Precipitation Behavior during Aging Operations in an Ultrafine-Grained Al–Cu–Mg Alloy Produced by High-Strain-Rate Processing
Abstract
1. Introduction
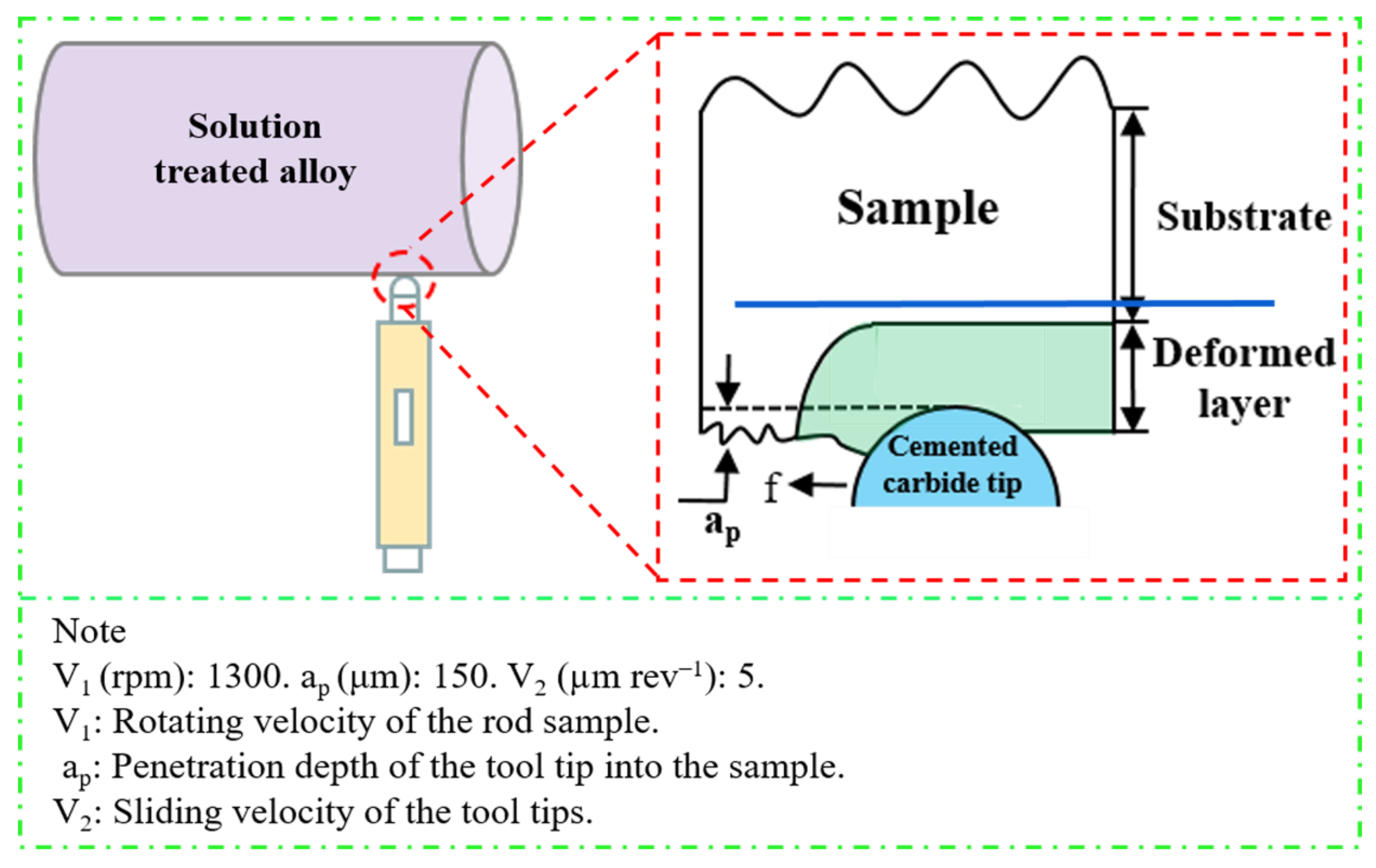
2. Experimental Procedures
2.1. Materials and Processing
2.2. Microstructure Characterization
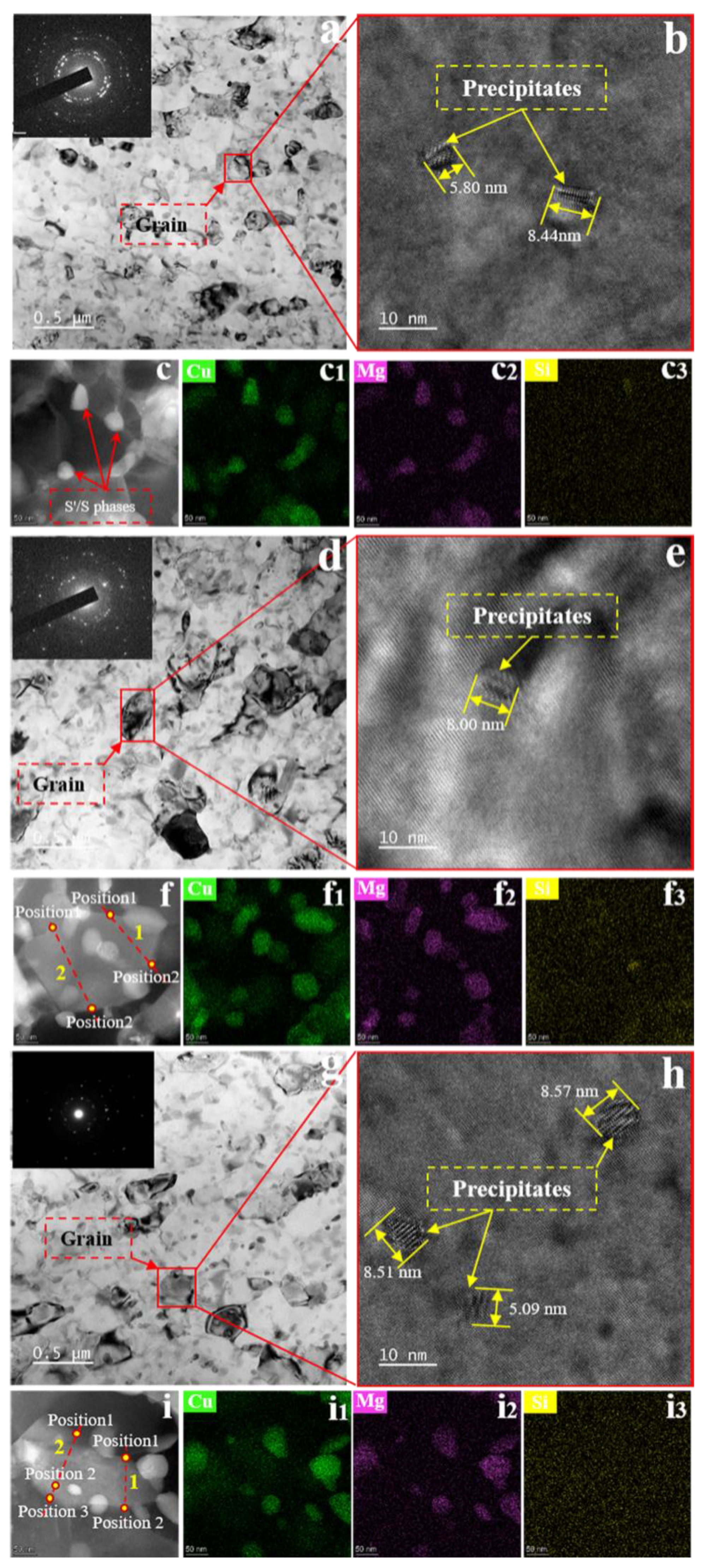
3. Results and Analysis
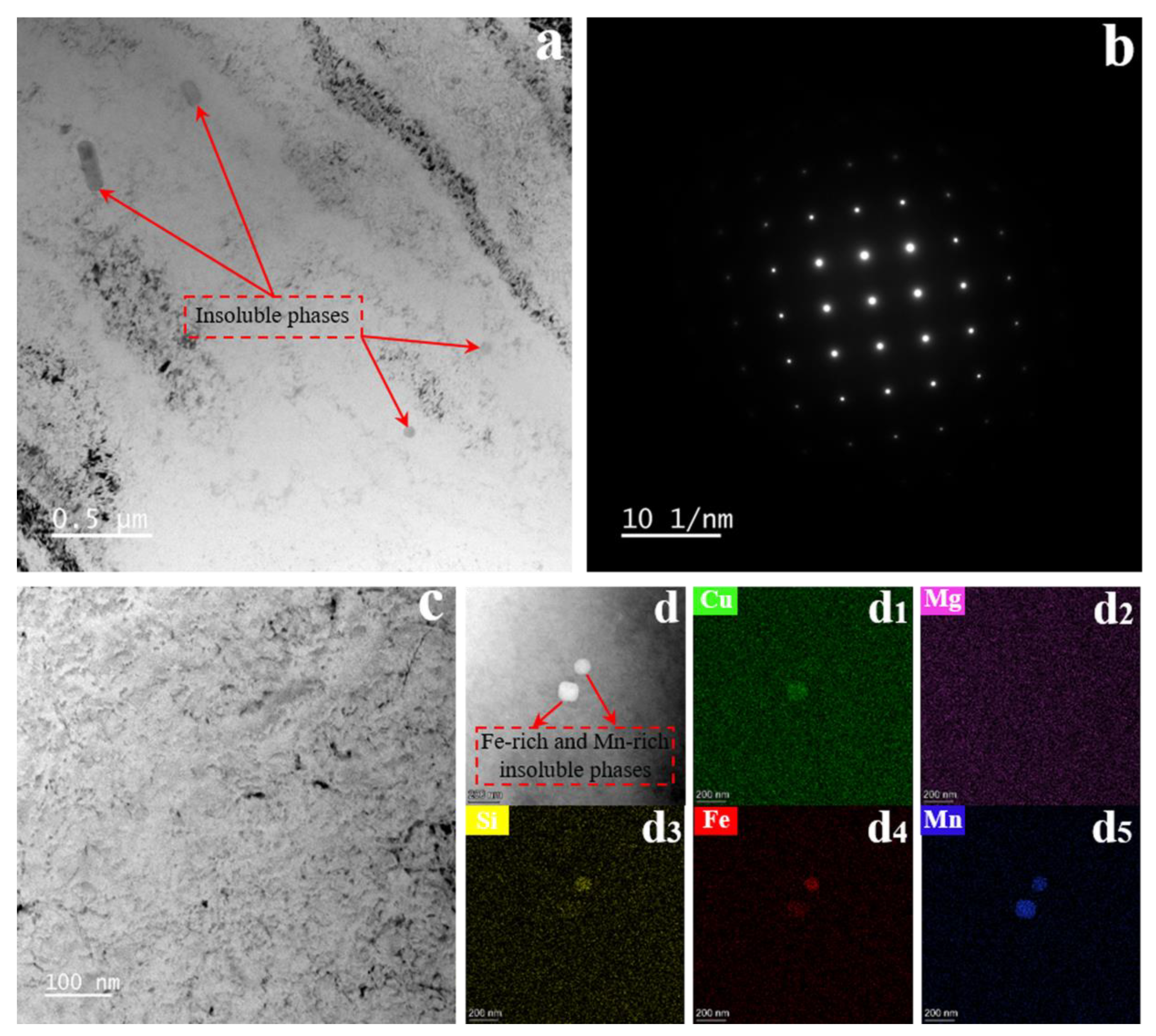
3.1. Microstructure in the Initial State
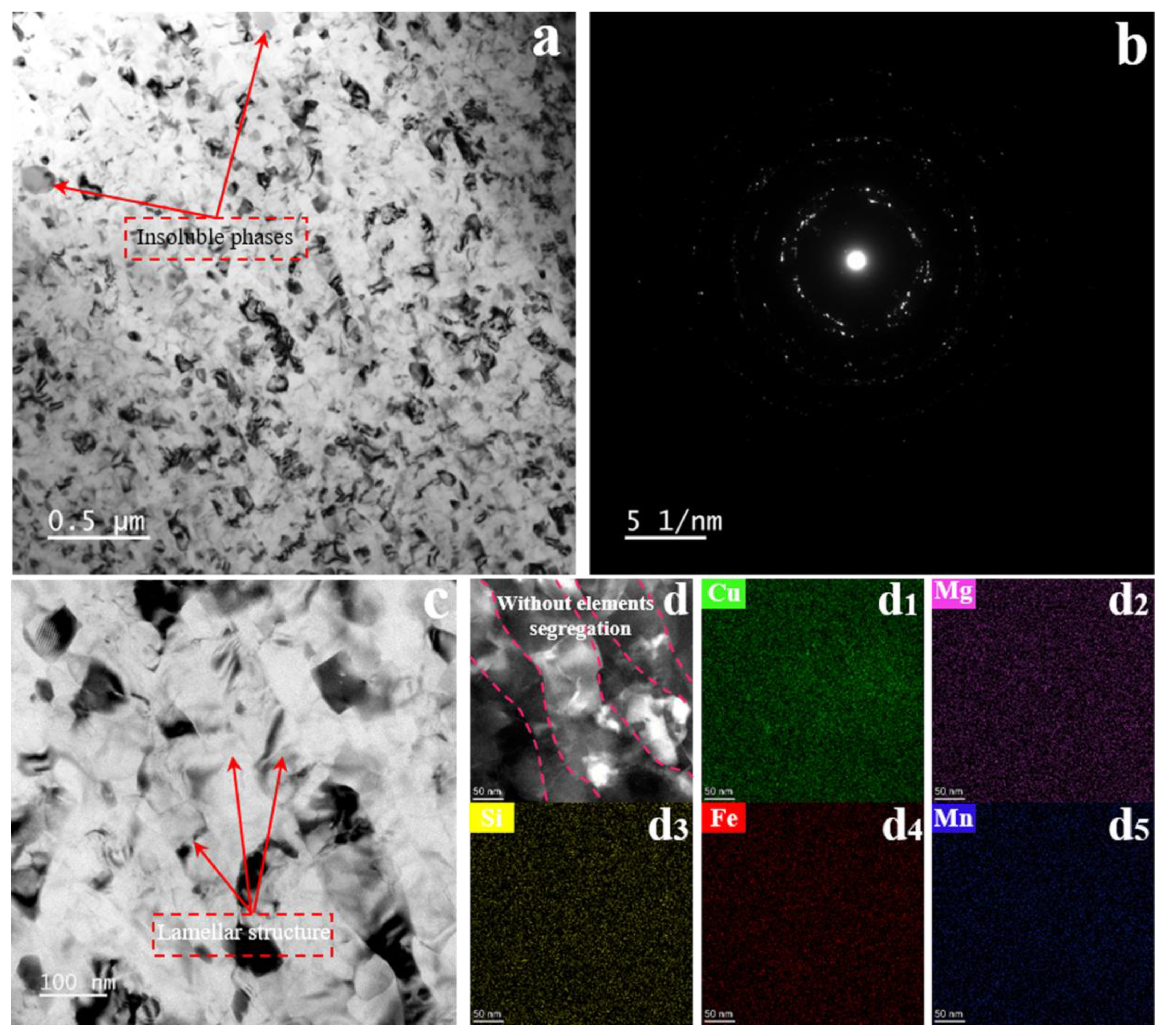
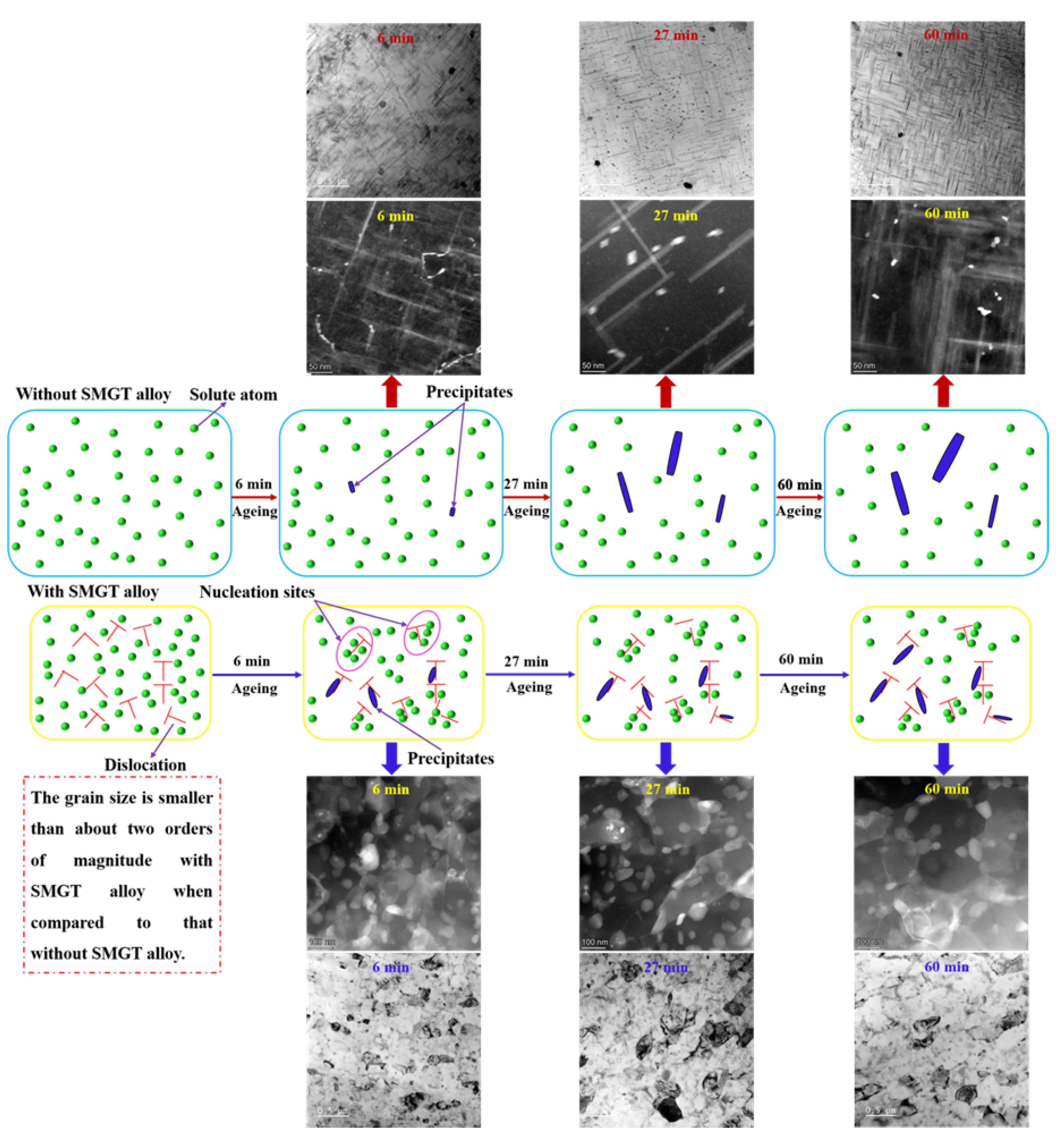
3.2. Precipitation with and without SMGT Alloys
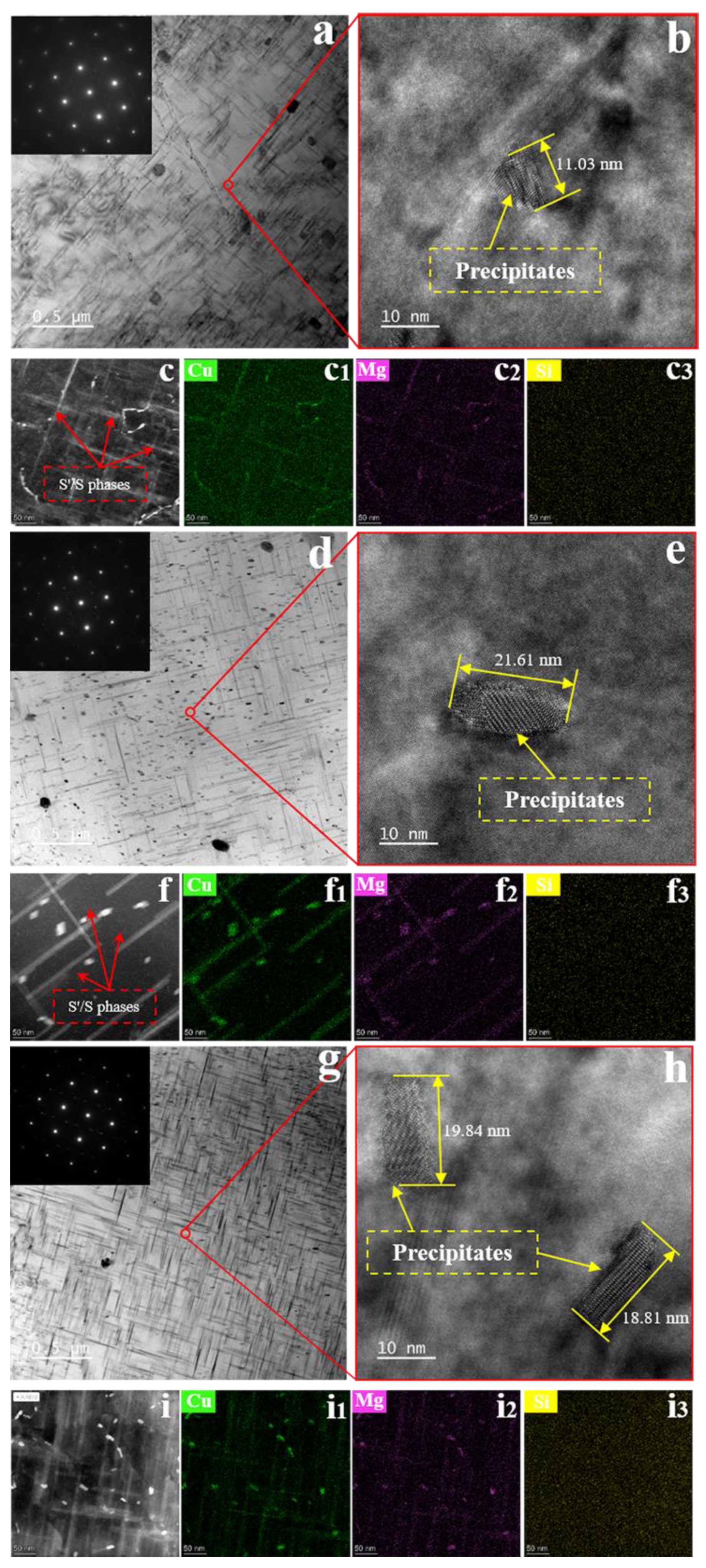
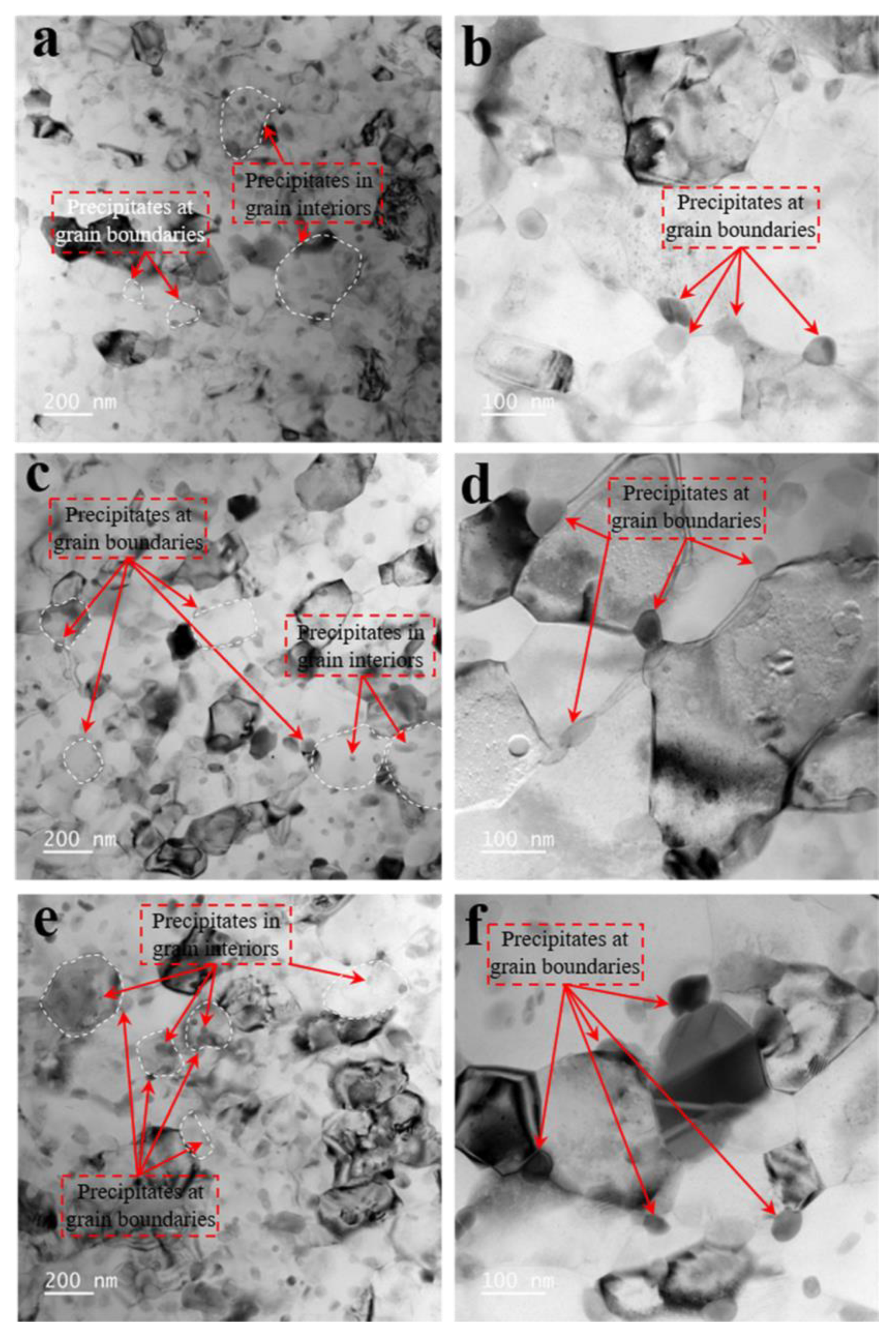
3.3. Evolution of Precipitate Size
4. Discussion
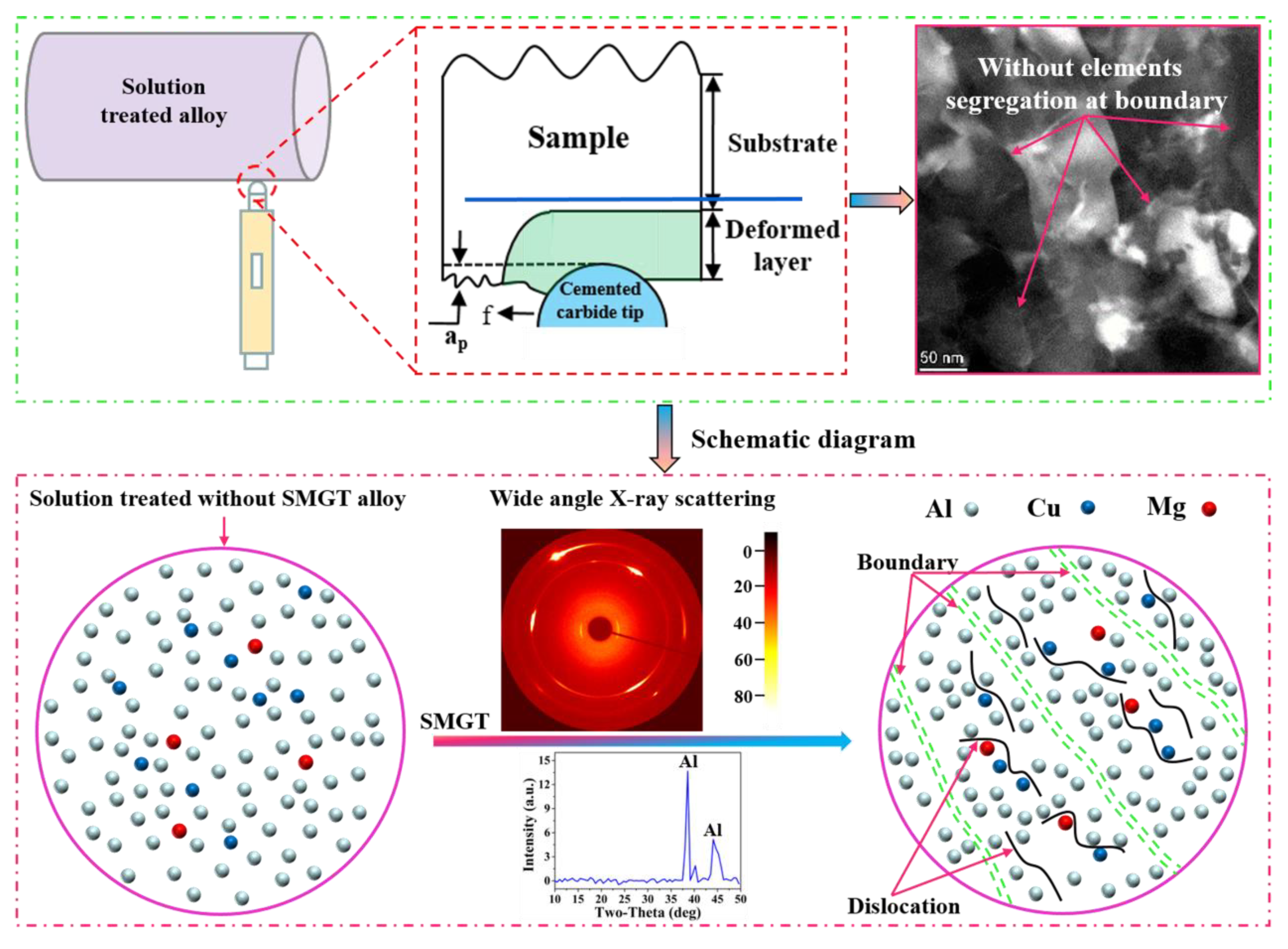
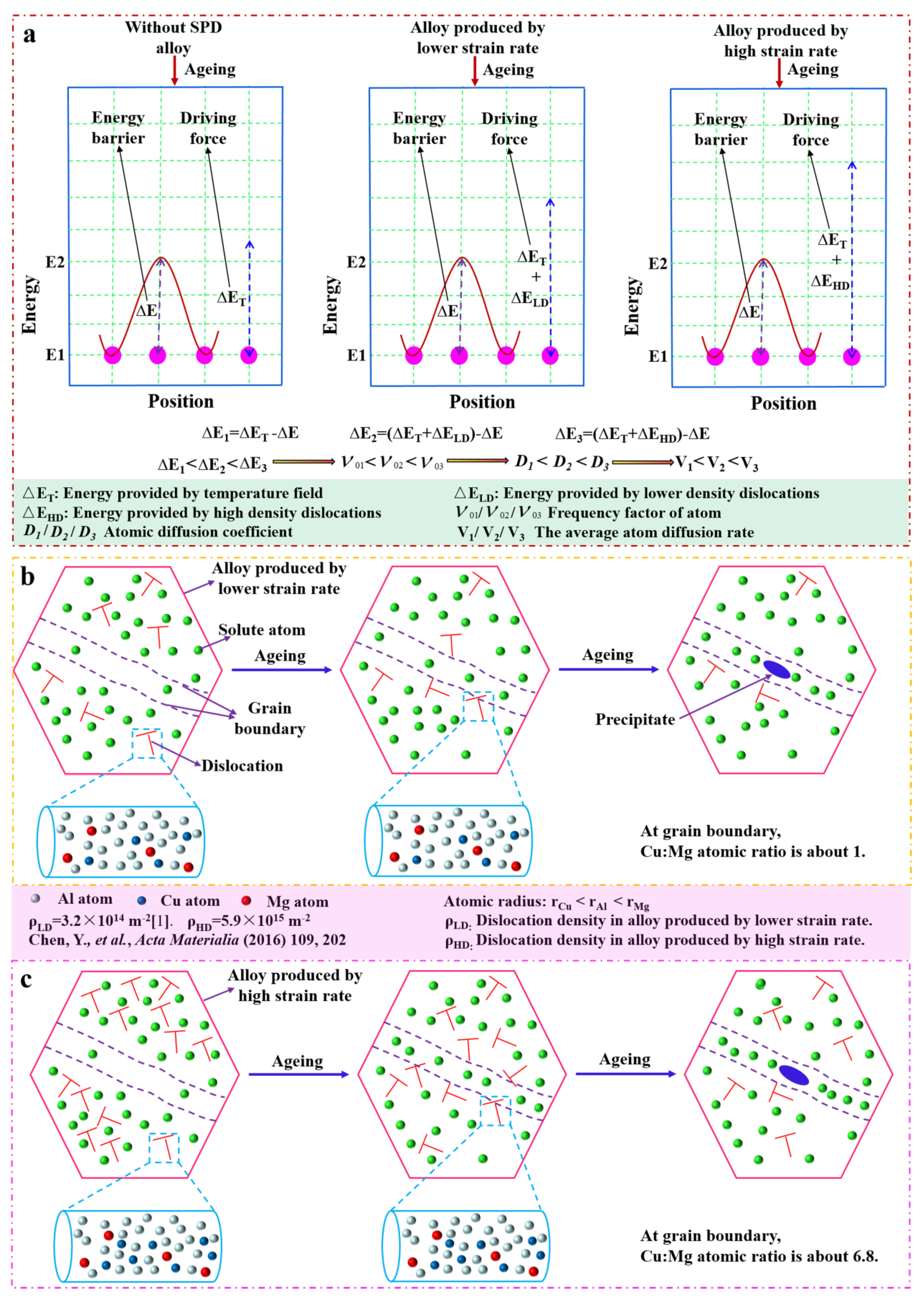
4.1. Microstructure Characteristics during SMGT
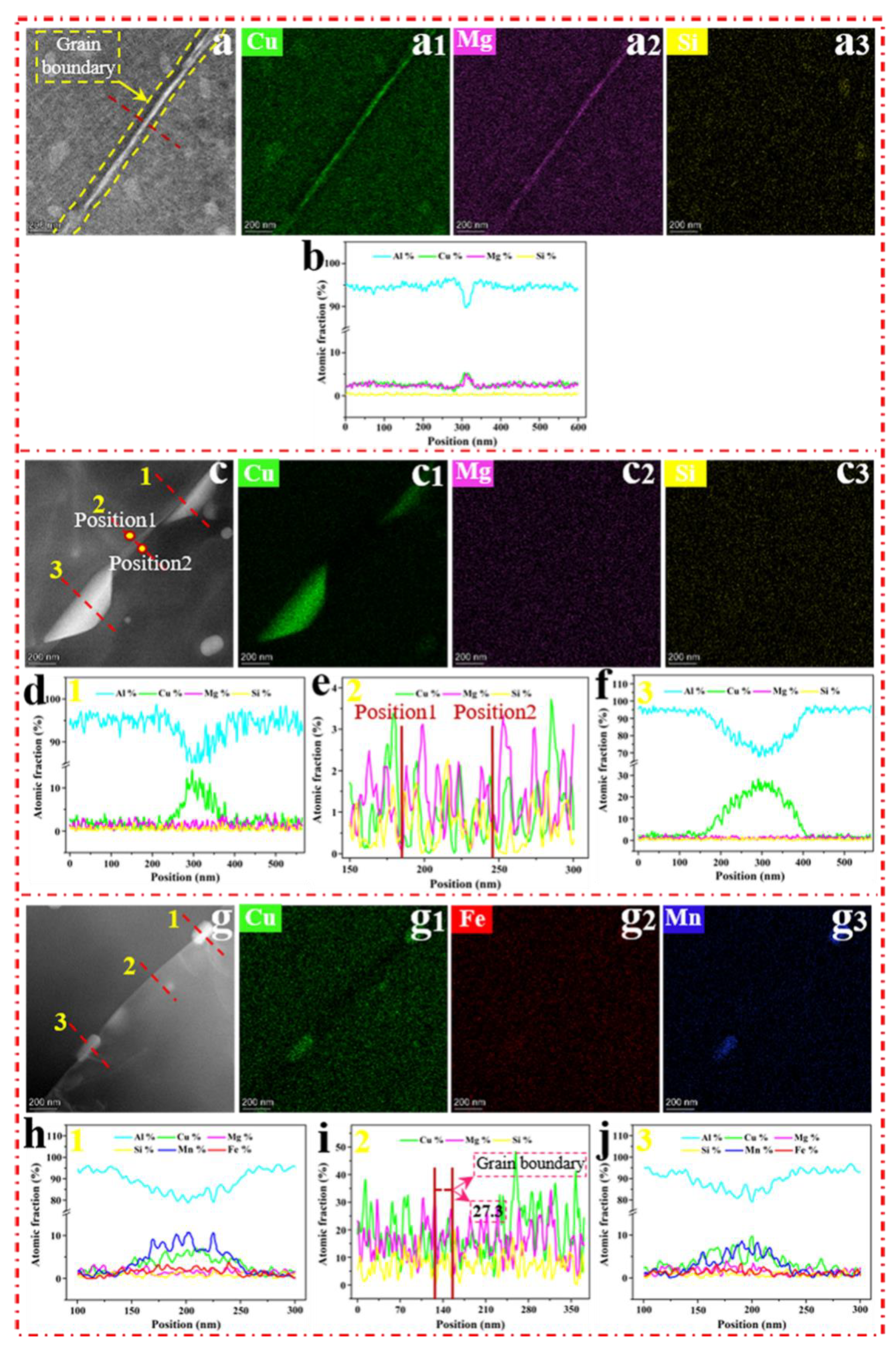
4.2. Diffusion Characteristics near the Grain Boundary after Aging
4.3. Precipitates’ Thermal Stability with SMGT Alloys
5. Conclusions
- The segregation of the elements at the boundary was rarely observed in the SMGT-processed sample before aging, since the solute atoms were hard to segregate at the boundary; segregation was impeded by dislocations produced during the SMGT processing.
- During early aging, the atomic fraction of Cu at the grain boundary was approximately 12.5% with SMGT alloys, while it was approximately 5.3% without SMGT alloys. Meanwhile, the diffusion rate of the Cu atoms from the grain toward the grain boundaries was accelerated with SMGT alloys, because Cu atoms were trapped by the dislocation core, which created a higher local elastic stress and further provided a driving force for the diffusion of Cu atoms. In addition, high-density dislocations acted as a diffusion path.
- The combined action in terms of the composition of the alloy, atomic radius, diffusion path, and diffusion driving force provided by high-density dislocations with SMGT alloys led to the Cu/Mg atomic ratio being approximately 6.8 during early aging, while stabilizing at approximately 2 during later aging at the grain boundary.
- The thermal stability of the precipitates was much higher with SMGT alloys than that without SMGT alloys. During early aging, the average sizes of the precipitates inside the grain were approximately 2 and 10 times larger than those formed after later aging with and without SMGT alloys, respectively, due to more nucleation sites at the dislocation located inside the grain having attracted and captured numerous solute atoms, and the grain size with SMGT alloys being too small to restrain the growth of the precipitates during the aging process.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, L.; Guo, Y.; Chen, L.; Zhao, G. Effects of solution and aging treatments on the microstructure and mechanical properties of cold rolled 2024 Al alloy sheet. J. Mater. Res. Technol. 2021, 12, 1126–1142. [Google Scholar] [CrossRef]
- Styles, M.J.; Marceau, R.K.W.; Bastow, T.J.; Brand, H.E.A.; Gibson, M.A.; Hutchinson, C.R. The competition between metastable and equilibrium S (Al2CuMg) phase during the decomposition of Al Cu Mg alloys. Acta Mater. 2015, 98, 64–80. [Google Scholar] [CrossRef]
- Medrano, S.; Zhao, H.; De Geuser, F.; Gault, B.; Stephenson, L.T.; Deschamps, A.; Ponge, D.; Raabe, D.; Sinclair, C.W. Cluster hardening in Al-3Mg triggered by small Cu additions. Acta Mater. 2018, 161, 12–20. [Google Scholar] [CrossRef]
- Bai, S.; Yi, X.; Liu, Z.; Wang, J.; Zhao, J.; Ying, P. The influence of preaging on the strength and precipitation behavior of a deformed Al-Cu-Mg-Ag alloy. J. Alloy Compd. 2018, 764, 62–72. [Google Scholar] [CrossRef]
- Gazizov, M.; Kaibyshev, R. Precipitation structure and strengthening mechanisms in an Al-Cu-Mg-Ag alloy. Mater. Sci. Eng. A 2017, 702, 29–40. [Google Scholar] [CrossRef]
- Gazizov, M.; Marioara, C.D.; Friis, J.; Wenner, S.; Holmestad, R.; Kaibyshev, R. Precipitation behavior in an Al–Cu–Mg–Si alloy during ageing. Mater. Sci. Eng. A 2019, 767, 138369. [Google Scholar] [CrossRef]
- Gazizov, M.R.; Boev, A.O.; Marioara, C.D.; Andersen, S.J.; Holmestad, R.; Kaibyshev, R.O.; Aksyonov, D.A.; Krasnikov, V.S. The unique hybrid precipitate in a peak-aged Al-Cu-Mg-Ag alloy. Scr. Mater. 2021, 194, 113669. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, F.; Cao, F.H.; Yin, Z.X.; Jiang, Y.; Wang, S.B.; Shen, P.K. Defective GP-zones and their evolution in an Al-Cu-Mg alloy during high-temperature aging. J. Alloy Compd. 2019, 774, 988–996. [Google Scholar] [CrossRef]
- Bahl, S.; Xiong, L.; Allard, L.F.; Michi, R.A.; Poplawsky, J.D.; Chuang, A.C.; Singh, D.; Watkins, T.R.; Shin, D.; Haynes, J.A.; et al. Aging behavior and strengthening mechanisms of coarsening resistant metastable θ’ precipitates in an Al–Cu alloy. Mater. Des. 2021, 198, 109378. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Staron, P.; Fischer, T.; Robson, J.D.; Kostka, A.; Colegrove, P.; Wang, H.; Hilgert, J.; Bergmann, L.; Hütsch, L.L.; et al. Understanding precipitate evolution during friction stir welding of Al-Zn-Mg-Cu alloy through in-situ measurement coupled with simulation. Acta Mater. 2018, 148, 163–172. [Google Scholar] [CrossRef]
- Fu, S.; Liu, H.; Qi, N.; Wang, B.; Jiang, Y.; Chen, Z.; Hu, T.; Yi, D. On the electrostatic potential assisted nucleation and growth of precipitates in Al-Cu alloy. Scr. Mater. 2018, 150, 13–17. [Google Scholar] [CrossRef]
- Gorbatov, O.I.; Stroev, A.Y.; Gornostyrev, Y.N.; Korzhavyi, P.A. Effective cluster interactions and pre–precipitate morphology in binary Al-based alloys. Acta Mater. 2019, 179, 70–84. [Google Scholar] [CrossRef]
- Gumbmann, E.; De Geuser, F.; Sigli, C.; Deschamps, A. Influence of Mg, Ag and Zn minor solute additions on the precipitation kinetics and strengthening of an Al-Cu-Li alloy. Acta Mater. 2017, 133, 172–185. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Koh, D.-H.; Kim, H.-W.; Ahn, Y.-S. Improved bake-hardening response of Al-Zn-Mg-Cu alloy through pre-aging treatment. Scr. Mater. 2018, 147, 45–49. [Google Scholar] [CrossRef]
- Chung, T.-F.; Yang, Y.-L.; Huang, B.-M.; Shi, Z.; Lin, J.; Ohmura, T.; Yang, J.-R. Transmission electron microscopy investigation of separated nucleation and in-situ nucleation in AA7050 aluminium alloy. Acta Mater. 2018, 149, 377–387. [Google Scholar] [CrossRef]
- Fallah, V.; Langelier, B.; Ofori-Opoku, N.; Raeisinia, B.; Provatas, N.; Esmaeili, S. Cluster evolution mechanisms during aging in Al–Mg–Si alloys. Acta Mater. 2016, 103, 290–300. [Google Scholar] [CrossRef]
- Miyoshi, H.; Kimizuka, H.; Ishii, A.; Ogata, S. Temperature-dependent nucleation kinetics of Guinier-Preston zones in Al–Cu alloys: An atomistic kinetic Monte Carlo and classical nucleation theory approach. Acta Mater. 2019, 179, 262–272. [Google Scholar] [CrossRef]
- Yi, G.; Littrell, K.C.; Poplawsky, J.D.; Cullen, D.A.; Sundberg, E.; Free, M.L. Characterization of the effects of different tempers and aging temperatures on the precipitation behavior of Al-Mg (5.25at.%)-Mn alloys. Mater. Des. 2017, 118, 22–35. [Google Scholar] [CrossRef]
- Ivanov, R.; Deschamps, A.; De Geuser, F. High throughput evaluation of the effect of Mg concentration on natural ageing of Al-Cu-Li-(Mg) alloys. Scr. Mater. 2018, 150, 156–159. [Google Scholar] [CrossRef]
- Zhang, F.; Levine, L.E.; Allen, A.J.; Campbell, C.E.; Creuziger, A.A.; Kazantseva, N.; Ilavsky, J. In situ structural characterization of ageing kinetics in aluminum alloy 2024 across angstrom-to-micrometer length scales. Acta Mater. 2016, 111, 385–398. [Google Scholar] [CrossRef]
- Lin, Y.C.; Xia, Y.-C.; Jiang, Y.-Q.; Li, L.-T. Precipitation in Al–Cu–Mg alloy during creep exposure. Mater. Sci. Eng. A 2012, 556, 796–800. [Google Scholar] [CrossRef]
- Lin, Y.C.; Xia, Y.-C.; Jiang, Y.-Q.; Zhou, H.-M.; Li, L.-T. Precipitation hardening of 2024-T3 aluminum alloy during creep aging. Mater. Sci. Eng. A 2013, 565, 420–429. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y.C.; Zhang, X.-C.; Jiang, Y.-Q. Effects of two-stage creep-aging on precipitates of an Al–Cu–Mg alloy. Mater. Sci. Eng. A 2014, 614, 45–53. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, N.; Sha, G.; Ringer, S.P.; Starink, M.J. Microstructural evolution, strengthening and thermal stability of an ultrafine-grained Al–Cu–Mg alloy. Acta Mater. 2016, 109, 202–212. [Google Scholar] [CrossRef]
- Huang, T.; Shuai, L.; Wakeel, A.; Wu, G.; Hansen, N.; Huang, X. Strengthening mechanisms and Hall-Petch stress of ultrafine grained Al-0.3%Cu. Acta Mater. 2018, 156, 369–378. [Google Scholar] [CrossRef]
- Huang, Y.; Robson, J.D.; Prangnell, P.B. The formation of nanograin structures and accelerated room-temperature theta precipitation in a severely deformed Al–4wt.% Cu alloy. Acta Mater. 2010, 58, 1643–1657. [Google Scholar] [CrossRef]
- Jia, H.; Bjørge, R.; Cao, L.; Song, H.; Marthinsen, K.; Li, Y. Quantifying the grain boundary segregation strengthening induced by post-ECAP aging in an Al-5Cu alloy. Acta Mater. 2018, 155, 199–213. [Google Scholar] [CrossRef]
- Chrominski, W.; Lewandowska, M. Precipitation phenomena in ultrafine grained Al–Mg–Si alloy with heterogeneous microstructure. Acta Mater. 2016, 103, 547–557. [Google Scholar] [CrossRef]
- Pandey, V.; Chattopadhyay, K.; Srinivas, N.C.S.; Singh, V. Thermal and microstructural stability of nanostructured surface of the aluminium alloy 7075. Mater. Charact. 2019, 151, 242–251. [Google Scholar] [CrossRef]
- Zuo, J.; Hou, L.; Shi, J.; Cui, H.; Zhuang, L.; Zhang, J. Effect of deformation induced precipitation on grain refinement and improvement of mechanical properties AA 7055 aluminum alloy. Mater. Charact. 2017, 130, 123–134. [Google Scholar] [CrossRef]
- Xu, P.; Luo, H.; Han, Z.; Zou, J. Tailoring a gradient nanostructured age-hardened aluminum alloy using high-gradient strain and strain rate. Mater. Des. 2015, 85, 240–247. [Google Scholar] [CrossRef]
- Duchaussoy, A.; Sauvage, X.; Deschamps, A.; De Geuser, F.; Renou, G.; Horita, Z. Complex interactions between precipitation, grain growth and recrystallization in a severely deformed Al-Zn-Mg-Cu alloy and consequences on the mechanical behavior. Materialia 2021, 15, 101028. [Google Scholar] [CrossRef]
- Wang, S.-S.; Jiang, J.-T.; Fan, G.-H.; Panindre, A.M.; Frankel, G.S.; Zhen, L. Accelerated precipitation and growth of phases in an Al-Zn-Mg-Cu alloy processed by surface abrasion. Acta Mater. 2017, 131, 233–245. [Google Scholar] [CrossRef]
- Deschamps, A.; De Geuser, F.; Horita, Z.; Lee, S.; Renou, G. Precipitation kinetics in a severely plastically deformed 7075 aluminium alloy. Acta Mater. 2014, 66, 105–117. [Google Scholar] [CrossRef]
- Dhal, A.; Panigrahi, S.K.; Shunmugam, M.S. Precipitation phenomena, thermal stability and grain growth kinetics in an ultra-fine grained Al 2014 alloy after annealing treatment. J. Alloy Compd. 2015, 649, 229–238. [Google Scholar] [CrossRef]
- Zuiko, I.; Kaibyshev, R. Effect of plastic deformation on the ageing behaviour of an Al–Cu–Mg alloy with a high Cu/Mg ratio. Mater. Sci. Eng. A 2018, 737, 401–412. [Google Scholar] [CrossRef]
- Yang, X.; Li, K.; An, X.; Ni, S.; Wei, W.; Du, Y.; Song, M. Influence of deformation microstructure on the precipitation behaviors of an Al-4Mg-0.3Cu alloy. J. Alloy Compd. 2017, 695, 2238–2245. [Google Scholar] [CrossRef]
- Jiang, F.; Tang, L.; Huang, J.; Cai, Y.; Yin, Z. Influence of equal channel angular pressing on the evolution of microstructures, aging behavior and mechanical properties of as-quenched Al-6.6Zn-1.25Mg alloy. Mater. Charact. 2019, 153, 1–13. [Google Scholar] [CrossRef]
- Majchrowicz, K.; Pakieła, Z.; Chrominski, W.; Kulczyk, M. Enhanced strength and electrical conductivity of ultrafine-grained Al-Mg-Si alloy processed by hydrostatic extrusion. Mater. Charact. 2018, 135, 104–114. [Google Scholar] [CrossRef]
- Sun, Q.; Han, Q. Surface segregation phenomenon of surface severe plastic deformed Al–Zn–Mg–Cu alloys. Materialia 2020, 11, 100741. [Google Scholar] [CrossRef]
- Chrominski, W.; Kulczyk, M.; Lewandowska, M.; Kurzydlowski, K.J. Precipitation strengthening of ultrafine-grained Al–Mg–Si alloy processed by hydrostatic extrusion. Mater. Sci. Eng. A 2014, 609, 80–87. [Google Scholar] [CrossRef]
- Zuiko, I.S.; Kaibyshev, R. Ageing response of cold-rolled Al–Cu–Mg alloy. Mater. Sci. Eng. A 2020, 781, 139148. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Yang, Z.Q.; Zhang, Z.; Su, G.Y.; Ma, X.L. Double-peak age strengthening of cold-worked 2024 aluminum alloy. Acta Mater. 2013, 61, 1624–1638. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.K.; Cheng, P.M.; Liu, G.; Wang, R.H.; Chen, B.A.; Zhang, J.Y.; Sun, J.; Yang, M.X.; Yang, G. Microalloying ultrafine grained Al alloys with enhanced ductility. Sci. Rep. 2014, 4, 3605. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, X.; Enikeev, N.; Valiev, R.; Nasedkina, Y.; Murashkin, M. Atomic-scale analysis of the segregation and precipitation mechanisms in a severely deformed Al–Mg alloy. Acta Mater. 2014, 72, 125–136. [Google Scholar] [CrossRef]
- Hu, T.; Ma, K.; Topping, T.D.; Schoenung, J.M.; Lavernia, E.J. Precipitation phenomena in an ultrafine-grained Al alloy. Acta Mater. 2013, 61, 2163–2178. [Google Scholar] [CrossRef]
- Liu, X.C.; Zhang, H.W.; Lu, K. Formation of nano-laminated structure in nickel by means of surface mechanical grinding treatment. Acta Mater. 2015, 96, 24–36. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Du, K.; Li, X.Y.; Lu, K. Thermally stable nanostructured Al-Mg alloy with relaxed grain boundaries. Acta Mater. 2022, 226, 117640. [Google Scholar] [CrossRef]
- Wang, J.J.; Tao, N.R.; Lu, K. Revealing the deformation mechanisms of nanograins in gradient nanostructured Cu and CuAl alloys under tension. Acta Mater. 2019, 180, 231–242. [Google Scholar] [CrossRef]
- Wang, P.F.; Han, Z.; Lu, K. Enhanced tribological performance of a gradient nanostructured interstitial-free steel. Wear 2018, 402–403, 100–108. [Google Scholar] [CrossRef]
- Han, K.; Li, X.; Liu, X.; Li, Y.; Li, D. Bending compensated surface mechanical grinding treatment overcoming the strength-ductility trade-off in thin copper sheet. Mater. Sci. Eng. A 2022, 832, 142391. [Google Scholar] [CrossRef]
- Gray, G.T. High-Strain-Rate Deformation: Mechanical Behavior and Deformation Substructures Induced. Annu. Rev. Mater. Res. 2012, 42, 285–303. [Google Scholar] [CrossRef]
- Ren, X.; Chen, G.-Q.; Zhou, W.-L.; Wu, C.-W.; Zhang, J.-S. Effect of high magnetic field on intermetallic phase growth in Ni–Al diffusion couples. J. Alloy Compd. 2009, 472, 525–529. [Google Scholar] [CrossRef]
- Luo, J.; Luo, H.; Liu, C.; Zhao, T.; Wang, R.; Ma, Y. Effect of magnetic field on precipitation kinetics of an ultrafine grained Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2020, 798, 139990. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Yang, Y.Q.; Huang, B.; Luo, X.; Li, M.H.; Han, M.; Fu, M.S. Variant selection and the strengthening effect of S precipitates at dislocations in Al–Cu–Mg alloy. Acta Mater. 2011, 59, 2412–2422. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Luo, H. Precipitation Behavior during Aging Operations in an Ultrafine-Grained Al–Cu–Mg Alloy Produced by High-Strain-Rate Processing. Materials 2022, 15, 8687. https://doi.org/10.3390/ma15238687
Zhang L, Luo H. Precipitation Behavior during Aging Operations in an Ultrafine-Grained Al–Cu–Mg Alloy Produced by High-Strain-Rate Processing. Materials. 2022; 15(23):8687. https://doi.org/10.3390/ma15238687
Chicago/Turabian StyleZhang, Linyan, and Hongyun Luo. 2022. "Precipitation Behavior during Aging Operations in an Ultrafine-Grained Al–Cu–Mg Alloy Produced by High-Strain-Rate Processing" Materials 15, no. 23: 8687. https://doi.org/10.3390/ma15238687
APA StyleZhang, L., & Luo, H. (2022). Precipitation Behavior during Aging Operations in an Ultrafine-Grained Al–Cu–Mg Alloy Produced by High-Strain-Rate Processing. Materials, 15(23), 8687. https://doi.org/10.3390/ma15238687




