Synthesis and Structure of 2-Hydroxypropyl Methacrylate-Capped Isophorone Diisocyanate and Poly(Propylene Glycol) Urethane Mixtures and the Properties of their UV-Cured Co-Networks with Isobornyl Methacrylate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
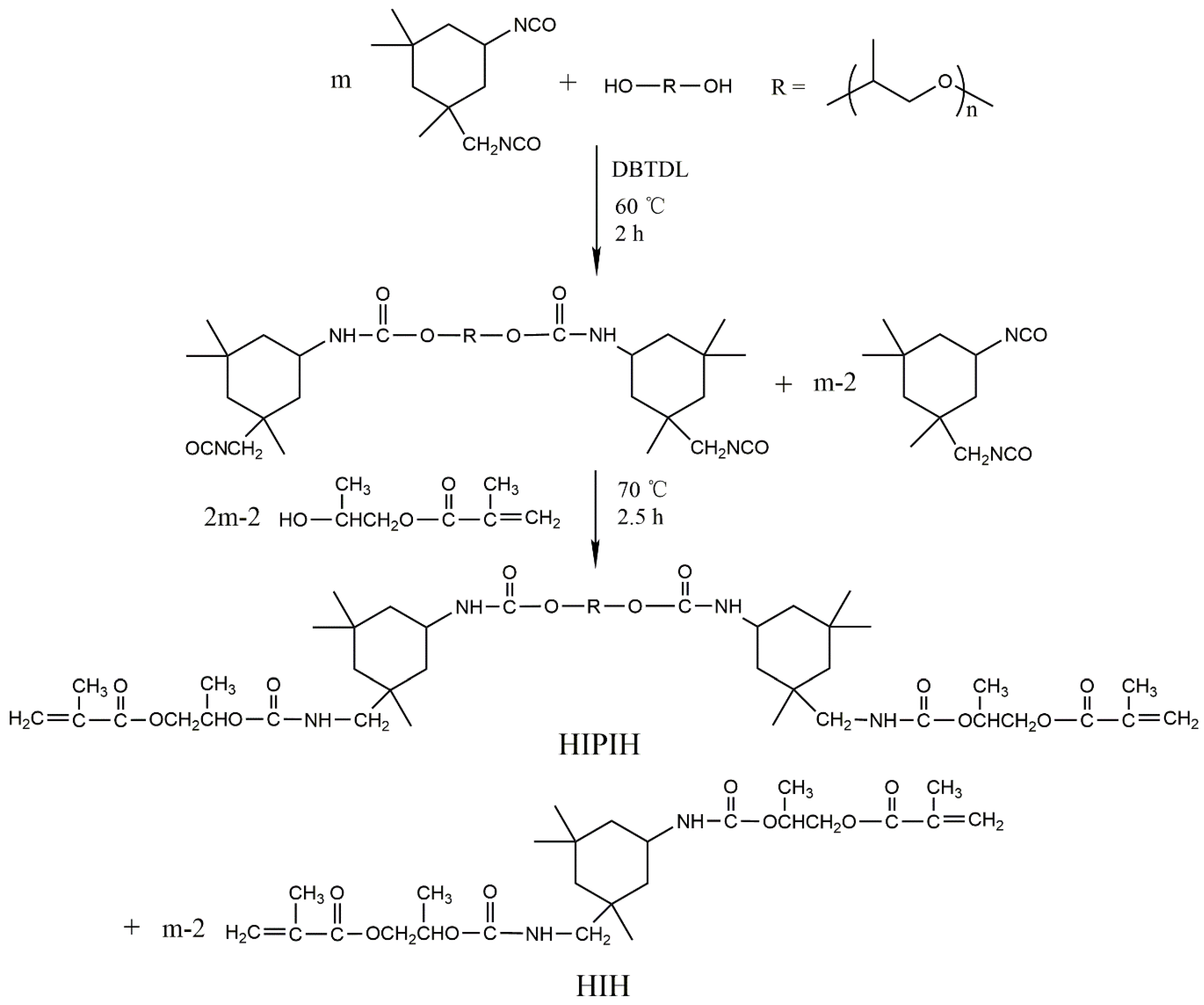
2.2. Preparation of Prepolymer Resins
2.3. Preparation of Cured Materials
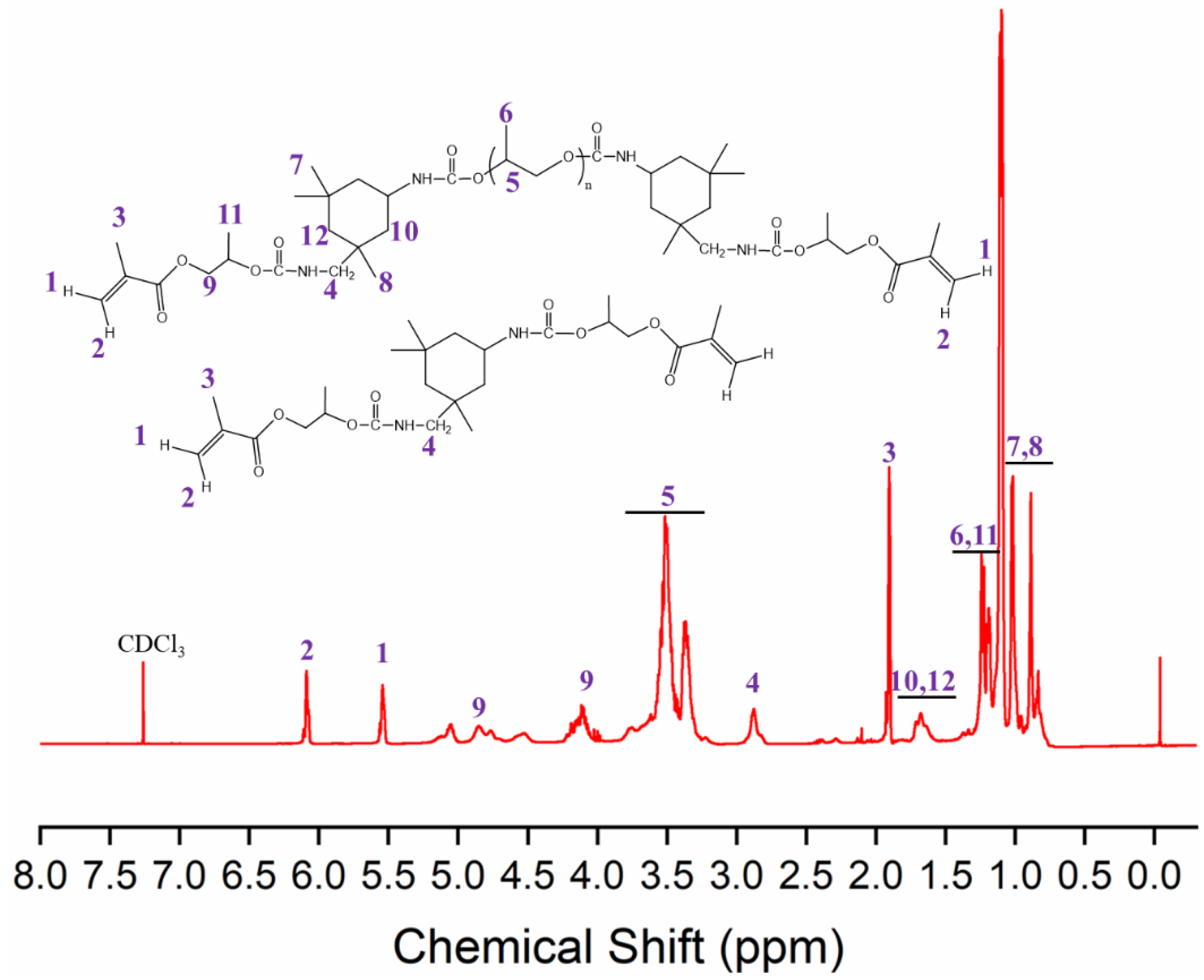
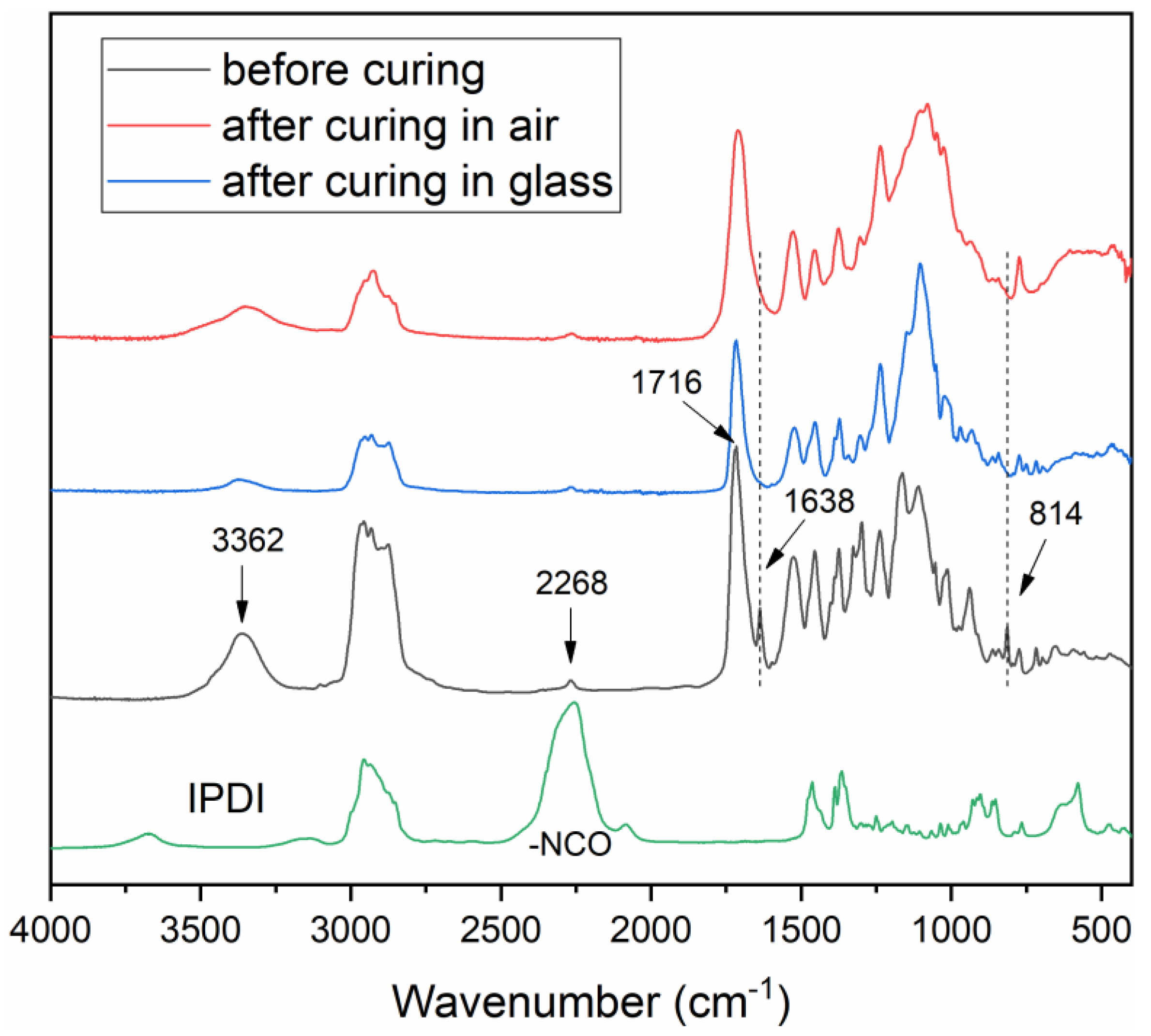
2.4. Characterization
2.5. Analysis on Properties
3. Results and Discussion
3.1. Synthesis, UV Curing, and Characterization
3.2. Performances of Cured Materials
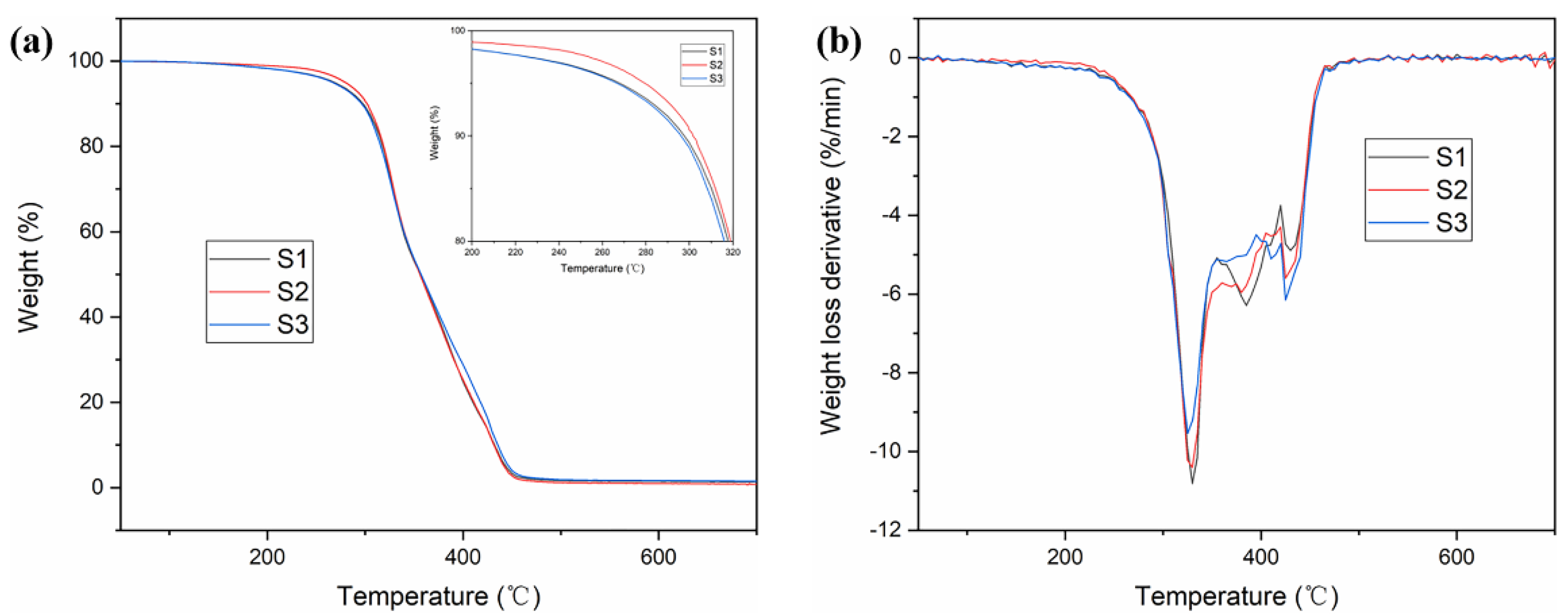
3.3. Thermo Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, M.; Hirner, S.; Wiesbrock, F.; Fuchs, P. A Review on Modeling Cure Kinetics and Mechanisms of Photopolymerization. Polymers 2022, 14, 2074. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the development of polymeric and multifunctional photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, W.; Yang, Y.; Wu, Y.; Wang, C.; Wu, X.; Zhang, X.; Yuan, Y.; Tang, Y.; Chen, Y. High-Performance and Highly Safe Solvate Ionic Liquid-Based Gel Polymer Electrolyte by Rapid UV-Curing for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 43397–43406. [Google Scholar] [CrossRef]
- Czachor-Jadacka, D.; Pilch-Pitera, B. Progress in development of UV curable powder coatings. Prog. Org. Coat. 2021, 158, 106355. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Wu, Y.; Guan, T.; Sun, N.; Ren, B. Self-healing UV light-curable resins containing disulfide group: Synthesis and application in UV coatings. Prog. Org. Coat. 2019, 133, 289–298. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liang, H.B.; Yang, H.T.; Xiong, L.; Zhou, J.P.; Huang, S.M.; Zhao, C.H.; Zhong, J.; Fan, X.F. UV-curable self-healing polyurethane coating based on thiol-ene and Diels-Alder double click reactions. Prog. Org. Coat. 2019, 137, 105282. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Arafa, A.A.; Zahran, M.K.; Abdou, L.A.W. Characterisation and application of pigmented UV-curable inkjet inks. Pigment. Resin Technol. 2018, 47, 164–172. [Google Scholar] [CrossRef]
- Saleh, E.; Woolliams, P.; Clarke, B.; Gregory, A.; Greedy, S.; Smartt, C.; Wildman, R.; Ashcroft, I.; Hague, R.; Dickens, P.; et al. 3D inkjet-printed UV-curable inks for multi-functional electromagnetic applications. Addit. Manuf. 2017, 13, 143–148. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Kowalczyk, A. UV-curable epoxy varnishes modified with polyvinyl resins. Prog. Org. Coat. 2015, 89, 100–105. [Google Scholar] [CrossRef]
- Feng, X.; Li, G. UV curable, flame retardant, and pressure-sensitive adhesives with two-way shape memory effect. Polymer 2022, 249, 124835. [Google Scholar] [CrossRef]
- Lim, W.B.; Bae, J.H.; Seo, M.J.; Min, J.G.; Lee, J.H.; Jung, Y.S.; Huh, P. A novel UV-curable acryl-polyurethane for flexural 3D printing architectures. Addit. Manuf. 2022, 51, 102625. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Zhang, L.; Protsak, I.; Jamali, R.; Shu, Y.; Pal, P.; Wang, Z.; Yang, J.; Zhang, D. Fast-cured UV-LED polymer materials filled with high mineral contents as wear-resistant, antibacterial coatings. Chem. Eng. J. 2020, 382, 122927. [Google Scholar] [CrossRef]
- Noe, C.; Iannucci, L.; Malburet, S.; Graillot, A.; Sangermano, M.; Grassini, S. New UV-Curable Anticorrosion Coatings from Vegetable Oils. Macromol. Mater. Eng. 2021, 306, 2100029. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Linden, L.A.; Rabek, J.F. Modelling the kinetics of photoinitiated polymerization of di(meth)acrylates. Polym. Int. 1997, 42, 179–187. [Google Scholar] [CrossRef]
- Hermann, A.; Burr, D.; Landry, V. Comparative study of the impact of additives against oxygen inhibition on pendulum hardness and abrasion resistance for UV-curable wood finishes. Prog. Org. Coat. 2021, 156, 106266. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husar, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to Reduce Oxygen Inhibition in Photoinduced Polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, B.; Wei, X.; Shen, H. Study on Shrinkage of Cured Volume for UV-Curing Coatings. Appl. Mech. Mater. 2015, 731, 588–592. [Google Scholar] [CrossRef]
- Decker, C. New developments in UV radiation curing of protective coatings. Surf. Coat. Int. Part B Coat. Trans. 2005, 88, 9–17. [Google Scholar] [CrossRef]
- Malik, M.S.; Schloegl, S.; Wolfahrt, M.; Sangermano, M. Review on UV-Induced Cationic Frontal Polymerization of Epoxy Monomers. Polymers 2020, 12, 2146. [Google Scholar] [CrossRef]
- Fu, J.; Wang, L.; Yu, H.; Haroon, M.; Haq, F.; Shi, W.; Wu, B.; Wang, L. Research progress of UV-curable polyurethane acrylate-based hardening coatings. Prog. Org. Coat. 2019, 131, 82–99. [Google Scholar] [CrossRef]
- Jiao, X.; Liu, J.; Jin, J.; Cheng, F.; Fan, Y.; Zhang, L.; Lai, G.; Hua, X.; Yang, X. UV-cured transparent silicone materials with high tensile strength prepared from hyperbranched silicon-containing polymers and polyurethane-acrylates. ACS Omega 2021, 6, 2890–2898. [Google Scholar] [CrossRef]
- Li, P.; Chu, Z.; Chen, Y.; Yuan, T.; Yang, Z. One-pot and solvent-free synthesis of castor oil-based polyurethane acrylate oligomers for UV-curable coatings applications. Prog. Org. Coat. 2021, 159, 106398. [Google Scholar] [CrossRef]
- Cheng, F.; Fan, Y.; He, N.; Song, Y.; Shen, J.; Gong, Z.; Tong, X.; Yang, X. Castor oil based high transparent UV cured silicone modified polyurethane acrylate coatings with outstanding tensile strength and good chemical resistance. Prog. Org. Coat. 2022, 163, 106624. [Google Scholar] [CrossRef]
- Noreen, A.; Mahmood, S.; Khalid, A.; Takriff, S.; Anjum, M.; Riaz, L.; Ditta, A.; Mahmood, T. Synthesis and characterization of bio-based UV curable polyurethane coatings from algal biomass residue. Biomass Convers. Biorefin. 2022, 1–17. [Google Scholar] [CrossRef]
- Czech, Z.; Kabatc, J.; Bartkowiak, M.; Mozelewska, K.; Kwiatkowska, D. Influence of an Alkoxylation Grade of Acrylates on Shrinkage of UV-Curable Compositions. Polymers 2020, 12, 2617. [Google Scholar] [CrossRef]
- Pasztor, S.; Becsei, B.; Szarka, G.; Thomann, Y.; Thomann, R.; Muehlhaupt, R.; Ivan, B. The Scissors Effect in Action: The Fox-Flory Relationship between the Glass Transition Temperature of Crosslinked Poly(Methyl Methacrylate) and Mc in Nanophase Separated Poly(Methyl Methacrylate)-l-Polyisobutylene Conetworks. Materials 2020, 13, 4822. [Google Scholar] [CrossRef]
- Fodor, C.; Stumphauser, T.; Thomann, R.; Thomann, Y.; Ivan, B. Poly(N-vinylimidazole)-l-poly(propylene glycol) amphiphilic conetworks and gels: Molecularly forced blends of incompatible polymers with single glass transition temperatures of unusual dependence on the composition. Polym. Chem. 2016, 7, 5375–5385. [Google Scholar] [CrossRef]
- Stumphauser, T.; Kasza, G.; Domjan, A.; Wacha, A.; Varga, Z.; Thomann, Y.; Thomann, R.; Pasztoi, B.; Troetschler, T.M.; Kerscher, B.; et al. Nanoconfined Crosslinked Poly(ionic liquid)s with Unprecedented Selective Swelling Properties Obtained by Alkylation in Nanophase-Separated Poly(1-vinylimidazole)-l-poly(tetrahydrofuran) Conetworks. Polymers 2020, 12, 2292. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Tang, J.; Zhang, J.; Wang, C.; Shang, Q.; Feng, G.; Liu, C.; Zhou, Y.; Lei, W. High-performance soybean-oil-based epoxy acrylate resins:“Green” synthesis and application in UV-curable coatings. ACS Sustain. Chem. Eng. 2018, 6, 8340–8349. [Google Scholar] [CrossRef]
- Prabhakar, A.; Chattopadhyay, D.; Jagadeesh, B.; Raju, K. Structural investigations of polypropylene glycol (PPG) and isophorone diisocyanate (IPDI)-based polyurethane prepolymer by 1D and 2D NMR spectroscopy. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1196–1209. [Google Scholar] [CrossRef]
- Romero-Sabat, G.; Angel Granda, L.; Medel, S. Synthesis of UV-curable polyurethane-acrylate hybrids with tuneable hardness and viscoelastic properties on-demand. Mater. Adv. 2022, 3, 5118–5130. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Zhang, T.; Dai, Y.; Yang, D.; Qiu, F.; Yu, Z.; Yang, P. Synthesis of UV-curing waterborne polyurethane-acrylate coating and its photopolymerization kinetics using FT-IR and photo-DSC methods. Prog. Org. Coat. 2018, 122, 10–18. [Google Scholar] [CrossRef]
- Yang, Z.; Shan, J.; Huang, Y.; Dong, X.; Zheng, W.; Jin, Y.; Zhou, W. Preparation and mechanism of free-radical/cationic hybrid photosensitive resin with high tensile strength for three-dimensional printing applications. J. Appl. Polym. Sci. 2021, 138, 49881. [Google Scholar] [CrossRef]
- Fei, M.; Liu, T.; Zhao, B.; Otero, A.; Chang, Y.-C.; Zhang, J. From Glassy Plastic to Ductile Elastomer: Vegetable Oil-Based UV-Curable Vitrimers and Their Potential Use in 3D Printing. ACS Appl. Polym. Mater. 2021, 3, 2470–2479. [Google Scholar] [CrossRef]
- Sun, B.; Yang, K.; Lei, Q.; Shi, H.; Li, Y.; Hu, N.; Fu, S. High residual mechanical properties at elevated temperatures of carbon fiber/acetylene-functional benzoxazine composite. Compos. Part A Appl. Sci. Manuf. 2018, 112, 11–17. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, H.; She, Y.; Jiang, S.; Cao, M.; Zhai, L.; Jiang, S. Synthesis, characterization, and properties of acrylate-modified tung-oil waterborne insulation varnish. J. Appl. Polym. Sci. 2015, 132, 41608. [Google Scholar] [CrossRef]




| Sample | PPG (g; mmol) | IPDI (g; mmol) | HPMA (g; mmol) | IBOMA (g; mmol) | PI 1173 (g) | HQ (g) | DBTDL (g) | Viscosity (cp) |
|---|---|---|---|---|---|---|---|---|
| S1 | 41.241 | 18.311 | 11.877 | 28.571 | 5 | 0.05 | 0.1 | 2700 |
| 41.241 | 82.482 | 82.479 | 128.70 | |||||
| S2 | 35.948 | 19.951 | 15.530 | 28.571 | 5 | 0.05 | 0.1 | 3000 |
| 35.948 | 89.869 | 107.847 | 128.70 | |||||
| S3 | 31.859 | 21.218 | 18.351 | 28.571 | 5 | 0.05 | 0.1 | 3100 |
| 31.859 | 95.577 | 127.438 | 128.70 |
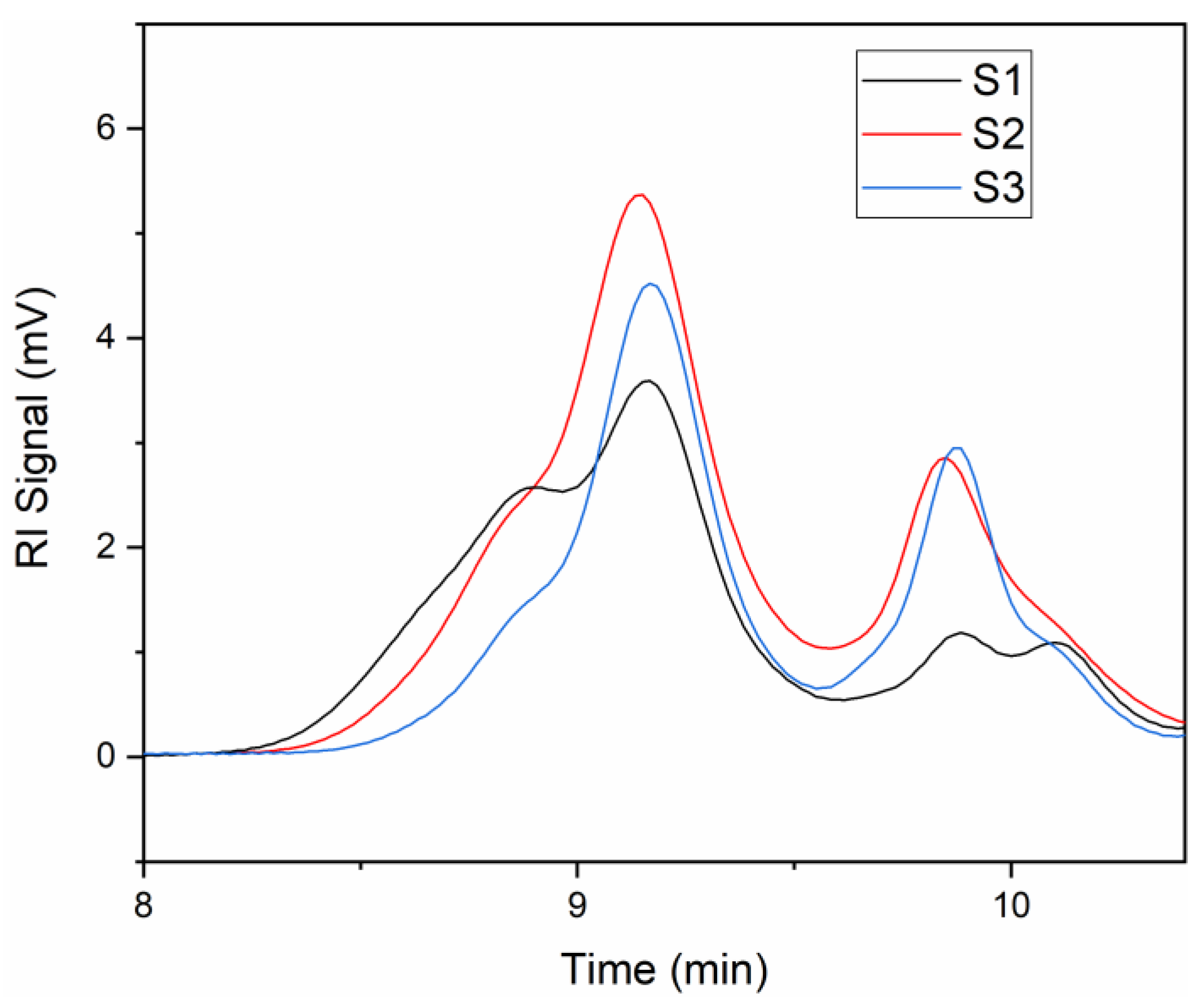
| Sample | Results at Short Retention Time | Results at Long Retention Time | ||||
|---|---|---|---|---|---|---|
| PDI | PDI | |||||
| S1 | 3060 | 4532 | 1.48 | -- | -- | -- |
| S2 | 2911 | 4071 | 1.40 | 608 | 633 | 1.04 |
| S3 | 2578 | 3198 | 1.24 | 587 | 633 | 1.08 |
| Sample | Gel Content (%) | Swelling Degree (%) | Volume Shrinkage (%) | Gloss | ||
|---|---|---|---|---|---|---|
| Water | Ethanol | Acetone | ||||
| S1 | 93.90 | 1.98 | 50.22 | 57.50 | 4.8 | 142.0 |
| S2 | 96.98 | 1.21 | 31.45 | 45.99 | 4.5 | 138.6 |
| S3 | 97.84 | 1.17 | 25.55 | 39.25 | 4.7 | 133.0 |
| Sample | Shore Hardness | Pencil Hardness | Adhesion Level | Flexibility (mm) | E 1 (MPa) | 2 (MPa) | 3 (%) |
|---|---|---|---|---|---|---|---|
| S1 | 66 | HB | 0 | 1 | 410.8 | 11.1 | 37.1 |
| S2 | 76 | 2H | 0 | 1 | 697.0 | 22.8 | 9.1 |
| S3 | 82 | 3H | 3 | 1 | 994.9 | 24.8 | 4.5 |
| Sample | Tg 1 (°C) | E’25 2 (MPa) | T5% 3 (°C) | Tp 4 (°C) |
|---|---|---|---|---|
| S1 | 71.2 | 3358.7 | 268.1 | 332.5 |
| S2 | 94.2 | 1453.7 | 279.6 | 327.2 |
| S3 | 110.9 | 1011.2 | 266.8 | 325.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Tang, L. Synthesis and Structure of 2-Hydroxypropyl Methacrylate-Capped Isophorone Diisocyanate and Poly(Propylene Glycol) Urethane Mixtures and the Properties of their UV-Cured Co-Networks with Isobornyl Methacrylate. Materials 2022, 15, 8586. https://doi.org/10.3390/ma15238586
Zhou J, Tang L. Synthesis and Structure of 2-Hydroxypropyl Methacrylate-Capped Isophorone Diisocyanate and Poly(Propylene Glycol) Urethane Mixtures and the Properties of their UV-Cured Co-Networks with Isobornyl Methacrylate. Materials. 2022; 15(23):8586. https://doi.org/10.3390/ma15238586
Chicago/Turabian StyleZhou, Junhao, and Liming Tang. 2022. "Synthesis and Structure of 2-Hydroxypropyl Methacrylate-Capped Isophorone Diisocyanate and Poly(Propylene Glycol) Urethane Mixtures and the Properties of their UV-Cured Co-Networks with Isobornyl Methacrylate" Materials 15, no. 23: 8586. https://doi.org/10.3390/ma15238586
APA StyleZhou, J., & Tang, L. (2022). Synthesis and Structure of 2-Hydroxypropyl Methacrylate-Capped Isophorone Diisocyanate and Poly(Propylene Glycol) Urethane Mixtures and the Properties of their UV-Cured Co-Networks with Isobornyl Methacrylate. Materials, 15(23), 8586. https://doi.org/10.3390/ma15238586







