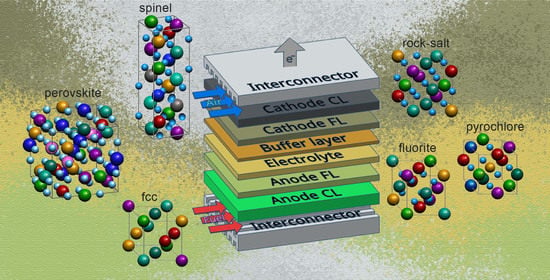
High-Entropy Materials in SOFC Technology: Theoretical Foundations for Their Creation, Features of Synthesis, and Recent Achievements
Abstract
1. Introduction
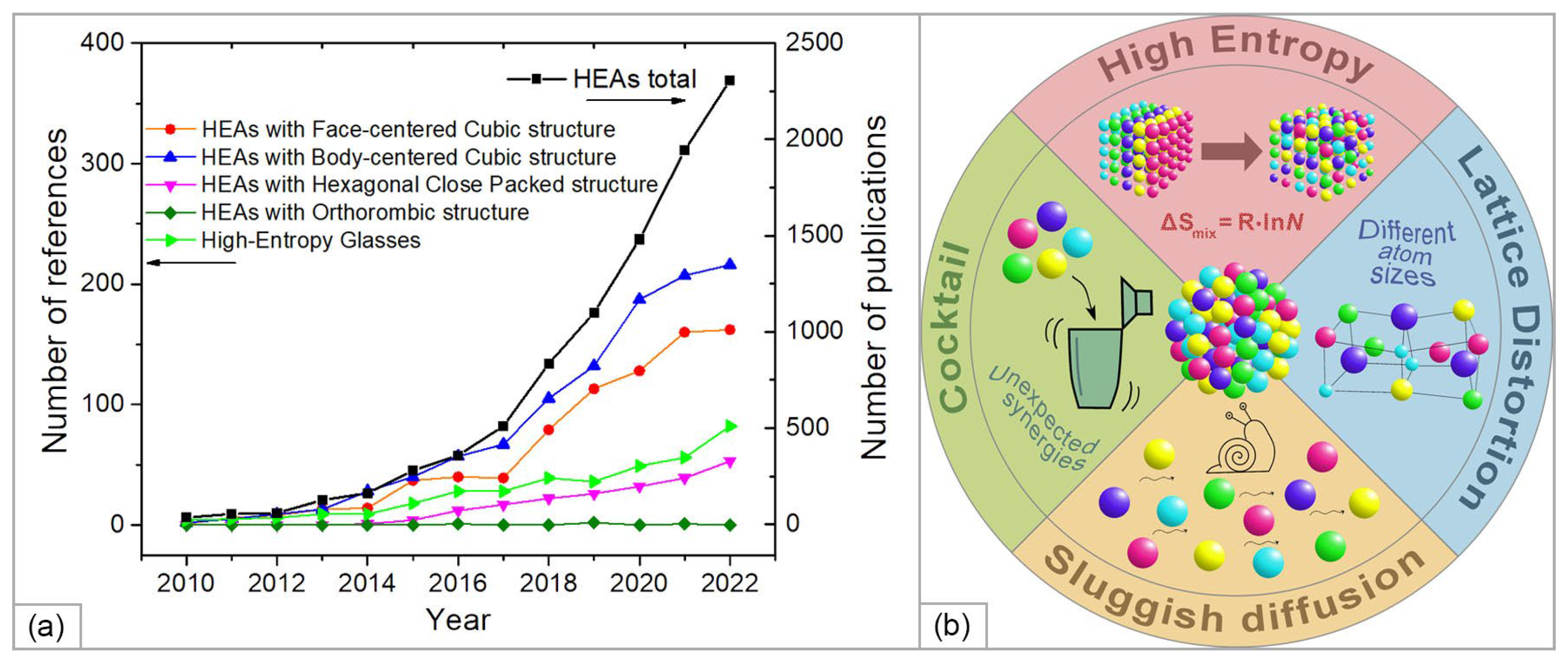
2. High-Entropy Alloys: Theory, Achievements, and Prospects for Use in SOFC Technology
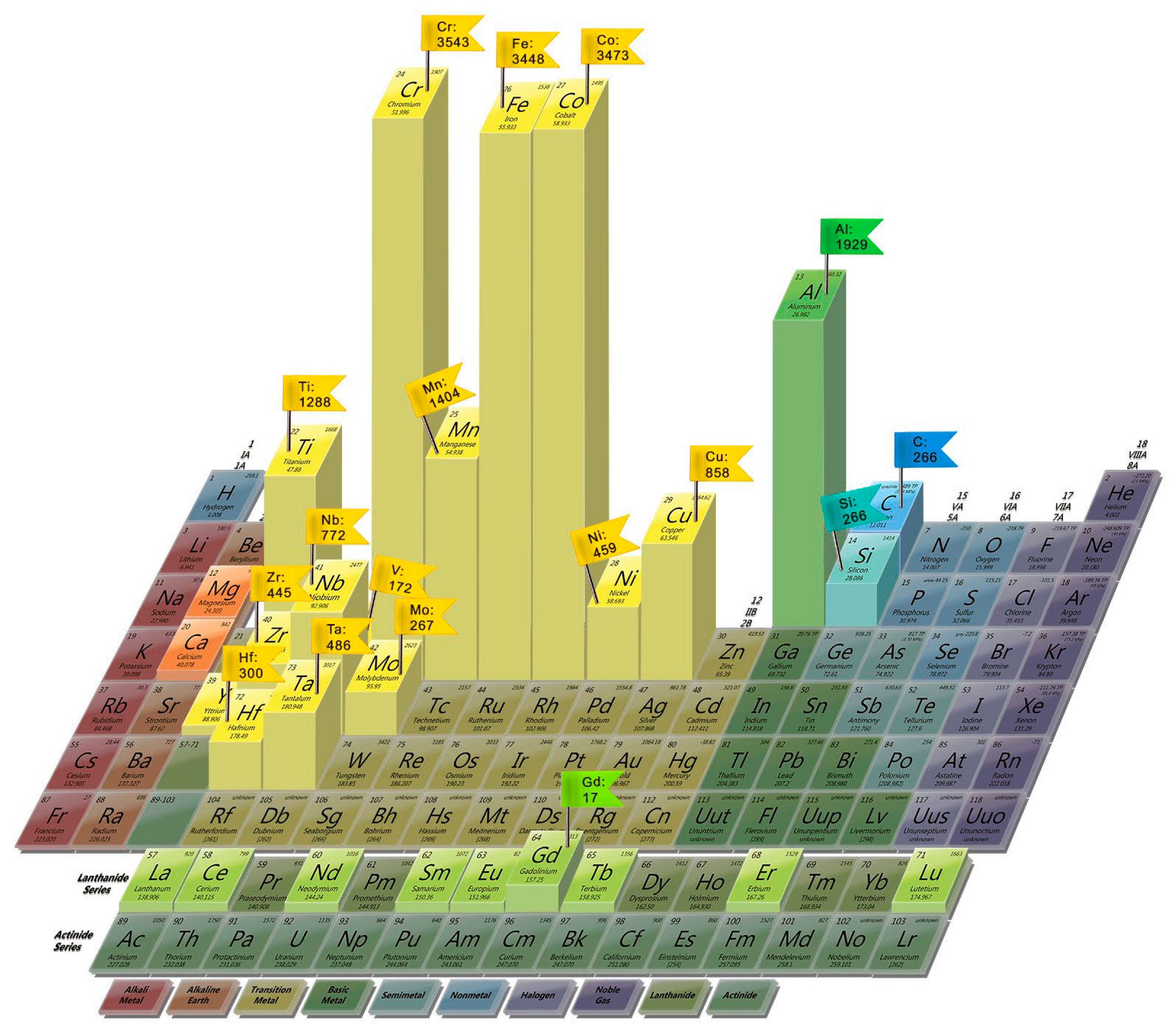
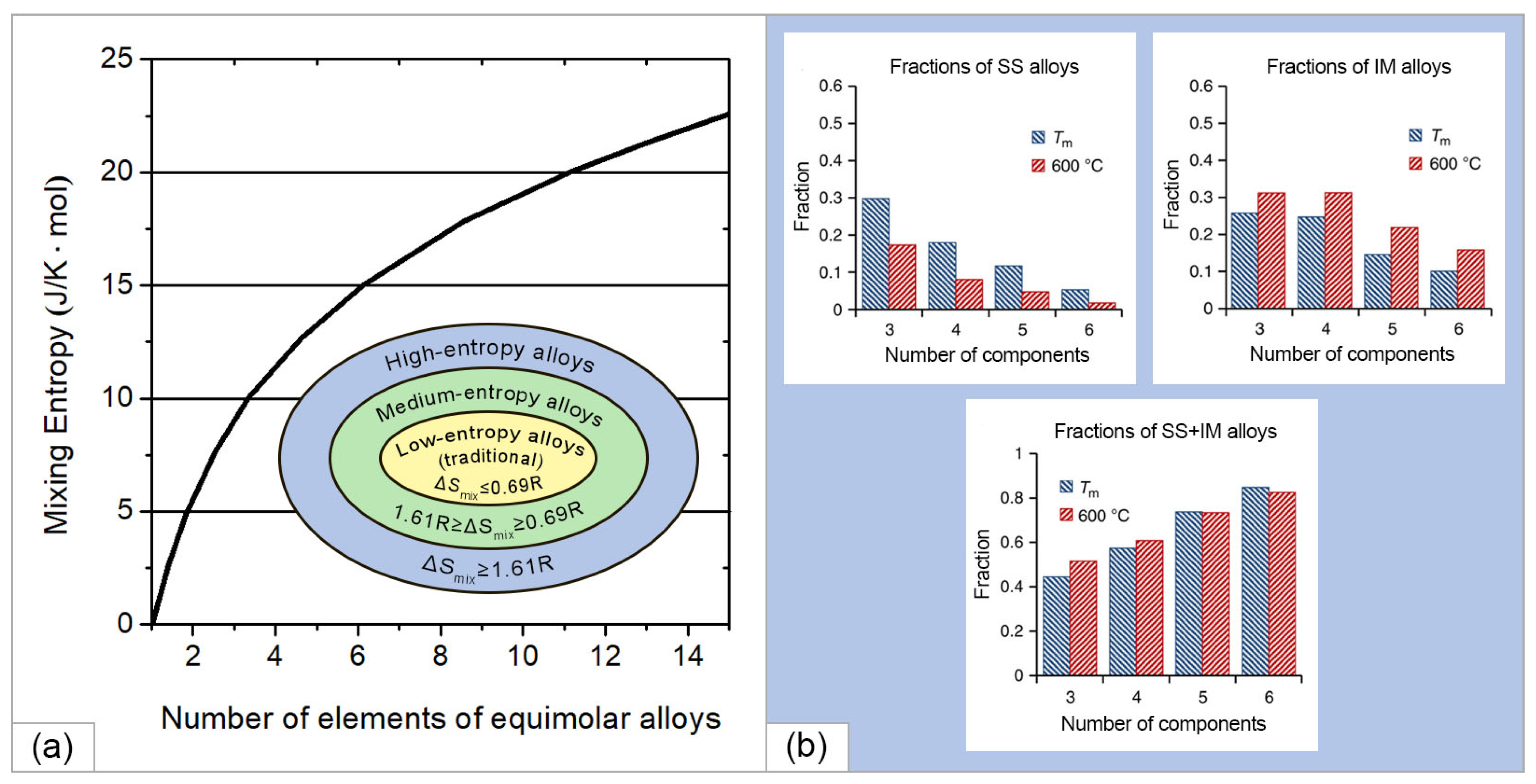
2.1. Theoretical Founditions and Recent Advances in the Development of High-Entropy Alloys
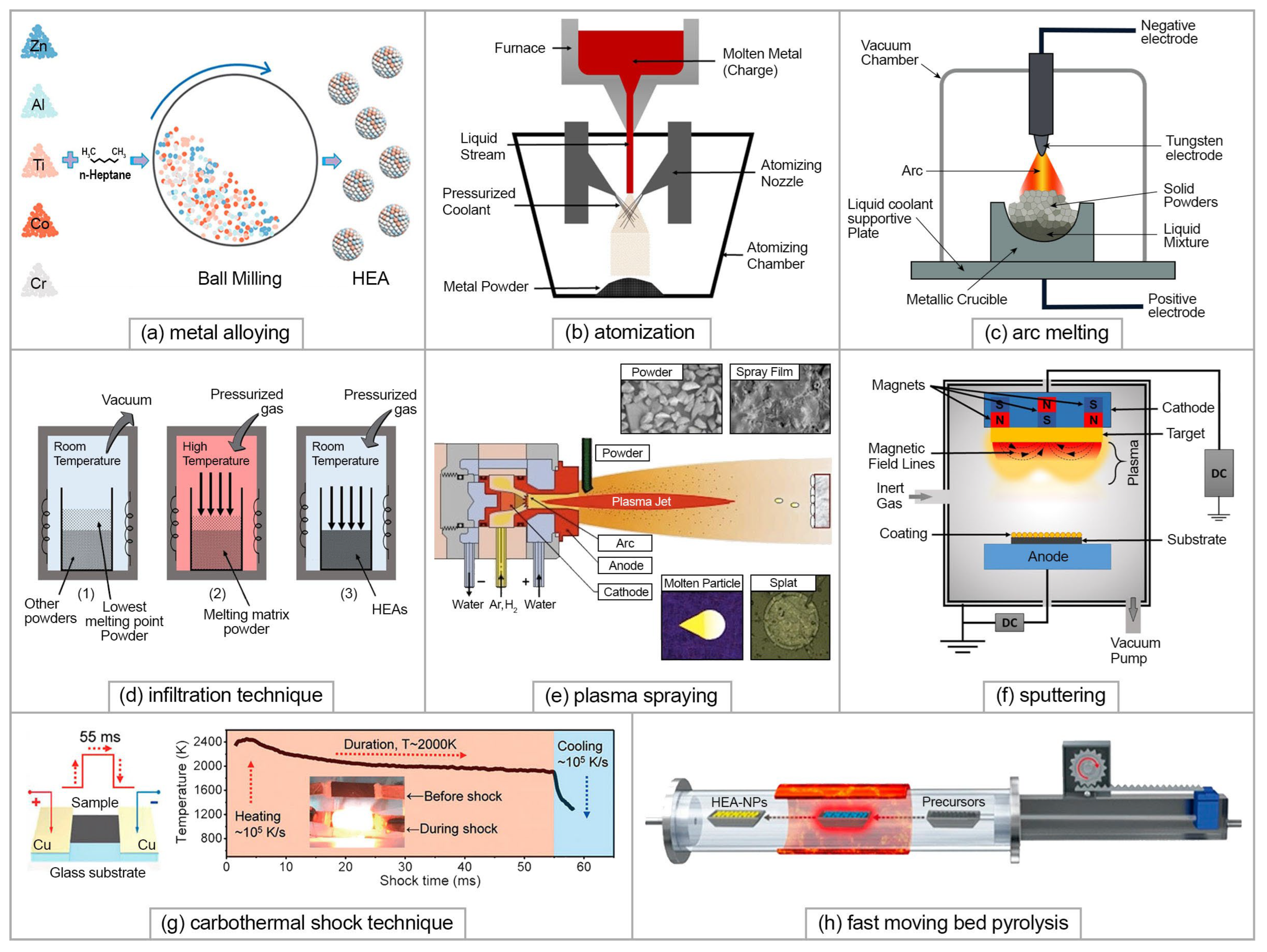
2.2. HEA Synthesis Methods
2.3. HEAs Applications
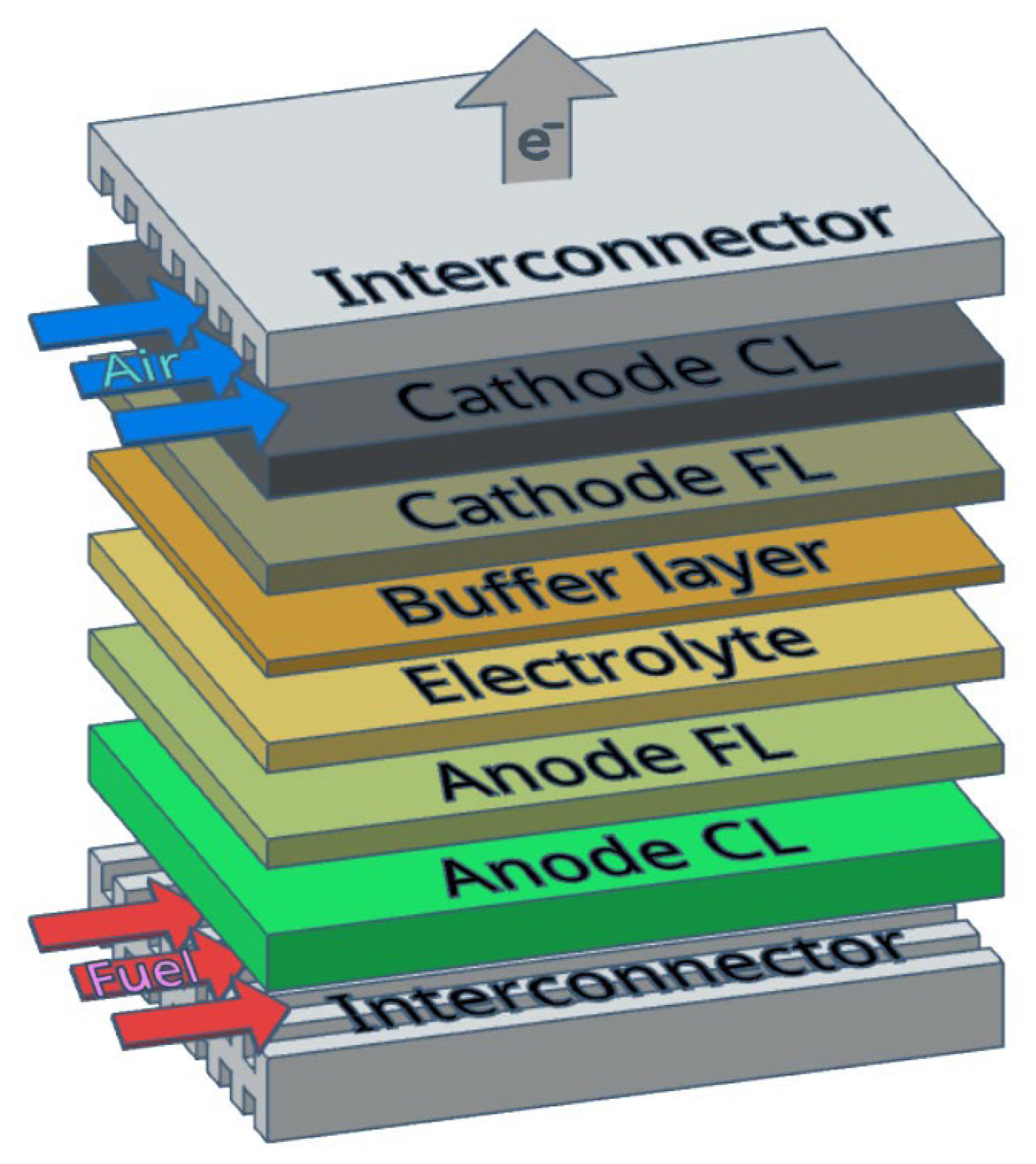
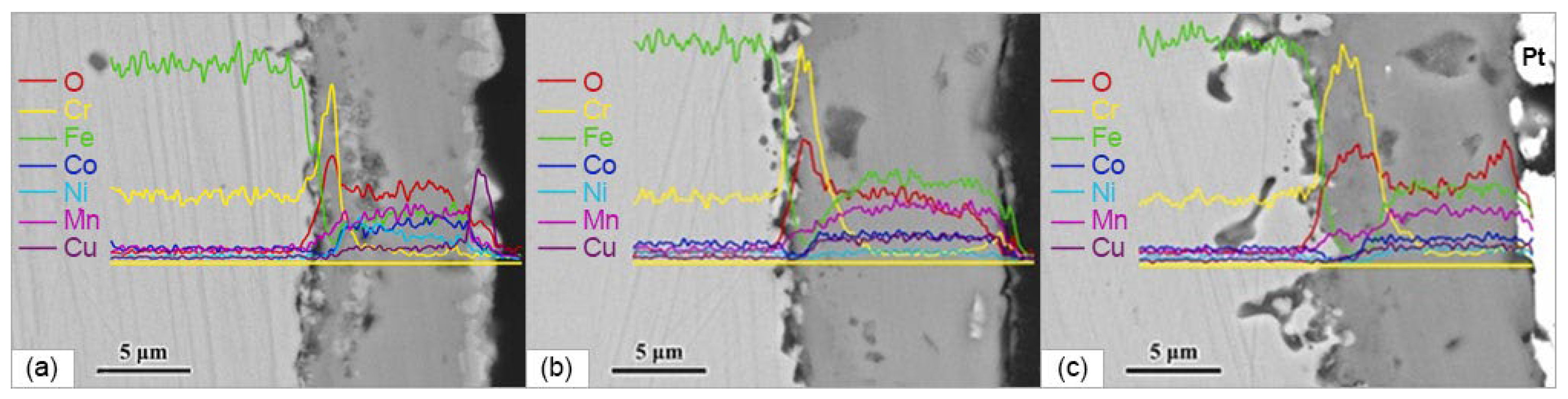
2.3.1. SOFC Interconnectors
- (1)
- High electrical (preferably electronic) conductivity under the SOFC operating temperatures (minimum value of 1 S/cm);
- (2)
- Sufficient chemical and phase, as well as microstructural stability in both reducing and oxidizing atmospheres;
- (3)
- Gas-tightness to prevent direct combination of oxidant and fuel during fuel operation;
- (4)
- Good thermal conductivity (minimum value of 5 W/(m K);
- (5)
- Thermomechanical compatibility with electrodes and electrolytes (Thermal expansion coefficient (TEC) value close to (10 × 10−6 1/K in the 25–1000 °C range) to minimize thermal stresses during stack startup and shutdown;
- (6)
- No reaction or inter diffusion between interconnectors and its adjoining components (anode and cathode);
- (7)
- Excellent resistance to oxidation, sulfidation and carbonization, and adequate strength and creep resistance at elevated temperatures;
- (8)
- Easy fabrication and low cost.
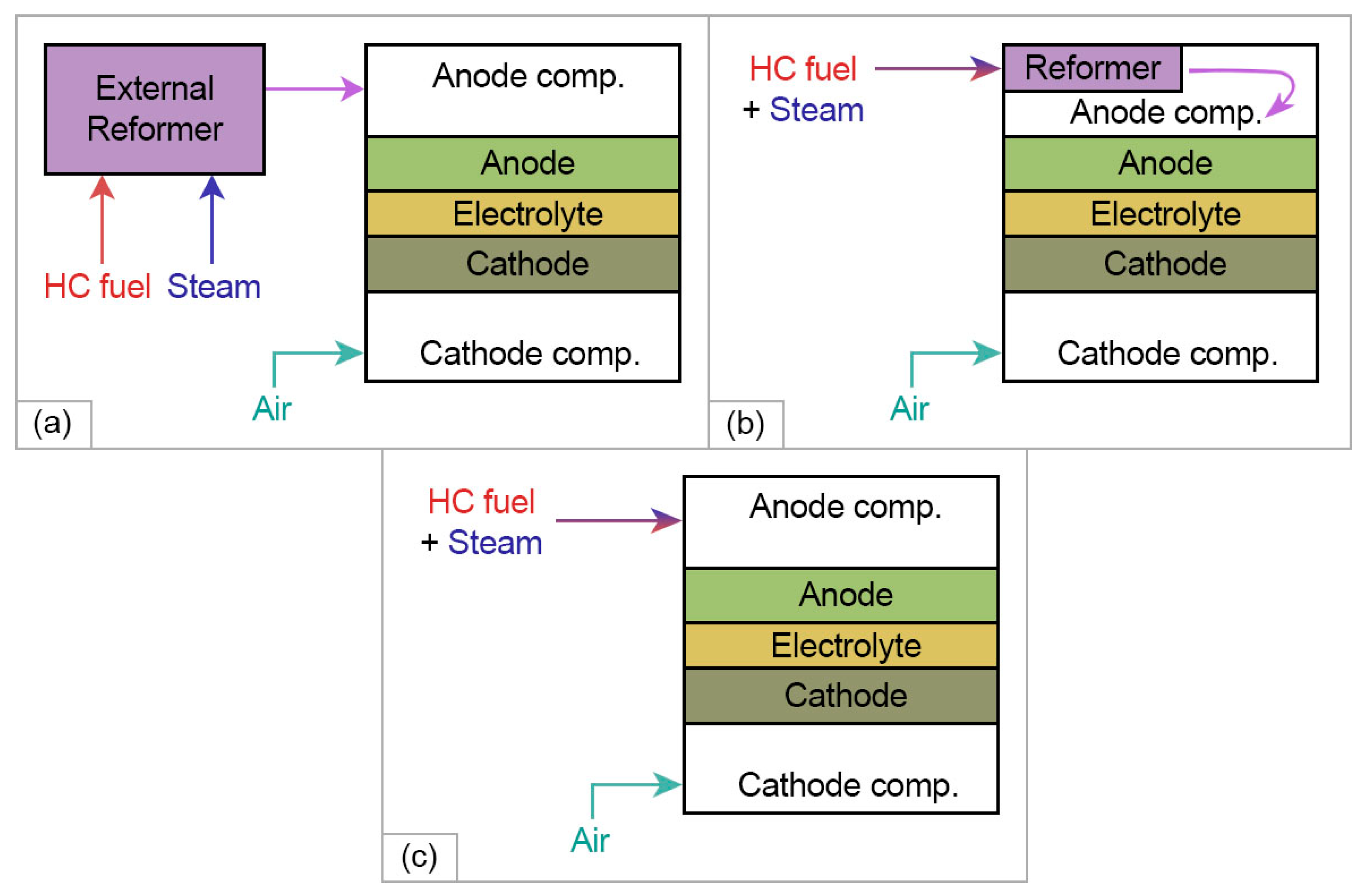
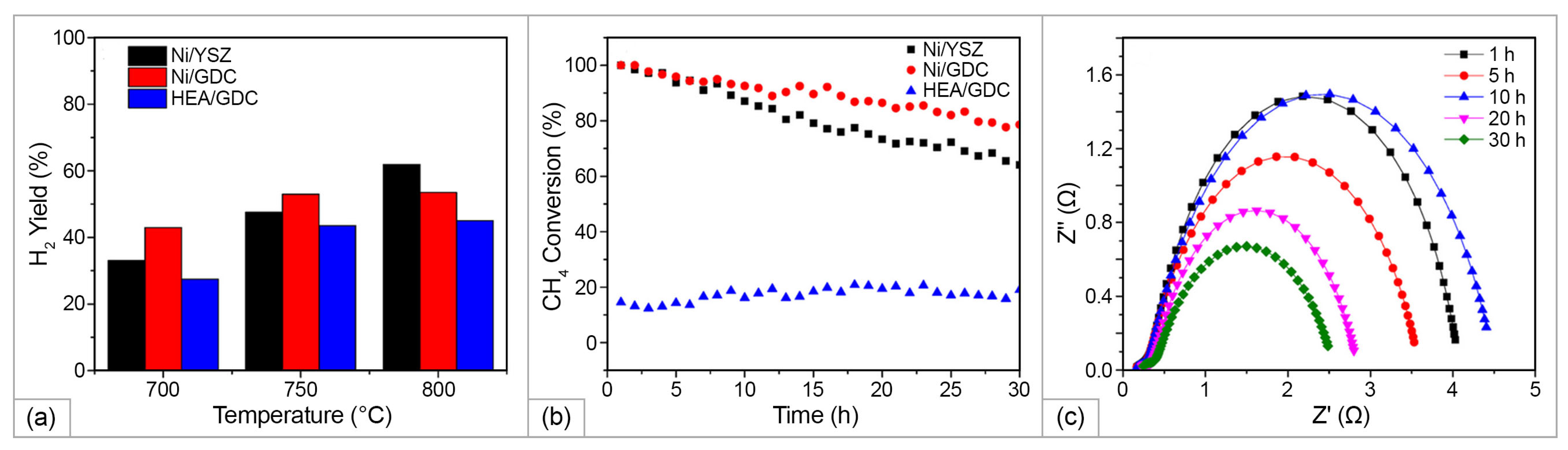
2.3.2. HEAs Application in Hydrocarbon-Fueled SOFCs
3. High-Entropy Ceramic Materials: Theoretical Aspects, Synthesis, Applications, and Use in SOFC Technology
3.1. Entropy Stabilization Approach to the Creation of HEOs
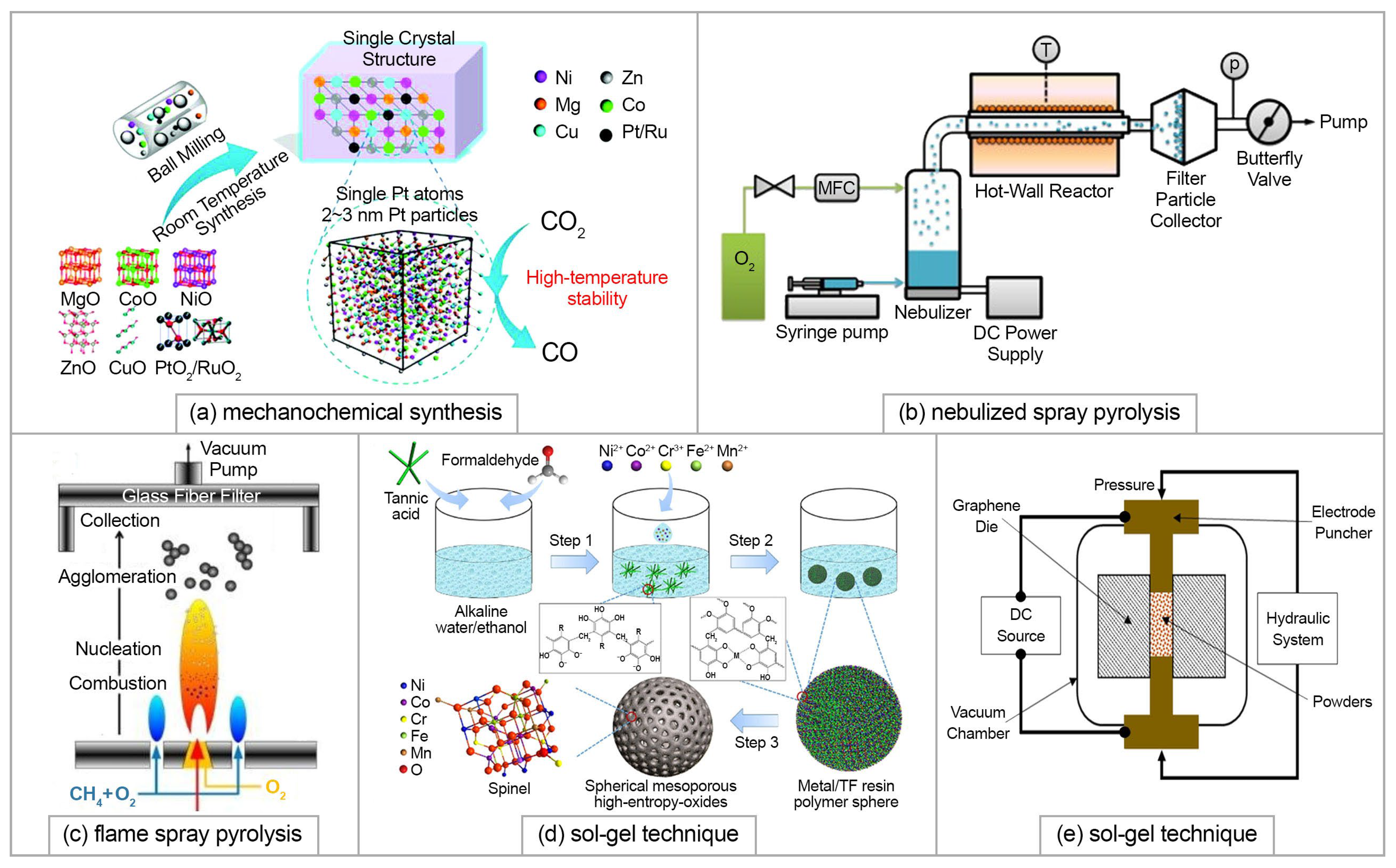
3.2. Production Methods for the Formation of HEO Materials, Bulk Ceramics and Films
3.3. SOFC-Related HEO Applications
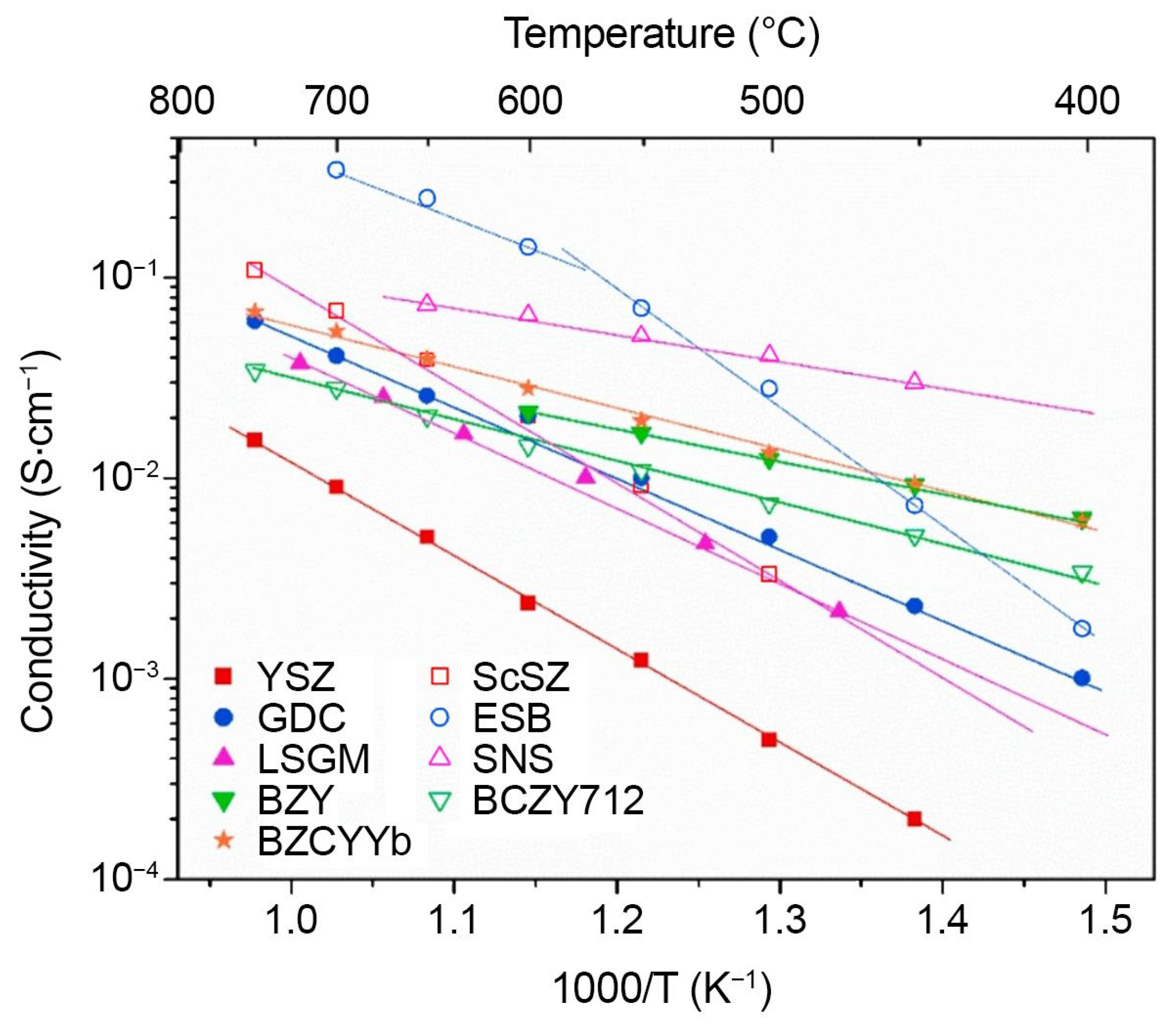
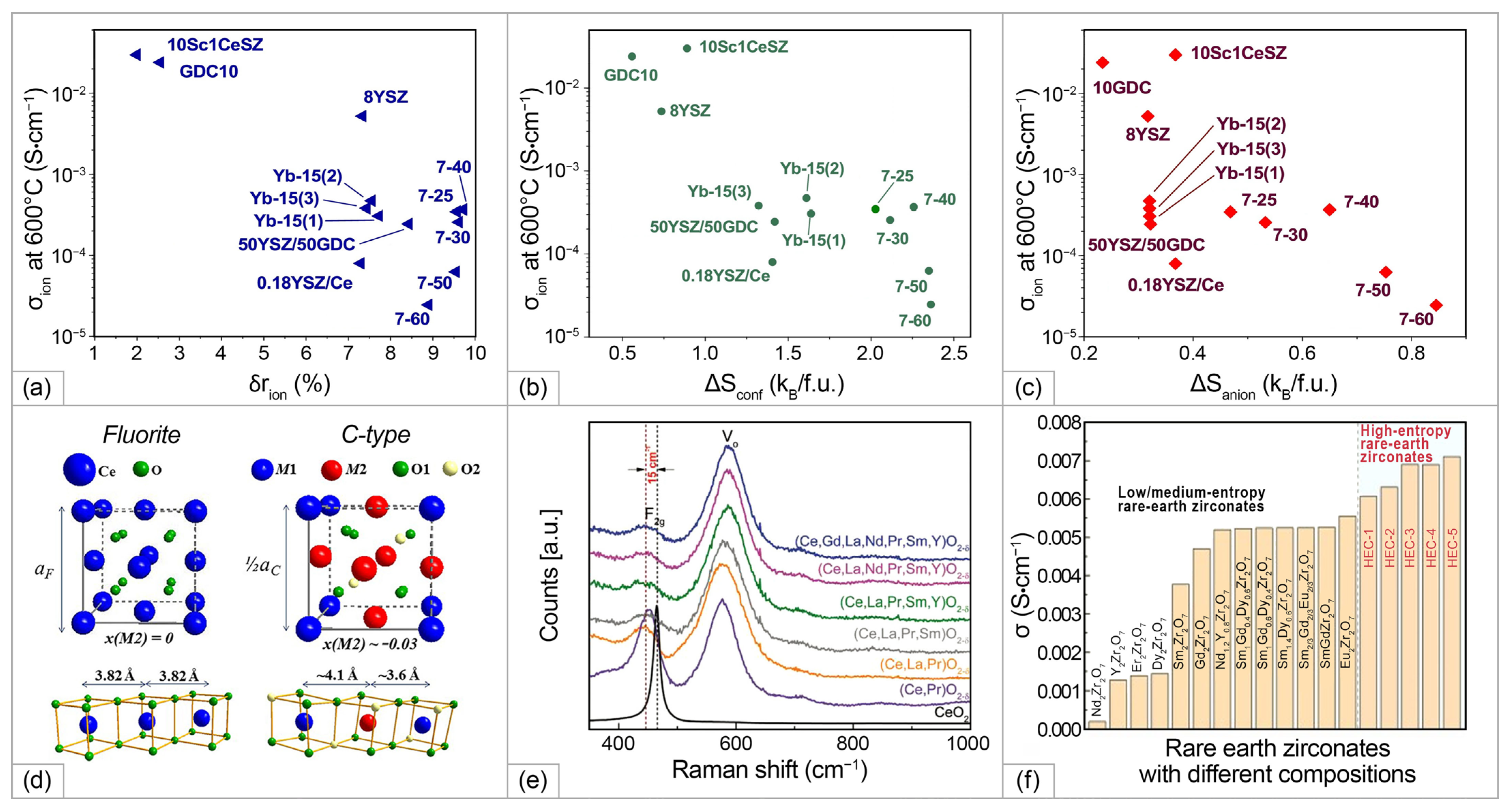
3.3.1. HEOs’ Application as SOFC Electrolytes
- (1)
- Gas-tightness;
- (2)
- High level of ionic conductivity (0.1 S/cm at the operating conditions) and low ohmic resistance (0.2 Ω cm2);
- (3)
- Pure ionic conductivity both in oxidizing and reducing atmospheres;
- (4)
- High chemical stability over a wide range of oxygen partial pressures.
3.3.2. HEOs’ Application as SOFC Cathodes
- (1)
- Electrical conductivity of no less than 100 S/cm under the oxidizing conditions;
- (2)
- Thermal expansion compatible with electrolyte and interconnector materials;
- (3)
- Absence of chemical interactions between the electrolyte and interconnector materials;
- (4)
- Sufficient porosity (usually no less than 35%) for oxygen migration through the cathode to the cathode/electrolyte interface;
- (5)
- Phase stability under oxidizing conditions;
- (6)
- High catalytic activity for the oxygen reduction reaction (ORR);
- (7)
- Easy production and low cost.
3.3.3. HEOs’ Application as SOFC Anodes
- (1)
- Large surface area of triple phase boundary for maximizing the anodic reactions;
- (2)
- Enhanced porosity (approximately 50% for supporting anode layer and 35% for functional anode layer) facilitating a gas transport;
- (3)
- Stability in reducing atmospheres;
- (4)
- High electronic conductivity;
- (5)
- Thermal compatibility with other construction materials of the cell;
- (6)
- High electro-catalytic activity for hydrogen oxidation reaction.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H.; Hajimolana, S. Optimization Strategies for Solid Oxide Fuel Cell (SOFC) Application: A Literature Survey. Renew. Sustain. Energy Rev. 2017, 76, 460–484. [Google Scholar] [CrossRef]
- Lin, G.; Wang, X.; Rezazadeh, A. Electrical Energy Storage from a Combined Energy Process Based on Solid Oxide Fuel Cell and Use of Waste Heat. Sustain. Energy Technol. Assess. 2021, 48, 101663. [Google Scholar] [CrossRef]
- Rupiper, L.N.; Skabelund, B.B.; Ghotkar, R.; Milcarek, R.J. Impact of Fuel Type on the Performance of a Solid Oxide Fuel Cell Integrated with a Gas Turbine. Sustain. Energy Technol. Assess. 2022, 51, 101959. [Google Scholar] [CrossRef]
- Ampah, J.D.; Afrane, S.; Agyekum, E.B.; Adun, H.; Yusuf, A.A.; Bamisile, O. Electric Vehicles Development in Sub-Saharan Africa: Performance Assessment of Standalone Renewable Energy Systems for Hydrogen Refuelling and Electricity Charging Stations (HRECS). J. Clean. Prod. 2022, 376, 134238. [Google Scholar] [CrossRef]
- Mendonça, C.; Ferreira, A.; Santos, D.M.F. Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels 2021, 2, 393–419. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced Materials for SOFC Application: Strategies for the Development of Highly Conductive and Stable Solid Oxide Proton Electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Solid Oxide Fuel Cells Based on Ceramic Membranes with Mixed Conductivity: Improving Efficiency. Russ. Chem. Rev. 2021, 90, 703–749. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef]
- Gelchinski, B.R.; Balyakin, I.A.; Yuryev, A.A.; Rempel, A.A. High-Entropy Alloys: Properties and Prospects of Application as Protective Coatings. Russ. Chem. Rev. 2022, 91, RCR5023. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-Entropy Ceramics: Review of Principles, Production and Applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Gazda, M.; Miruszewski, T.; Jaworski, D.; Mielewczyk-Gryń, A.; Skubida, W.; Wachowski, S.; Winiarz, P.; Dzierzgowski, K.; Łapiński, M.; Szpunar, I.; et al. Novel Class of Proton Conducting Materials—High Entropy Oxides. ACS Mater. Lett. 2020, 2, 1315–1321. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Accardo, G.; Frattini, D.; Dell’Agli, G. Entropy-Stabilized Oxides Owning Fluorite Structure Obtained by Hydrothermal Treatment. Materials 2020, 13, 558. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, F.; Cheng, C.; Guo, M.; Liu, Y.; Miao, Y.; Gao, F.; Wang, X. Preparation and Electrical Conductivity of (Zr, Hf, Pr, Y, La) O High Entropy Fluorite Oxides. J. Mater. Sci. Technol. 2022, 105, 122–130. [Google Scholar] [CrossRef]
- Shijie, Z.; Na, L.; Liping, S.; Qiang, L.; Lihua, H.; Hui, Z. A Novel High-Entropy Cathode with the A2BO4-Type Structure for Solid Oxide Fuel Cells. J. Alloy. Compd. 2022, 895, 162548. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Adamczyk, J.; Stępień, A.; Zajusz, M.; Bar, K.; Berent, K.; Świerczek, K. Synthesis and Properties of the Gallium-Containing Ruddlesden-Popper Oxides with High-Entropy B-Site Arrangement. Materials 2022, 15, 6500. [Google Scholar] [CrossRef]
- Han, X.; Yang, Y.; Fan, Y.; Ni, H.; Guo, Y.; Chen, Y.; Ou, X.; Ling, Y. New Approach to Enhance Sr-Free Cathode Performance by High-Entropy Multi-Component Transition Metal Coupling. Ceram. Int. 2021, 47, 17383–17390. [Google Scholar] [CrossRef]
- Ma, G.; Chen, D.; Ji, S.; Bai, X.; Wang, X.; Huan, Y.; Dong, D.; Hu, X.; Wei, T. Medium-Entropy SrV1/3Fe1/3Mo1/3O3 with High Conductivity and Strong Stability as SOFCs High-Performance Anode. Materials 2022, 15, 2298. [Google Scholar] [CrossRef]
- Zhao, Q.; Geng, S.; Zhang, Y.; Chen, G.; Zhu, S.; Wang, F. High-Entropy FeCoNiMnCu Alloy Coating on Ferritic Stainless Steel for Solid Oxide Fuel Cell Interconnects. J. Alloy. Compd. 2022, 908, 164608. [Google Scholar] [CrossRef]
- Huang, K.H.; Yeh, J.W. A Study on the Multicomponent Alloy Systems Containing Equal-Mole Elements. Master’s Thesis, National Tsing Hua University, Hsinchu, Taiwan, 1996. [Google Scholar]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A Review on Fundamental of High Entropy Alloys with Promising High–Temperature Properties. J. Alloy. Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-Entropy Alloy: Challenges and Prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R. High-Entropy Alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Marques, F.; Balcerzak, M.; Winkelmann, F.; Zepon, G.; Felderhoff, M. Review and Outlook on High-Entropy Alloys for Hydrogen Storage. Energy Environ. Sci. 2021, 14, 5191–5227. [Google Scholar] [CrossRef]
- Xin, Y.; Li, S.; Qian, Y.; Zhu, W.; Yuan, H.; Jiang, P.; Guo, R.; Wang, L. High-Entropy Alloys as a Platform for Catalysis: Progress, Challenges, and Opportunities. ACS Catal. 2020, 10, 11280–11306. [Google Scholar] [CrossRef]
- Cheng, H.; Pan, Z.; Fu, Y.; Wang, X.; Wei, Y.; Luo, H.; Li, X. Review—Corrosion-Resistant High-Entropy Alloy Coatings: A Review. J. Electrochem. Soc. 2021, 168, 111502. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent Progress in High-Entropy Alloys. Eur. J. Control 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated Exploration of Multi-Principal Element Alloys with Solid Solution Phases. Nat. Commun. 2015, 6, 6529. [Google Scholar] [CrossRef]
- Gorsse, S.; Couzinié, J.-P.; Miracle, D.B. From High-Entropy Alloys to Complex Concentrated Alloys. Comptes Rendus Phys. 2018, 19, 721–736. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, D.; Jin, X.; Zhang, L.; Du, X.; Li, B. Design of Non-Equiatomic Medium-Entropy Alloys. Sci. Rep. 2018, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Łoński, W.; Spilka, M.; Kądziołka-Gaweł, M.; Gębara, P.; Radoń, A.; Warski, T.; Młynarek-Żak, K.; Babilas, R. The Effect of Cooling Rate on the Structure and Selected Properties of AlCoCrFeNiSix (x = 0; 0.25; 0.5; 0.75) High Entropy Alloys. J. Alloy. Compd. 2022, 905, 164074. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy Design and Properties Optimization of High-Entropy Alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Toda-Caraballo, I.; Rivera-Díaz-del-Castillo, P.E.J. A Criterion for the Formation of High Entropy Alloys Based on Lattice Distortion. Intermetallics 2016, 71, 76–87. [Google Scholar] [CrossRef]
- Poletti, M.G.; Battezzati, L. Electronic and Thermodynamic Criteria for the Occurrence of High Entropy Alloys in Metallic Systems. Acta Mater. 2014, 75, 297–306. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Ma, D.; Grabowski, B.; Körmann, F.; Neugebauer, J.; Raabe, D. Ab Initio Thermodynamics of the CoCrFeMnNi High Entropy Alloy: Importance of Entropy Contributions beyond the Configurational One. Acta Mater. 2015, 100, 90–97. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, T.; Hou, H.; Zhao, Y. The Magnetic, Electronic, and Thermodynamic Properties of High Entropy Alloy CrMnFeCoNi: A First-Principles Study. Phys. Status Solidi B 2018, 255, 1800306. [Google Scholar] [CrossRef]
- Haas, S.; Mosbacher, M.; Senkov, O.; Feuerbacher, M.; Freudenberger, J.; Gezgin, S.; Völkl, R.; Glatzel, U. Entropy Determination of Single-Phase High Entropy Alloys with Different Crystal Structures over a Wide Temperature Range. Entropy 2018, 20, 654. [Google Scholar] [CrossRef]
- Li, Z.; Raabe, D. Strong and Ductile Non-Equiatomic High-Entropy Alloys: Design, Processing, Microstructure, and Mechanical Properties. JOM 2017, 69, 2099–2106. [Google Scholar] [CrossRef]
- Kaushik, N.; Meena, A.; Mali, H.S. High Entropy Alloy Synthesis, Characterisation, Manufacturing & Potential Applications: A Review. Mater. Manuf. Process. 2022, 37, 1085–1109. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-Entropy Alloys Fabricated via Powder Metallurgy. A Critical Review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Rajendrachari, S. An Overview of High-Entropy Alloys Prepared by Mechanical Alloying Followed by the Characterization of Their Microstructure and Various Properties. Alloys 2022, 1, 116–132. [Google Scholar] [CrossRef]
- Průša, F.; Šenková, A.; Vojtěch, D.; Čapek, J.; Bernatiková, A. High Entropy Alloys Prepared by Combination of Mechanical Alloying and Spark Plasma Sintering. Manuf. Technol. 2016, 16, 1350–1354. [Google Scholar] [CrossRef]
- Wang, P.; Huang, P.; Ng, F.L.; Sin, W.J.; Lu, S.; Nai, M.L.S.; Dong, Z.; Wei, J. Additively Manufactured CoCrFeNiMn High-Entropy Alloy via Pre-Alloyed Powder. Mater. Des. 2019, 168, 107576. [Google Scholar] [CrossRef]
- Ryltsev, R.E.; Estemirova, S.K.; Gaviko, V.S.; Yagodin, D.A.; Bykov, V.A.; Sterkhov, E.V.; Cherepanova, L.A.; Sipatov, I.S.; Balyakin, I.A.; Uporov, S.A. Structural Evolution in TiZrHfNb High-Entropy Alloy. Materialia 2022, 21, 101311. [Google Scholar] [CrossRef]
- Uporov, S.A.; Ryltsev, R.E.; Sidorov, V.A.; Estemirova, S.K.; Sterkhov, E.V.; Balyakin, I.A.; Chtchelkatchev, N.M. Pressure Effects on Electronic Structure and Electrical Conductivity of TiZrHfNb High-Entropy Alloy. Intermetallics 2022, 140, 107394. [Google Scholar] [CrossRef]
- Alshataif, Y.A.; Sivasankaran, S.; Al-Mufadi, F.A.; Alaboodi, A.S.; Ammar, H.R. Manufacturing Methods, Microstructural and Mechanical Properties Evolutions of High-Entropy Alloys: A Review. Met. Mater. Int. 2020, 26, 1099–1133. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Fu, Y. High-Entropy Alloys: Emerging Materials for Advanced Functional Applications. J. Mater. Chem. A 2021, 9, 663–701. [Google Scholar] [CrossRef]
- Shojaei, Z.; Khayati, G.R.; Darezereshki, E. Review of Electrodeposition Methods for the Preparation of High-Entropy Alloys. Int. J. Miner. Metall. Mater. 2022, 29, 1683–1696. [Google Scholar] [CrossRef]
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High Entropy Alloy as a Highly Active and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2018, 279, 19–23. [Google Scholar] [CrossRef]
- Dai, W.; Lu, T.; Pan, Y. Novel and Promising Electrocatalyst for Oxygen Evolution Reaction Based on MnFeCoNi High Entropy Alloy. J. Power Sources 2019, 430, 104–111. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.; Nie, A.; Pu, T.; Rehwoldt, M.; et al. Carbothermal Shock Synthesis of High-Entropy-Alloy Nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-F.; Wu, P.-W.; Lin, P.; Chao, C.-G.; Yeh, K.-Y. Sputter Deposition of Multi-Element Nanoparticles as Electrocatalysts for Methanol Oxidation. Jpn. J. Appl. Phys. 2008, 47, 5755–5761. [Google Scholar] [CrossRef]
- Bondesgaard, M.; Broge, N.L.N.; Mamakhel, A.; Bremholm, M.; Iversen, B.B. General Solvothermal Synthesis Method for Complete Solubility Range Bimetallic and High-Entropy Alloy Nanocatalysts. Adv. Funct. Mater. 2019, 29, 1905933. [Google Scholar] [CrossRef]
- Gao, S.; Hao, S.; Huang, Z.; Yuan, Y.; Han, S.; Lei, L.; Zhang, X.; Shahbazian-Yassar, R.; Lu, J. Synthesis of High-Entropy Alloy Nanoparticles on Supports by the Fast Moving Bed Pyrolysis. Nat. Commun. 2020, 11, 2016. [Google Scholar] [CrossRef]
- Qiao, H.; Saray, M.T.; Wang, X.; Xu, S.; Chen, G.; Huang, Z.; Chen, C.; Zhong, G.; Dong, Q.; Hong, M.; et al. Scalable Synthesis of High Entropy Alloy Nanoparticles by Microwave Heating. ACS Nano 2021, 15, 14928–14937. [Google Scholar] [CrossRef]
- Shih, C.-Y.; Streubel, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmidt, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two Mechanisms of Nanoparticle Generation in Picosecond Laser Ablation in Liquids: The Origin of the Bimodal Size Distribution. Nanoscale 2018, 10, 6900–6910. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Yu, X.; Cao, Y.; Gao, L.; Wu, C.; Yao, Y.; Lin, Z.; Zou, Z. General Synthesis of High-Entropy Alloy and Ceramic Nanoparticles in Nanoseconds. Nat. Synth. 2022, 1, 138–146. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. Applications and Future Directions. In High Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–169. [Google Scholar]
- Straumal, B.; Korneva, A.; Kuzmin, A.; Klinger, L.; Lopez, G.A.; Vershinin, N.; Straumal, A.; Gornakova, A. High Entropy Alloys for Energy Conversion and Storage: A Review of Grain Boundary Wetting Phenomena. Energies 2022, 15, 7130. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Mohan, M.; Kumar, V.; Sharma, A.; Ahn, B. A Comprehensive Review of the Prospects for Future Hydrogen Storage in Materials-application and Outstanding Issues. Int. J. Energy Res. 2022, 46, 16150–16177. [Google Scholar] [CrossRef]
- Wang, K.; Huang, J.; Chen, H.; Wang, Y.; Yan, W.; Yuan, X.; Song, S.; Zhang, J.; Sun, X. Recent Progress in High Entropy Alloys for Electrocatalysts. Electrochem. Energy Rev. 2022, 5, 17. [Google Scholar] [CrossRef]
- Glasscott, M.W. Classifying and Benchmarking High-Entropy Alloys and Associated Materials for Electrocatalysis: A Brief Review of Best Practices. Curr. Opin. Electrochem. 2022, 34, 100976. [Google Scholar] [CrossRef]
- Sarac, B.; Zadorozhnyy, V.; Ivanov, Y.P.; Spieckermann, F.; Klyamkin, S.; Berdonosova, E.; Serov, M.; Kaloshkin, S.; Greer, A.L.; Sarac, A.S.; et al. Transition Metal-Based High Entropy Alloy Microfiber Electrodes: Corrosion Behavior and Hydrogen Activity. Corros. Sci. 2021, 193, 109880. [Google Scholar] [CrossRef]
- Huo, X.; Yu, H.; Xing, B.; Zuo, X.; Zhang, N. Review of High Entropy Alloys Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reaction. Chem. Rec. 2022, e202200175. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.A.; Yan, D.; Skjegstad, L.E.J.; Rossmeisl, J. Surface Electrocatalysis on High-Entropy Alloys. Curr. Opin. Electrochem. 2021, 26, 100651. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Guo, Z.; Zhang, J.; Wang, L.; Yan, G. Advance in and Prospect of Moderator Materials for Space Nuclear Reactors. Int. J. Energy Res. 2021, 45, 11493–11509. [Google Scholar] [CrossRef]
- Shi, T.; Lei, P.-H.; Yan, X.; Li, J.; Zhou, Y.-D.; Wang, Y.-P.; Su, Z.-X.; Dou, Y.-K.; He, X.-F.; Yun, D.; et al. Current Development of Body-Centered Cubic High-Entropy Alloys for Nuclear Applications. Tungsten 2021, 3, 197–217. [Google Scholar] [CrossRef]
- Pickering, E.J.; Carruthers, A.W.; Barron, P.J.; Middleburgh, S.C.; Armstrong, D.E.J.; Gandy, A.S. High-Entropy Alloys for Advanced Nuclear Applications. Entropy 2021, 23, 98. [Google Scholar] [CrossRef]
- Sturman, J.W.; Baranova, E.A.; Abu-Lebdeh, Y. Review: High-Entropy Materials for Lithium-Ion Battery Electrodes. Front. Energy Res. 2022, 10, 862551. [Google Scholar] [CrossRef]
- Zheng, S.-M.; Tian, Y.-R.; Liu, Y.-X.; Wang, S.; Hu, C.-Q.; Wang, B.; Wang, K.-M. Alloy Anodes for Sodium-Ion Batteries. Rare Met. 2021, 40, 272–289. [Google Scholar] [CrossRef]
- Dixit, S.; Rodriguez, S.; Jones, M.R.; Buzby, P.; Dixit, R.; Argibay, N.; DelRio, F.W.; Lim, H.H.; Fleming, D. Refractory High-Entropy Alloy Coatings for High-Temperature Aerospace and Energy Applications. J. Therm. Spray Technol. 2022, 31, 1021–1031. [Google Scholar] [CrossRef]
- Chen, D.; He, H.; Zhang, D.; Wang, H.; Ni, M. Percolation Theory in Solid Oxide Fuel Cell Composite Electrodes with a Mixed Electronic and Ionic Conductor. Energies 2013, 6, 1632–1656. [Google Scholar] [CrossRef]
- Zhu, W.; Yan, M. Perspectives on the Metallic Interconnects for Solid Oxide Fuel Cells. J. Zheijang Univ.-Sci. 2004, 5, 1471–1503. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic Interconnects for Solid Oxide Fuel Cell: A Review on Protective Coating and Deposition Techniques. Int. J. Hydrog. Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A Review of Recent Progress in Coatings, Surface Modifications and Alloy Developments for Solid Oxide Fuel Cell Ferritic Stainless Steel Interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Zhu, J.H.; Chesson, D.A.; Yu, Y.T. Review—(Mn,Co)3O4 -Based Spinels for SOFC Interconnect Coating Application. J. Electrochem. Soc. 2021, 168, 114519. [Google Scholar] [CrossRef]
- Zhao, M.; Geng, S.; Chen, G.; Wang, F. FeCoNi Converting Coating for Solid Oxide Fuel Cell Steel Interconnect Application. J. Power Sources 2019, 414, 530–539. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Sanad, M.M.S.; Mattar, T.; El-Shahat, M.F.; Rossignol, C.; Dessemond, L.; Zaidat, K.; Obbade, S. The Structural, Thermal and Electrochemical Properties of MnFe1−x−yCuxNiyCoO4 Spinel Protective Layers in Interconnects of Solid Oxide Fuel Cells (SOFCs). J. Alloy. Compd. 2022, 923, 166351. [Google Scholar] [CrossRef]
- Stygar, M.; Dąbrowa, J.; Moździerz, M.; Zajusz, M.; Skubida, W.; Mroczka, K.; Berent, K.; Świerczek, K.; Danielewski, M. Formation and Properties of High Entropy Oxides in Co-Cr-Fe-Mg-Mn-Ni-O System: Novel (Cr,Fe,Mg,Mn,Ni)3O4 and (Co,Cr,Fe,Mg,Mn)3O4 High Entropy Spinels. J. Eur. Ceram. Soc. 2020, 40, 1644–1650. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Meena, A.K.; Dragoe, N. Room Temperature Lithium Superionic Conductivity in High Entropy Oxides. J. Mater. Chem. A 2016, 4, 9536–9541. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, N.; Wu, B.; Yang, Z.; Sun, S.; Wang, Y. A New Spinel High-Entropy Oxide (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 with Fast Reaction Kinetics and Excellent Stability as an Anode Material for Lithium Ion Batteries. RSC Adv. 2020, 10, 9736–9744. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Patra, J.; Chang, J.-K.; Ting, J.-M. High Entropy Spinel Oxide Nanoparticles for Superior Lithiation–Delithiation Performance. J. Mater. Chem. A 2020, 8, 18963–18973. [Google Scholar] [CrossRef]
- Senthil Kumar, S.; Aruna, S.T. Hydrocarbon Compatible SOFC Anode Catalysts and Their Syntheses: A Review. Sustain. Chem. 2021, 2, 707–763. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.A.; Bagger, A.; Rossmeisl, J. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions. ACS Catal. 2020, 10, 2169–2176. [Google Scholar] [CrossRef]
- Kumar Katiyar, N.; Biswas, K.; Yeh, J.-W.; Sharma, S.; Sekhar Tiwary, C. A Perspective on the Catalysis Using the High Entropy Alloys. Nano Energy 2021, 88, 106261. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Katiyar, N.K.; Kumar, R.; Parui, A.; Malviya, K.D.; Pradeep, K.G.; Singh, A.K.; Sharma, S.; Tiwary, C.S.; Biswas, K. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions: Experimental Realization. ACS Catal. 2020, 10, 3658–3663. [Google Scholar] [CrossRef]
- Löffler, T.; Meyer, H.; Savan, A.; Wilde, P.; Garzón Manjón, A.; Chen, Y.-T.; Ventosa, E.; Scheu, C.; Ludwig, A.; Schuhmann, W. Discovery of a Multinary Noble Metal-Free Oxygen Reduction Catalyst. Adv. Energy Mater. 2018, 8, 1802269. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Fang, G.; Wen, Y.; Liu, P.; Xie, G.; Liu, X.; Sun, S. Nanoporous High-Entropy Alloys for Highly Stable and Efficient Catalysts. J. Mater. Chem. A 2019, 7, 6499–6506. [Google Scholar] [CrossRef]
- Chen, T.; Wang, W.G.; Miao, H.; Li, T.; Xu, C. Evaluation of Carbon Deposition Behavior on the Nickel/Yttrium-Stabilized Zirconia Anode-Supported Fuel Cell Fueled with Simulated Syngas. J. Power Sources 2011, 196, 2461–2468. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Z.; Jin, Y.; Liu, C.; Lei, Z.; Chen, F.; Peng, S. Progress Report on the Catalyst Layers for Hydrocarbon-Fueled SOFCs. Int. J. Hydrog. Energy 2021, 46, 39369–39386. [Google Scholar] [CrossRef]
- Lee, K.X.; Hu, B.; Dubey, P.K.; Anisur, M.R.; Belko, S.; Aphale, A.N.; Singh, P. High-Entropy Alloy Anode for Direct Internal Steam Reforming of Methane in SOFC. Int. J. Hydrog. Energy 2022, 47, 38372–38385. [Google Scholar] [CrossRef]
- Navrotsky, A.; Kleppa, O.J. The Thermodynamics of Cation Distributions in Simple Spinels. J. Inorg. Nucl. Chem. 1967, 29, 2701–2714. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare Earth and Transition Metal Based Entropy Stabilised Perovskite Type Oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Chen, K.; Pei, X.; Tang, L.; Cheng, H.; Li, Z.; Li, C.; Zhang, X.; An, L. A Five-Component Entropy-Stabilized Fluorite Oxide. J. Eur. Ceram. Soc. 2018, 38, 4161–4164. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-Entropy Ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Musicó, B.L.; Gilbert, D.; Ward, T.Z.; Page, K.; George, E.; Yan, J.; Mandrus, D.; Keppens, V. The Emergent Field of High Entropy Oxides: Design, Prospects, Challenges, and Opportunities for Tailoring Material Properties. APL Mater. 2020, 8, 040912. [Google Scholar] [CrossRef]
- Albedwawi, S.H.; AlJaberi, A.; Haidemenopoulos, G.N.; Polychronopoulou, K. High Entropy Oxides-Exploring a Paradigm of Promising Catalysts: A Review. Mater. Des. 2021, 202, 109534. [Google Scholar] [CrossRef]
- Amiri, A.; Shahbazian-Yassar, R. Recent Progress of High-Entropy Materials for Energy Storage and Conversion. J. Mater. Chem. A 2021, 9, 782–823. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Fluorine and Chlorine Doping in Oxygen-Deficient Perovskites: A Strategy for Improving Chemical Stability. Comptes Rendus. Chim. 2019, 22, 363–368. [Google Scholar] [CrossRef]
- Rák, Z.; Maria, J.-P.; Brenner, D.W. Evidence for Jahn-Teller Compression in the (Mg,Co,Ni,Cu,Zn)O Entropy-Stabilized Oxide: A DFT Study. Mater. Lett. 2018, 217, 300–303. [Google Scholar] [CrossRef]
- Anand, G.; Wynn, A.P.; Handley, C.M.; Freeman, C.L. Phase Stability and Distortion in High-Entropy Oxides. Acta Mater. 2018, 146, 119–125. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent Equiatomic Rare Earth Oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef]
- Zhong, Y.; Sabarou, H.; Yan, X.; Yang, M.; Gao, M.C.; Liu, X.; Sisson, R.D. Exploration of High Entropy Ceramics (HECs) with Computational Thermodynamics—A Case Study with LaMnO3±δ. Mater. Des. 2019, 182, 108060. [Google Scholar] [CrossRef]
- Hillert, M.; Jansson, B.; Sundman, B. Application of the Compound-Energy Model to Oxide Systems. Int. J. Mater. Res. 1988, 79, 81–87. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Huang, C.; Nieto, A.; Chen, R.; Luo, J. From High-Entropy Ceramics to Compositionally-Complex Ceramics: A Case Study of Fluorite Oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef]
- Wright, A.J.; Luo, J. A Step Forward from High-Entropy Ceramics to Compositionally Complex Ceramics: A New Perspective. J. Mater. Sci. 2020, 55, 9812–9827. [Google Scholar] [CrossRef]
- Desissa, T.D.; Meja, M.; Andoshe, D.; Olu, F.; Gochole, F.; Bekele, G.; Zelekew, O.A.; Temesgen, T.; Brehane, B.; Kuffi, K.D.; et al. Synthesis and Characterizations of (Mg,Co,Ni,Cu,Zn)O High-Entropy Oxides. SN Appl. Sci. 2021, 3, 733. [Google Scholar] [CrossRef]
- Chen, H.; Lin, W.; Zhang, Z.; Jie, K.; Mullins, D.R.; Sang, X.; Yang, S.-Z.; Jafta, C.J.; Bridges, C.A.; Hu, X.; et al. Mechanochemical Synthesis of High Entropy Oxide Materials under Ambient Conditions: Dispersion of Catalysts via Entropy Maximization. ACS Mater. Lett. 2019, 1, 83–88. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Usharani, N.J.; Sanghvi, K.P.; Chakravadhanula, V.S.K.; Gandhi, A.S.; Hahn, H.; Bhattacharya, S.S. Nanocrystalline Multicomponent Entropy Stabilised Transition Metal Oxides. J. Eur. Ceram. Soc. 2017, 37, 747–754. [Google Scholar] [CrossRef]
- Djenadic, R.; Botros, M.; Benel, C.; Clemens, O.; Indris, S.; Choudhary, A.; Bergfeldt, T.; Hahn, H. Nebulized Spray Pyrolysis of Al-Doped Li7La3Zr2O12 Solid Electrolyte for Battery Applications. Solid State Ion. 2014, 263, 49–56. [Google Scholar] [CrossRef]
- Zhang, T.; Go, M.A.; Stricker, C.; Daria, V.R.; Tricoli, A. Low-Cost Photo-Responsive Nanocarriers by One-Step Functionalization of Flame-Made Titania Agglomerates with l-Lysine. J. Mater. Chem. B 2015, 3, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qin, J.; Feng, Y.; Feng, B.; Yang, S.; Wang, Z.; Zhao, Y.; Wei, J. Sol–Gel Synthesis of Spherical Mesoporous High-Entropy Oxides. ACS Appl. Mater. Interfaces 2020, 12, 45155–45164. [Google Scholar] [CrossRef]
- Fan, L. Solid-State Electrolytes for SOFC. In Solid Oxide Fuel Cells; Zhu, B., Raza, R., Fan, L., Sun, C., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 35–78. ISBN 978-3-527-34411-6. [Google Scholar]
- Sarkar, A.; Loho, C.; Velasco, L.; Thomas, T.; Bhattacharya, S.S.; Hahn, H.; Djenadic, R. Multicomponent Equiatomic Rare Earth Oxides with a Narrow Band Gap and Associated Praseodymium Multivalency. Dalton Trans. 2017, 46, 12167–12176. [Google Scholar] [CrossRef]
- Pianassola, M.; Loveday, M.; McMurray, J.W.; Koschan, M.; Melcher, C.L.; Zhuravleva, M. Solid-state Synthesis of Multicomponent Equiatomic Rare-earth Oxides. J. Am. Ceram. Soc. 2020, 103, 2908–2918. [Google Scholar] [CrossRef]
- Tseng, K.; Yang, Q.; McCormack, S.J.; Kriven, W.M. High-entropy, Phase-constrained, Lanthanide Sesquioxide. J Am. Ceram. Soc. 2020, 103, 569–576. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A New Class of High-Entropy Perovskite Oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and Structures of High-Entropy Pyrochlore Oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Zeng, J.; Deng, T.; Luo, B.; Zhang, H.; Huang, X. Preparation of (La0.2Nd0.2Sm0.2Gd0.2Yb0.2)2Zr2O7 High-Entropy Transparent Ceramic Using Combustion Synthesized Nanopowder. J. Alloy. Compd. 2020, 817, 153328. [Google Scholar] [CrossRef]
- Biesuz, M.; Fu, S.; Dong, J.; Jiang, A.; Ke, D.; Xu, Q.; Zhu, D.; Bortolotti, M.; Reece, M.J.; Hu, C.; et al. High Entropy Sr((Zr0.94Y0.06)0.2Sn0.2Ti0.2Hf0.2Mn0.2)O3−x Perovskite Synthesis by Reactive Spark Plasma Sintering. J. Asian Ceram. Soc. 2019, 7, 127–132. [Google Scholar] [CrossRef]
- Sharma, Y.; Musico, B.L.; Gao, X.; Hua, C.; May, A.F.; Herklotz, A.; Rastogi, A.; Mandrus, D.; Yan, J.; Lee, H.N.; et al. Single-Crystal High Entropy Perovskite Oxide Epitaxial Films. Phys. Rev. Mater. 2018, 2, 060404. [Google Scholar] [CrossRef]
- Meisenheimer, P.B.; Kratofil, T.J.; Heron, J.T. Giant Enhancement of Exchange Coupling in Entropy-Stabilized Oxide Heterostructures. Sci. Rep. 2017, 7, 13344. [Google Scholar] [CrossRef]
- Kotsonis, G.N.; Rost, C.M.; Harris, D.T.; Maria, J.-P. Epitaxial Entropy-Stabilized Oxides: Growth of Chemically Diverse Phases via Kinetic Bombardment. MRS Commun. 2018, 8, 1371–1377. [Google Scholar] [CrossRef]
- Einert, M.; Mellin, M.; Bahadorani, N.; Dietz, C.; Lauterbach, S.; Hofmann, J.P. Mesoporous High-Entropy Oxide Thin Films: Electrocatalytic Water Oxidation on High-Surface-Area Spinel (Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)3O4 Electrodes. ACS Appl. Energy Mater. 2022, 5, 717–730. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, B.; Dong, H.; Wang, D. Electronic Structure and Transport Properties of Sol-Gel-Derived High-Entropy Ba(Zr0.2Sn0.2Ti0.2Hf0.2Nb0.2)O3 Thin Films. Ceram. Int. 2021, 47, 20196–20200. [Google Scholar] [CrossRef]
- Liu, J.; Shao, G.; Liu, D.; Chen, K.; Wang, K.; Ma, B.; Ren, K.; Wang, Y. Design and Synthesis of Chemically Complex Ceramics from the Perspective of Entropy. Mater. Today Adv. 2020, 8, 100114. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, J.-X.; Tu, T.-Z.; Wang, W.; Zhang, G.-J. High-Entropy Oxides for Catalysis: A Diamond in the Rough. Chem. Eng. J. 2022, 451, 138659. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y. New Trends in the Development of Electrophoretic Deposition Method in the Solid Oxide Fuel Cell Technology: Theoretical Approaches, Experimental Solutions and Development Prospects. Russ. Chem. Rev. 2019, 88, 1179–1219. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Liang, Z.; Ning, H.; Fu, X.; Xu, Z.; Qiu, T.; Xu, W.; Yao, R.; Peng, J. High-Entropy Oxides: Advanced Research on Electrical Properties. Coatings 2021, 11, 628. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte Materials for Intermediate-Temperature Solid Oxide Fuel Cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Zakaria, Z.; Abu Hassan, S.H.; Shaari, N.; Yahaya, A.Z.; Boon Kar, Y. A Review on Recent Status and Challenges of Yttria Stabilized Zirconia Modification to Lowering the Temperature of Solid Oxide Fuel Cells Operation. Int. J. Energy Res. 2020, 44, 631–650. [Google Scholar] [CrossRef]
- Carda, M.; Budáč, D.; Paidar, M.; Bouzek, K. Current Trends in the Description of Lanthanum Strontium Manganite Oxygen Electrode Reaction Mechanism in a High-Temperature Solid Oxide Cell. Curr. Opin. Electrochem. 2022, 31, 100852. [Google Scholar] [CrossRef]
- He, S.; Jiang, S.P. Electrode/Electrolyte Interface and Interface Reactions of Solid Oxide Cells: Recent Development and Advances. Prog. Nat. Sci. Mater. Int. 2021, 31, 341–372. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of Lanthanum Strontium Cobalt Ferrite Perovskite Electrodes of Solid Oxide Fuel Cells—A Review. Int. J. Hydrog. Energy 2019, 44, 7448–7493. [Google Scholar] [CrossRef]
- Shabri, H.A.; Othman, M.H.D.; Mohamed, M.A.; Kurniawan, T.A.; Jamil, S.M. Recent Progress in Metal-Ceramic Anode of Solid Oxide Fuel Cell for Direct Hydrocarbon Fuel Utilization: A Review. Fuel Process. Technol. 2021, 212, 106626. [Google Scholar] [CrossRef]
- Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells. Materials 2021, 14, 1715. [Google Scholar] [CrossRef]
- Tao, Z.; Fu, M.; Liu, Y. A Mini-Review of Carbon-Resistant Anode Materials for Solid Oxide Fuel Cells. Sustain. Energy Fuels 2021, 5, 5420–5430. [Google Scholar] [CrossRef]
- Maiti, T.K.; Majhi, J.; Maiti, S.K.; Singh, J.; Dixit, P.; Rohilla, T.; Ghosh, S.; Bhushan, S.; Chattopadhyay, S. Zirconia- and Ceria-Based Electrolytes for Fuel Cell Applications: Critical Advancements toward Sustainable and Clean Energy Production. Environ. Sci. Pollut. Res. 2022, 29, 64489–64512. [Google Scholar] [CrossRef]
- Abd Aziz, A.J.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Review of Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cell Applications. Ceram. Int. 2020, 46, 23314–23325. [Google Scholar] [CrossRef]
- Ndubuisi, A.; Abouali, S.; Singh, K.; Thangadurai, V. Recent Advances, Practical Challenges, and Perspectives of Intermediate Temperature Solid Oxide Fuel Cell Cathodes. J. Mater. Chem. A 2022, 10, 2196–2227. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, S.H.; Chen, R.S.; Ismail, A.F.; Hazan, R.; Baharuddin, N.A. Review on Recent Advancement in Cathode Material for Lower and Intermediate Temperature Solid Oxide Fuel Cells Application. Int. J. Hydrog. Energy 2022, 47, 1103–1120. [Google Scholar] [CrossRef]
- Shuk, P. Oxide Ion Conducting Solid Electrolytes Based on Bi2O3. Solid State Ion. 1996, 89, 179–196. [Google Scholar] [CrossRef]
- Sammes, N.M.; Tompsett, G.A.; Näfe, H.; Aldinger, F. Bismuth Based Oxide Electrolytes—Structure and Ionic Conductivity. J. Eur. Ceram. Soc. 1999, 19, 1801–1826. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Shubin, K.; Ermakova, L.; Eremeev, N.; Farlenkov, A.; Khrustov, A.; Filonova, E.; Sadykov, V. Development of Composite LaNi0.6Fe0.4O3−δ-Based Air Electrodes for Solid Oxide Fuel Cells with a Thin-Film Bilayer Electrolyte. Int. J. Hydrog. Energy 2021, 46, 16947–16964. [Google Scholar] [CrossRef]
- Wang, H.; Lei, Z.; Jiang, W.; Xu, X.; Jing, J.; Zheng, Z.; Yang, Z.; Peng, S. High-Conductivity Electrolyte with a Low Sintering Temperature for Solid Oxide Fuel Cells. Int. J. Hydrog. Energy 2022, 47, 11279–11287. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, H.; Park, J.; Kim, Y.-B. Low-Temperature Constrained Sintering of YSZ Electrolyte with Bi2O3 Sintering Sacrificial Layer for Anode-Supported Solid Oxide Fuel Cells. Ceram. Int. 2022, 48, 9673–9680. [Google Scholar] [CrossRef]
- Zhigachev, A.O.; Rodaev, V.V.; Zhigacheva, D.V.; Lyskov, N.V.; Shchukina, M.A. Doping of Scandia-Stabilized Zirconia Electrolytes for Intermediate-Temperature Solid Oxide Fuel Cell: A Review. Ceram. Int. 2021, 47, 32490–32504. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Advanced Modification of Scandia-stabilized Zirconia Electrolytes for Solid Oxide Fuel Cells Application—A Review. Int. J. Energy Res. 2021, 45, 4871–4887. [Google Scholar] [CrossRef]
- Filonova, E.; Medvedev, D. Recent Progress in the Design, Characterisation and Application of LaAlO3- and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes. Nanomaterials 2022, 12, 1991. [Google Scholar] [CrossRef] [PubMed]
- Raza, R.; Zhu, B.; Rafique, A.; Naqvi, M.R.; Lund, P. Functional Ceria-Based Nanocomposites for Advanced Low-Temperature (300–600 °C) Solid Oxide Fuel Cell: A Comprehensive Review. Mater. Today Energy 2020, 15, 100373. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Upadhyay, S.; Parkash, O. A Brief Review on Ceria Based Solid Electrolytes for Solid Oxide Fuel Cells. J. Alloy. Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Dunyushkina, L.A. Solid Oxide Fuel Cells with a Thin Film Electrolyte: A Review on Manufacturing Technologies and Electrochemical Characteristics. Electrochem. Mater. Technol. 2022, 1, 20221006. [Google Scholar] [CrossRef]
- Wei, T.; Singh, P.; Gong, Y.; Goodenough, J.B.; Huang, Y.; Huang, K. Sr3−3xNa3xSi3O9−1.5x (x = 0.45) as a Superior Solid Oxide-Ion Electrolyte for Intermediate Temperature-Solid Oxide Fuel Cells. Energy Environ. Sci. 2014, 7, 1680–1684. [Google Scholar] [CrossRef]
- Medvedev, D. Trends in Research and Development of Protonic Ceramic Electrolysis Cells. Int. J. Hydrog. Energy 2019, 44, 26711–26740. [Google Scholar] [CrossRef]
- Zvonareva, I.; Fu, X.-Z.; Medvedev, D.; Shao, Z. Electrochemistry and Energy Conversion Features of Protonic Ceramic Cells with Mixed Ionic-Electronic Electrolytes. Energy Environ. Sci. 2022, 15, 439–465. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z.; Liu, M. Enhanced Sulfur and Coking Tolerance of a Mixed Ion Conductor for SOFCs: BaZr0.1Ce0.7Y0.2−xYbxO3−δ. Science 2009, 326, 126–129. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Zhu, B. State of the Art Ceria-Carbonate Composites (3C) Electrolyte for Advanced Low Temperature Ceramic Fuel Cells (LTCFCs). Int. J. Hydrog. Energy 2012, 37, 19417–19425. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-Entropy Fluorite Oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Wang, X.; Yu, J.; Li, L. A Review of Zirconia-Based Solid Electrolytes. Ionics 2016, 22, 2249–2262. [Google Scholar] [CrossRef]
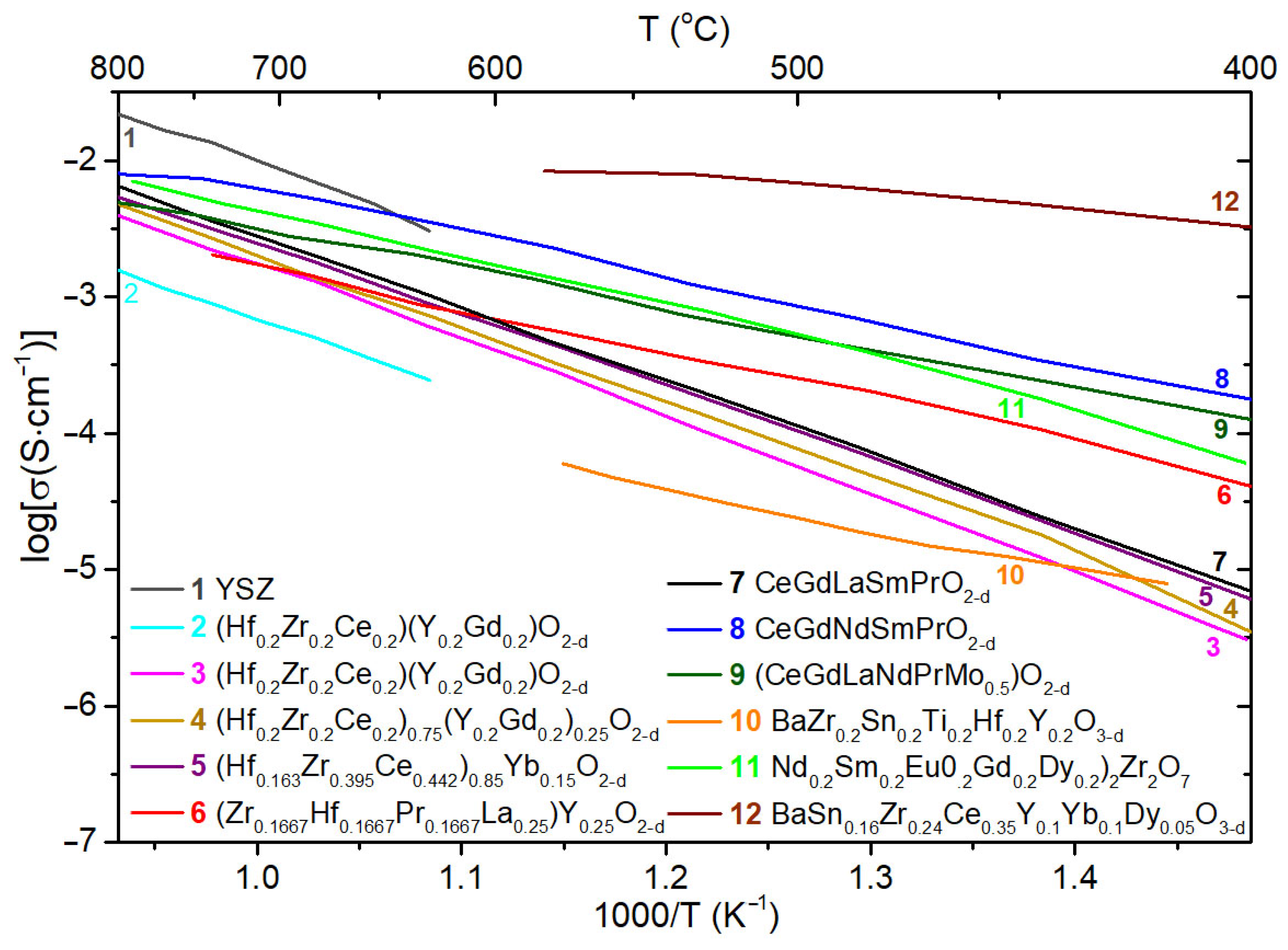
- Bonnet, E.; Grenier, J.C.; Bassat, J.M.; Jacob, A.; Delatouche, B.; Bourdais, S. On the Ionic Conductivity of Some Zirconia-Derived High-Entropy Oxides. J. Eur. Ceram. Soc. 2021, 41, 4505–4515. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Kim, D.-J. Lattice Parameters, Ionic Conductivities, and Solubility Limits in Fluorite-Structure MO2 Oxide [M = Hf4+, Zr4+, Ce4+, Th4+, U4+] Solid Solutions. J. Am. Ceram. Soc. 1989, 72, 1415–1421. [Google Scholar] [CrossRef]
- Tsoga, A. Total Electrical Conductivity and Defect Structure of ZrO2–CeO2–Y2O3–Gd2O3 Solid Solutions. Solid State Ion. 2000, 135, 403–409. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, P.V.; Venkatramani, N.; Rohatgi, V.K.; Momin, A.C.; Venkateswarlu, K.S. Structure and Ionic Conductivity of Solid Solutions in the System 0.9{(ZrO2)1−x-(CeO2)x}-0.1(Y2O3). J. Eur. Ceram. Soc. 1990, 6, 111–117. [Google Scholar] [CrossRef]
- Coduri, M.; Checchia, S.; Longhi, M.; Ceresoli, D.; Scavini, M. Rare Earth Doped Ceria: The Complex Connection Between Structure and Properties. Front. Chem. 2018, 6, 526. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.-J.; Ding, Z.-Y.; Cao, G.; Liu, Z.-G.; Wei, T.; Ouyang, J.-H.; Wang, Y.-M.; Wang, Y.-J. Microstructure and Electrical Properties of New High-Entropy Rare-Earth Zirconates. J. Alloy. Compd. 2022, 906, 164331. [Google Scholar] [CrossRef]
- Van Herle, J.; Seneviratne, D.; McEvoy, A.J. Lanthanide Co-Doping of Solid Electrolytes: AC Conductivity Behaviour. J. Eur. Ceram. Soc. 1999, 19, 837–841. [Google Scholar] [CrossRef]
- Yapryntsev, M.N.; Sudzhanskaya, I.V.; Lyubushkin, R.A.; Yapryntseva, E.N. Synthesis and Electric Transportation Properties of High-Entropy Oxides Based on Cerium Oxide. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1014, 012059. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Szymczak, M.; Zajusz, M.; Mikuła, A.; Moździerz, M.; Berent, K.; Wytrwal-Sarna, M.; Bernasik, A.; Stygar, M.; Świerczek, K. Stabilizing Fluorite Structure in Ceria-Based High-Entropy Oxides: Influence of Mo Addition on Crystal Structure and Transport Properties. J. Eur. Ceram. Soc. 2020, 40, 5870–5881. [Google Scholar] [CrossRef]
- Guo, R.; He, T. High-Entropy Perovskite Electrolyte for Protonic Ceramic Fuel Cells Operating below 600 °C. ACS Mater. Lett. 2022, 4, 1646–1652. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, L.; Cao, X. Chemical Stability and Electrical Property of Ba1.03Ce0.6Zr0.2Yb0.2O3−α Ceramic. J. Rare Earths 2011, 29, 678–682. [Google Scholar] [CrossRef]
- Amsif, M.; Marrero-López, D.; Ruiz-Morales, J.C.; Savvin, S.N.; Núñez, P. The Effect of Zn Addition on the Structure and Transport Properties of BaCe0.9−xZrxY0.1O3−δ. J. Eur. Ceram. Soc. 2014, 34, 1553–1562. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode Materials for Solid Oxide Fuel Cells: A Review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, H.; Ni, H.; Ou, X.; Wang, S.; Lin, B.; Feng, P.; Ling, Y. A Novel Facile Strategy to Suppress Sr Segregation for High-Entropy Stabilized La0.8Sr0.2MnO3−δ Cathode. J. Power Sources 2021, 482, 228959. [Google Scholar] [CrossRef]
- Shi, Y.; Ni, N.; Ding, Q.; Zhao, X. Tailoring High-Temperature Stability and Electrical Conductivity of High Entropy Lanthanum Manganite for Solid Oxide Fuel Cell Cathodes. J. Mater. Chem. A 2022, 10, 2256–2270. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, G.; Wu, H.; Beshiwork, B.A.; Tian, D.; Zhu, S.; Yang, Y.; Lu, X.; Ding, Y.; Ling, Y.; et al. A High-Entropy Perovskite Cathode for Solid Oxide Fuel Cells. J. Alloy. Compd. 2021, 872, 159633. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Olszewska, A.; Falkenstein, A.; Schwab, C.; Szymczak, M.; Zajusz, M.; Moździerz, M.; Mikuła, A.; Zielińska, K.; Berent, K.; et al. An Innovative Approach to Design SOFC Air Electrode Materials: High Entropy La1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (x = 0, 0.1, 0.2, 0.3) Perovskites Synthesized by the Sol–Gel Method. J. Mater. Chem. A 2020, 8, 24455–24468. [Google Scholar] [CrossRef]
- Li, Z.; Guan, B.; Xia, F.; Nie, J.; Li, W.; Ma, L.; Li, W.; Zhou, L.; Wang, Y.; Tian, H.; et al. High-Entropy Perovskite as a High-Performing Chromium-Tolerant Cathode for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 24363–24373. [Google Scholar] [CrossRef]
- Zhao, Z.; Rehder, L.; Steinbach, F.; Feldhoff, A. High-Entropy Perovskites Pr1−xSrx(Cr,Mn,Fe,Co,Ni)O3−δ (x = 0–0.5): Synthesis and Oxygen Permeation Properties. Membranes 2022, 12, 1123. [Google Scholar] [CrossRef]
- Ling, Y.; Lin, T.; Wang, X.; Ou, X.; Wang, S. Stable High-Entropy Double Perovskite Cathode SmBa(Mn0.2Fe0.2Co0.2Ni0.2Cu0.2)2O5+δ for Intermediate-Temperature Solid Oxide Fuel Cells. Kuei Suan Jen Hsueh Pao/J. Chin. Ceram. Soc. 2022, 50, 219–225. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Stępień, A.; Szymczak, M.; Zajusz, M.; Czaja, P.; Świerczek, K. High-Entropy Approach to Double Perovskite Cathode Materials for Solid Oxide Fuel Cells: Is Multicomponent Occupancy in (La,Pr,Nd,Sm,Gd)BaCo2O5+δ Affecting Physicochemical and Electrocatalytic Properties? Front. Energy Res. 2022, 10, 899308. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Bi, L. A High-Entropy Spinel Ceramic Oxide as the Cathode for Proton-Conducting Solid Oxide Fuel Cells. J. Adv. Ceram. 2022, 11, 794–804. [Google Scholar] [CrossRef]
- Shen, L.; Du, Z.; Zhang, Y.; Dong, X.; Zhao, H. Medium-Entropy Perovskites Sr(FeαTiβCoγMnζ)O3−δ as Promising Cathodes for Intermediate Temperature Solid Oxide Fuel Cell. Appl. Catal. B 2021, 295, 120264. [Google Scholar] [CrossRef]
- Krawczyk, P.A.; Jurczyszyn, M.; Pawlak, J.; Salamon, W.; Baran, P.; Kmita, A.; Gondek, Ł.; Sikora, M.; Kapusta, C.; Strączek, T.; et al. High-Entropy Perovskites as Multifunctional Metal Oxide Semiconductors: Synthesis and Characterization of (Gd0.2Nd0.2La0.2Sm0.2Y0.2)CoO3. ACS Appl. Electron. Mater. 2020, 2, 3211–3220. [Google Scholar] [CrossRef]
- Miyazaki, K.; Ding, Y.; Muroyama, H.; Matsui, T.; Eguchi, K. La0.6Sr0.4Co0.2Fe0.8O3−δ-Ba(Ce,Co,Y)O3−δ Composite Cathodes for Proton-Conducting Ceramic Fuel Cells. Electrochemistry 2020, 88, 28–33. [Google Scholar] [CrossRef]
- Bogdanovich, N.M.; Bronin, D.I.; Vdovin, G.K.; Yaroslavtsev, I.Y.; Kuzin, B.L. Effect of Bi0.75Y0.25O1.5 Electrolyte Additive in Collector Layer to Properties of Bilayer Composite Cathodes of Solid Oxide Fuel Cells Based on La(Sr)MnO3 and La(Sr)Fe(Co)O3 Compounds. Russ. J. Electrochem. 2009, 45, 456–464. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Wei, Z.; Zhang, X.; Meng, J.; Liu, X.; Meng, J. La0.6Sr0.4Co0.2Fe0.8O3−δ/CeO2 Heterostructured Composite Nanofibers as a Highly Active and Robust Cathode Catalyst for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 26830–26841. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Li, J.; Guo, X.; Hu, Q.; Yang, Z.; Yu, F.; Li, G. Modified La0.6Sr0.4Co0.2Fe0.8O3−δ Cathodes with the Infiltration of Er0.4Bi1.6O3 for Intermediate-Temperature Solid Oxide Fuel Cells. Int. J. Hydrog. Energy 2021, 46, 22932–22941. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Zielińska, K.; Stępień, A.; Zajusz, M.; Nowakowska, M.; Moździerz, M.; Berent, K.; Szymczak, M.; Świerczek, K. Formation of Solid Solutions and Physicochemical Properties of the High-Entropy Ln1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (Ln = La, Pr, Nd, Sm or Gd) Perovskites. Materials 2021, 14, 5264. [Google Scholar] [CrossRef] [PubMed]
- Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Punda, A.Y.; Valiulina, A.N.; Gavrilyak, A.M.; Zaitseva, O.V.; Tishkevich, D.I.; Zubar, T.I.; Sun, Z.; et al. Impact of the A-Site Rare-Earth Ions (Ln3+—Sm3+, Eu3+, Gd3+) on Structure and Electrical Properties of the High Entropy LnCr0.2Mn0.2Fe0.2Co0.2Ni0.2O3 Perovskites. Ceram. Int. 2022, 48, 9239–9247. [Google Scholar] [CrossRef]
- Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Pashkeev, I.Y.; Punda, A.Y.; Gavrilyak, M.; Zaitseva, O.V.; Taskaev, S.V.; Podgornov, F.V.; Darwish, M.A.; et al. Polysubstituted High-Entropy [LaNd](Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 Perovskites: Correlation of the Electrical and Magnetic Properties. Nanomaterials 2021, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Filonova, E.A.; Demina, A.N.; Kleibaum, E.A.; Gavrilova, L.Y.; Petrov, A.N. Phase Equilibria in the System LaMnO3+δ-SrMnO3-LaFeO3-SrFeO3−d. Inorg. Mater. 2006, 42, 443–447. [Google Scholar] [CrossRef]
- Filonova, E.A.; Demina, A.N.; Petrov, A.N. Regions of Stability of Variable-Composition Perovskite Phases La1−xSrxMn1−yMyO3 (M = Ti, Ni). Russ. J. Phys. Chem. 2007, 81, 1591–1594. [Google Scholar] [CrossRef]
- Aksenova, T.V.; Gavrilova, L.Y.; Cherepanov, V.A. Phase Equilibria at 1100 °C in Air and Crystal Structure of Solid Solutions in the System LaCoO3-SrCoO2.5-“SrNiO3”-“LaNiO3”. Inorg. Mater. 2004, 40, 1336–1340. [Google Scholar] [CrossRef]
- Filonova, E.A.; Demina, A.N.; Petrov, A.N. Phase Equilibria in the System LaMnO3-SrMnO3-SrCrO3-LaCrO3. Russ. J. Inorg. Chem. 2007, 52, 771–774. [Google Scholar] [CrossRef]
- Cherepanov, V.A.; Filonova, E.A.; Voronin, V.I.; Berger, I.F.; Barkhatova, L.Y. Phase Equilibria in the LaCoO3–LaMnO3–SrCoO2.5–SrMnO3 System. Mat. Res. Bull. 1999, 34, 1481–1489. [Google Scholar] [CrossRef]
- Aksenova, T.V.; Anan’ev, M.V.; Gavrilova, L.Y.; Cherepanov, V.A. Phase Equilibria and Crystal Structures of Solid Solutions in the System LaCoO3−δ-SrCoO2.5±δ-SrFeO3−δ-LaFeO3−δ. Inorg. Mater. 2007, 43, 296–300. [Google Scholar] [CrossRef]
- Demina, A.N.; Polovnikova, K.P.; Filonova, E.A.; Petrov, A.N.; Demin, A.K.; Pikalova, E.Y. Thermal Expansion and Electrical Conductivity of La0.7Sr0.3Mn1−yCryO3. Inorg. Mater. 2007, 43, 430–435. [Google Scholar] [CrossRef]
- Filonova, E.A.; Gilev, A.R.; Skutina, L.S.; Vylkov, A.I.; Kuznetsov, D.K.; Shur, V.Y. Double Sr2Ni1−xMgxMoO6 Perovskites (x = 0, 0.25) as Perspective Anode Materials for LaGaO3-Based Solid Oxide Fuel Cells. Solid State Ion. 2018, 314, 112–118. [Google Scholar] [CrossRef]
- Pikalov, S.M.; Vedmid’, L.B.; Filonova, E.A.; Pikalova, E.Y.; Lyagaeva, J.G.; Danilov, N.A.; Murashkina, A.A. High-Temperature Behavior of Calcium Substituted Layered Neodymium Nickelates. J. Alloy. Compd. 2019, 801, 558–567. [Google Scholar] [CrossRef]
- Maksimchuk, T.; Filonova, E.; Mishchenko, D.; Eremeev, N.; Sadovskaya, E.; Bobrikov, I.; Fetisov, A.; Pikalova, N.; Kolchugin, A.; Shmakov, A.; et al. High-Temperature Behavior, Oxygen Transport Properties, and Electrochemical Performance of Cu-Substituted Nd1.6Ca0.4NiO4+δ Electrode Materials. Appl. Sci. 2022, 12, 3747. [Google Scholar] [CrossRef]
- Kravchenko, E.; Zakharchuk, K.; Viskup, A.; Grins, J.; Svensson, G.; Pankov, V.; Yaremchenko, A. Impact of Oxygen Deficiency on the Electrochemical Performance of K2NiF4—Type (La1−xSrx)2NiO4−δ Oxygen Electrodes. ChemSusChem 2017, 10, 600–611. [Google Scholar] [CrossRef]
- Kol’chugin, A.A.; Pikalova, E.Y.; Bogdanovich, N.M.; Bronin, D.I.; Filonova, E.A. Electrochemical Properties of Doped Lantanum–Nickelate-Based Electrodes. Russ. J. Electrochem. 2017, 53, 826–833. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kolchugin, A.A.; Sadykov, V.A.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Bogdanovich, N.M. Structure, Transport Properties and Electrochemical Behavior of the Layered Lanthanide Nickelates Doped with Calcium. Int. J. Hydrog. Energy 2018, 43, 17373–17386. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Malyshkin, D.A.; Sukhanov, K.S.; Cherepanov, V.A. Hydration Effect on Properties of the La2−xAxNi1−yFeyO4+δ (A = Ca, Sr) Cathode Materials for H+-SOFCs. J. Alloy. Compd. 2021, 860, 158452. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Hurtado, J.; Losilla, E.R.; Marrero-López, D. Stability and Electrochemical Performance of Nanostructured La2CuO4+δ Cathodes. J. Alloy. Compd. 2019, 788, 565–572. [Google Scholar] [CrossRef]
- Musicó, B.L.; Wright, Q.; Delzer, C.; Ward, T.Z.; Rawn, C.J.; Mandrus, D.G.; Keppens, V. Synthesis Method Comparison of Compositionally Complex Rare Earth-based Ruddlesden–Popper n = 1 T′-type Cuprates. J. Am. Ceram. Soc. 2021, 104, 3750–3759. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Cherepanov, V.A. Performance of the Lanthanum Gallate Based Solid Oxide Fuel Cells with the La2−xCaxNi1−yFeyO4+δ Cathodes and Sr2Ni0.75Mg0.25MoO6−δ Anode. Solid State Ion. 2019, 339, 115001. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, M.; Xia, C.; Bouwmeester, H.J.M. Bismuth-Doped La1.75Sr0.25NiO4+δ as a Novel Cathode Material for Solid Oxide Fuel Cells. J. Mater. Chem. A 2017, 5, 14012–14019. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Wu, S.; Yu, S.; Bi, L. Enhancing the Performance of Traditional La2NiO4+x Cathode for Proton-Conducting Solid Oxide Fuel Cells with Zn-Doping. Ceram. Int. 2022, 48, 19626–19632. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Eremeev, N.F.; Pikalova, E.Y.; Bogdanovich, N.M.; Filonova, E.A.; Krieger, T.A.; Fedorova, Y.E.; Krasnov, A.V.; Skriabin, P.I.; et al. Novel Materials for Solid Oxide Fuel Cells Cathodes and Oxygen Separation Membranes: Fundamentals of Oxygen Transport and Performance. Carbon Resour. Convers. 2020, 3, 112–121. [Google Scholar] [CrossRef]
- Pikalova, E.; Eremeev, N.; Sadovskaya, E.; Sadykov, V.; Tsvinkinberg, V.; Pikalova, N.; Kolchugin, A.; Vylkov, A.; Baynov, I.; Filonova, E. Influence of the Substitution with Rare Earth Elements on the Properties of Layered Lanthanum Nickelate—Part 1: Structure, Oxygen Transport and Electrochemistry Evaluation. Solid State Ion. 2022, 379, 115903. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Eremeev, N.F.; Sadovskaya, E.M.; Shlyakhtina, A.V.; Pikalova, E.Y.; Osinkin, D.A.; Yaremchenko, A.A. Design of Materials for Solid Oxide Fuel Cells, Permselective Membranes, and Catalysts for Biofuel Transformation into Syngas and Hydrogen Based on Fundamental Studies of Their Real Structure, Transport Properties, and Surface Reactivity. Curr. Opin. Green Sustain. Chem. 2022, 33, 100558. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, J.G.; Medvedev, D.A.; Bi, L.; Yaremchenko, A.A. Recent Advances in Layered Ln2NiO4+δ Nickelates: Fundamentals and Prospects of Their Applications in Protonic Ceramic Fuel and Electrolysis Cells. J. Mater. Chem. A 2021, 9, 154–195. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Baratov, S.A.; Medvedev, D.A. Modernized Synthesis Technique of Pr2NiO4+δ-Based Complex Oxides Using Low-Temperature Salt Melts. Materials 2022, 15, 6148. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, Y.G.; Vylkov, A.I.; Gorshkov, M.Y.; Vdovin, G.K.; Medvedev, D.A. Performance of Pr2(Ni,Cu)O4+δ Electrodes in Protonic Ceramic Electrochemical Cells with Unseparated and Separated Gas Spaces. J. Mater. Sci. Technol. 2021, 93, 157–168. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Koroleva, M.; Vdovin, G.; Farlenkov, A.; Medvedev, D. Functionality of an Oxygen Ca3Co4O9+δ Electrode for Reversible Solid Oxide Electrochemical Cells Based on Proton-Conducting Electrolytes. J. Power Sources 2019, 438, 226996. [Google Scholar] [CrossRef]
- Filonova, E.A.; Tokareva, E.S.; Pikalova, N.S.; Vylkov, A.I.; Bogdanovich, N.M.; Pikalova, E.Y. Assessment of Prospective Cathodes Based on (1−x)Ca3Co4O9+δ-xBaCe0.5Zr0.3Y0.1Yb0.1O3−δ Composites for Protonic Ceramic Electrochemical Cells. J. Solid State Electrochem. 2020, 24, 1509–1521. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Sukhanov, K.S.; Korona, D.V.; Cherepanov, V.A. Evaluation of La2−x(Ca/Sr)xNi1−yFeyO4+δ (x = 0.5, 0.6; y = 0.4, 0.5) as Cathodes for Proton-Conducting SOFC Based on Lanthanum Tungstate. Electrochim. Acta 2022, 421, 140479. [Google Scholar] [CrossRef]
- Kasyanova, A.; Tarutin, A.; Lyagaeva, J.; Fu, X.-Z.; Medvedev, D. Double-Doped YFeO3 as New Electrodes for Protonic Ceramic Fuel Cells. Ceram. Int. 2021, 47, 22821–22829. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K.; Yang, Y.; Song, W.; Ma, Z.; Chen, H.; Ou, X.; Zhao, L.; Khan, M.; Ling, Y. Investigation of Fe-Substituted in BaZr0.8Y0.2O3−δ Proton Conducting Oxides as Cathode Materials for Protonic Ceramics Fuel Cells. J. Alloy. Compd. 2020, 814, 152220. [Google Scholar] [CrossRef]
- Baiutti, F.; Chiabrera, F.; Acosta, M.; Diercks, D.; Parfitt, D.; Santiso, J.; Wang, X.; Cavallaro, A.; Morata, A.; Wang, H.; et al. A High-Entropy Manganite in an Ordered Nanocomposite for Long-Term Application in Solid Oxide Cells. Nat. Commun. 2021, 12, 2660. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Sun, C.; Ni, Q.; Wang, C.; Jin, H. High Entropy Spinel-Structure Oxide for Electrochemical Application. Chem. Eng. J. 2022, 431, 133448. [Google Scholar] [CrossRef]
- Grzesik, Z.; Smoła, G.; Miszczak, M.; Stygar, M.; Dąbrowa, J.; Zajusz, M.; Świerczek, K.; Danielewski, M. Defect Structure and Transport Properties of (Co,Cr,Fe,Mn,Ni)3O4 Spinel-Structured High Entropy Oxide. J. Eur. Ceram. Soc. 2020, 40, 835–839. [Google Scholar] [CrossRef]
- Mao, A.; Quan, F.; Xiang, H.-Z.; Zhang, Z.-G.; Kuramoto, K.; Xia, A.-L. Facile Synthesis and Ferrimagnetic Property of Spinel (CoCrFeMnNi)3O4 High-Entropy Oxide Nanocrystalline Powder. J. Mol. Struct. 2019, 1194, 11–18. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and Microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 High Entropy Oxide Characterized by Spinel Structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Zhu, H.; Xie, H.; Zhao, Y.; Dai, S.; Li, M.; Wang, X. Structure and Magnetic Properties of a Class of Spinel High-Entropy Oxides. J. Magn. Magn. Mater. 2021, 535, 168063. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Wang, S.-Y.; Kuo, C.-H.; Huang, S.-C.; Lin, M.-H.; Li, C.-H.; Chen, H.-Y.T.; Wang, C.-C.; Liao, Y.-F.; Lin, C.-C.; et al. In Operando Synchrotron X-ray Studies of a Novel Spinel (Ni0.2Co0.2Mn0.2Fe0.2Ti0.2)3O4 High-Entropy Oxide for Energy Storage Applications. J. Mater. Chem. A 2020, 8, 21756–21770. [Google Scholar] [CrossRef]
- Wang, B.; Yao, J.; Wang, J.; Chang, A. Spinel-Type High-Entropy (Co0.2Mn0.2Fe0.2Zn0.2Ti0.2)3O4 Oxides Constructed from Disordered Cations and Oxygen Vacancies. J. Alloy. Compd. 2022, 897, 163188. [Google Scholar] [CrossRef]
- Dai, S.; Li, M.; Wang, X.; Zhu, H.; Zhao, Y.; Wu, Z. Fabrication and Magnetic Property of Novel (Co,Zn,Fe,Mn,Ni)3O4 High-Entropy Spinel Oxide. J. Magn. Magn. Mater. 2021, 536, 168123. [Google Scholar] [CrossRef]
- Sarkar, A.; Eggert, B.; Witte, R.; Lill, J.; Velasco, L.; Wang, Q.; Sonar, J.; Ollefs, K.; Bhattacharya, S.S.; Brand, R.A.; et al. Comprehensive Investigation of Crystallographic, Spin-Electronic and Magnetic Structure of (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4: Unraveling the Suppression of Configuration Entropy in High Entropy Oxides. Acta Mater. 2022, 226, 117581. [Google Scholar] [CrossRef]
- Wang, K.; Ma, B.; Li, T.; Xie, C.; Sun, Z.; Liu, D.; Liu, J.; An, L. Fabrication of High-Entropy Perovskite Oxide by Reactive Flash Sintering. Ceram. Int. 2020, 46, 18358–18361. [Google Scholar] [CrossRef]
- Yu, P.; Ma, Z.; Liu, C.; Liu, W.; Zhao, S.; Han, Z.; Shao, T.; Gao, P.; Jiang, B.; Gu, X. Preparation of High Entropy (Ba0.2Mg0.2Ca0.2Sr0.2Pb0.2)TiO3 Perovskite Oxide Powders by a Sol-Hydrothermal Method. Ceram. Int. 2022, 48, 15992–15999. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, S.; Li, T.; Xie, B.; Guo, K.; Lu, J. Microstructure and Ferroelectric Properties of High-Entropy Perovskite Oxides with A-Site Disorder. Ceram. Int. 2021, 47, 33039–33046. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, X.; Yuan, M.; Gao, J.; Wang, Z.; Abdalla, A.M.; Azad, A.K.; Xu, L.; Lv, Z.; Wei, B. A SrCo0.9Ta0.1O3−δ Derived Medium-Entropy Cathode with Superior CO2 Poisoning Tolerance for Solid Oxide Fuel Cells. J. Power Sources 2022, 540, 231661. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of Solid Oxide Fuel Cell Materials: Cathode, Anode, and Electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Kolchugin, A.A.; Bogdanovich, N.M.; Beresnev, S.M. Performance and Redox Stability of a Double–Layer Sr2Fe1.5Mo0.5O6−δ—Based Electrode for Solid State Electrochemical Application. Electrochim. Acta 2020, 361, 137058. [Google Scholar] [CrossRef]
- Tóthová, E.; Düvel, A.; Witte, R.; Brand, R.A.; Sarkar, A.; Kruk, R.; Senna, M.; Da Silva, K.L.; Menzel, D.; Girman, V.; et al. A Unique Mechanochemical Redox Reaction Yielding Nanostructured Double Perovskite Sr2FeMoO6 With an Extraordinarily High Degree of Anti-Site Disorder. Front. Chem. 2022, 10, 846910. [Google Scholar] [CrossRef]
- Afroze, S.; Karim, A.; Cheok, Q.; Eriksson, S.; Azad, A.K. Latest Development of Double Perovskite Electrode Materials for Solid Oxide Fuel Cells: A Review. Front. Energy 2019, 13, 770–797. [Google Scholar] [CrossRef]
- Huan, Y.; Li, Y.; Yin, B.; Ding, D.; Wei, T. High Conductive and Long-Term Phase Stable Anode Materials for SOFCs: A2FeMoO6 (A = Ca, Sr, Ba). J. Power Sources 2017, 359, 384–390. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Fu, R.; Feng, T.; Shen, Y.; Liu, J.; He, T. Pd-Impregnated Sr1.9VMoO6−δ Double Perovskite as an Efficient and Stable Anode for Solid-Oxide Fuel Cells Operating on Sulfur-Containing Syngas. Electrochim. Acta 2018, 274, 91–102. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Liang, G.; Croft, M.; Lehtimäki, M.; Karppinen, M.; Goodenough, J.B. Double-Perovskite Anode Materials Sr2MMoO6 (M = Co, Ni) for Solid Oxide Fuel Cells. Chem. Mater. 2009, 21, 2319–2326. [Google Scholar] [CrossRef]
- Wei, T.; Ji, Y.; Meng, X.; Zhang, Y. Sr2NiMoO6−δ as Anode Material for LaGaO3-Based Solid Oxide Fuel Cell. Electrochem. Commun. 2008, 10, 1369–1372. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Dass, R.I.; Denyszyn, J.C.; Goodenough, J.B. Synthesis and Characterization of Sr2MgMoO6−δ. J. Electrochem. Soc. 2006, 153, A1266. [Google Scholar] [CrossRef]
- Thomas, T.; Qi, H.; Liu, X.; Zondlo, J.W.; Sabolsky, E.M.; Hart, R. Effect of Mg/Mo Ratio in Stoichiometric Sr2MgMoO6−δ (SMM) Redox-Stable Anode. ECS Trans. 2017, 78, 1205–1215. [Google Scholar] [CrossRef]
- Filonova, E.A.; Dmitriev, A.S.; Pikalov, P.S.; Medvedev, D.A.; Pikalova, E.Y. The Structural and Electrical Properties of Sr2Ni0.75Mg0.25MoO6 and Its Compatibility with Solid State Electrolytes. Solid State Ion. 2014, 262, 365–369. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Du, Z.; Chen, T.; Chen, N. Electrical, Chemical, and Electrochemical Properties of Double Perovskite Oxides Sr2Mg1−xNixMoO6−δ as Anode Materials for Solid Oxide Fuel Cells. J. Phys. Chem. C 2014, 118, 18853–18860. [Google Scholar] [CrossRef]
- Skutina, L.S.; Vylkov, A.I.; Medvedev, D.A.; Filonova, E.A. Features of Structural, Thermal and Electrical Properties of Mo-Based Composite Materials as Fuel Electrodes for High-Temperature Applications. J. Alloy. Compd. 2017, 705, 854–861. [Google Scholar] [CrossRef]
- Filonova, E.A.; Russkikh, O.V.; Skutina, L.S.; Kochetova, N.A.; Korona, D.V.; Ostroushko, A.A. Influence of Synthesis Conditions on Phase Formation and Functional Properties of Prospective Anode Material Sr2Ni0.75Mg0.25MoO6−δ. J. Alloy. Compd. 2018, 748, 671–678. [Google Scholar] [CrossRef]
- Filonova, E.A.; Russkikh, O.V.; Skutina, L.S.; Vylkov, A.I.; Maksimchuk, T.Y.; Ostroushko, A.A. Sr2Ni0.7Mg0.3MoO6−δ: Correlation between Synthesis Conditions and Functional Properties as Anode Material for Intermediate-Temperature SOFCs. Int. J. Hydrog. Energy 2021, 46, 35910–35922. [Google Scholar] [CrossRef]
- Tietz, F. Thermal Expansion of SOFC Materials. Ionics 1999, 5, 129–139. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Sereda, V.V.; Malyshkin, D.A.; Ivanov, I.L.; Zuev, A.Y. Chemical Lattice Strain in Nonstoichiometric Oxides: An Overview. J. Mater. Chem. A 2022, 10, 6351–6375. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Gabás, M.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Redox Behaviour, Chemical Compatibility and Electrochemical Performance of Sr2MgMoO6−δ as SOFC Anode. Solid State Ion. 2010, 180, 1672–1682. [Google Scholar] [CrossRef]


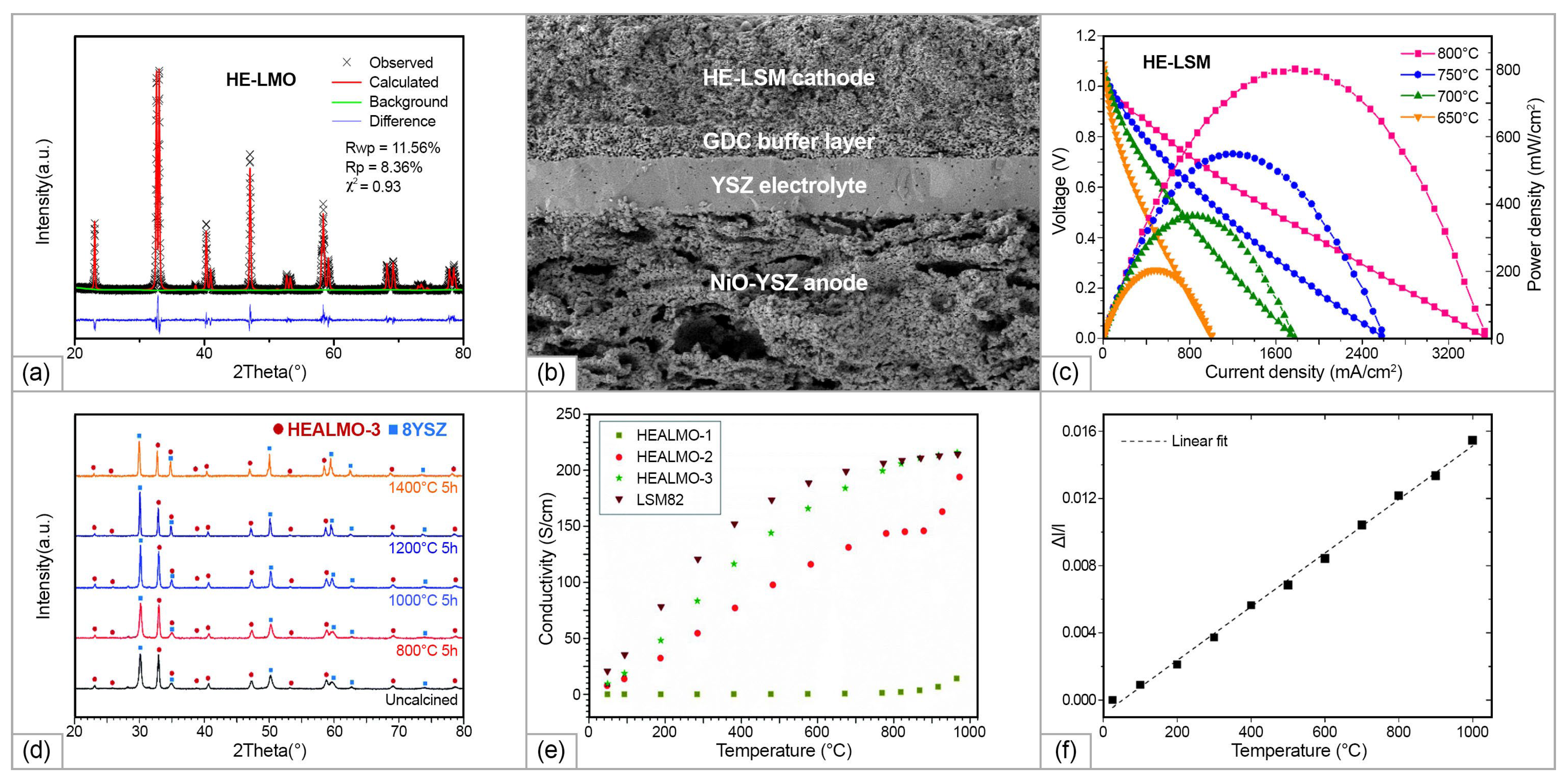
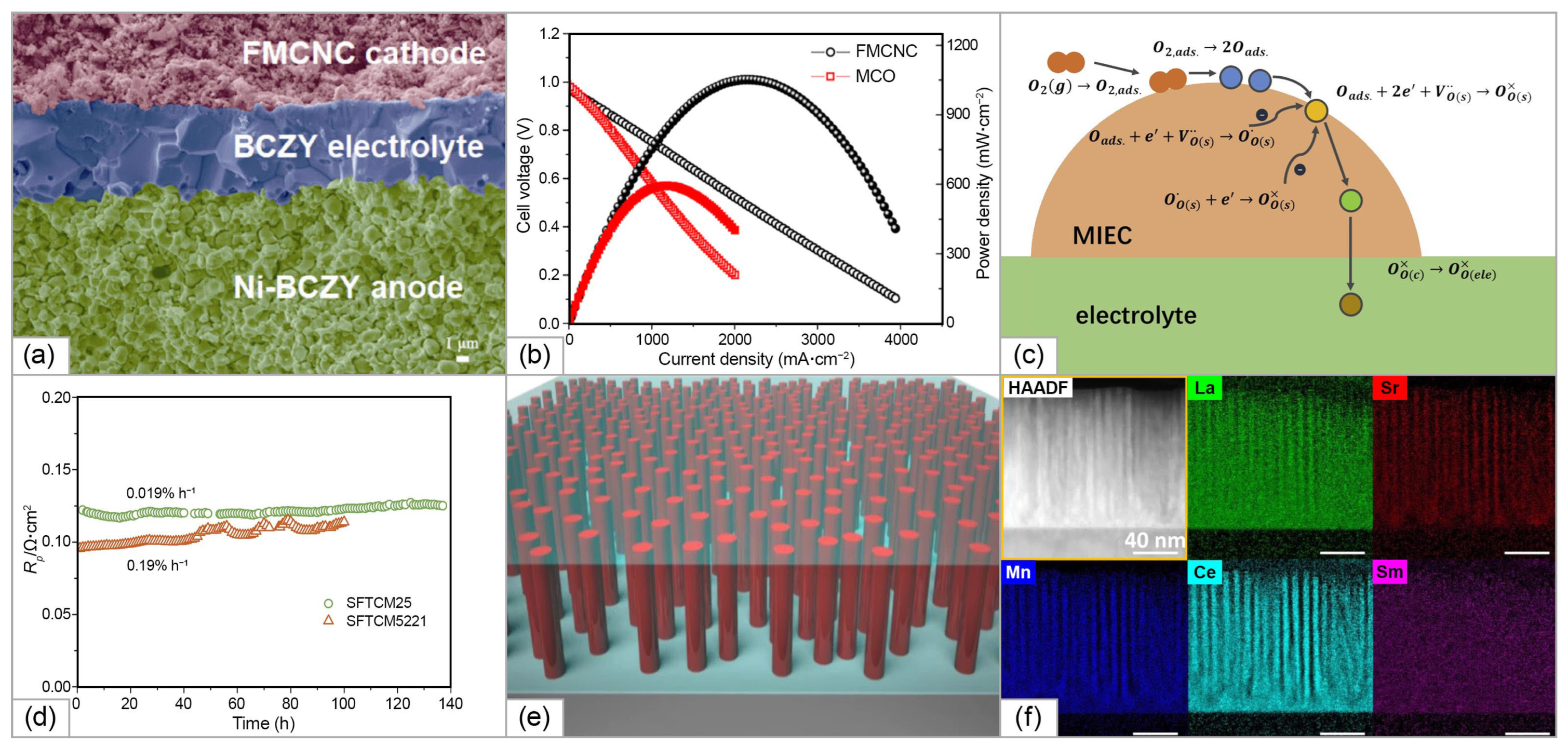
| Anode | Buffer Layer| Electrolyte| Buffer Layer | Cathode | T, °C | MPD, mW/cm2 | Ref. |
|---|---|---|---|---|---|
| NiO-YSZ | |YSZ|CGO | LaMn0.2Fe0.2Co0.2Ni0.2Cu0.2O3-δ | 800 | 551 | [17] |
| NiO-YSZ | |YSZ|CGO | La0.2Pr0.2Nd0.2Sm0.2Sr0.2MnO3-δ | 800 | 801 | [179] |
| NiO-YSZ | |YSZ|GDC | La0.2Pr0.2Nd0.2Sm0.2Ba0.1Sr0.1Co0.2Fe0.6Ni0.1Cu0.1O3-δ | 800 | 714 | [181] |
| NiO-GDC | GDC|LSGM8282| | La0.7Sr0.3Co0.2Cr0.2Fe0.2Mn0.2Ni0.2O3-δ | 900 | 550 | [182] |
| NiO-YSZ | |YSZ|GDC | La0.2Sr0.2Pr0.2Y0.2Ba0.2Co0.2Fe0.8O3-δ | 750 | 1006 | [183] |
| NiO-YSZ | |YSZ|GDC | SmBa(Mn0.2Fe0.2Co0.2Ni0.2Cu0.2)2O5+δ | 800 | 684 | [185] |
| NiO-YSZ | |YSZ|GDC | SmBa(Mn0.2Fe0.2Co0.2Ni0.2Cu0.2)2O5+δ-GDC | 800 | 839 | [185] |
| NiO-YSZ | |YSZ|CGO | (La0.2Pr0.2Nd0.2Sm0.2Gd0.2)2CuO4 | 700 | 528 | [15] |
| NiO-BCZY | |BCZY| | Fe0.6Mn0.6Co0.6Ni0.6Cr0.6O4 | 700 | 1052 | [187] |
| NiO-CGO | LDC|LSGM8282| | SrFe0.25Ti0.25Co0.25Mn0.25O3-δ | 800 | 850 | [188] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikalova, E.Y.; Kalinina, E.G.; Pikalova, N.S.; Filonova, E.A. High-Entropy Materials in SOFC Technology: Theoretical Foundations for Their Creation, Features of Synthesis, and Recent Achievements. Materials 2022, 15, 8783. https://doi.org/10.3390/ma15248783
Pikalova EY, Kalinina EG, Pikalova NS, Filonova EA. High-Entropy Materials in SOFC Technology: Theoretical Foundations for Their Creation, Features of Synthesis, and Recent Achievements. Materials. 2022; 15(24):8783. https://doi.org/10.3390/ma15248783
Chicago/Turabian StylePikalova, Elena Y., Elena G. Kalinina, Nadezhda S. Pikalova, and Elena A. Filonova. 2022. "High-Entropy Materials in SOFC Technology: Theoretical Foundations for Their Creation, Features of Synthesis, and Recent Achievements" Materials 15, no. 24: 8783. https://doi.org/10.3390/ma15248783
APA StylePikalova, E. Y., Kalinina, E. G., Pikalova, N. S., & Filonova, E. A. (2022). High-Entropy Materials in SOFC Technology: Theoretical Foundations for Their Creation, Features of Synthesis, and Recent Achievements. Materials, 15(24), 8783. https://doi.org/10.3390/ma15248783