Effect of Nd Element of Mg-Nd Binary Alloy on the Corrosion Resistance in Sulfate-Reducing Bacteria Solution
Abstract
:1. Introduction
2. Experimental Process
2.1. Material Preparation
2.2. Material Characterization
2.3. Immersion Test
2.4. Electromechanical Corrosion Test
3. Results
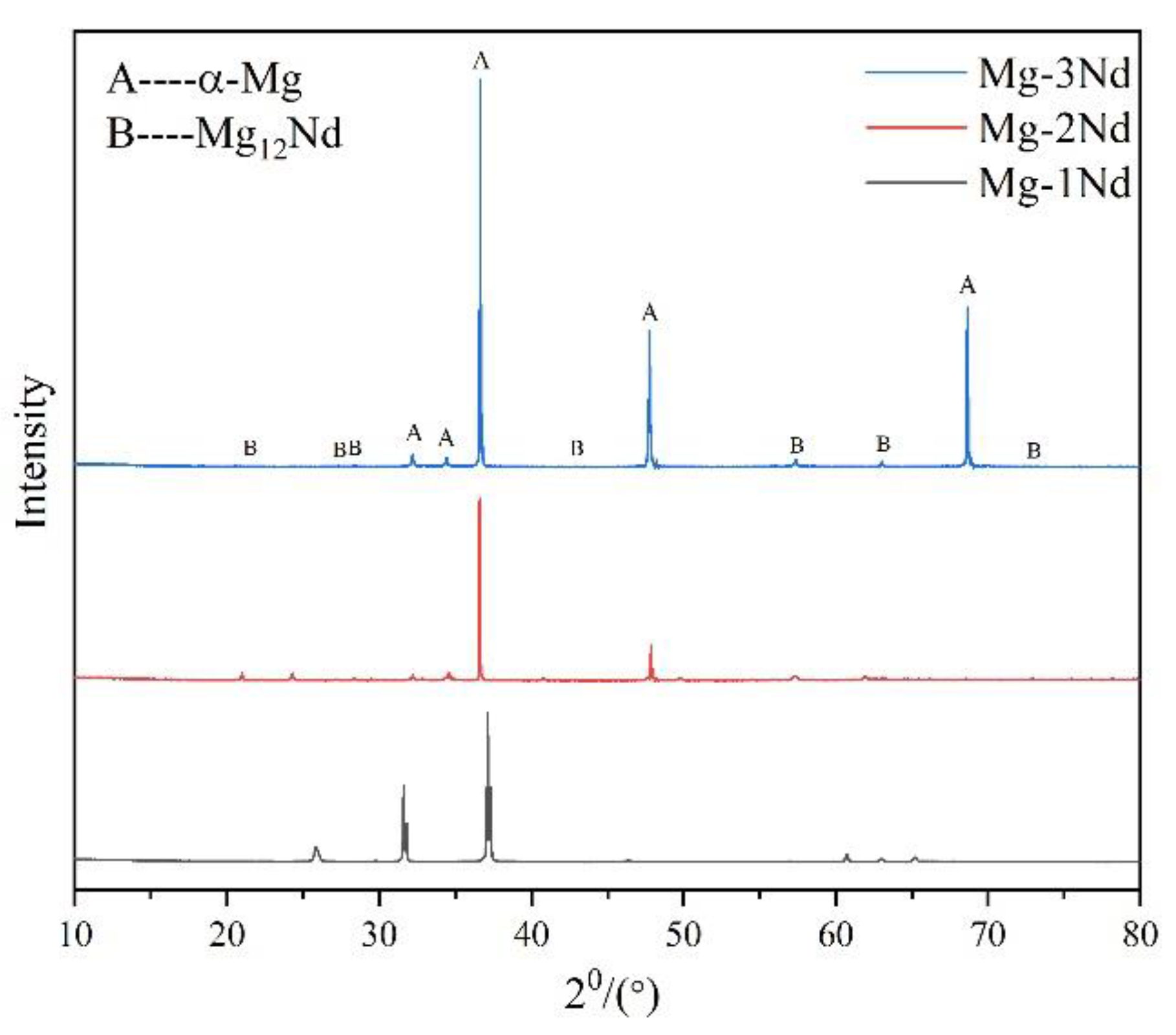
3.1. Microstructure Characteristics
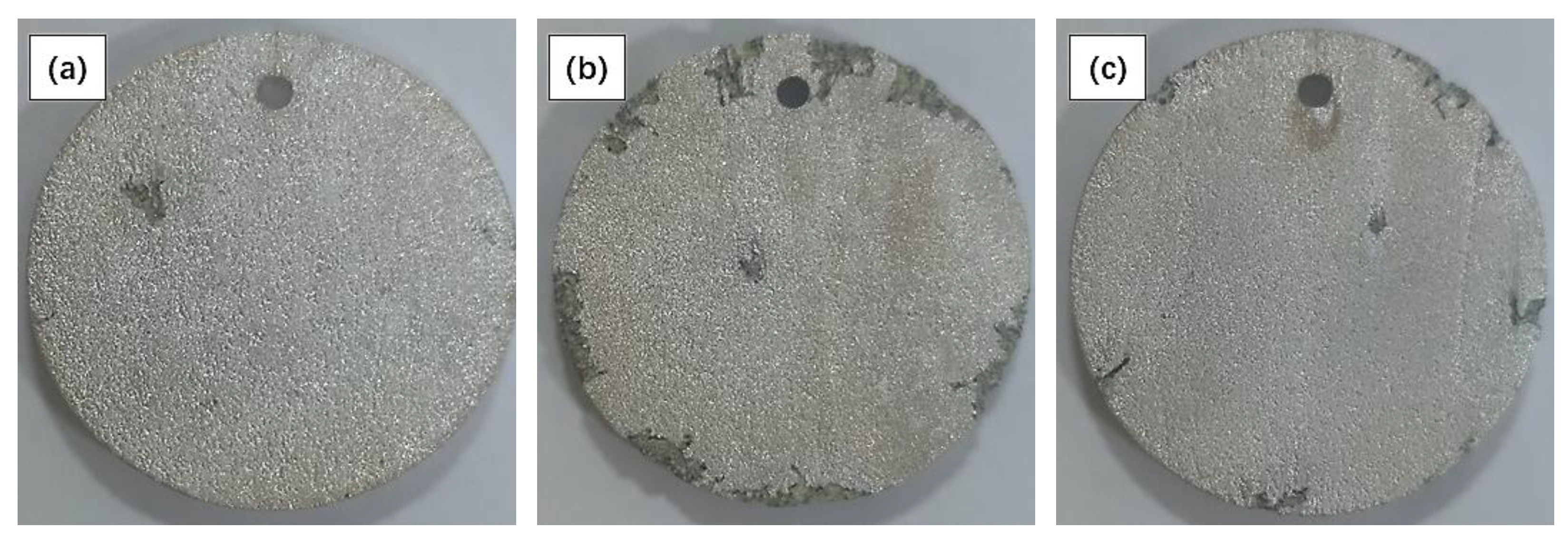
3.2. Immersion Test
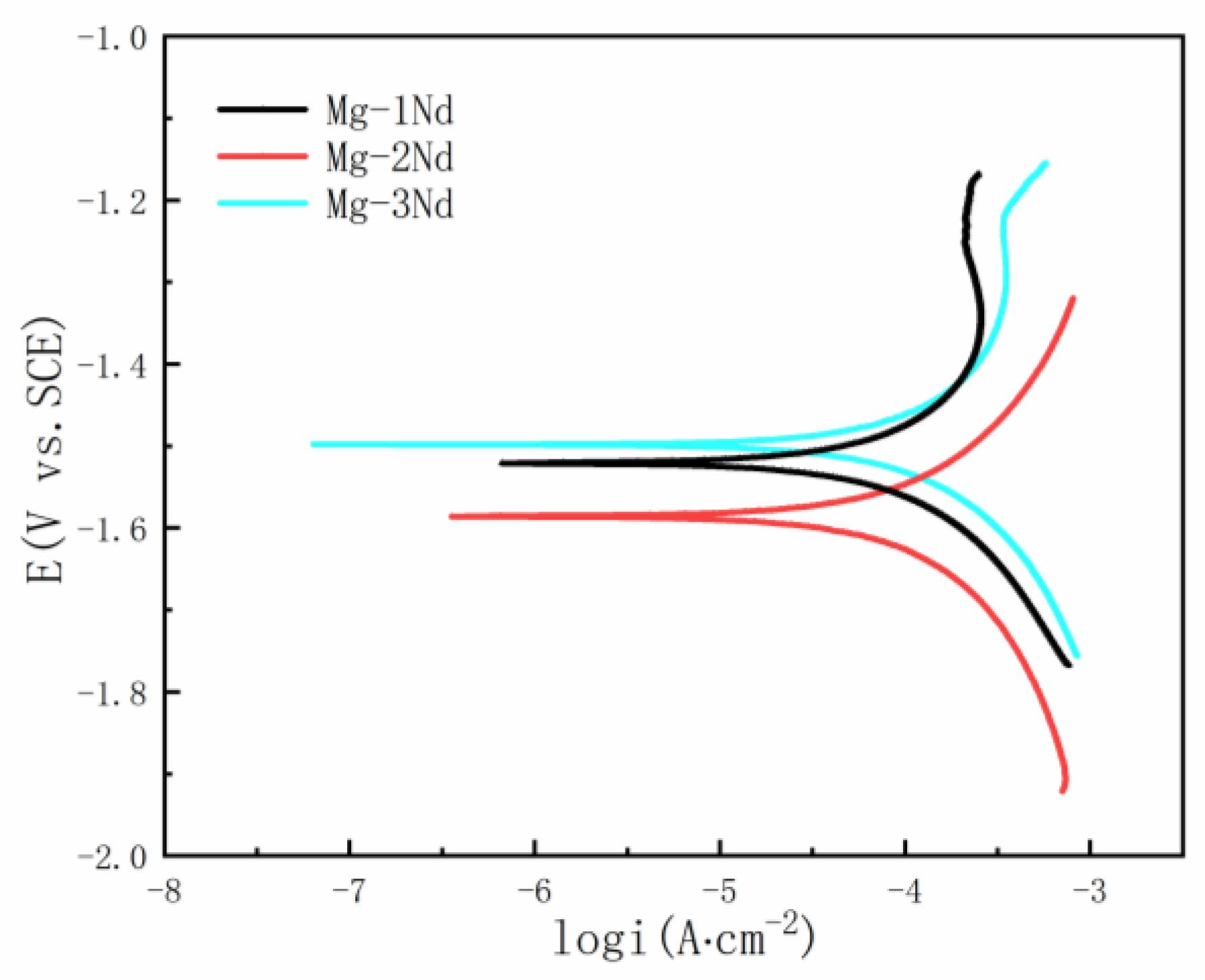
3.3. Potentiodynamic Polarization Analysis
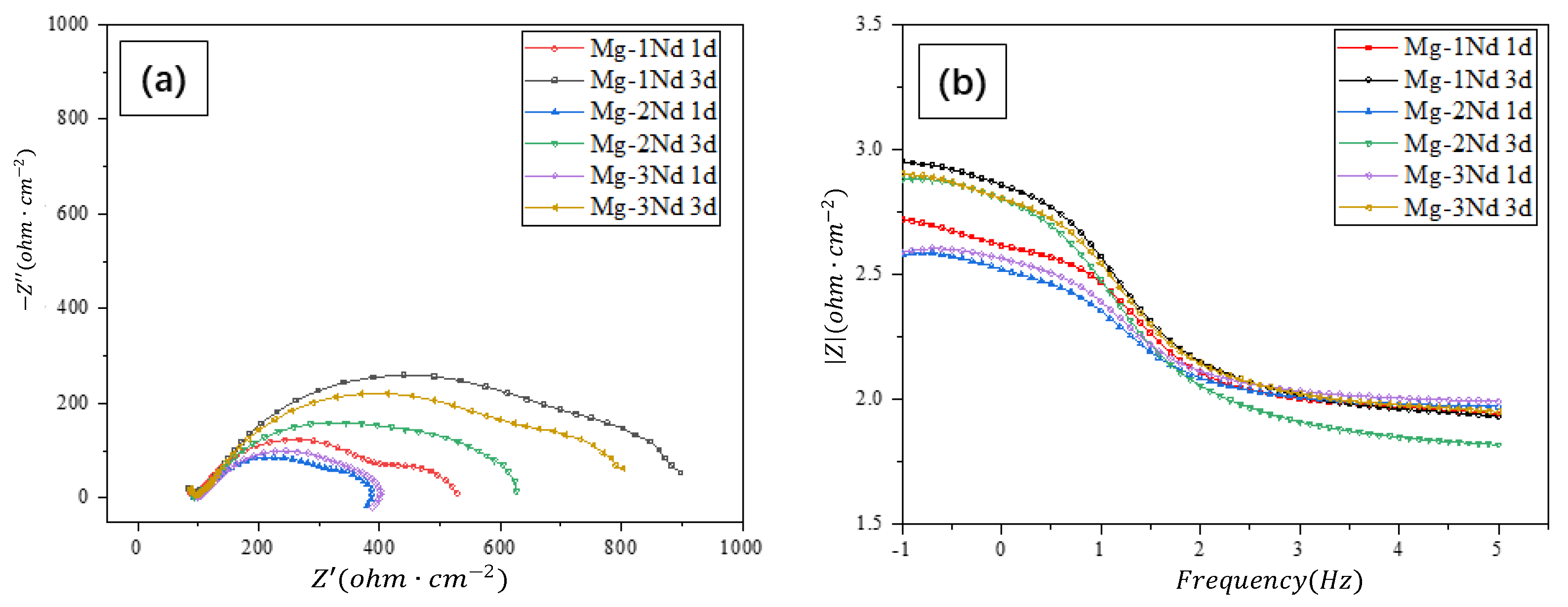
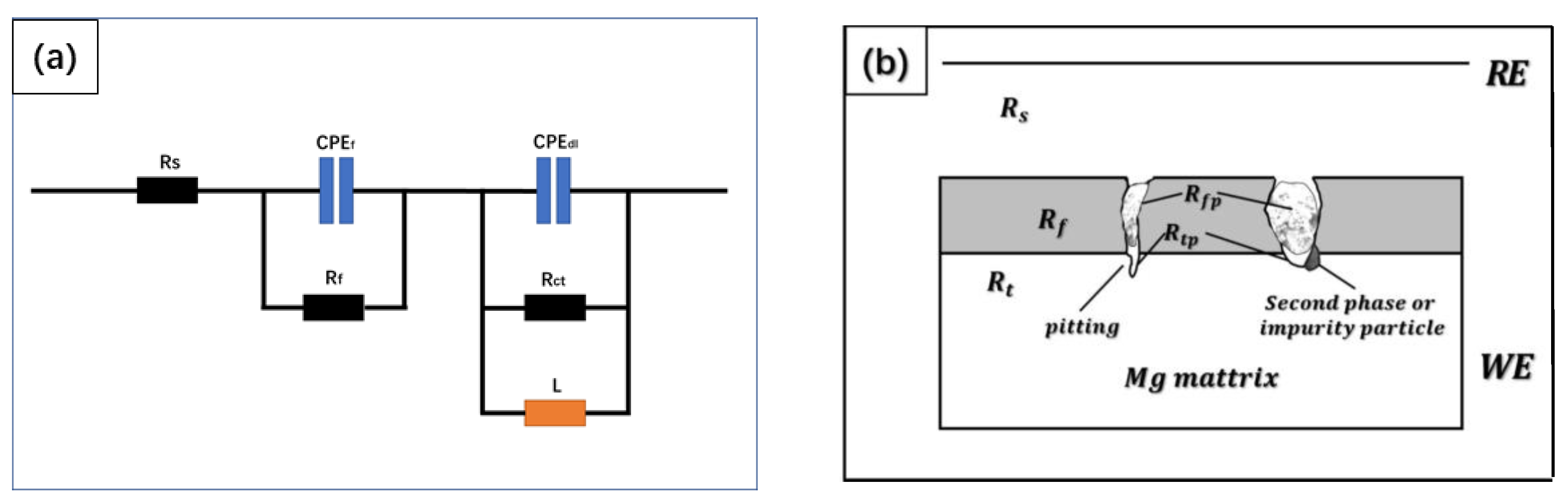
3.4. Electrochemical Impendence Spectroscopy
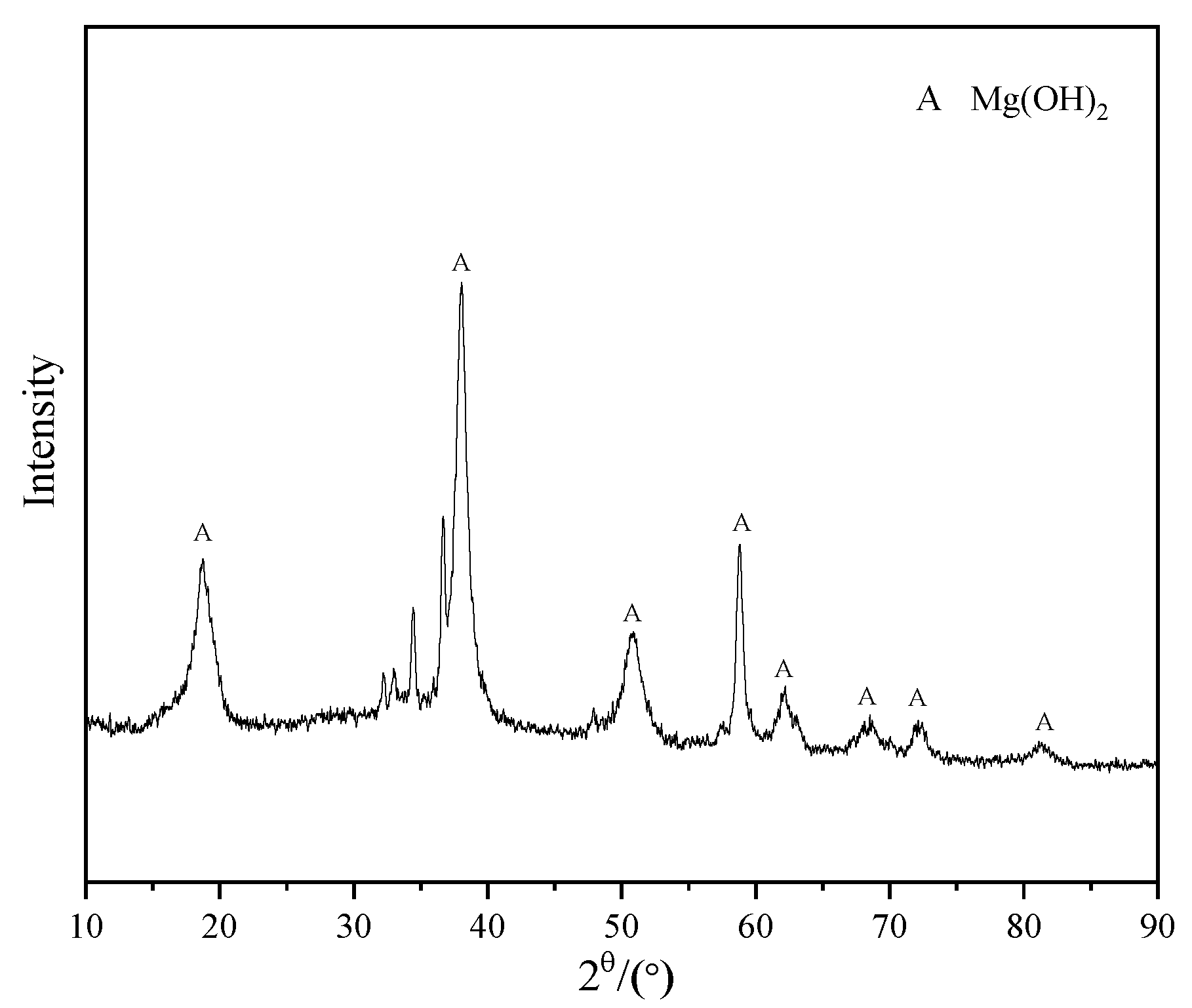
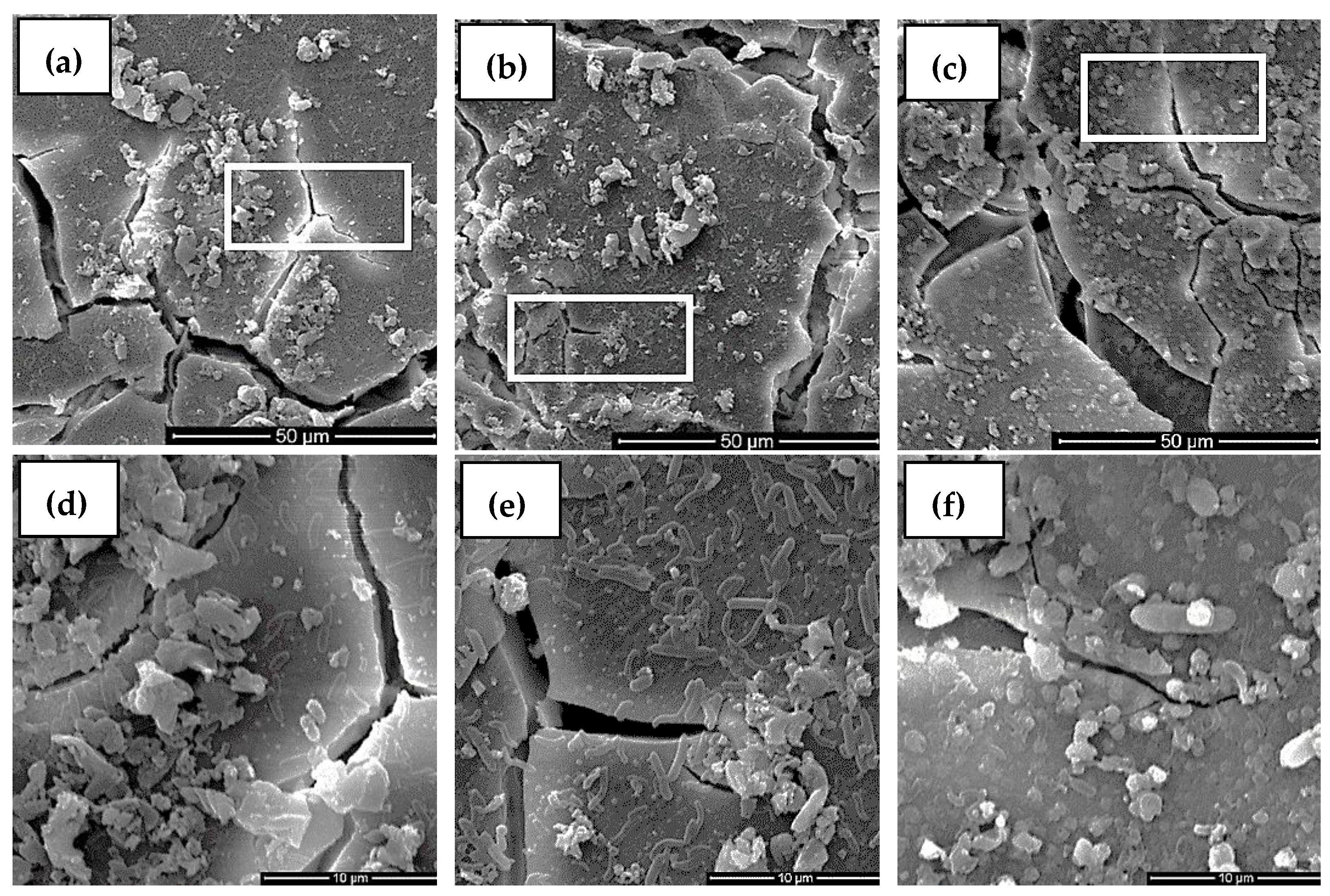
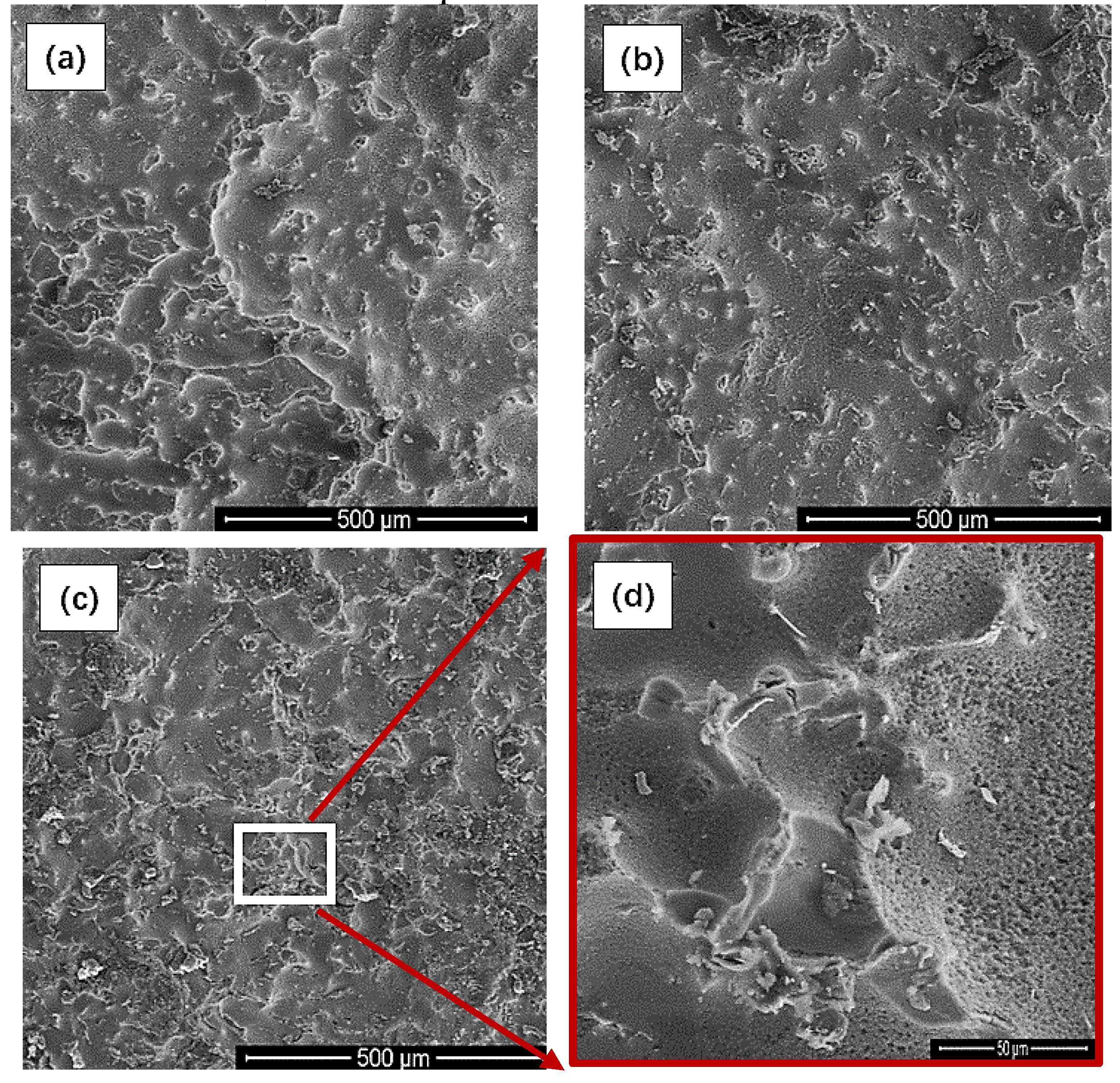
3.5. Surface Morphology Analysis
4. Discussion
5. Conclusions
- (1)
- With the increase in the Nd element, the average corrosion rate of the Mg-Nd binary alloy in the SRB medium increases first and then decreases. This is related to the distribution of the second phase. The corrosion process is accelerated only when the distribution of the second phase is discontinuous. However, the continuous network distribution of the second phase can improve the corrosion resistance.
- (2)
- The corrosion characteristics of the Mg-Nd binary alloys in the SRB medium pit the corrosion caused by galvanic corrosion. A biofilm can be formed on the surface, and the Nd element affects the protection of the biofilm.
- (3)
- There are two functions for the biofilm formed on the surface. One is to slow down the process of corrosion by blocking the penetration of erosive ions. The other function is the biological metabolism of the HS− ions and organic acids generated by SRB, which corrode the alloy matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aghion, E.; Gueta, Y.; Moscovitch, N.; Bronfin, B. Effect of yttrium additions on the properties of grain-refined Mg-3%Nd alloy. J. Mater. Sci. 2008, 43, 4870–4875. [Google Scholar] [CrossRef]
- Sun, J.; Jin, L.; Dong, J.; Ding, W.; Luo, A.A. Microscopic deformation compatibility during monotonic loading in a Mg-Gd-Y alloy. Mater. Charact. 2016, 119, 195–199. [Google Scholar] [CrossRef]
- Takenaka, T.; Ono, T.; Narazaki, Y.; Naka, Y.; Kawakami, M. Improvement of corrosion resistance of magnesium metal by rare earth elements. Electrochim. Acta 2007, 53, 117–121. [Google Scholar] [CrossRef]
- Jia, R.; Unsal, T.; Xu, D.K.; Lekbach, Y.; Gu, T.Y. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Li, Y.C.; Xu, D.K.; Chen, C.F.; Li, X.G.; Jia, R.; Zhang, D.W.; Sand, W.; Wang, F.H.; Gu, T.Y. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Fan, Y.X.; Chen, C.Y.; Zhang, Y.X.; Liu, H.X.; Liu, H.W.; Liu, H.F. Early corrosion behavior of X80 pipeline steel in a simulated soil solution containing Desulfovibrio desulfuricans. Bioelectrochemistry 2021, 141, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Xu, D.K.; Yang, K.; Liu, H.F.; Cheng, Y.F. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corros. Sci. 2018, 132, 46–55. [Google Scholar] [CrossRef]
- Anandkumar, B.; George, R.P.; Maruthamuthu, S.; Parvathavarthini, N.; Mudali, U.K. Corrosion characteristics of sulfate-reducing bacteria (SRB) and the role of molecular biology in SRB studies: An overview. Corros. Rev. 2016, 34, 41–63. [Google Scholar] [CrossRef]
- Marciales, A.; Peralta, Y.; Haile, T.; Crosby, T.; Wolodko, J. Mechanistic microbiologically influenced corrosion modeling—A review. Corros. Sci. 2019, 146, 99–111. [Google Scholar] [CrossRef]
- Gu, T.Y.; Jia, R.; Unsal, T.; Xu, D.K. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Chang, J.W.; Guo, X.W.; Fu, P.H.; Peng, L.M.; Ding, W.J. Relationship between heat treatment and corrosion behaviour of Mg-3.0%Nd-0.4%Zr magnesium alloy. Trans. Nonferrous Met. Soc. China 2007, 17, 1152–1157. [Google Scholar] [CrossRef]
- Yan, J.L.; Sun, Y.S.; Xue, F.; Xue, S.; Tao, W.J. Microstructure and mechanical properties in cast magnesium-neodymium binary alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2008, 476, 366–371. [Google Scholar] [CrossRef]
- Wang, C.; Han, P.D.; Zhang, L.; Zhang, C.L.; Xu, B.S. First-Principles Study on the Stabilities of the Intermetallic Compounds in Mg-Nd Alloys. Rare Met. Mater. Eng. 2011, 40, 590–594. [Google Scholar] [CrossRef]
- Chang, J.-W.; Guo, X.-W.; Fu, P.-H.; Peng, L.-M.; Ding, W.-J.J.E.A. Effect of heat treatment on corrosion and electrochemical behaviour of Mg–3Nd–0.2 Zn–0.4 Zr (wt.%) alloy. Electrochim. Acta 2007, 52, 3160–3167. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Jiang, B.; Yang, Q.S.; Huang, D.; Tang, A.T.; Pan, F.S.; Han, Q.Y. Role of second phases on the corrosion resistance of Mg-Nd-Zr alloys. J. Alloys Compd. 2020, 849, 11. [Google Scholar] [CrossRef]
- Salvarezza, R.C.; Videla, H.A.; Arvía, A.J. The electrochemical behaviour of mild steel in phosphate-borate-sulphide solutions. Corros. Sci. 1983, 23, 717–732. [Google Scholar] [CrossRef]

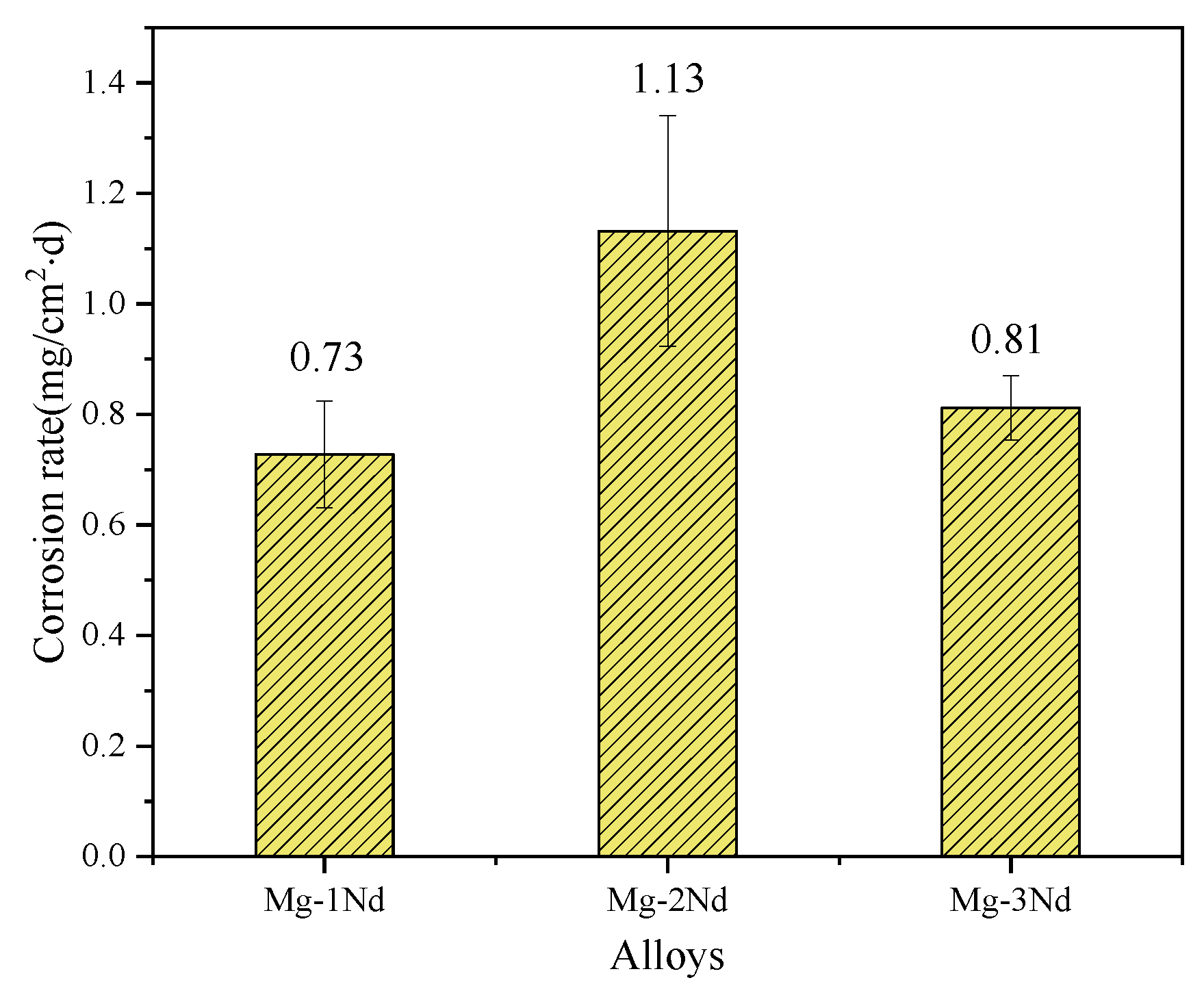

| Alloy Code | Chemical Composition, wt.% | ||
|---|---|---|---|
| Nd | Fe | Mg | |
| Mg-1Nd | 1.172 | 0.012 | margin |
| Mg-2Nd | 1.997 | 0.031 | margin |
| Mg-3Nd | 2.968 | 0.025 | margin |
| Alloys | Ecorr (V) | Icorr (μA·cm−2) | Corrosion Rate (mmpy) |
|---|---|---|---|
| Mg-1Nd | −1.521 | 107 | 1.242 |
| Mg-2Nd | −1.586 | 175.9 | 2.041 |
| Mg-3Nd | −1.498 | 130.4 | 1.514 |
| Time (day) | Rs (Ω/cm2) | Rf (Ω/cm2) | CPEf (mF/cm2) | nf | Rct (Ω/cm2) | CPEdl (mF/cm2) | ndl | L (H/cm−2) |
|---|---|---|---|---|---|---|---|---|
| Mg-1Nd | ||||||||
| 1 | 54.61 | 448.5 | 190.2 × 10−6 | 631.1 × 10−3 | 35.43 | 30.56 × 10−9 | 946.6 × 10−3 | 38.46 × 10−3 |
| 3 | 14.06 | 900.4 | 123.5 × 10−6 | 671.3 × 10−3 | 76.38 | 31.69 × 10−9 | 880.0 × 10−3 | 245.5 × 10−3 |
| Mg-2Nd | ||||||||
| 1 | 73.32 | 319.4 | 287.0 × 10−6 | 638.9 × 10−3 | 21.60 | 439.5 × 10−9 | 725.8 × 10−3 | 38.20 × 10−3 |
| 3 | 52.73 | 600.7 | 230.3 × 10−6 | 638.3 × 10−3 | 41.73 | 4.454 × 10−6 | 468.6 × 10−3 | 114.4 × 10−3 |
| Mg-3Nd | ||||||||
| 1 | 70.64 | 334.6 | 178.0 × 10−6 | 708.9 × 10−3 | 33.10 | 93.32 × 10−6 | 325.9 × 10−3 | 99.10 × 10−3 |
| 3 | 32.23 | 784.7 | 146.6 × 10−6 | 655.6 × 10−3 | 60.58 | 17.33 × 10−9 | 954.6 × 10−3 | 153.7 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Zhang, Z.; Zhou, Y.; Xu, J.; Zheng, X.; Sun, M.; Wang, F.; Zhang, Z.; Hu, Q. Effect of Nd Element of Mg-Nd Binary Alloy on the Corrosion Resistance in Sulfate-Reducing Bacteria Solution. Materials 2022, 15, 8788. https://doi.org/10.3390/ma15248788
Chu Z, Zhang Z, Zhou Y, Xu J, Zheng X, Sun M, Wang F, Zhang Z, Hu Q. Effect of Nd Element of Mg-Nd Binary Alloy on the Corrosion Resistance in Sulfate-Reducing Bacteria Solution. Materials. 2022; 15(24):8788. https://doi.org/10.3390/ma15248788
Chicago/Turabian StyleChu, Zhenhua, Zhixin Zhang, Yuanqing Zhou, Jingxiang Xu, Xingwei Zheng, Ming Sun, Fang Wang, Zheng Zhang, and Qingsong Hu. 2022. "Effect of Nd Element of Mg-Nd Binary Alloy on the Corrosion Resistance in Sulfate-Reducing Bacteria Solution" Materials 15, no. 24: 8788. https://doi.org/10.3390/ma15248788
APA StyleChu, Z., Zhang, Z., Zhou, Y., Xu, J., Zheng, X., Sun, M., Wang, F., Zhang, Z., & Hu, Q. (2022). Effect of Nd Element of Mg-Nd Binary Alloy on the Corrosion Resistance in Sulfate-Reducing Bacteria Solution. Materials, 15(24), 8788. https://doi.org/10.3390/ma15248788








