Abstract
We present a smart and efficient methodology for the synthesis of a variety of fluorinated silsesquioxanes (SQs) with diverse Si-O-Si core architecture. The protocol is based on an easy-to-handle and selective hydrosilylation reaction. An investigation on the placement of the reactive Si-HC=CH2 vs. Si-H in the silsesquioxane, as well as silane vs. olefin structure, respectively, on the progress and selectivity of the hydrosilylation process, was studied. Two alternative synthetic pathways for obtaining a variety of fluorine-functionalized silsesquioxanes were developed. As a result, a series of mono- and octa- T8 SQs, tri- ‘open-cage’ T7 SQs, in addition to di- and tetrafunctionalized double-decker silsesquioxane (DDSQ) derivatives, were obtained selectively with high yields. All products were characterized by spectroscopic (NMR, FTIR) techniques. Selected samples were subjected to the measurements revealing their dielectric permittivity in a wide range of temperatures (from −100 °C to 100 °C) and electric field frequencies (100–106 Hz).
1. Introduction
Novel insulating materials have become an urging field of research due to their importance in microelectronics [1]. Along with this development, new challenges for the size reduction of the individual components of these devices to correspond with the increase in the number of transistors have been appearing. This is correlated with some difficulties related to signal delay and the energy consumption that have become significant. The energy consumption is raised because the systems are functioning at higher frequencies. This dependence can be described by Equation (1):
where: P—energy consumption; α—conductor activity; C—capacitance of the output and input of transistors and the cable, f—current frequency and V—voltage of the power source.
P = αCfV2
The requirements for the insulating materials also concern their appropriate mechanical properties (resistance to moisture, thermal stability) [2]. As a result of those needs, the use of silicon-based materials has gained a predominant significance [3]. It corresponds to the employment of not only simple poly-[4] or cyclosiloxanes [5] but organosilicons namely described as hybrid materials [6]. Within this group, a particularly important role possesses silsesquioxanes (POSS®, SQs). This is due to their physical properties and relatively simple and practically unlimited modification possibilities that affect their use as scaffolds for the modelling of new materials with a low dielectric constant (κ) under various conditions. This physical constant is also connected with a relative electric permittivity (ε) to describe their electrical properties, i.e., one of the crucial parameters when designing materials for the microelectronic industry [1]. Low- or ultra-low κ materials are of significant importance from the application point of view [7].
There are many materials tested in terms of their permittivity and they are often classified by their dielectric constant value as follows [8]:
- κ > 3.3–3.4—e.g., SiO2, fluorosilicate glass [1,8,9];
- 3.3 > κ > 2.7, e.g., organics: DLC (Diamond Like Carbon) and FDLC (Fluorinated Diamond-Like Carbon), SiCOH, parylenes, polynaphthalenes, silicon-substituted polyimides, polyquinoxalines, quinolines, norbornenes, benzocyclobutenes, indanes, perfluorocyclobutanes [8,10,11,12];
- 2.7 > κ > 2.2 and κ < 2.0—ultra-low κ materials, e.g., porous SiLK (Silicon Low κ), boron nitride nanotube (BNNT) material, porogen-free systems, aerogels, xerogels and f-xerogels, organosilicates) [8,13,14,15].
The above-mentioned silsesquioxanes are a group of organosilicon compounds of the general formula (RSiO3/2)n. The chemical formula does not reflect the multiplicity of their core architectures, varying from random, ladder, partial cage to cage, i.a. T6, T8 or T10 to even T18 discovered recently, and also so-called double-decker SQ (DDSQ) [16,17]. The variety of rigid core structures is also defined by the diversity of the amount of reactive/inert substituents anchored to the Si-O-Si core, from one to eight or more. For instance, the DDSQ compounds are known in the area of their double- and tetrafunctionalized derivatives [18,19,20]. These systems exhibit the organic–inorganic, i.e., hybrid nature of well-defined nano sizes (diameter of ca. 0.3–0.4 nm) and specific properties, i.a. oxidation resistance and non-toxicity, thermal stability, biocompatibility, etc. [16,21,22]. As a result, they may be applied in the chemistry of materials (as nanofillers or polymer modifiers), [23,24,25] in optoelectronics, [26,27] catalysis [28,29] or even medicine (e.g., drug delivery systems) [30,31,32]. The application potential of silsesquioxanes lies in the ease of their modifications and the adjustments of functional groups anchored to the inorganic Si-O-Si core. The crucial issue in this matter is the proper selection of the prefunctional moiety at SQs core for the selection of the respective reaction, usually catalytic, for its modification. Among the variety of catalytic processes, a few are of special importance due to their effectiveness and selectivity, i.e., hydrosilylation, cross-metathesis, coupling processes, e.g., Heck or Sonogashira, dehydrogenative coupling of Friedel-Crafts reaction [26,27,33,34,35,36,37].
Among the application studies is the introduction of polyhedral oligomeric silsesquioxane into polymers as their components and modifiers have been considered of significant importance. These materials have already shown potential applications, including low- κ insulators with dielectric constant values in a range of ca. 1.5–3 [38,39,40]. They may be obtained in several ways, e.g., hydrolytic condensation, hydrosilylation, thermal polymerization or curing and casting are some of these methods combinations [38,39,40,41,42,43,44,45]. Moreover, there are some reports on the dependence of dielectric constant value on the type and number of functional/inert groups in T8 and T7 open-cage SQs, e.g., alkyl, alkenyl, cycloalkyl, methacryl, ester or epoxide moieties, varying in the range of 2.4–3.0. The methods for their film formation include, e.g., chemical vapor deposition (CVD) also enhanced by plasma, spin-on deposition (SOD) or electrospun [46,47]. Interestingly, the SQs-based materials, in most cases, exhibited lower dielectric constants than the host polymers, and what is more, the dielectric constants can be tuned by adjusting the content of SQs. In general, there is a tendency for the dielectric constant value to decrease with the increasing content of SQs [42,48,49,50,51]. The fluorinated silsesquioxanes, mainly based on octasubstituted-T8, but also DDSQs systems, are groups of compounds being building blocks of low-κ hybrid materials [42,44]. Within this group, modified polyimides, also with compounds containing fluorine, may be distinguished as being utilized for dielectric layers formation and hence appliance in the microelectronic industry [41,52,53,54,55,56,57,58].
However, the reports on the values of dielectric constant measured only for bare silsesquioxanes without any modification in their deposition are strongly limited. Therefore, these studies concern the elaboration of a smart methodology for the synthesis of a series of silsesquioxanes with a diverse amount of organofluorine substituents as well as the SQ’s core structure. Additionally, part of this research was the determination of the dielectric permittivity for selected samples measured in a wide range of temperature and field frequencies.
2. Materials and Methods
The experimental procedures, analytical data and NMR spectra of all isolated products are available in the ESI file.
2.1. Materials
The chemicals were purchased from the following sources: Sigma-Aldrich (St. Louis, MO, USA) for toluene, chloroform-d, chloroform, Karstedt’s catalyst—2% xylene solution [Pt2(dvs)3)] and silica gel 60. The following silsesquioxanes, monoT8SiH, monoT8SiVi, triT7SiH, triT7SiVi, DDSQ-2SiH, DDSQ-2SiVi, DDSQ-2OSiVi, DDSQ-4OSiH, DDSQ-4OSiVi, octaT8SiH and octaT8SiVi, were prepared according to the literature procedures [59,60,61] and so was the olefin CH2=CHCH2OCH2(CF2)3CHF2 (F1) and silane HSiMe2OSiMe2CH2CH2CH2OCH2(CF2)3CHF2 (F5) [62,63]. All solvents were dried over CaH2 before use and stored under argon over 4Å molecular sieves. All liquid substrates were also dried and degassed by bulb-to-bulb distillation. All syntheses were conducted under an argon atmosphere using standard Schlenk-line and vacuum techniques.
2.2. General Synthetic Procedures
2.2.1. General Synthetic Procedure for Fluorinated Silsesquioxanes Obtained via Hydrosilylation of SQ-H with Olefins (F1, F2)
The procedure for the synthesis of monoT8(F1) is described as an example. To a two-neck round-bottom flask equipped with a condenser and magnetic stirrer, monoT8SiH (0.1009 g, 0.098 mmol) was placed in argon atmosphere along with anhydrous toluene (3.0 mL), 1.1 equiv. of olefin F1 (0.0298 mL, 0.106 mmol) and 10−4 equiv. of [Pt2(dvs)3] (0.113 µL 9.8 × 10−9 mol). The reaction mixture was kept at 95 °C for 24 h. After cooling it to room temperature, the excess of olefin and solvent was evaporated under a vacuum and the crude product was purified using column chromatography (silica gel 60) using chloroform as an eluent. Evaporation of eluent gave an analytically pure sample of monoT8(F1) in 96% yield.
2.2.2. General Synthetic Procedure for Fluorinated Silsesquioxanes Obtained via Hydrosilylation of SQ-Vi with Silanes (F3–F5)
The procedure for the synthesis of monoT8(F5) is described as an example. It is analogous to obtaining monoT8(F1). The stoichiometry of respective reagents is as follows: [monoT8SiVi][F5][Pt2(dvs)3] = 1:1.2:10−4. MonoT8(F5) was obtained in 94% yield.
2.3. Measurements
2.3.1. Nuclear Magnetic Resonance (NMR)
1H, 13C, and 29Si Nuclear Magnetic Resonance (NMR) were performed on Brucker Ultra Shield 600, 400 and 300 spectrometers, using CDCl3 as a solvent. Chemical shifts are reported in ppm with reference to the residual solvent (CHCl3) peaks for 1H and 13C and to TMS for 29Si NMR.
2.3.2. FTIR Spectroscopy
Fourier-transform infrared (FTIR) spectra were recorded on a Nicolet iS5 (Thermo Scientific, Waltham, MA, USA) spectrophotometer equipped with a diamond ATR unit. In all cases, 16 scans at a resolution of 2 cm−1 were collected, to record the spectra in a range of 4000–650 cm−1.
2.3.3. Elemental Analysis (EA)
Elemental analyses were performed using a Vario EL III instrument.
2.3.4. Dielectric Relaxation Spectra (DRS)
Dielectric spectroscopy (DS) measurements were performed using an Alpha-A Novocontrol High-Performance Broadband Dielectric Spectrometer (Novocontrol GmbH, Montabaur, Germany) in a frequency range from 1 Hz to 106 Hz and with voltage oscillations of ±1 volts. For dielectric measurements, the powder material was formed into the form of a pellet (p = 30 MPa, 293 K). The surfaces of the samples were covered with a silver paste (Hans Wolbring, Höhr-Grenzhausen, Germany).
To check the thermal stability of the powdered materials, the dielectric properties were studied using the following temperature procedure: in the beginning, the studied material was measured continuously at 20 °C for 5 h. Later, the sample was measured during cooling down to −100 °C with the continuous step of −0.45 K/min. After cooling, the sample was measured continuously during heating to 100 °C with a step of 0.45 K/min, and later, the measured material was cooled to 20 °C. After cooling, the sample was measured at a temperature of 20 °C for another hour. The temperature was controlled using a Quatro Cryosystem (Montabaur, Germany) with a stabilization temperature of ±0.05 K.
The dielectric measurements were also performed for the materials in the form of films of the selected compounds. The films were obtained by sputtering the solution of the material on the steel plates. Thin films in one layer were prepared by a spin-coating technique using a 5–10 wt% solution of compounds in DCM or DCM/THF (1:1) that was deposited on the steel plates (spin speed of 100 rpm for 40 s.) to form homogeneous surface. The film thickness was in the range of 500–1500 nm. The measurements were performed at 20 °C for 5 h, using the same equipment as in the case of the powder sample.
3. Results and Discussion
3.1. Synthetic Methodology to form Fluorinated Silsesquioxanes
Silsesquioxanes are known to be used as components of materials exhibiting low permittivity (low-κ materials), especially using fluoro-organosilicon derivatives [8,46,47]. The synthetic strategy for these compounds may be not straight and is limited to the functional group attached to the SQ’s core. Therefore, these studies concern the elaboration of a smart methodology for the synthesis of a series of silsesquioxanes with a diverse amount of organofluorine substituents as well as the SQ’s core structure. The synthetic protocol is based on a hydrosilylation reaction performed in a two-way form (Scheme 1):
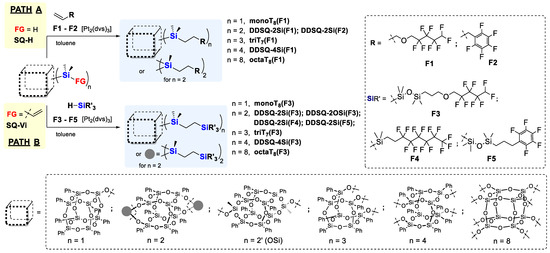
Scheme 1.
The reaction path for the synthesis of fluorinated silsesquioxanes via hydrosilylation of SQ-H with olefins (F1, F2) or SQ-Vi with silanes (F3–F5) leading to, respectively, substituted products varying in the SQ-core architecture and number of fluorinated substituents.
- PATH A—concerns the hydrosilylation of fluoroolefins with SQs possessing reactive Si-H (SQ-H) moiety varied from 1 to 8 (F1, F2);
- PATH B—is connected with the use of silsesquioxanes with Si-HC=CH2 group(s) (SQ-Vi) (from 1 to 8) to be hydrosilylated with silanes bearing fluorine atoms (F3–F5)
For the studies, the diverse type of SQs was applied, i.e., varying not only in the core type but also the amount of functional Si-H or Si-HC=CH2 groups from one (monoT8), two (DDSQ-2Si or DDSQ-2OSi), three (triT7), four (DDSQ-4Si) or eight (octaT8). Additionally, the reagents containing the fluorine atoms were also diverse, i.e., olefins: with aliphatic chain (F1) and aromatic group (F2), as well as respective silanes with analogous substituents (aliphatic: F3, F5 and aromatics: F4). The synthetic procedure was based on Pt-Karstedt’s catalyst ([Pt2(dvds)3]) used in 10−4 mol of Pt loading per 1 mol of Si-H group and performed at 95 °C for 24 h. This enabled the >99% consumption of SQs applied in the studies, i.e., SQ-H and SQ-Vi, and its result was monitored using the FTIR and 1H NMR spectroscopy. The absence of band at FTIR spectra characteristics for stretching vibrations of Si-H bonds (at ῡ = 895 cm−1) and the respective doublet of doublets at 1H NMR spectra (δ in a range of ca. 5.5–6.5 ppm for CDCl3, depending on the SQs core type and amount of vinyl moieties). The reagents’ stoichiometry was maintained at equivalent. We noted previously that alkene excess may affect the reaction time, but mainly in its initial time and does not significantly reduce the total reaction time [64]. As it is known, hydrosilylation may occur with side reactions, e.g., hydrogenation of olefins, and dehydrogenative silylation. It also might not be selective and a mixture of α- and β-hydrosilylation products may be formed. It should be noted that the studied hydrosilylation reaction of SQ-H with 5-allyl-1,1,2,2,3,3,4,4-octafluoropentyl ether (F1) and 1-allyl-2,3,4,5,6-pentafluorobenzene (F2) were regioselective towards the formation of β-hydrosilylation products. The selectivity of the reaction was monitored via 1H NMR of the product and verification of CH2 moiety presence—characteristics of β-hydrosilylation compound and absence of CH and CH3 groups—the features of α-hydrosilylation products (see Supporting Information). This observation was verified for PATH A, i.e., the hydrosilylation of fluorinated olefins (F1, F2) by respective silsesquioxanes SQ-H (monoT8H, DDSQ-2SiH, triT7H, DDSQ-4SiH, octaT8H, Scheme 1).
It is worth noting that the changes in the chemical surrounding of the functional Si atoms affect their resonance lines placement in 29Si NMR spectra. Interestingly, the amount of the fluorine atoms located in the fragment anchored to the SQs core insignificantly influences the shift of the Si peak, which is shown for DDSQ-2Si(F1) and DDSQ-2Si(F5) 29Si NMR spectra (Figure 1). The electronic impact of those moieties, regardless of the aliphatic of aromatic units present or even though possessing highly electronegative fluorine atoms, is rather limited. The functional SiD atoms connected with the respective fluorosubstituted groups are placed at ca. −17.64 and −17.08 ppm, respectively. Additional resonance lines at 6.80 and 8.77 ppm correspond with the SiM atoms in the additional siloxy moiety in the fluoroalkyl chain of F5.
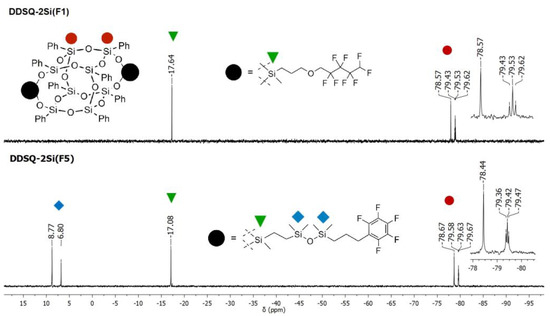
Figure 1.
Selected range of stacked 29Si NMR spectra of DDSQ-2Si(F1) and DDSQ-2Si(F5).
It should be emphasized that a more important aspect should be taken into consideration, i.e., the order of the silicon atoms. It may visualize how the placement of the SiM, SiD, SiT and SiQ atoms in 29Si NMR changes in the obtained products varying in the SQs core type (Figure 2 and Figure 3). In the case of monoT8(F1), triT7(F1) and octaT8(F1) beside the typical placement of SiT and SiQ peaks, i.e., in the range of −77.36 to −78.37 ppm for monoT8(F1), triT7(F1) and −109 vs. −108.92 ppm, respectively, octaT8(F1) and monoT8(F1), the functional SiM was located at ca. from 11.85 to 13.07 ppm (Figure 2).
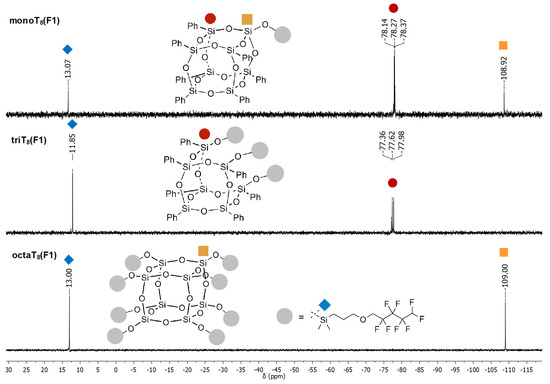
Figure 2.
Selected range of stacked 29Si NMR spectra of monoT8(F1), triT8(F1) and octaT8(F1).
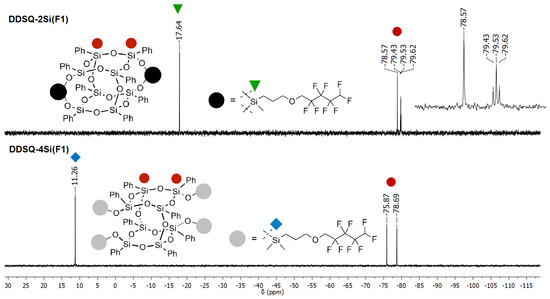
Figure 3.
Selected range of stacked 29Si NMR spectra of DDSQ-2Si(F1) and DDSQ-4Si(F1).
More visible changes could be noticed in the case of DDSQs products, i.e., DDSQ-2Si(F1) and DDSQ-4Si(F1), where the SiT DDSQ-derived signals were present in a typical range of −75.87 to −79.62 ppm. Interestingly, and regardless of fluorosubtituent, the SiD resonance lines were up-field shifted to −17.64 ppm for DDSQ-2Si(F1) when compared to SiM down-field shifted to 11.26 ppm (Figure 3).
Additionally, the methodology for the synthesis of the fluorinated silsesquioxanes was also verified in terms of the reverse Si-H and Si-CH=CH2 reactive groups placement, PATH B, i.e., hydrosilylation of SQ-Vi (monoT8SiVi, triT7SiVi, DDSQ-2SiVi, DDSQ-2OSiVi, DDSQ-4OSiVi, octaT8SiVi) by three types of silanes containing respective fluorosubstituents to olefins used before (F3–F5) (Scheme 1). The reaction conditions were also analogous to the previous ones. In this case, the process was also regioselective towards the formation of only β-hydrosilylation products.
Interestingly, in the case of DDSQ-2SiVi to react with F4 and F5 silanes, the mixture of cis- and trans-geometric isomers was isolated (e.g., DDSQ core resonance lines for DDSQ-2Si(F4): δ = −78.60, −79.57 (cis), −79.64 (trans), −79.72 (cis) and DDSQ-2Si(F5): δ = −78.67, −79.58 (cis), −79.63 (trans), −79.67 (cis)), which was observed in 29Si NMR analysis, respectively. When silane F3, i.e., with an additional Si-O-Si bridge between the Si-H reactive group and the fluorosubstituted moiety was applied, the trans-isomer of DDSQ-2Si(F3) was isolated and proved by the presence of respective DDSQ core resonance lines at 29Si NMR: δ = −78.69, −79.64 (trans) (Figure 4). It should be mentioned that, for this product, both cis- and trans-isomers are postulated to be formed. However, due to the different physicochemical properties of these isomeric structures, their separation may be performed [65,66]. Herein, the trans-isomer of DDSQ-2Si(F3) was isolated exclusively during purification on column chromatography, as the amount of cis-isomer was residual.
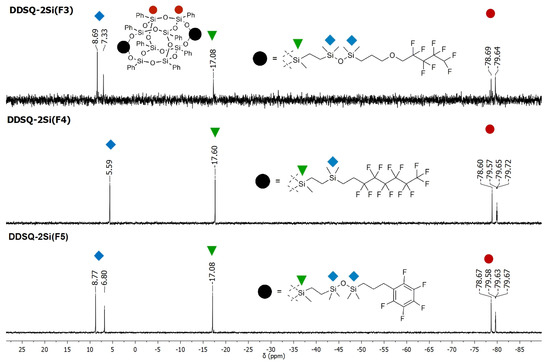
Figure 4.
Selected range of stacked 29Si-NMR spectra of DDSQ-2Si(F3), DDSQ-2Si(F4) and DDSQ-2Si(F5).
The observations concerning the appearance of the 29Si NMR spectra and placement of peaks derived from the respective type of Si atoms in the obtained products are analogous to the compounds obtained via PATH A. In this way, the resonance lines corresponding with the SiD atoms are up-field-shifted (DDSQ-2Si(F3)) when compared with the down-field placement of SiM atoms in DDSQ-2OSi(F3), DDSQ-4Si(F3) and monoT8(F3), triT8(F3) and octaT8(F3), respectively (Figures S44 and S45 in Supplementary Information).
All products based on silsesquioxanes are air-stable solids or oils or waxy, white solids, which are depicted in Figure 5. They were obtained in very high yields in a range of 87–96%. They are soluble in organic solvents like DCM, CDCl3, THF and toluene but not in, e.g., methanol, or MeCN. They were isolated and characterized using spectroscopic methods (NMR, FTIR) and elemental analysis.
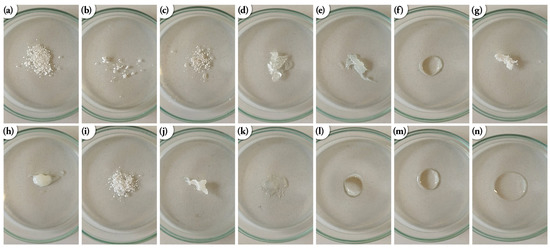
Figure 5.
Images of physical state of the obtained compounds (a) monoT8(F1), (b) DDSQ-2Si(F1), (c) DDSQ-2Si(F2), (d) triT7(F1), (e) DDSQ-4Si(F1), (f) octaT8(F1), (g) monoT8(F3), (h) DDSQ-2Si(F5), (i) DDSQ-2Si(F3), (j) DDSQ-2Si(F4), (k) DDSQ-2OSi(F3), (l) triT7(F3), (m) DDSQ-4Si(F3), (n) octaT8(F3).
3.2. Dielectric Properties of Fluorinated Silsesquioxanes
Fluorinated silsesquioxanes have been promising candidates for their use as interlayer dielectric or intermetal dielectric materials with great application potential in the microelectronic industry [47,58,67]. The dielectric properties of silsesquioxanes and their derivatives were measured in a frequency range of 1 Hz to 1 MHz and in a wide temperature range (from −100 °C to 100 °C). Figure 6 shows an example of the time and temperature dependence of the real part of permittivity of the DDSQ-2SiVi sample, measured at a fixed frequency of 100 Hz (more data are presented in the Supplementary Information). In the first cycle, measurements were carried out at RT temperature for 300 min and the value of dielectric permittivity was stable and had a value of ~18.95. During the next cycle, the sample was cooled to T = −100 °C and one could observe an increase of dielectric permittivity, which reached a maximum value of ~19.92 at T = −100 °C. The following third cycle presented heating of the measured material from −100 °C to 100 °C, and the permittivity decreased to the same value as observed at the beginning of the experiment. However, after coming back to the RT temperature (last temperature cycle), dielectric permittivity slightly increased to a value of ~19.07 (less than 1% change compared to the pristine sample).
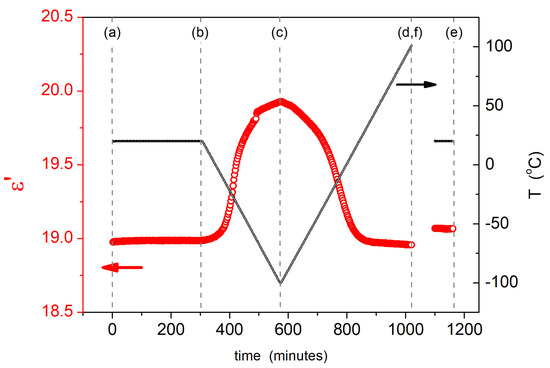
Figure 6.
Typical time and temperature dependences of the real part of complex permittivity ε’ of DDSQ-2SiVi compound, measured at 100 Hz. Letters denote characteristics temperatures: (a) 20 °C (pristine sample, before thermal studies); (b) 20 °C (after 300 min, at T = 20 °C), (c) the minimum temperature at −100 °C, (d,f) maximum temperature at 100 °C and (e) 20 °C (after thermal treatment).
Figure 7 presents a comparison of the dielectric properties of the powdered samples of fluorinated silsesquioxanes for selected temperatures. Dielectric permittivity measured for the pristine initial material (Figure 7a) is the highest from the compared derivatives, with a broad plateau in the 20 < ν < 20 × 105 Hz range and value of ~20.18 and reaches a maximum value at the highest frequency of 20.38 (at ν = 2 × 106 Hz). The lowest value of ε’ for pristine material (before thermal treatment) is observed for the DDSQ2Si(F3) (16.1 at maximum and 15.68 at minimum). After 300 min of measuring at T = 20 °C (Figure 7b), the frequency dependences are the same, indicating the very good stability of the materials. Data, collected at T = −100 °C, show that changes caused by the cooling process are almost negligible (Figure 7c). The opposite effect is observed at the highest measured temperature, T = 100 °C (Figure 7d,e), as in the case of samples DDSQ-2Si(F1) and DDSQ2Si(F3) dielectric permittivity increases, and reaches the maximum value of 25.8 and 65.6 at 1Hz, respectively. After cooling back to 20 °C (Figure 7e), the dielectric permittivity of DDSQ-2Si(F1) and DDSQ2Si(F3) samples decreases. However, the measured values of ε’ are higher, approximately ~12% and 45% higher than for the pristine material. In contrast, the characteristics of the initial material (DDSQ) are almost the same at all stages of thermal studies.
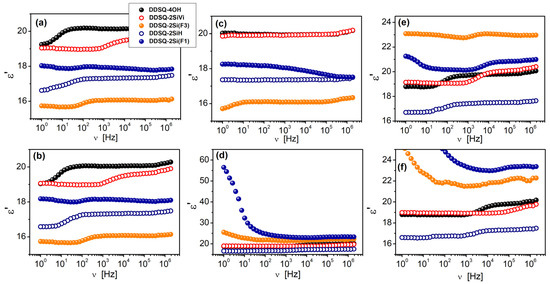
Figure 7.
Frequency dependence of dielectric permittivity measured for respective compounds: DDSQ-4OH, DDSQ-2SiVi, DDSQ2Si(F3), DDSQ-2SiH, DDSQ-2Si(F1) at different temperatures: (a) 20 °C (pristine sample, before thermal studies), (b) 20 °C (after 300 min, at T = 20 °C), (c) −100 °C (minimum temperature), (d) 100 °C (maximum temperature) and (e) 20 °C (after thermal treatment). Plot (f) shows parts of the data collected at T = 100 °C.
The values of dielectric permittivity, which depended on the frequency for measurements performed at 20 °C, are collected in Table 1. The values of dielectric permittivity at −100 °C and 85 °C, respectively, are presented in Tables S2 and S3 in the Supplementary Information.

Table 1.
Values of dielectric permittivity, measured at 20 °C at 100 × 106 Hz.
In addition to the studies of the powdered materials, measurements were also performed of the thin films of the selected DDSQ-2Si(F1)-f and DDSQ2Si(F3)-f materials for 300 min at temperature T = 20 °C. Results obtained at the beginning (1 min), at the middle (150 min) and at the end of the studies (300 min) are presented in Figure 8.
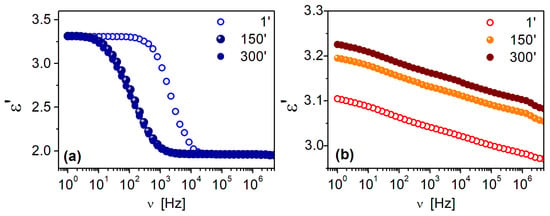
Figure 8.
Frequency dependence of dielectric permittivity, measured for DDSQ-2Si(F1)-f sample (a) and DDSQ-2Si(F3)-f sample (b) in form of thin films. Presented data were collected after 1 min, 150 min and 300 min at T = 20 °C.
Materials in the form of thin films show remarkably smaller values of ε’. The stability of the materials is satisfying. The frequency dependence of the DDSQ-2Si(F1)-f sample shows dispersion, as permittivity decreases with increasing frequency, to ~1.96 at ν = 2 × 104 Hz and above at the first measured point (see Figure 8a). With time, the transition region from the high to low values of ε’ moves to lower frequencies (to 3 × 103 Hz). In the case of sample DDSQ-2Si(F3)-f (Figure 8b), permittivity is frequency-dependent in all measuring frequency windows, although the difference between maximum and minimum is small, ~3.11 at ν = 1 Hz and ~2.97 at ν = 5 × 106 Hz, respectively. With time, permittivity increases but the shape of the characteristics are the same. After 300 min, ε’ reaches the maximum value of 3.23 at ν = 1 Hz and the minimum of 3.09 at ν = 5 × 106 Hz. The temperature dependence of dielectric permittivity, respective samples: DDSQ-4OH, DDSQ-2SiVi, DDSQ-2SiH, DDSQ2Si(F3), DDSQ-2Si(F1), are presented as charts in Figures S46–S50 in the Supplementary Information. What is worth noting, the values of dielectric permittivity of DDSQ-4OH, DDSQ-2SiVi and DDSQ-2SiH is decreasing with the temperature raise to maintain the plateau (Figures S46–S48). In contrast, the solid samples DDSQ2Si(F3) and DDSQ-2Si(F1) exhibited a reverse trend and a significant increase in dielectric permittivity, which is especially visible above 50 °C (Figures S49–S50). However, the temperature dependence of dielectric permittivity measured for the film samples DDSQ-2Si(F1)-f and DDSQ-2Si(F3)-f exhibit noteworthy behavior. For DDSQ-2Si(F1)-f, the value of ε’ is constant (ca. ε’ = 3.1) with the temperature raised from −100 °C to +100 °C in a whole range of field frequencies. The DDSQ-2Si(F3)-f displays a constant value of ε’ (ca. ε’ = 2.9) from −100 °C to ca. 30 °C, and its substantial increase may be noted further on with the temperature raise to +100 °C. It is graphically depicted in Figure 9 and Figure 10.
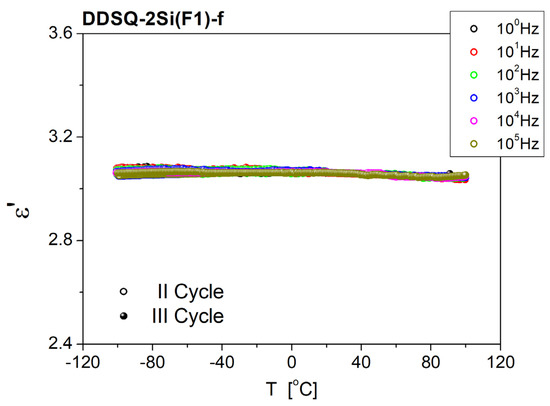
Figure 9.
Temperature dependence of dielectric permittivity, measured for a thin film of DDSQ-2Si(F1)-f. II cycle—sample was cooled from RT to T = −100 °C; III cycle—sample heated from −100 °C to 100 °C.
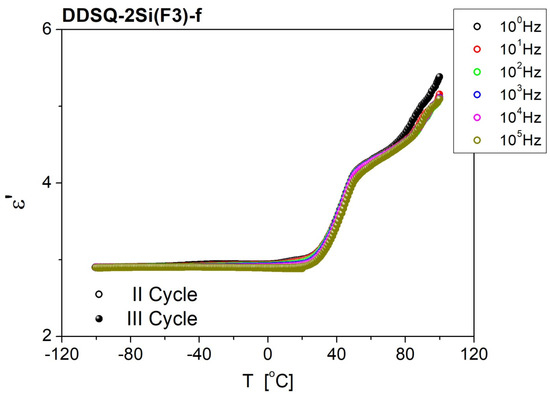
Figure 10.
Temperature dependence of dielectric permittivity, measured for a thin film of DDSQ-2Si(F3)-f. II cycle—sample was cooled from RT to T = −100 °C; III cycle—sample heated from −100 °C to 100 °C.
Commonly, dielectric permittivity decreases with increasing frequency, as in the case of DDSQ-2Si(F1) pellet and DDSQ-2Si(F1)-f and DDSQ2Si(F3)-f films. The observed effect in the DDSQ-2Si(F3) material is probably caused by some parasite effect, although before each measurement, the calibration was performed according to the proposed Novocontrol apparatus. One of the possible explanations for this effect can be the so-called “fringe effect”. The measured sample is placed between the plates of the sample holder and forms a capacitor. A voltage applied between these plates results in a dielectric field between them. Of course, this electric field exists not just directly between the plates but also extends some distance away. This extending field is known as a fringing field. This additional outside field may cause changes in capacitance (i.e., increasing the permittivity). This effect is not observed in the film, as the ratio of sample diameter and thickness is high. It is worth noticing, that the difference between the plateau region in the lower-frequency part and the high-frequency part where the permittivity increases are less than 3.5%, and it does not change the main observations and conclusions of the dielectric measurements. However, the issue of dielectric permittivity value dependence on the sample morphology or the method of its preparation seems to be very interesting [42,58,68]. It will be a subject of our further research.
4. Conclusions
Herein, we showed a hydrosilylation-based synthetic protocol for the formation of a variety of fluorinated silsesquioxanes (SQs). The elaborated conditions enable the regioselective formation of β-hydrosilylation products regardless of the placement of reactive Si-H vs. Si-HC=CH2 moiety at the SQ’s core. Diverse SQ core architectures, i.e., mono- and octa- T8, tri- ‘open-cage’ T7 SQs and di- and tetra- DDSQs, possessing varied amounts of reactive groups, were tested. As a consequence, a group of, respectively, fluorosubstituted silsesquioxane derivatives were obtained selectively and with high yields that were thoroughly examined using spectroscopic (NMR, FTIR) techniques. Interesting observations concerning the 29Si NMR analysis of the obtained products were made in terms of the tendency of resonance line shifts affected more by the type of the functional silicon atom (SiM, SiD, SiT) than the presence of fluorine atoms. Additionally, the samples susceptible to the form of pellets or thin films deposited on steel plates (obtained by spin-coating technique) were subjected to dielectric permittivity measurements. The analysis was also performed for respective substrates, i.e., DDSQ-4OH, DDSQ-2SiVi and DDSQ-2SiH. It may be noticed that fluorosubstituted groups insensibly decrease the value of dielectric permittivity for solid samples that are not changing highly in applied field frequencies from 100 to 106 Hz. Interestingly, for the samples analyzed as films, a significant decrease in the values of ε’ may be noted for the values of ca. ε’ = 3. It is visible, especially for the DDSQ-2Si(F1)-f sample, that this behavior is constant regardless of temperature and field frequency changes. This observation may be valuable for materials chemists in terms of the potential applications of the presented compounds in the modification of polymeric matrixes to be applied to microelectronics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma15248997/s1, list of compounds (Table S1), spectroscopic (NMR and FT-IR) spectra of obtained compounds (Figures S1–S45) as well as charts of dielectric permittivity for compounds (Figures S46–S50, Tables S2 and S3). Ref. [69] was cited in the Supplementary Information.
Author Contributions
Conceptualization and supervision, B.D.; synthesis and characterization of the reported compounds, J.D., K.M., M.R., B.S. and R.J.; funding acquisition, J.D. and B.D.; dielectric permittivity measurements and description, P.Ł.; writing—original draft preparation, editing, J.D., P.Ł., B.D. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National Science Centre (Poland) Project OPUS UMO 2016/23/B/ST5/00201 and grant no. POWR.03.02.00-00-I026/16 co-financed by the European Union through the European Social Fund under the Operational Program Knowledge Education Development.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Information file.
Acknowledgments
The authors are grateful to Jan Jarożek for the project of graphical abstract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maex, K.; Baklanov, M.R.; Shamiryan, D.; Iacopi, F.; Brongersma, S.H.; Yanovitskaya, Z.S. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Hyeon Lee, J.; Lyu, Y.Y.; Lee, M.S.; Yim, J.H.; Kim, S.Y. Characterization of Nanoporous Low Dielectric Polysilsesquioxane Thin Films. Key Eng. Mater. 2005, 277–279, 907–911. [Google Scholar] [CrossRef]
- Zheng, P.; McCarthy, T.J. Rediscovering silicones: Molecularly smooth, low surface energy, unfilled, UV/Vis-transparent, extremely cross-linked, thermally stable, hard, elastic PDMS. Langmuir 2010, 26, 18585–18590. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.; Jin, K.; Wang, L.; Sun, J.; Fang, Q. A New Fluorinated Polysiloxane with Good Optical Properties and Low Dielectric Constant at High Frequency Based on Easily Available Tetraethoxysilane (TEOS). Macromolecules 2017, 50, 9394–9402. [Google Scholar] [CrossRef]
- Ha, J.W.; Kim, Y.; Roh, J.; Park, J.I.; Kwak, J.; Lee, C.; Hwang, D.H. Thermally curable polymers consisting of alcohol-functionalized cyclotetrasiloxane and melamine derivatives for use as insulators in OTFTs. Org. Electron. 2014, 15, 3666–3673. [Google Scholar] [CrossRef]
- Sasikala, T.S.; Nair, B.P.; Pavithran, C.; Sebastian, M.T. at the Interface. Langmuir 2012, 28, 9742–9747. [Google Scholar] [CrossRef]
- Coonrod, J. Understanding the variables of dielectric constant for PCB materials used at microwave frequencies. In Proceedings of the 2011 41st European Microwave Conference, Manchester, UK, 9–14 October 2011; pp. 938–944. [Google Scholar]
- Volksen, W.; Miller, R.D.; Dubois, G. Low dielectric constant materials. Chem. Rev. 2010, 110, 56–110. [Google Scholar] [CrossRef]
- Guyer, E.P.; Patz, M.; Dauskardt, R.H. Fracture of nanoporous methyl silsesquioxane thin-film glasses. J. Mater. Res. 2006, 21, 882–894. [Google Scholar] [CrossRef]
- Song, Z.R.; Yu, Y.H.; Li, C.L.; Zou, S.C.; Zhang, F.M.; Wang, X.; Shen, D.S.; Luo, E.Z.; Sundaravel, B.; Wong, S.P.; et al. Tetrahedral amorphous-carbon thin films for silicon-on-insulator application. Appl. Phys. Lett. 2002, 80, 743–745. [Google Scholar] [CrossRef]
- Grill, A.; Patel, V. Low dielectric constant films prepared by plasma-enhanced chemical vapor deposition from tetramethylsilane. J. Appl. Phys. 1999, 85, 3314–3318. [Google Scholar] [CrossRef]
- Grill, A. Amorphous carbon based materials as the interconnect dielectric in ULSI chips. Diam. Relat. Mater. 2001, 10, 234–239. [Google Scholar] [CrossRef]
- Tada, M.; Yamamoto, H.; Ito, F.; Takeuchi, T.; Furutake, N.; Hayashi, Y. Chemical Structure Effects of Ring-Type Siloxane Precursors on Properties of Plasma-Polymerized Porous SiOCH Films. J. Electrochem. Soc. 2007, 154, D354–D361. [Google Scholar] [CrossRef]
- Gorman, B.P.; Orozco-Teran, R.A.; Roepsch, J.A.; Dong, H.; Reidy, R.F.; Mueller, D.W. High strength, low dielectric constant fluorinated silica xerogel films. Appl. Phys. Lett. 2001, 79, 4010–4012. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.; Herrmann, N.; Ramsahye, N.; Totée, C.; Carcel, C.; Unno, M.; Bartlett, J.R.; Wong Chi Man, M. Large Polyhedral Oligomeric Silsesquioxane Cages: The Isolation of Functionalized POSS with an Unprecedented Si18O27 Core. Angew. Chem.—Int. Ed. 2021, 60, 3022–3027. [Google Scholar] [CrossRef]
- Morimoto, Y.; Watanabe, K.; Ootake, N.; Inagaki, J.; Yoshida, K.; Ohguma, K. Silsesquioxane Derivative Production Process For The Same. U.S. 7449539 B2, 11 November 2008. [Google Scholar]
- Yoshida, T.; Hattori, T.; Ootake, N.; Tanaka, R.; Matsumonto, H. Silsesquioxane-Based Polymers: Synthesis of Phenylsilsesquioxanes with Double-Decker Structure and Their Polymers. Silicon Based Polym. 2008, 206, 205–211. [Google Scholar]
- Dudziec, B.; Marciniec, B. Double-decker Silsesquioxanes: Current Chemistry and Applications. Curr. Org. Chem. 2017, 21, 2794–2813. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: London, UK; New York, NY, USA, 2011; ISBN 978-90-481-3786-2. [Google Scholar]
- Soldatov, M.; Liu, H. Hybrid porous polymers based on cage-like organosiloxanes: Synthesis, properties and applications. Prog. Polym. Sci. 2021, 119, 101419. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, H.; Xu, J. Cubic polyhedral oligomeric silsesquioxane based functional materials: Synthesis, assembly, and applications. Chem.—An Asian J. 2016, 11, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Dudziec, B.; Zak, P.; Marciniec, B. Synthetic routes to silsesquioxane-based systems as photoactive materials and their precursors. Polymers 2019, 11, 504. [Google Scholar] [CrossRef]
- Laine, R.M. Unconventional conjugation in macromonomers and polymers. Chem. Commun. 2022, 58, 10596–10618. [Google Scholar] [CrossRef]
- Au-Yeung, H.-L.; Leung, S.Y.-L.; Yam, V.W.-W. Supramolecular assemblies of dinuclear alkynylplatinum(II) terpyridine complexes with double-decker silsesquioxane nano-cores: The role of isomerism in constructing nano-structures. Chem. Commun. 2018, 54, 4128–4131. [Google Scholar] [CrossRef]
- Calabrese, C.; Aprile, C.; Gruttadauria, M.; Giacalone, F. POSS nanostructures in catalysis. Catal. Sci. Technol. 2020, 10, 7415–7447. [Google Scholar] [CrossRef]
- Filion, T.M.; Xu, J.; Prasad, M.L.; Song, J. In vivo tissue responses to thermal-responsive shape memory polymer nanocomposites. Biomaterials 2011, 32, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Teoh, G.Z.; Crowley, C.; Birchall, M.A.; Seifalian, A.M. Development of resorbable nanocomposite tracheal and bronchial scaffolds for paediatric applications. Br. J. Surg. 2015, 102, e140–e150. [Google Scholar] [CrossRef]
- Shi, H.; Yang, J.; You, M.; Li, Z.; He, C. Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Hybrid Soft Gels: Molecular Design, Material Advantages, and Emerging Applications. ACS Mater. Lett. 2020, 2, 296–316. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like Silsesquioxanes-based Hybrid Materials. Dalt. Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, J.; Kuciński, K.; Hreczycho, G. Highly Efficient Catalytic Route for the Synthesis of Functionalized Silsesquioxanes. Inorg. Chem. 2017, 56, 9337–9342. [Google Scholar] [CrossRef] [PubMed]
- Vautravers, N.R.; André, P.; Slawin, A.M.Z.; Cole-Hamilton, D.J. Synthesis and characterization of photoluminescent vinylbiphenyl decorated polyhedral oligomeric silsesquioxanes. Org. Biomol. Chem. 2009, 7, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Stefanowska, K.; Szyling, J.; Walkowiak, J.; Franczyk, A. Alkenyl-Functionalized Open-Cage Silsesquioxanes (RSiMe2O)3R′7Si7O9: A Novel Class of Building Nanoblocks. Inorg. Chem. 2021, 60, 11006–11013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, X.H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Eckstorff, F.; Zhu, Y.; Maurer, R.; Müller, T.E.; Scholz, S.; Lercher, J.A. Materials with tunable low-k dielectric constant derived from functionalized octahedral silsesquioxanes and spherosilicates. Polymer 2011, 52, 2492–2498. [Google Scholar] [CrossRef]
- Su, R.Q.; Müller, T.E.; Procházka, J.; Lercher, J.A. A new type of low-κ dielectric films based on polysilsesquioxanes. Adv. Mater. 2002, 14, 1369–1373. [Google Scholar] [CrossRef]
- Mendiratta, S.; Usman, M.; Lu, K.L. Expanding the dimensions of metal–organic framework research towards dielectrics. Coord. Chem. Rev. 2018, 360, 77–91. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Zhou, J.; Jin, K.; Fang, Q. Fluorinated and Thermo-Cross-Linked Polyhedral Oligomeric Silsesquioxanes: New Organic—Inorganic Hybrid Materials for High- Performance Dielectric Application. ACS Appl. Mater. Interfaces 2017, 9, 12782–12790. [Google Scholar] [CrossRef]
- Sharma, B.; Verma, R.; Baur, C.; Bykova, J.; Mabry, M. Ultra low dielectric, self-cleansing and highly oleophobic POSS-PFCP aryl ether polymer composites. J. Mater. Chem. C 2013, 1, 7222–7227. [Google Scholar] [CrossRef]
- Lee, A.S.; Choi, S.; Oh, S.Y.; Lee, H.S.; Kim, B.; Hwang, S.S.; Baek, K. Incompletely condensed POSS-based spin-on-glass networks for impeccable ultra. J. Mater. Chem. C 2015, 3, 11605–11611. [Google Scholar] [CrossRef]
- Geng, Z.; Huo, M.; Mu, J.; Zhang, S.; Lu, Y.; Luan, J.; Huo, P.; Du, Y.; Wang, G. Ultra low dielectric constant soluble polyhedral oligomeric silsesquioxane (POSS)-poly(aryl ether ketone) nanocomposites with excellent thermal and mechanical properties. J. Mater. Chem. C 2014, 2, 1094–1103. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N. Fluoro-silsesquioxane-urethane hybrid for thin film applications. ACS Appl. Mater. Interfaces 2009, 1, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Hogle, R.A.; Helly, P.J.; Ma, C.; Miller, L.J. Methods for Forming Low-K Delectric Films. U.S. 6.936.537 B2, 30 August 2005. [Google Scholar]
- Kuehnle, A.; Jost, C.; Rauleder, H.; Rentrop, C.; Van Dam, R.; Timmer, K.; Fischer, H. Process for Producing Low-K Delectricfilms. U.S. 7.410.914 B2, 2008. [Google Scholar]
- Lee, Y.; Huang, J.; Kuo, S.; Chang, F. Low-dielectric, nanoporous polyimide films prepared from PEO—POSS nanoparticles. Polymer 2005, 46, 10056–10065. [Google Scholar] [CrossRef]
- Leu, C.; Chang, Y.; Wei, K. Synthesis and Dielectric Properties of Polyimide-Tethered Polyhedral. Macromolecules 2003, 36, 9122–9127. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Fan, H.; Fu, Q.; Gu, Y.; Li, X.; Fan, H.; Fu, Q.; Gu, Y. For Table of Contents use only Multifunctional Benzoxazine Copolymerize with Epoxy. Mater. Chem. Phys. 2019, 223, 260–267. [Google Scholar] [CrossRef]
- Hyeon-Lee, J.; Lyu, Y.Y.; Lee, M.S.; Hahn, J.H.; Rhee, J.H.; Mah, S.K.; Yim, J.H.; Kim, S.Y. Nanoporous low dielectric cyclosiloxane bearing polysilsesquioxane thin films templated by poly(ε-caprolactone). Macromol. Mater. Eng. 2004, 289, 164–173. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Mittal, K.L.; Ghosh, M.K.; Mittal, K.L. (Eds.) Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996; pp. 1–673. [Google Scholar]
- Lv, P.; Dong, Z.; Dai, X.; Qiu, X. Flexible Polydimethylsiloxane-Based Porous Polyimide Films with an Ultralow Dielectric Constant and Remarkable Water Resistance. ACS Appl. Polym. Mater. 2019, 1, 2597–2605. [Google Scholar] [CrossRef]
- Mei-Hui Tsai; Wha-Tzong Whang Low dielectric polyimide/poly(silsesquioxane)-like nanocomposite material. Polymer 2001, 42, 4197–4207. [CrossRef]
- Wu, S.; Hayakawa, T.; Kikuchi, R.; Grunzinger, S.J.; Kakimoto, M.; Oikawa, H. Synthesis and Characterization of Semiaromatic Polyimides Containing POSS in Main Chain Derived from Double-Decker-Shaped Silsesquioxane. Macromolecules 2007, 40, 5698–5705. [Google Scholar] [CrossRef]
- Iyoku, Y.; Kakimoto, M.; Imai, Y. The preparation of new poly(phenylsilsesquioxane)-polyimide hybrid films by the sol-gel process and their properties. High Perform. Polym. 1994, 6, 43–52. [Google Scholar] [CrossRef]
- Wu, S.; Hayakawa, T.; Kakimoto, M.A.; Oikawa, H. Synthesis and characterization of organosoluble aromatic polyimides containing POSS in main chain derived from double-decker-shaped silsesquioxane. Macromolecules 2008, 41, 3481–3487. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, W.; Hao, J.; Wei, Y.; Mu, J.; Jiang, Z. A unique “cage–cage” shaped hydrophobic fluoropolymer film derived from a novel double-decker structural POSS with a low dielectric constant. J. Mater. Chem. C 2015, 3, 11729–11734. [Google Scholar] [CrossRef]
- Mituła, K.; Duszczak, J.; Brząkalski, D.; Dudziec, B.; Kubicki, M.; Marciniec, B. Tetra-functional double-decker silsesquioxanes as anchors for reactive functional groups and potential synthons for hybrid materials. Chem. Commun. 2017, 53, 10370–10373. [Google Scholar] [CrossRef] [PubMed]
- Mituła, K.; Dutkiewicz, M.; Duszczak, J.; Rzonsowska, M.; Dudziec, B. Preparation of Tri(alkenyl)functional Open-Cage Silsesquioxanes as Specific Polymer Modifiers. Polymers 2020, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Januszewski, R.; Majchrzak, M.; Kubicki, M.; Dudziec, B.; Marciniec, B. Unusual cis- and trans- architecture of dihydrofunctional double-decker shaped silsesquioxane—Design and construction of its ethyl bridged π-conjugated arene derivatives. New J. Chem. 2017, 41, 3290–3296. [Google Scholar] [CrossRef]
- Januszewski, R.; Kownacki, I.; Maciejewski, H.; Marciniec, B. An efficient catalytic and solvent-free method for the synthesis of mono-organofunctionalized 1,1,3,3-tetramethyldisiloxane derivatives. J. Organomet. Chem. 2017, 846, 263–268. [Google Scholar] [CrossRef]
- Mituła, K.; Dutkiewicz, M.; Dudziec, B.; Marciniec, B.; Czaja, K. A library of monoalkenylsilsesquioxanes as potential comonomers for synthesis of hybrid materials. J. Therm. Anal. Calorim. 2018, 132, 1545–1555. [Google Scholar] [CrossRef]
- Duszczak, J.; Mituła, K.; Januszewski, R.; Żak, P.; Dudziec, B.; Marciniec, B. Highly efficient route for the synthesis of a novel generation of tetraorganofunctional double-decker type of silsesquioxanes. ChemCatChem 2019, 11, 1086–1091. [Google Scholar] [CrossRef]
- Schoen, B.W.; Lira, C.T.; Lee, A. Separation and Solubility of Cis and Trans Isomers in Nanostructured Double-Decker Silsequioxanes. J. Chem. Eng. Data 2014, 59, 1483–1493. [Google Scholar] [CrossRef]
- Władyczyn, A.; Gągor, A.; Ślepokura, K.; John, Ł. Hydroxyalkyl-substituted double-decker silsesquioxanes: Effective separation of cis and trans isomers. Inorg. Chem. Front. 2022, 9, 3999–4008. [Google Scholar] [CrossRef]
- Khan, H.; Amin, M.; Ahmad, A. Characteristics of silicone composites for high voltage insulations. Rev. Adv. Mater. Sci. 2018, 56, 91–123. [Google Scholar] [CrossRef]
- Barbier, B.; Combettes, C.; Guillemet-Fritsch, S.; Chartier, T.; Rossignol, F.; Rumeau, A.; Lebey, T.; Dutarde, E. CaCu3Ti4O12 ceramics from co-precipitation method: Dielectric properties of pellets and thick films. J. Eur. Ceram. Soc. 2009, 29, 731–735. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Maciejewski, H.; Marciniec, B.; Karasiewicz, J. New fluorocarbofunctional sphe-rosilicates: Synthesis and characterization. Organometallics 2011, 30, 2149–2153. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).