet | Violet | Violet | Violet | Purple | ||
| 1.5 | L* | 47.92 | 48.86 | 60.12 | 58.20 | 41.39 | 36.24 |
| a* | 5.35 | −2.54 | 15.05 | 17.52 | 26.21 | 18.62 | |
| b* | −20.70 | −38.37 | −23.98 | −24.11 | −25.70 | −18.33 | |
| Colour | Violet | Blue | Violet | Violet | Violet | Purple | |
| 2.0 | L* | 40.20 | 60.85 | 59.14 | 58.97 | 49.49 | 31.33 |
| a* | −1.83 | −10.40 | 14.03 | 16.26 | 24.25 | 30.93 | |
| b* | −41.74 | −30.24 | −23.17 | −22.42 | −24.39 | −22.79 | |
| Colour | Blue | Blue | Violet | Violet | Violet | Purple | |
| 2.5 | L* | 54.41 | 64.37 | 68.93 | 68.73 | 56.60 | 38.86 |
| a* | −2.81 | −24.25 | 3.77 | 7.54 | 20.30 | 31.58 | |
| b* | −36.03 | −29.37 | −14.87 | −15.13 | −21.20 | −24.17 | |
| Colour | Blue | Light blue | Lilac | Lilac | Violet | Purple | |
| 3.0 | L* | 93.89 | 93.31 | 95.35 | 95.38 | 94.90 | 94.24 |
| a* | −0.10 | −0.04 | −0.15 | −0.10 | 0.29 | 0.35 | |
| b* | +0.38 | +1.12 | 0.16 | 0.05 | −0.48 | −0.54 | |
| Colour | White | White | White | White | White | White |
| x | L* | a* | b* | Observed Colour |
|---|---|---|---|---|
| 1.5 | 18.53 | −0.24 | −26.18 | Dark blue |
| 2.0 | 20.56 | +0.00 | −20.20 | Dark blue |
| 2.5 | 27.05 | +0.55 | −15.95 | Blue |
| 3.0 | 68.24 | +13.22 | +26.94 | Beige |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tena, M.Á.; Mendoza, R.; Trobajo, C.; García-Granda, S. Cobalt Minimisation in Violet Co3P2O8 Pigment. Materials 2022, 15, 1111. https://doi.org/10.3390/ma15031111
Tena MÁ, Mendoza R, Trobajo C, García-Granda S. Cobalt Minimisation in Violet Co3P2O8 Pigment. Materials. 2022; 15(3):1111. https://doi.org/10.3390/ma15031111
Chicago/Turabian StyleTena, Mª Ángeles, Rafael Mendoza, Camino Trobajo, and Santiago García-Granda. 2022. "Cobalt Minimisation in Violet Co3P2O8 Pigment" Materials 15, no. 3: 1111. https://doi.org/10.3390/ma15031111
APA StyleTena, M. Á., Mendoza, R., Trobajo, C., & García-Granda, S. (2022). Cobalt Minimisation in Violet Co3P2O8 Pigment. Materials, 15(3), 1111. https://doi.org/10.3390/ma15031111







