Orthogonal Experimental Study on the Factors Affecting the Mechanical Properties of Alkali-Activated Slag Materials
Abstract
1. Introduction
2. Sample Preparation and Characterization Methods
2.1. Materials
- (a)
- Blast furnace slag (BFS) is a molten material with silicate and aluminosilicate as the main components after quenching and granulation when smelting pig iron in the blast furnace [10]. The BFS was provided by Dehang mineral product processing plant in Hebei Province, China. It is an S105 grade mineral powder. This gray powder has a specific surface area of 480 m2/kg, a density of 3.1 g/cm3, and a loss on ignition of 0.84%. The X-ray fluorescence spectrum showed that the BFS contains many active components, such as SiO2 and Al2O3. Table 1 lists the specific chemical compositions. In particular, the chemical composition of the raw materials in Table 1 is obtained by XRF testing and is provided by the material supplier.
- (b)
- Metakaolin (MK) is an Aluminum Source Additive in Tests. It is a highly active mineral mainly composed of amorphous aluminum silicate that is formed by calcining superfine kaolin at low temperatures [17]. The MK was provided by Jinshan mineral product processing plant in Henan Province, China. This white powder, through a 4000-mesh sieve, has an activity index greater than 110 and a loss on ignition of 0.29%. The content of the active components SiO2 and Al2O3 in MK is more than 90%, and its main chemical composition is shown in Table 1.
- (c)
- Sodium hydroxide (NaOH), white flake solid, pure in quality grade analysis, provided by Hengyuan chemical factory in Jiangsu, China.
- (d)
- Sodium silicate (Na2SiO3), industrial grade powdery instant sodium silicate with a modulus of 2, provided by Hengyuan chemical plant in Jiangsu, China.
2.2. Mix Design and Sample Preparation
2.3. Test and Characterization Methods
- (1)
- Uniaxial compression test: The uniaxial compressive strength test of the alkali-activated slag-based geopolymer was carried out in the uniaxial compression mode of the SYD0709 (Hangxing Instrument Manufacturing Co., Guangdong, China) Marshall stability tester (Figure 1a). The maximum range of the instrument is 50 KN, the loading rate is controlled to 1 mm/min, and the strength of the sample is tested at 7 d and 28 d. According to the test method standard of the physical and mechanical properties of concrete, the cubic compressive strength Rc of the sample is defined as the stress when the sample is destroyed.
- (2)
- XRD test: An Xpert Pro intelligent X-ray diffractometer (PANalytical B.V., Almelo, Netherlands) was used for the phase analysis of alkali-activated slag-based geopolymers (as shown in Figure 1b) with a scanning range of 20°~60°. The glass tube anode type of the machine is Cu, the test speed is set to 5°/min and the step length is 0.02°. The samples used for the XRD test were cured to 28 d, crushed to powder and sieved through 200 mesh.
- (3)
- SEM test: This paper uses the JSM-6501 scanning electron microscope (JEOL, Tokyo, Japan) to observe the samples. The test voltage is 5 kV, and the SE mode is adopted.
3. Results and Discussion
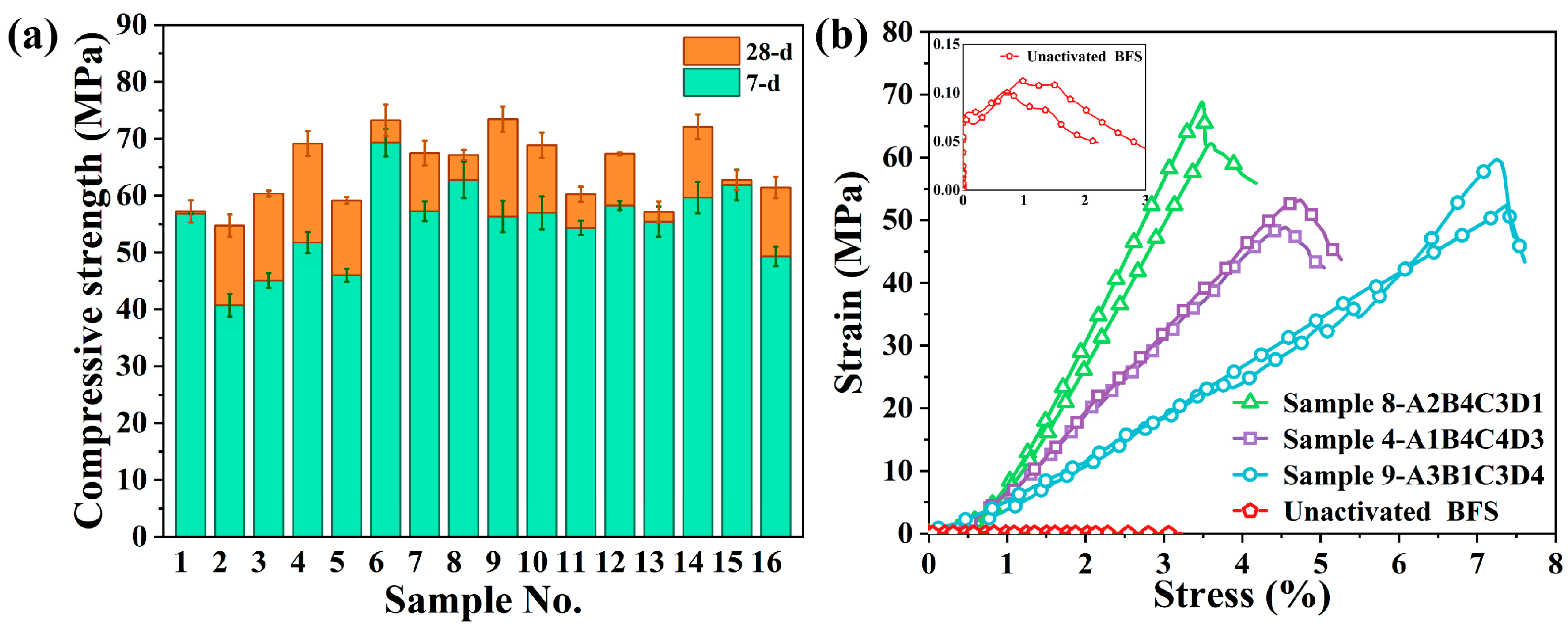
3.1. Uniaxial Compressive Strength of the Alkali-Activated Slag Sample
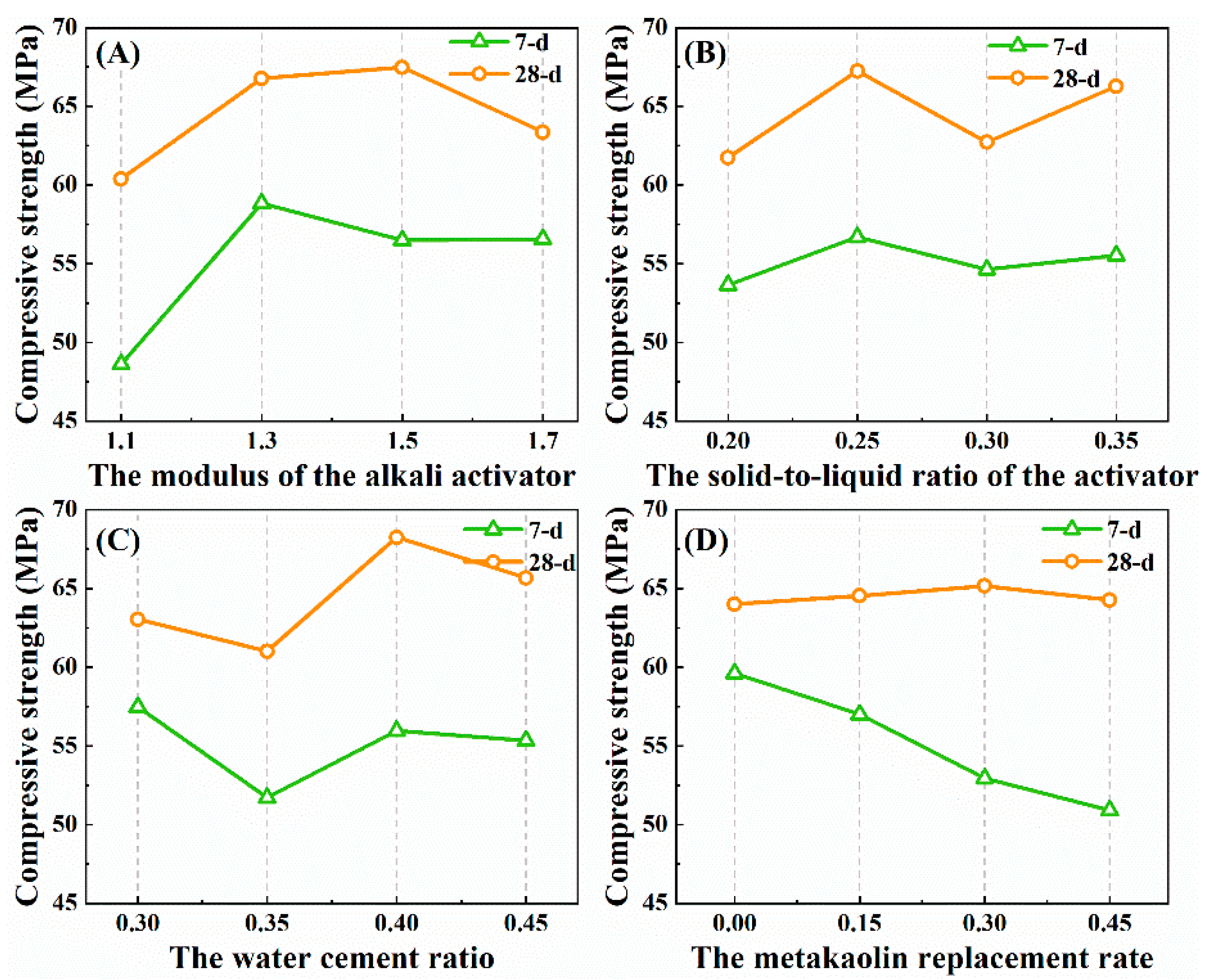
3.2. Range Analysis of the Uniaxial Compressive Strength
- (a)
- The modulus of the alkali activator
- (b) The solid-to-liquid ratio of the activator
- (c) The water–cement ratio
- (d) The Metakaolin replacement rate
3.3. Variance Analysis of Uniaxial Compressive Strength
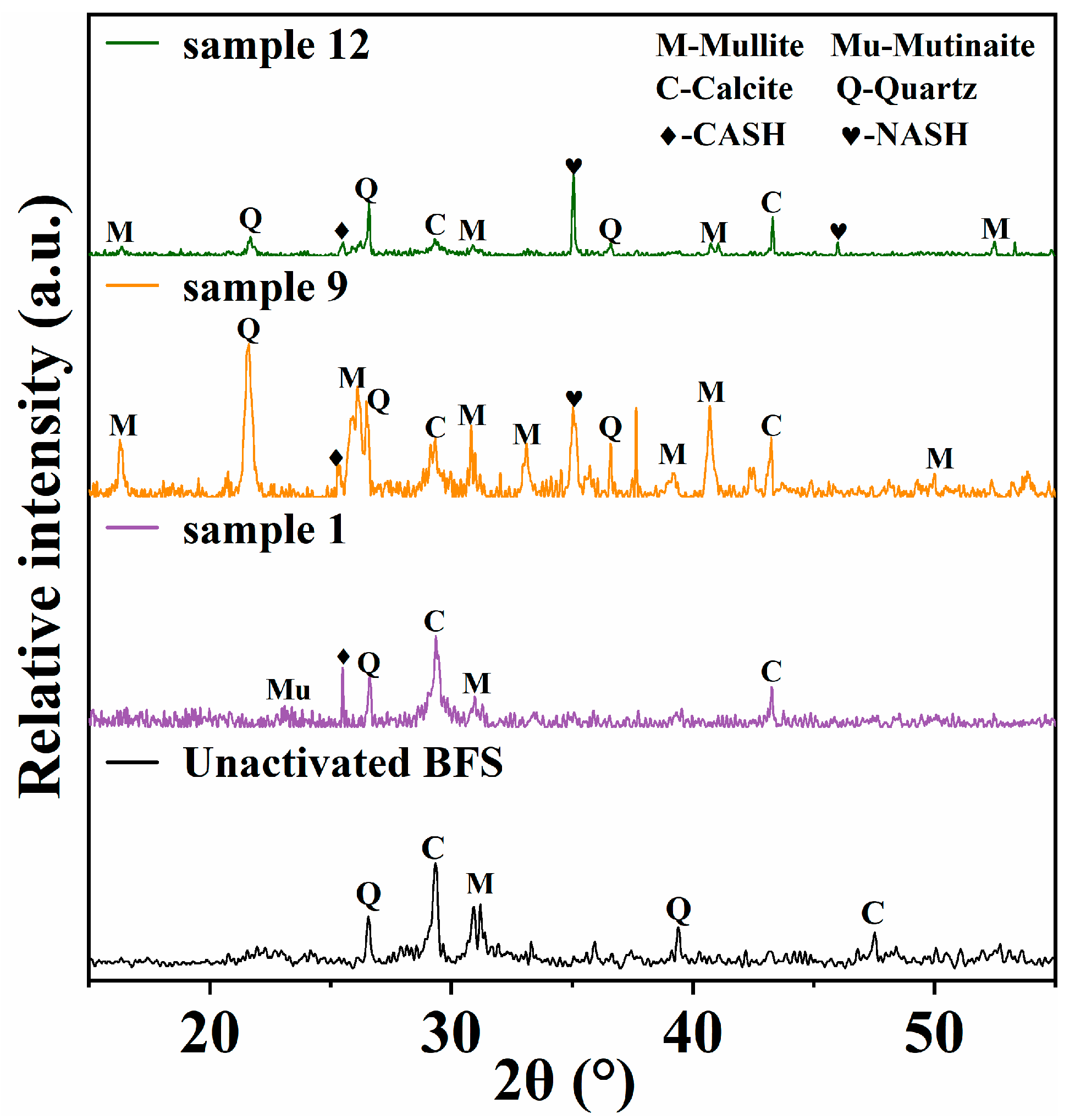
3.4. XRD Phase Analysis
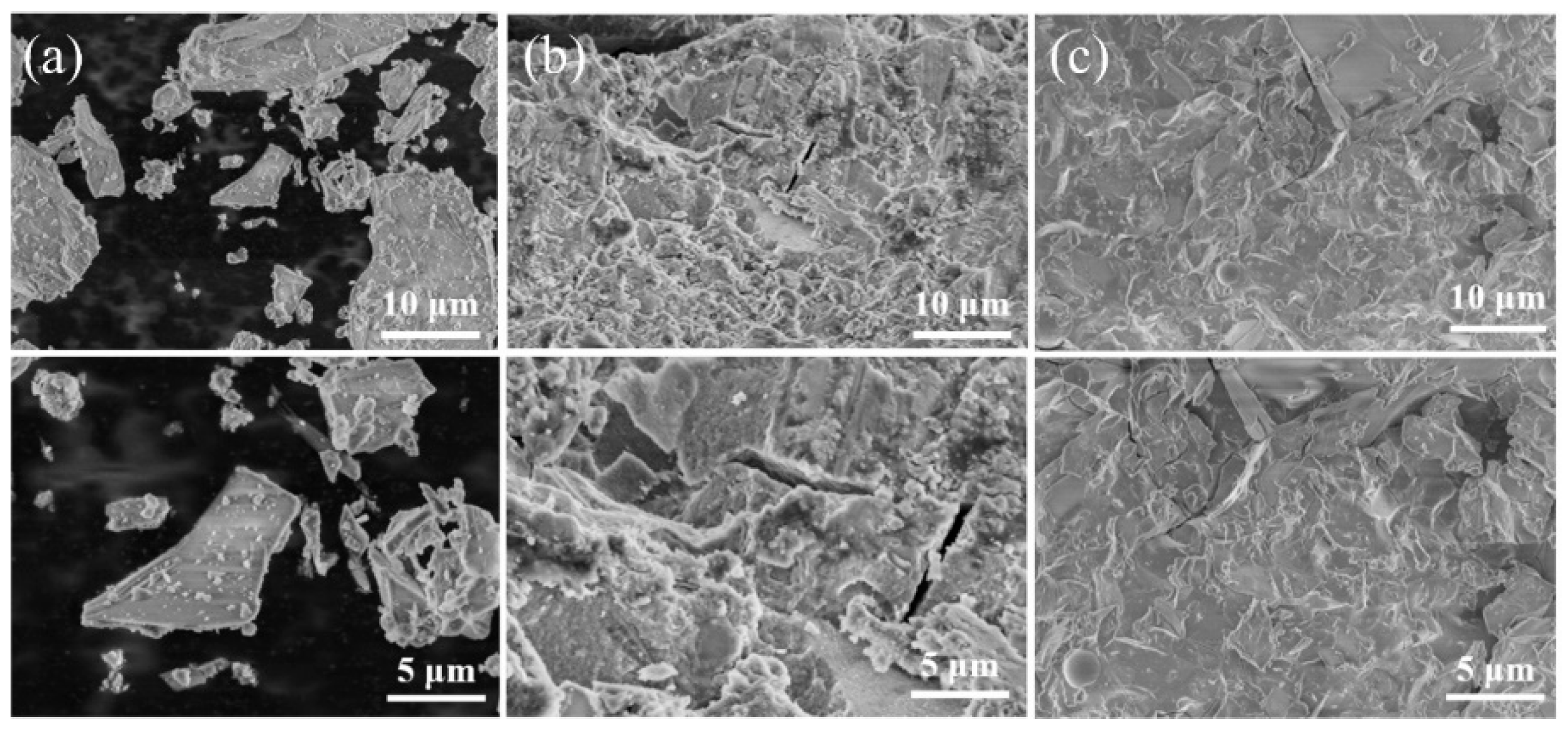
3.5. SEM Analysis
4. Conclusions
- (1)
- The significant degree of influence of each factor on the strength of the sample is as follows: At the early stage of curing, the modulus of the alkali activator > the Metakaolin replacement rate > the water–cement ratio > the solid-to-liquid ratio of the activator. In the middle and later period of curing, the modulus of the alkali activator > the water–cement ratio > the solid-to-liquid ratio of the activator > the Metakaolin replacement rate.
- (2)
- From the point of view of the alkali activator, the alkali activator mainly composed of sodium silicate and sodium hydroxide, on the one hand, serves as a catalyst in the alkali-activated reaction, and, on the other hand, it provides a large number of Si-components in the reaction system. The effect of its mix ratio on the strength of the sample is more complicated. Different levels of the alkali activator modulus, alkali activator solid–liquid ratio and water-cement ratio have little effect on the strength growth of the sample, so the specific ratio of the alkali activator determines the final strength level of the sample to a certain extent. In addition, through orthogonal analysis, we found that the samples with a higher strength can be prepared by setting the modulus of the alkali activator between 1.3 and 1.5 and the water–cement ratio to about 0.4. The test results also show that a higher content of the alkali activator in the system is not necessarily better. From the perspective of economy, the solid-to-liquid ratio is selected at about 0.25, which can obtain a higher strength at the same time.
- (3)
- From the perspective of element composition, in the alkali-activated slag system with high calcium and low aluminum levels, increasing the proportion of aluminum in the system by increasing the metakaolin replacement rate can significantly promote the late strength and toughness growth of the sample. In this process, the new gel phase NASH generated by the continuous reaction plays a key role.
- (4)
- From the above conclusions, we can prepare the sample according to the expected material properties. For example, to obtain early strength building materials, we can choose pure slag as a raw material. If it is necessary to ensure that the material strength can still increase effectively with time while ensuring high early strength, we can choose to use an appropriate amount of high-aluminum and low-calcium raw materials in the slag system to achieve the desired purpose. This can provide some reference for engineering applications.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farooq, F.; Jin, X.; Javed, M.F.; Akbar, A.; Shah, M.I.; Aslam, F.; Alyousef, R. Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Alhawat, M.; Ashour, A.; Yildirim, G.; Aldemir, A.; Sahmaran, M. Properties of geopolymers sourced from construction and demolition waste: A review. J. Build. Eng. 2022, 50, 104104. [Google Scholar] [CrossRef]
- Chan, C.L.; Zhang, M. Behaviour of strain hardening geopolymer composites at elevated temperatures. Cem. Concr. Compos. 2022, 132, 104634. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.; Lukey, G.; Kriven, W.; van Deventer, J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Huang, J.; Wan, C.; Yang, H.; Wan, C. Investigation on Potential Reactive Evaluation of Fly Ash for Geopolymer. Cailiao Daobao Mater. Rep. 2022, 36, 21010007–7. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Amran, Y.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Wang, P.Z.; Trettin, R.; Rudert, V. Effect of fineness and particle size distribution of granulated blast-furnace slag on the hydraulic reactivity in cement systems. Adv. Cem. Res. 2005, 17, 161–167. [Google Scholar] [CrossRef]
- Jun, W.U.; Xiyao, Z.; Aiwu, Y. Experimental study on the compressive strength of muddy clay solidified by the one-part slag-fly ash based geopolymer. Rock Soil Mech. 2021, 42, 647–655. [Google Scholar]
- Ma, C.; Bao, S.; Zhang, Y.; Luo, Y.; Gui, Y.; Ren, Y. Preparation of non-sintered sewage sludge based ceramsite by alkali-thermal activation and hydration mechanism. Ceram. Int. 2022, 48, 31606–31613. [Google Scholar] [CrossRef]
- Luo, X.C.; Wang C, A. Effect of calcia content on structure and Properties of metakaolin/blast furnace slag-based geopolymers. J. Chin. Ceram. Soc. 2015, 43, 1800. [Google Scholar]
- Yi, Y.L.; Qing, X.W.; Zhuang, Y.; Liu, S.Y.; Du, G.Y. Application of granulated blast furnace slag powder in soft soil solidification and its reinforcement mechanism. Chin. J. Geotech. Eng. 2013, 35 (Suppl. 2), 829–833. [Google Scholar]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Luo, X.C.; Wang, C.A. Preparation and properties of slag-based gelpolymer porous materials. J. Chin. Ceram. Soc. 2016, 44, 450–456. [Google Scholar]
- Cui, C.; Peng, H.; Liu, Y.; Zhang, J.; Cai, C.; Peng, A. Influence of GGBFS content and activator modulus on curing of metakaolin based geopolymer at ambient temperature. J. Build. Mater. 2017, 104, 535–542. [Google Scholar]
- Zheng, D.D.; Ji, T.; Wang, C.Q.; Zheng, W.Y.; Cheng, K.J.; Lin, X.J. Effect of alkali-activator on compression strength of alkali-activated slag cement mortar. J. Fuzhou Univ. (Nat. Sci. Ed.) 2016, 44, 593–597+603. [Google Scholar]
- Ma, Q.M.; HL, P.; Niu, Z.L.; Guo, R.X.; Yan, F.; Lin, Z.W.; Du, H.Y. Effect of Alkali Concentration and Modulus of Alkaline Activator on the Compressive Properties and Hydration Products of Alkali Activated Slag Cementitious Materials. Bull. Chin. Ceram. Soc. 2018, 37, 2002–2007. [Google Scholar]
- Ding, Z.; Hong, X.; Zhu, J.X.; Tian, B.Y.; Fang, Y. Alkali-activated red mud-slag cementitious materials. J. Chin. Electron Microsc. Soc. 2018, 37, 145–153. [Google Scholar]
- Peng, H.; Li, Y.; Luo, D.; Liu, Y.; Cai, C.S. Analysis of Reaction Level and Factors of Alkali Activated Metakaolin/GGBFS. J. Build. Mater. 2020, 23, 1390–1397. [Google Scholar]
- Chi, M.; Huang, R. Effects of dosage and modulus ratio of alkali-activated solution on the properties of slag mortars. Adv. Sci. Lett. 2012, 16, 7–12. [Google Scholar] [CrossRef]
- Chen, T.A.; Chen, J.H.; Huang, J.S. Effects of activator and aging process on the compressive strengths of alkali-activated glass inorganic binders. Cem. Concr. Compos. 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Falah, M.; Ohenoja, K.; Obenaus-Emler, R.; Kinnunen, P.; Illikainen, M. Improvement of mechanical strength of alkali-activated materials using micro low-alumina mine tailings. Constr. Build. Mater. 2020, 248, 118659. [Google Scholar] [CrossRef]
- Bernal, S.A. Effect of the activator dose on the compressive strength and accelerated carbonation resistance of alkali silicate-activated slag/metakaolin blended materials. Constr. Build. Mater. 2015, 98, 217–226. [Google Scholar] [CrossRef]
- Singh, A.; Bhadauria, S.S.; Mudgal, M.; Kushwah, S.S. Effect of alkali activator dosage on compressive and tensile strength of ground granulated blast furnace slag based geopolymer concrete. Can. J. Civ. Eng. 2022, 49, 73–82. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, Y.; Zhang, J.; Men, J.; Sun, T.; Zhao, H.; Wu, H.; Wang, H.; Dai, S. Orthogonal analysis and mechanism of compressive strength and microstructure of the metakaolin-fly ash geopolymer. Case Stud. Constr. Mater. 2022, 17, e01154. [Google Scholar] [CrossRef]
- Luo, Y.; Meng, J.; Wang, D.; Jiao, L.; Xue, G. Experimental study on mechanical properties and microstructure of metakaolin based geopolymer stabilized silty clay. Constr. Build. Mater. 2022, 316, 125662. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, T.; Gong, W.L.; Zhai, J.P.; Zhang, M. T Analysis of Reaction Factors Under Fly Ash Based Geopolymer System. Mater. Rep. 2016, 30, 118–123. [Google Scholar]
- Zhao, Y.; Yang, C.; Li, K.; Qu, F.; Yan, C.; Wu, Z. Toward understanding the activation and hydration mechanisms of composite activated coal gangue geopolymer. Constr. Build. Mater. 2022, 318, 125999. [Google Scholar] [CrossRef]
- Zhou, H.Y. Study on Mechanical Properties and Durability of Silt Soft Soil Solidified by Different Calcium Geopolymers. Master’s thesis, Zhejiang Sci-Tech University, Zhejiang, China, 2021. [Google Scholar]
- Wang, S.Y.; Li, Y.; Zhang, Y.K. Comparison of range analysis and variance analysis methods for orthogonal test results of concrete. Dev. Guide Build. Mater. 2016, 14, 44–48. [Google Scholar] [CrossRef]
- Sun, S.Y. Orthogonal experiment research on geopolymer synthesis by utilizing slag and fly ash as raw material. China Min. Mag. 2019, 28, 118–122+127. [Google Scholar]
- Mikhailova, O.; Del Campo, A.; Rovnanik, P.; Fernández, J.F.; Torres-Carrasco, M. In situ characterization of main reaction products in alkali-activated slag materials by Confocal Raman Microscopy. Cem. Concr. Compos. 2019, 99, 32–39. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers—Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]

| Raw Material | CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | MgO |
|---|---|---|---|---|---|---|
| Blast furnace slag (BFS) | 34.00 | 34.50 | 17.70 | 1.64 | 1.03 | 6.01 |
| Metakaolin (MK) | 0.17 | 55.06 | 43.02 | / | 0.76 | 0.06 |
| Level | A (The Modulus of the Alkali Activator) | B (The Solid-to-Liquid Ratio of the Activator) | C (The Water–Cement Ratio) | D (The Metakaolin Replacement Rate) |
|---|---|---|---|---|
| 1 | 1.1 | 0.20 | 0.30 | 0.00 |
| 2 | 1.3 | 0.25 | 0.35 | 0.15 |
| 3 | 1.5 | 0.30 | 0.40 | 0.30 |
| 4 | 1.7 | 0.35 | 0.45 | 0.45 |
| Mix No. | Mix Design | Mass Distribution (g) | Alkali Activator (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | BFS | MK | Water | Na2SiO3 | NaOH | |
| 1-A1B1C1D1 | 1.1 | 0.20 | 0.30 | 0% | 103.23 | 0.00 | 30.97 | 10 | 3.60 |
| 2-A1B2C2D4 | 1.1 | 0.25 | 0.35 | 45% | 77.86 | 63.71 | 49.55 | 20 | 7.19 |
| 3-A1B3C3D2 | 1.1 | 0.30 | 0.40 | 15% | 87.75 | 15.48 | 41.29 | 20 | 7.19 |
| 4-A1B4C4D3 | 1.1 | 0.35 | 0.45 | 30% | 55.06 | 23.60 | 35.39 | 20 | 7.19 |
| 5-A2B1C2D3 | 1.3 | 0.20 | 0.35 | 30% | 104.82 | 44.92 | 52.41 | 20 | 4.73 |
| 6-A2B2C1D2 | 1.3 | 0.25 | 0.30 | 15% | 118.79 | 20.96 | 41.93 | 20 | 4.73 |
| 7-A2B3C4D4 | 1.3 | 0.30 | 0.45 | 45% | 42.70 | 34.94 | 34.94 | 20 | 4.73 |
| 8-A2B4C3D1 | 1.3 | 0.35 | 0.40 | 0% | 74.87 | 0.00 | 29.95 | 20 | 4.73 |
| 9-A3B1C3D4 | 1.5 | 0.20 | 0.40 | 45% | 62.45 | 51.10 | 45.42 | 20 | 2.93 |
| 10-A3B2C4D1 | 1.5 | 0.25 | 0.45 | 0% | 80.75 | 0.00 | 36.34 | 20 | 2.93 |
| 11-A3B3C1D3 | 1.5 | 0.30 | 0.30 | 30% | 70.66 | 30.28 | 30.28 | 20 | 2.93 |
| 12-A3B4C2D2 | 1.5 | 0.35 | 0.35 | 15% | 63.03 | 11.12 | 25.95 | 20 | 2.93 |
| 13-A4B1C4D2 | 1.7 | 0.20 | 0.45 | 15% | 75.70 | 13.36 | 40.08 | 20 | 1.55 |
| 14-A4B2C3D3 | 1.7 | 0.25 | 0.40 | 30% | 56.11 | 24.05 | 32.06 | 20 | 1.55 |
| 15-A4B3C2D1 | 1.7 | 0.30 | 0.35 | 0% | 76.34 | 0.00 | 26.72 | 20 | 1.55 |
| 16-A4B4C1D4 | 1.7 | 0.35 | 0.30 | 45% | 41.99 | 34.35 | 22.90 | 20 | 1.55 |
| Mix No. | Mix Design | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | 7 Days | 28 Days | |
| 1-A1B1C1D1 | 1.1 | 0.20 | 0.30 | 0% | 56.88 | 57.25 |
| 2-A1B2C2D4 | 1.1 | 0.25 | 0.35 | 45% | 40.75 | 54.75 |
| 3-A1B3C3D2 | 1.1 | 0.30 | 0.40 | 15% | 45.08 | 60.38 |
| 4-A1B4C4D3 | 1.1 | 0.35 | 0.45 | 30% | 51.75 | 69.17 |
| 5-A2B1C2D3 | 1.3 | 0.20 | 0.35 | 30% | 46.00 | 59.17 |
| 6-A2B2C1D2 | 1.3 | 0.25 | 0.30 | 15% | 69.33 | 73.25 |
| 7-A2B3C4D4 | 1.3 | 0.30 | 0.45 | 45% | 57.25 | 67.50 |
| 8-A2B4C3D1 | 1.3 | 0.35 | 0.40 | 0% | 62.75 | 67.17 |
| 9-A3B1C3D4 | 1.5 | 0.20 | 0.40 | 45% | 56.33 | 73.42 |
| 10-A3B2C4D1 | 1.5 | 0.25 | 0.45 | 0% | 57.00 | 68.88 |
| 11-A3B3C1D3 | 1.5 | 0.30 | 0.30 | 30% | 54.33 | 60.25 |
| 12-A3B4C2D2 | 1.5 | 0.35 | 0.35 | 15% | 58.25 | 67.38 |
| 13-A4B1C4D2 | 1.7 | 0.20 | 0.45 | 15% | 55.42 | 57.17 |
| 14-A4B2C3D3 | 1.7 | 0.25 | 0.40 | 30% | 59.67 | 72.08 |
| 15-A4B3C2D1 | 1.7 | 0.30 | 0.35 | 0% | 61.88 | 62.75 |
| 16-A4B4C1D4 | 1.7 | 0.35 | 0.30 | 45% | 49.33 | 61.42 |
| Factors | Compressive Strength of 7 Days | Compressive Strength of 28 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |
| 194.458 | 214.625 | 229.875 | 238.500 | 241.542 | 247.000 | 252.167 | 256.042 | |
| 235.333 | 226.750 | 206.875 | 228.083 | 267.083 | 268.958 | 244.042 | 258.167 | |
| 225.917 | 218.542 | 223.833 | 211.750 | 269.917 | 250.875 | 273.042 | 260.667 | |
| 226.292 | 222.083 | 221.417 | 203.667 | 253.417 | 265.125 | 262.708 | 257.083 | |
| 48.615 | 53.656 | 57.469 | 59.625 | 60.385 | 61.750 | 63.042 | 64.010 | |
| 58.833 | 56.688 | 51.719 | 57.021 | 66.771 | 67.240 | 61.010 | 64.542 | |
| 56.479 | 54.635 | 55.958 | 52.938 | 67.479 | 62.719 | 68.260 | 65.167 | |
| 56.573 | 55.521 | 55.354 | 50.917 | 63.354 | 66.281 | 65.677 | 64.271 | |
| 10.219 | 3.031 | 5.750 | 8.708 | 7.094 | 5.490 | 7.250 | 0.625 | |
| Factors | Compressive Strength of 7 Days | Compressive Strength of 28 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| Sum of Squares | Degree of Freedom | Mean Square Sum | F | Sum of Squares | Degree of Freedom | Mean Square Sum | F | |
| A | 240.27 | 3 | 80.09 | 0.90 | 129.10 | 3 | 43.03 | 0.59 |
| B | 19.98 | 3 | 6.66 | 0.07 | 85.65 | 3 | 28.55 | 0.39 |
| C | 71.37 | 3 | 23.79 | 0.27 | 119.32 | 3 | 39.77 | 0.54 |
| D | 185.36 | 3 | 61.79 | 0.69 | 2.95 | 3 | 0.98 | 0.01 |
| Error | 267.93 | 3 | 89.31 | \ | 219.34 | 3 | 73.11 | \ |
| T | 784.91 | 15 | F(3,3)0.10 = 5.36 | 556.37 | 15 | F(3,3)0.10 = 5.36 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Lu, H.; Li, J.; Bai, H. Orthogonal Experimental Study on the Factors Affecting the Mechanical Properties of Alkali-Activated Slag Materials. Materials 2022, 15, 8795. https://doi.org/10.3390/ma15248795
Zhang K, Lu H, Li J, Bai H. Orthogonal Experimental Study on the Factors Affecting the Mechanical Properties of Alkali-Activated Slag Materials. Materials. 2022; 15(24):8795. https://doi.org/10.3390/ma15248795
Chicago/Turabian StyleZhang, Kai, Haifeng Lu, Jie Li, and Hao Bai. 2022. "Orthogonal Experimental Study on the Factors Affecting the Mechanical Properties of Alkali-Activated Slag Materials" Materials 15, no. 24: 8795. https://doi.org/10.3390/ma15248795
APA StyleZhang, K., Lu, H., Li, J., & Bai, H. (2022). Orthogonal Experimental Study on the Factors Affecting the Mechanical Properties of Alkali-Activated Slag Materials. Materials, 15(24), 8795. https://doi.org/10.3390/ma15248795








