Application of Nano-SiO2 Reinforced Urea-Formaldehyde Resin and Molecular Dynamics Simulation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Performance Test of UF Resin
2.3. Preparation and Performance Measurement of Plywood
2.4. Simulation of Configuration Construction
2.5. Simulation Method
3. Results
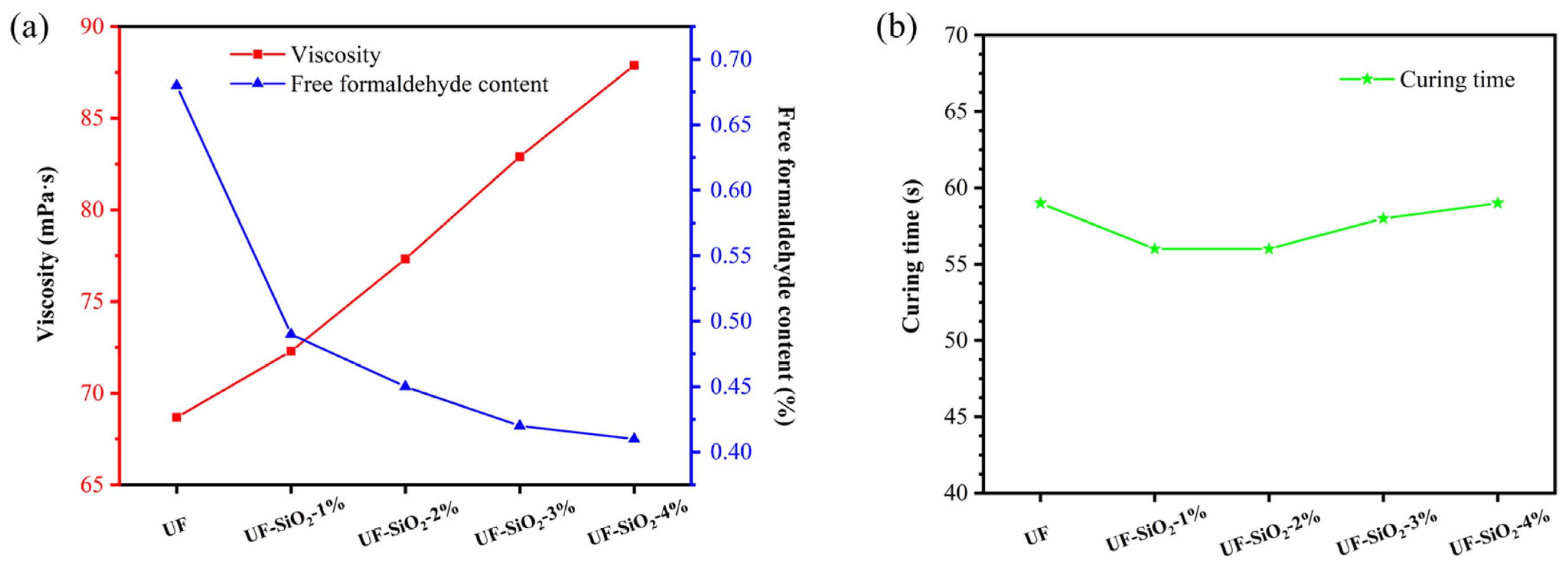
3.1. Effect of Nano-SiO2 Addition on the Properties of UF Resin
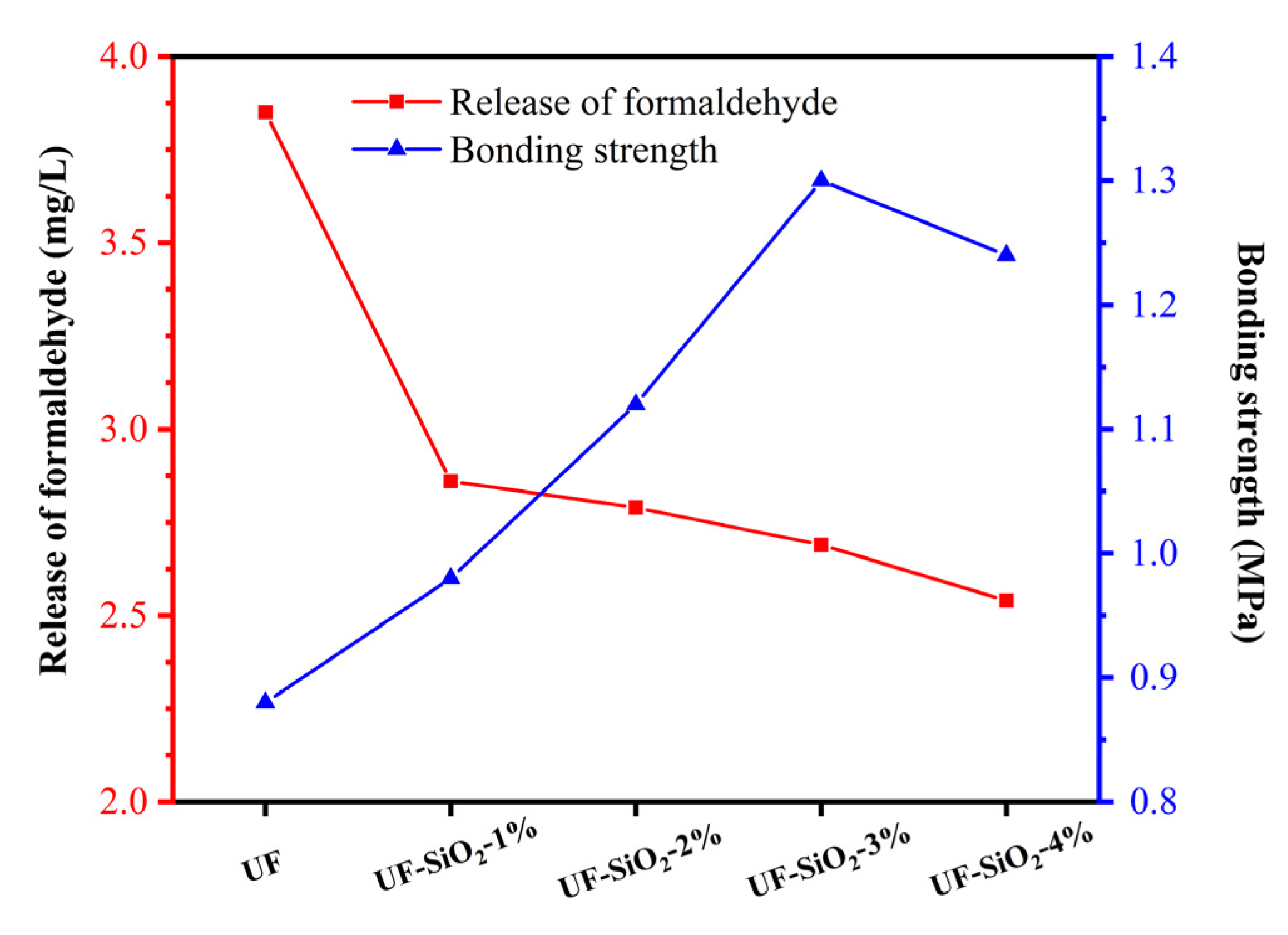
3.2. Effect of Nano-SiO2 Addition on the Performance of UF Resin Plywood
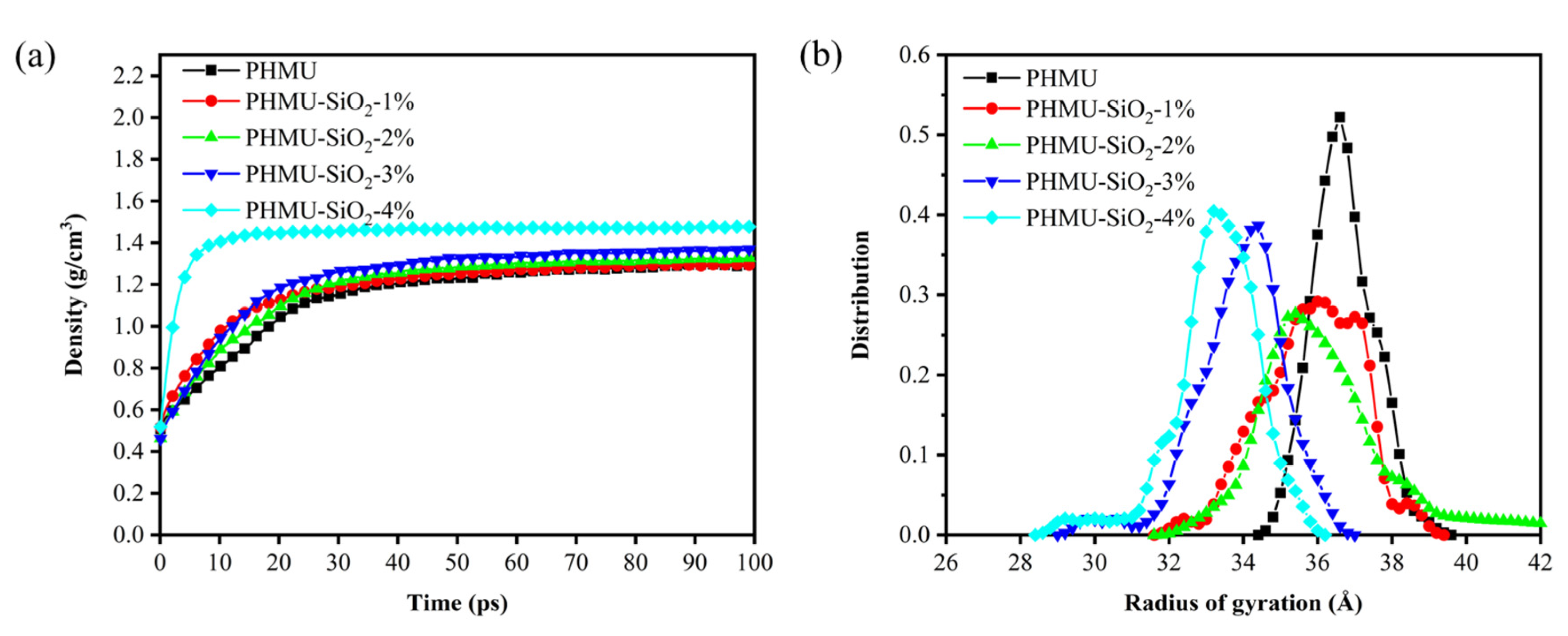
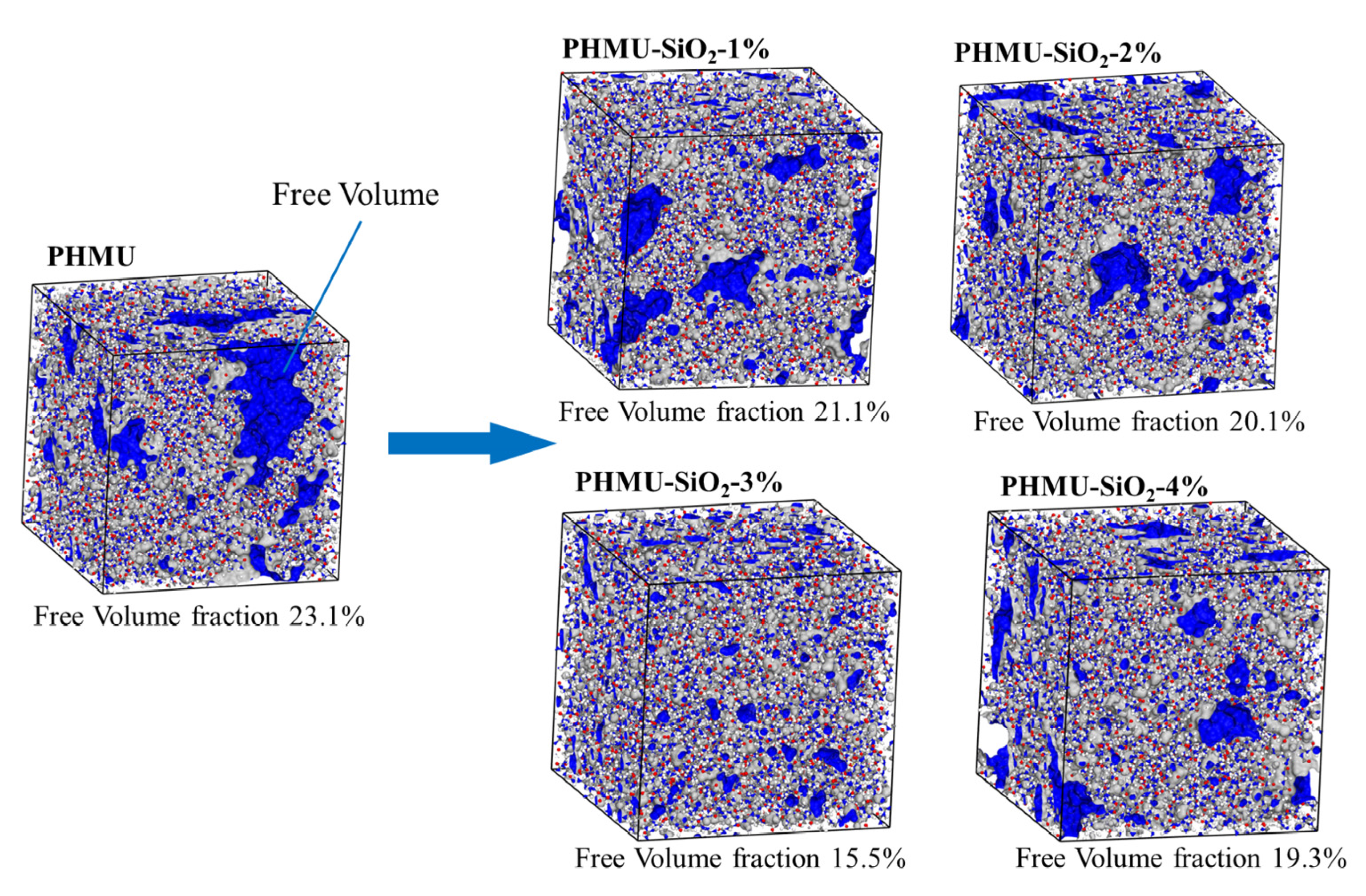
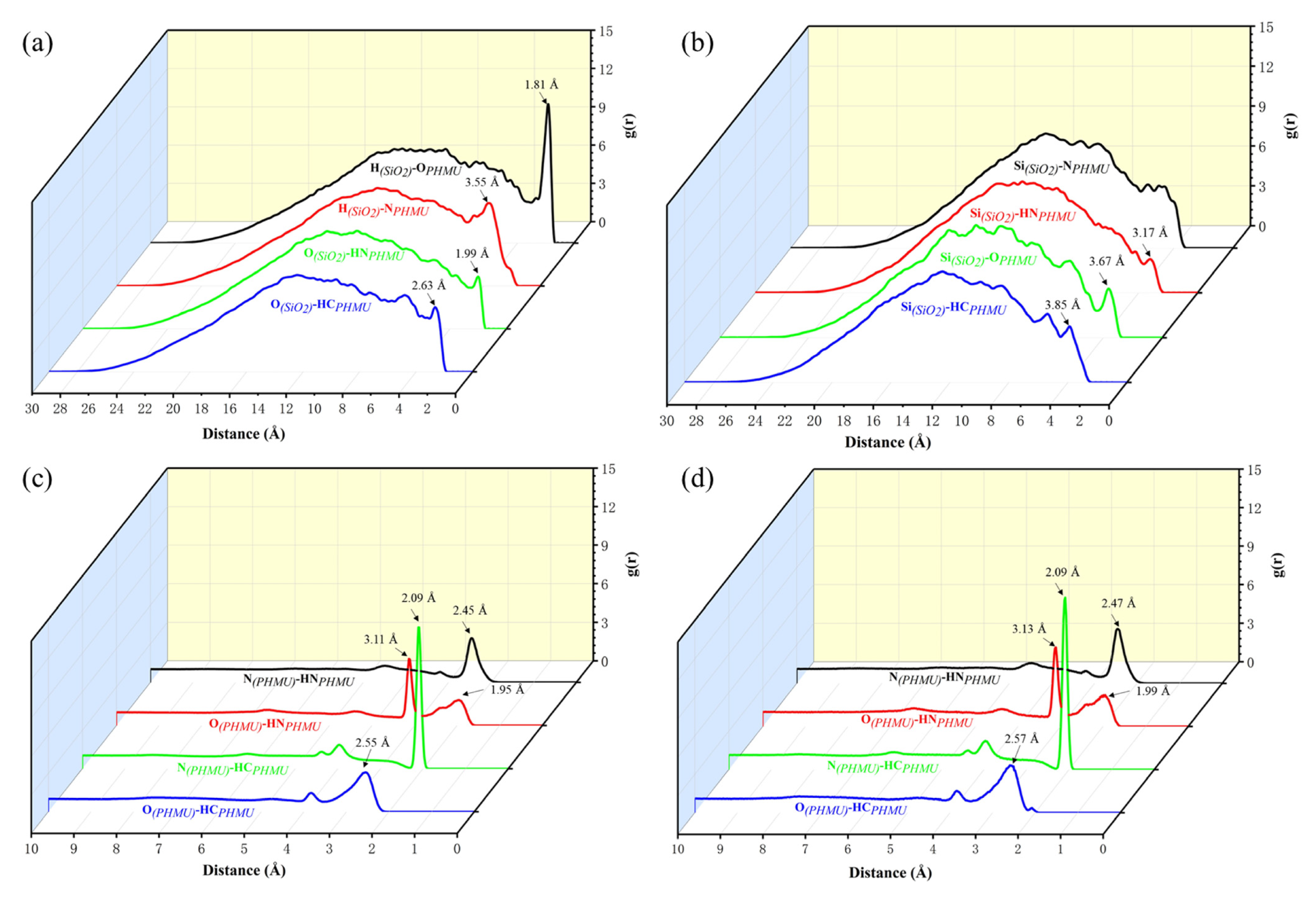
3.3. Molecular Dynamics Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wibowo, E.S.; Park, B.D.; Causin, V. Recent advances in urea-formaldehyde resins: Converting crystalline thermosetting polymers back to amorphous ones. Polym. Rev. 2022, 62, 722–756. [Google Scholar] [CrossRef]
- Que, Z.L.; Furuno, T.; Katoh, S.; Nishino, Y. Effects of urea-formaldehyde resin mole ratio on the properties of particleboard. Build. Environ. 2007, 42, 1257–1263. [Google Scholar] [CrossRef]
- Park, B.D.; Kim, J.W. Dynamic mechanical analysis of urea-formaldehyde resin adhesives with different formaldehyde-to-urea molar ratios. J. Appl. Polym. Sci. 2008, 108, 2045–2051. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Lee, Y.K.; Kwon, J.H.; Han, T.H.; Kim, H.J. Formaldehyde emission and VOCs from LVLs produced with three grades of urea-formaldehyde resin modified with nanocellulose. Build. Environ. 2016, 97, 82–87. [Google Scholar] [CrossRef]
- Dorieh, A.; Selakjani, P.P.; Shahavi, M.H.; Pizzi, A.; Movahed, S.G.; Pour, M.F.; Aghaei, R. Recent developments in the performance of micro/nanoparticle-modified urea-formaldehyde resins used as wood-based composite binders: A review. Int. J. Adhes. Adhes. 2022, 114, 103106. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Lubis, M.A.R.; Park, B.D.; Kim, J.S.; Causin, V. Converting crystalline thermosetting urea-formaldehyde resins to amorphous polymer using modified nanoclay. J. Ind. Eng. Chem. 2020, 87, 78–89. [Google Scholar] [CrossRef]
- Yadav, S.M.; Lubis, M.A.R.; Wibowo, E.S.; Park, B.D. Effects of nanoclay modification with transition metal ion on the performance of urea-formaldehyde resin adhesives. Polym. Bull. 2021, 78, 2375–2388. [Google Scholar] [CrossRef]
- Zahedsheijani, R.; Faezipour, M.; Tarmian, A.; Layeghi, M.; Yousefi, H. The effect of Na+ montmorillonite (NaMMT) nanoclay on thermal properties of medium density fiberboard (MDF). Eur. J. Wood Wood Prod. 2012, 70, 565–571. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.; Sharma, K.V. Thermal and mechanical properties of urea-formaldehyde (UF) resin combined with multiwalled carbon nanotubes (MWCNT) as nanofiller and fiberboards prepared by UF-MWCNT. Holzforschung 2015, 69, 199–205. [Google Scholar] [CrossRef]
- Saito, K.; Hirabayashi, Y.; Yamanaka, S. Reduction of formaldehyde emission from urea-formaldehyde resin with a small quantity of graphene oxide. RSC Adv. 2021, 11, 32830–32836. [Google Scholar] [CrossRef]
- Kawalerczyk, J.; Dziurka, D.; Mirski, R.; Siuda, J. The reduction of adhesive application in plywood manufacturing by using nanocellulose-reinforced urea-formaldehyde resin. J. Appl. Polym. Sci. 2021, 138, e49834. [Google Scholar] [CrossRef]
- Mahrdt, E.; Pinkl, S.; Schmidberger, C.; van Herwijnen, H.W.G.; Veigel, S.; Gindl-Altmutter, W. Effect of addition of microfibrillated cellulose to urea-formaldehyde on selected adhesive characteristics and distribution in particle board. Cellulose 2016, 23, 571–580. [Google Scholar] [CrossRef]
- Moslemi, A.; Koohi, M.Z.; Behzad, T.; Pizzi, A. Addition of cellulose nanofibers extracted from rice straw to urea formaldehyde resin; effect on the adhesive characteristics and medium density fiberboard properties. Int. J. Adhes. Adhes. 2020, 99, 102582. [Google Scholar] [CrossRef]
- Yu, X.; Qi, H.J.; Huang, Z.H.; Zhang, B.; Liu, S.X. Preparation and characterization of spherical beta-cyclodextrin/urea-formaldehyde microcapsules modified by nano-titanium oxide. RSC Adv. 2017, 7, 7857–7863. [Google Scholar] [CrossRef]
- Roumeli, E.; Papadopoulou, E.; Pavlidou, E.; Vourlias, G.; Bikiaris, D.; Paraskevopoulos, K.M.; Chrissafis, K. Synthesis, characterization and thermal analysis of urea-formaldehyde/nanoSiO2 resins. Thermochim. Acta 2012, 527, 33–39. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.; Sharma, K.V.; Gazali, S.B. Influence of Aluminum Oxide Nanoparticles on the Physical and Mechanical Properties of Wood Composites. Bioresources 2013, 8, 6231–6241. [Google Scholar] [CrossRef]
- Candan, Z.; Akbulut, T. Physical and mechanical properties of nanoreinforced particleboard composites. Maderas. Cienc. Tecnol. 2015, 17, 319–334. [Google Scholar] [CrossRef]
- Salari, A.; Tabarsa, T.; Khazaeian, A.; Saraeian, A. Improving some of applied properties of oriented strand board (OSB) made from underutilized low quality paulownia (Paulownia fortunie) wood employing nano-SiO2. Ind. Crops Prod. 2013, 42, 1–9. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Allen, M.P.; Quigley, D. Some comments on Monte Carlo and molecular dynamics methods. Mol. Phys. 2013, 111, 3442–3447. [Google Scholar] [CrossRef]
- Rutkai, G.; Kristóf, T. Dynamic Monte Carlo simulation in mixtures. J. Chem. Phys. 2010, 132, 104107. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V.; Purohit, R.; Sethumadhavan, R. In silico investigation of molecular mechanism of laminopathy caused by a point mutation (R482W) in lamin A/C protein. Amino Acids 2012, 43, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhardwaj, V.K.; Singh, R.; Das, P.; Purohit, R. Identification of acridinedione scaffolds as potential inhibitor of DENV-2 C protein: An in silico strategy to combat dengue. J. Cell. Biochem. 2022, 123, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Bhattacherjee, D.; Zyryanov, G.V.; Purohit, R. An insight from computational approach to explore novel, high-affinity phosphodiesterase 10A inhibitors for neurological disorders. J. Biomol. Struct. Dyn. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Støve, S.I.; Skjevik, Å.A.; Teigen, K.; Martinez, A. Inhibition of VMAT2 by β2-adrenergic agonists, antagonists, and the atypical antipsychotic ziprasidone. Commun. Biol. 2022, 5, 1283. [Google Scholar] [CrossRef] [PubMed]
- Borsatto, A.; Akkad, O.; Galdadas, I.; Ma, S.; Damfo, S.; Haider, S.; Kozielski, F.; Estarellas, C.; Gervasio, F.L. Revealing druggable cryptic pockets in the Nsp1 of SARS-CoV-2 and other β-coronaviruses by simulations and crystallography. eLife 2022, 11, e81167. [Google Scholar] [CrossRef]
- Tao, Z.; Zou, H.; Li, M.; Ren, S.; Xu, J.; Lin, J.; Yang, M.; Feng, Y.; Wang, G. Polypyrrole coated carbon nanotube aerogel composite phase change materials with enhanced thermal conductivity, high solar-/electro- thermal energy conversion and storage. J. Colloid Interface Sci. 2023, 629, 632–643. [Google Scholar] [CrossRef]
- Su, C.; Liu, K.; Guo, Y.; Li, H.; Zeng, Z.; Li, L. The role of pore structure and nitrogen surface groups in the adsorption behavior of formaldehyde on resin-based carbons. Surf. Interface Anal. 2021, 53, 330–339. [Google Scholar] [CrossRef]
- Peng, X.; Ji, H. Control mechanism of small organic molecules on methane adsorption capacity of coal. Fuel 2023, 331, 125904. [Google Scholar] [CrossRef]
- Yang, J.; Lou, W. Molecule Dynamics Simulation of the Effect of Oxidative Aging on Properties of Nitrile Rubber. Polymers 2022, 14, 226. [Google Scholar] [CrossRef]
- Hang, G.-y.; Wang, T.; Wang, J.-t.; Yu, W.-l.; Shen, H.-m. Theoretical research on performances of CL-20/HMX cocrystal explosive and its based polymer bonded explosives (PBXs) by molecular dynamics method. J. Mol. Model. 2022, 28, 385. [Google Scholar] [CrossRef] [PubMed]
- Morsch, S.; Wand, C.R.; Gibbon, S.; Irwin, M.; Siperstein, F.; Lyon, S. The effect of cross-linker structure on interfacial interactions, polymer dynamics and network composition in an epoxy-amine resin. Appl. Surf. Sci. 2023, 609, 155380. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, Y.; Liu, Y.; Qu, N.; Lai, Z.; Zhang, X.; Zhu, J. The mechanics and design of a local crystallization of amorphous for carbon material by molecular dynamics simulation. J. Non-Cryst. Solids 2023, 600, 121991. [Google Scholar] [CrossRef]
- Sangkhawasi, M.; Remsungnen, T.; Vangnai, A.S.; Poo-arporn, R.P.; Rungrotmongkol, T. All-Atom Molecular Dynamics Simulations on a Single Chain of PET and PEV Polymers. Polymers 2022, 14, 1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Zhou, X.C.; Kuang, F.M.; Zuo, H.X.; Huang, J. Mechanical and Tribological Properties of Nitrile Rubber Reinforced by Nano-SiO2: Molecular Dynamics Simulation. Tribol. Lett. 2021, 69, 54. [Google Scholar] [CrossRef]
- He, E.Q.; Wang, S.J.; Li, Y.L.; Wang, Q. Enhanced tribological properties of polymer composites by incorporation of nano-SiO2 particles: A molecular dynamics simulation study. Comput. Mater. Sci. 2017, 134, 93–99. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, J. Molecular dynamics and dissipative particle dynamics simulations for the miscibility of poly(ethylene oxide)/poly(vinyl chloride) blends. Polymer 2010, 51, 291–299. [Google Scholar] [CrossRef]
- Colmenero, J.; Alvarez, F.; Arbe, A. Self-motion and the alpha relaxation in a simulated glass-forming polymer: Crossover from Gaussian to non-Gaussian dynamic behavior. Phys. Rev. E 2002, 65, 041804. [Google Scholar] [CrossRef]
- Samoletov, A.A.; Dettmann, C.P.; Chaplain, M.A.J. Thermostats for “Slow” Configurational Modes. J. Stat. Phys. 2007, 128, 1321–1336. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Bardak, T.; Sozen, E.; Kayahan, K.; Bardak, S. The Impact of Nanoparticles and Moisture Content on Bonding Strength of Urea Formaldehyde Resin Adhesive. Drv. Ind. 2018, 69, 247–252. [Google Scholar] [CrossRef]
- Dazmiri, M.K.; Kiamahalleh, M.V.; Dorieh, A.; Pizzi, A. Effect of the initial F/U molar ratio in urea-formaldehyde resins synthesis and its influence on the performance of medium density fiberboard bonded with them. Int. J. Adhes. Adhes. 2019, 95, 102440. [Google Scholar] [CrossRef]
- Mohan, M.; Sale, K.L.; Kalb, R.S.; Simmons, B.A.; Gladden, J.M.; Singh, S. Multiscale Molecular Simulation Strategies for Understanding the Delignification Mechanism of Biomass in Cyrene. ACS Sustain. Chem. Eng. 2022, 10, 11016–11029. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Lu, F.; Zhang, L.; Wu, S. Molecular-level insight of hindered phenol AO-70/nitrile-butadiene rubber damping composites through a combination of a molecular dynamics simulation and experimental method. RSC Adv. 2016, 6, 85994–86005. [Google Scholar] [CrossRef]

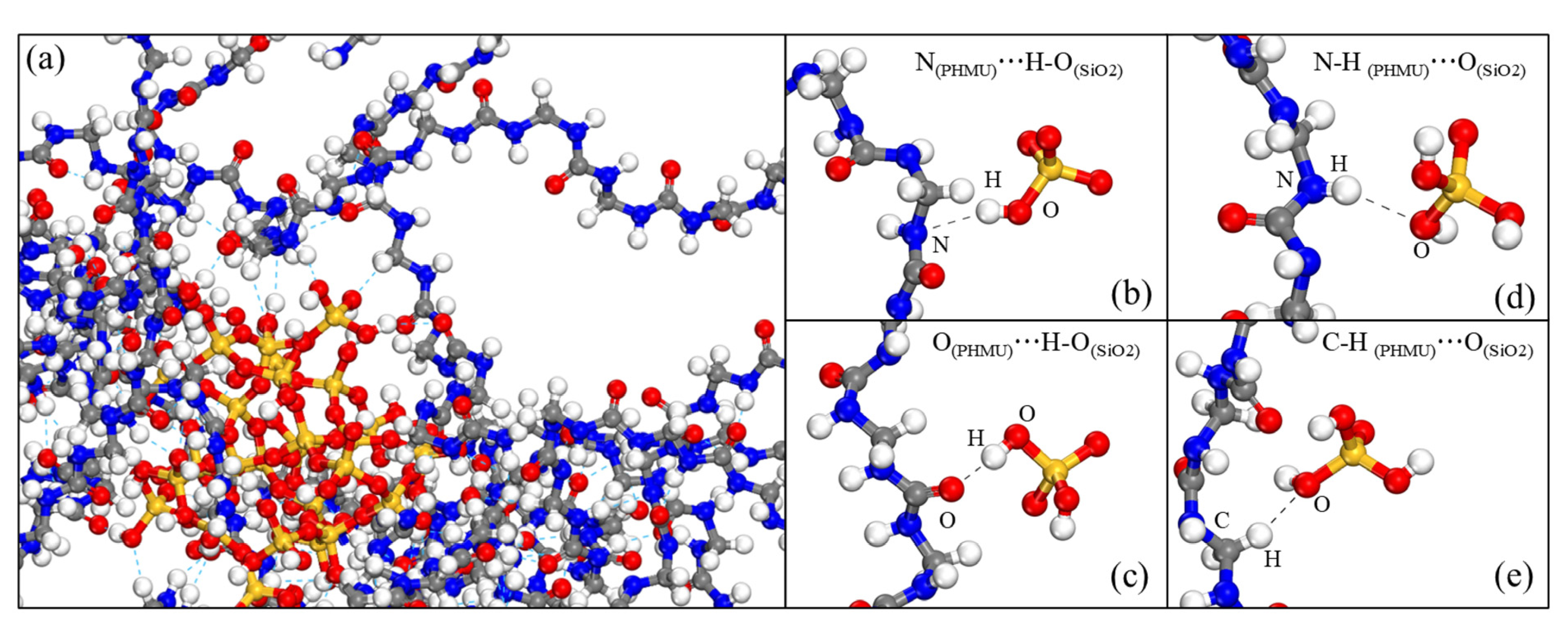
| System | Number of PHMU Chains | Number of Nano-SiO2 Clusters | Total Number of Atoms in the System |
|---|---|---|---|
| PHMU | 66 | - | 23,958 |
| PHMU-SiO2-1% | 66 | 1 | 24,102 |
| PHMU-SiO2-2% | 66 | 2 | 24,246 |
| PHMU-SiO2-3% | 66 | 3 | 24,390 |
| PHMU-SiO2-4% | 66 | 4 | 24,534 |
| System | C11 | C22 | C33 | C44 | C55 | C66 | λ/ GPa | μ/ GPa | E/ GPa |
|---|---|---|---|---|---|---|---|---|---|
| PHMU | 10.0935 | 10.2791 | 8.7662 | 2.8329 | 2.6627 | 2.7714 | 4.2015 | 2.7557 | 7.17 |
| PHMU-SiO2-1% | 11.3011 | 8.9072 | 9.6571 | 2.8828 | 2.9239 | 2.7462 | 4.2532 | 2.8510 | 7.41 |
| PHMU-SiO2-2% | 10.8068 | 10.1945 | 11.3171 | 2.8998 | 3.1195 | 2.8315 | 4.8723 | 2.9502 | 7.74 |
| PHMU-SiO2-3% | 13.3416 | 14.2885 | 14.6164 | 3.4910 | 3.0231 | 3.1938 | 7.6102 | 3.2360 | 8.74 |
| PHMU-SiO2-4% | 10.2779 | 10.0658 | 10.7602 | 3.0534 | 2.9722 | 3.0301 | 4.3309 | 3.0185 | 7.81 |
| System | Total Number of Hbonds | Average Length of Hbonds/(Å) | Hbonds/(SiO2-PHMU) |
|---|---|---|---|
| PHMU | 1888 | 2.088 | 0 |
| PHMU-SiO2-1% | 1904 | 2.080 | 26 |
| PHMU-SiO2-2% | 1933 | 2.075 | 48 |
| PHMU-SiO2-3% | 1966 | 2.054 | 69 |
| PHMU-SiO2-4% | 1953 | 2.072 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Guo, D.; Xia, C.; Li, T.; Lian, H. Application of Nano-SiO2 Reinforced Urea-Formaldehyde Resin and Molecular Dynamics Simulation Study. Materials 2022, 15, 8716. https://doi.org/10.3390/ma15248716
Xiao J, Guo D, Xia C, Li T, Lian H. Application of Nano-SiO2 Reinforced Urea-Formaldehyde Resin and Molecular Dynamics Simulation Study. Materials. 2022; 15(24):8716. https://doi.org/10.3390/ma15248716
Chicago/Turabian StyleXiao, Jun, Dingmeng Guo, Changlei Xia, Taohong Li, and Hailan Lian. 2022. "Application of Nano-SiO2 Reinforced Urea-Formaldehyde Resin and Molecular Dynamics Simulation Study" Materials 15, no. 24: 8716. https://doi.org/10.3390/ma15248716
APA StyleXiao, J., Guo, D., Xia, C., Li, T., & Lian, H. (2022). Application of Nano-SiO2 Reinforced Urea-Formaldehyde Resin and Molecular Dynamics Simulation Study. Materials, 15(24), 8716. https://doi.org/10.3390/ma15248716







