Phase Formation of Iron-Based Superconductors during Mechanical Alloying
Abstract
1. Introduction
2. Methods
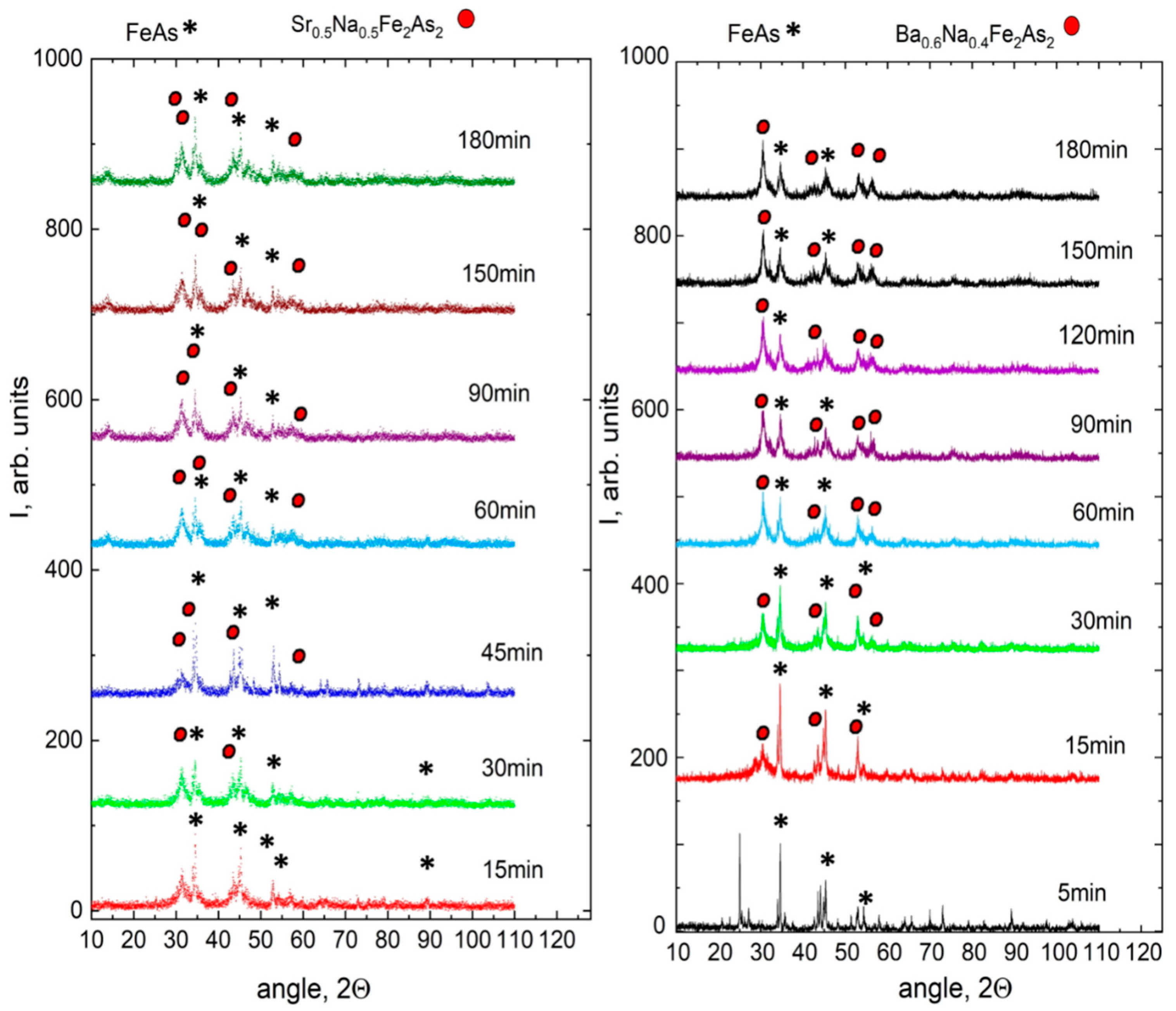
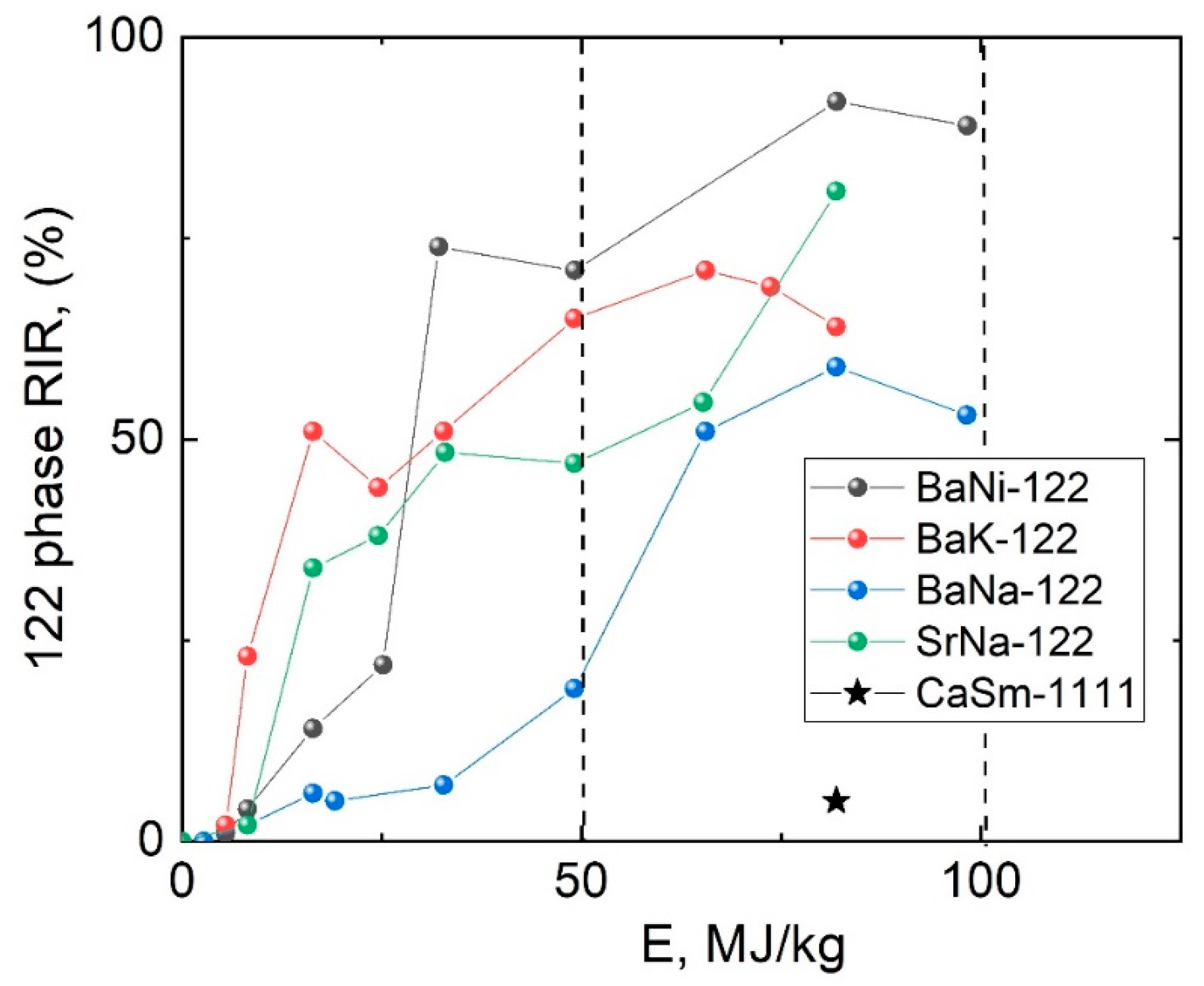
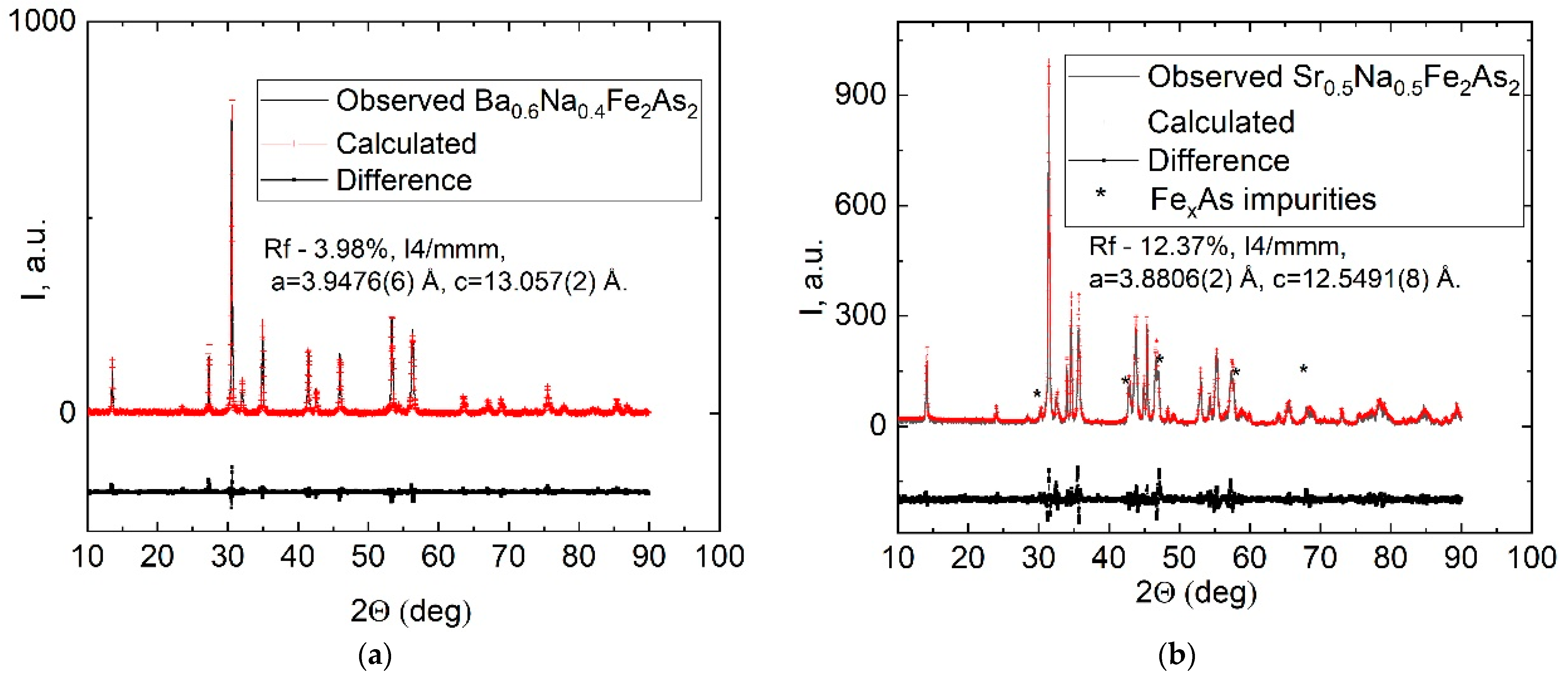
3. Results and Discussion
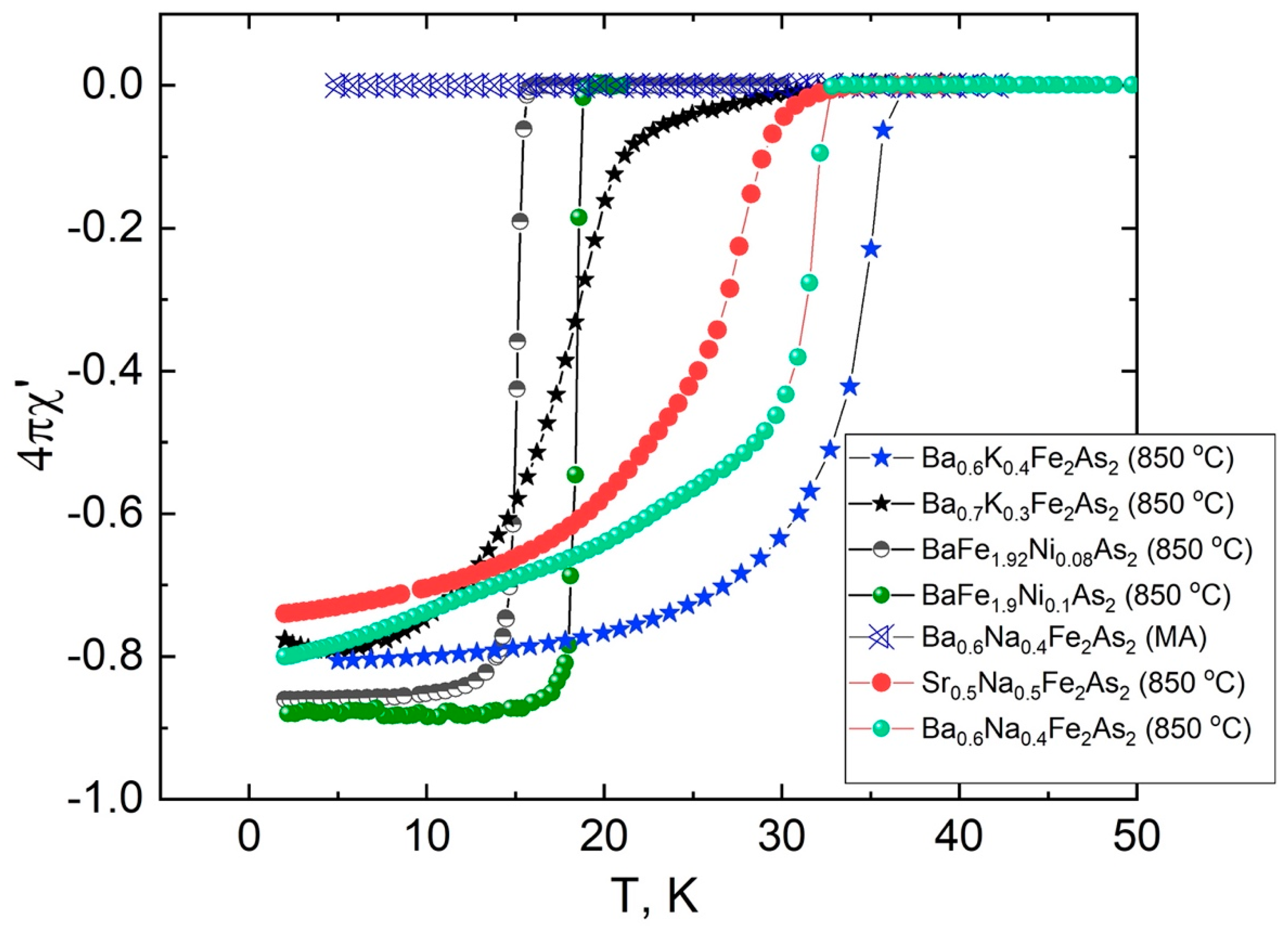
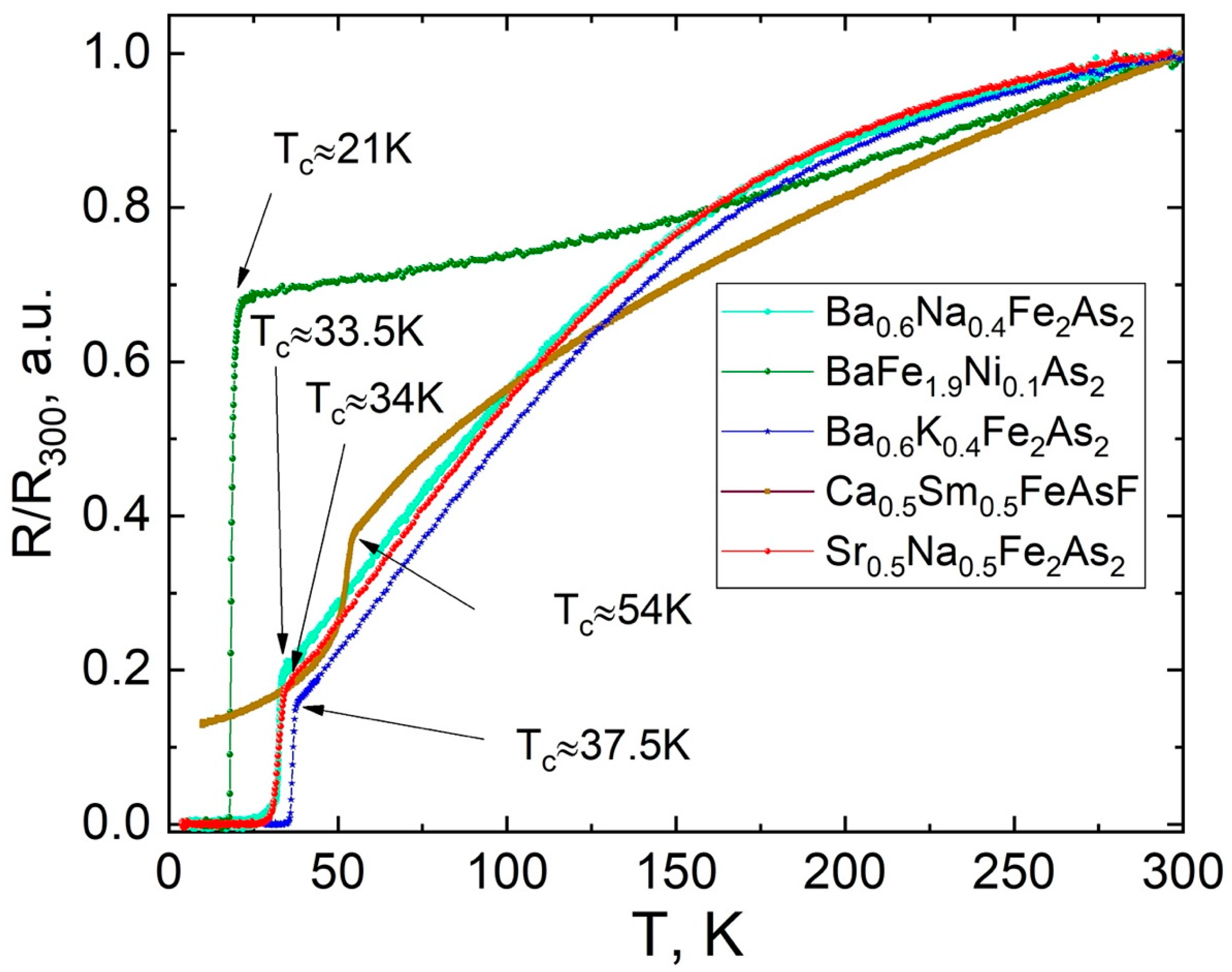
4. Superconducting Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamihara, Y.; Watanabe, T.; Hirano, M.; Hosono, H. Iron-based layered superconductor La[O1−xFx]FeAs (x = 0.05–0.12) with Tc = 26 K. J. Am. Chem. Soc. 2008, 130, 3296–3297. [Google Scholar] [CrossRef]
- Fujioka, M.; Denholme, S.J.; Ozaki, T.; Okazaki, H.; Deguchi, K.; Demura, S.; Hara, H.; Watanabe, T.; Takeya, H.; Yamaguchi, T.; et al. Phase diagram and superconductivity at 58.1 K in α-FeAs-free SmFeAsO1−xFx. Supercond. Sci. Technol. 2013, 26, 085023. [Google Scholar] [CrossRef]
- Koblischka, M.R.; Koblischka-Veneva, A.; Schmauch, J.; Murakami, M. Microstructure and flux pinning of reacted-and-pressed, polycrystalline Ba0.6K0.4Fe2As2 powders. Materials 2019, 12, 2173. [Google Scholar] [CrossRef]
- Yang, H.; Luo, H.; Wang, Z.; Wen, H.H. Fishtail effect and the vortex phase diagram of single crystal Ba0.6K0.4Fe2As2. Appl. Phys. Lett. 2008, 93, 142506. [Google Scholar] [CrossRef]
- Cheng, W.; Lin, H.; Shen, B.; Wen, H.H. Comparative study of vortex dynamics in CaKFe4As4 and Ba0.6K0.4Fe2As2 single crystals. Sci. Bull. 2019, 64, 81–90. [Google Scholar] [CrossRef]
- Pervakov, K.S.; Vlasenko, V.A.; Khlybov, E.P.; Zaleski, A.; Pudalov, V.M.; Eltsev, Y.F. Bulk magnetization and strong intrinsic pinning in Ni-doped BaFe2As2 single crystals. Supercond. Sci. Technol. 2012, 26, 015008. [Google Scholar] [CrossRef]
- Tarantini, C.; Pak, C.; Su, Y.F.; Hellstrom, E.E.; Larbalestier, D.C.; Kametani, F. Effect of heat treatments on superconducting properties and connectivity in K-doped BaFe2As2. Sci. Rep. 2021, 11, 3143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dong, C.; Yang, H.; Zhang, Q.; Awaji, S.; Gu, L.; Wen, H.H.; Ma, Y. Strengthened proximity effect at grain boundaries to enhance inter-grain supercurrent in Ba1−xKxFe2As2 superconductors. Mater. Today Phys. 2022, 28, 100848. [Google Scholar] [CrossRef]
- Fang, L.; Jia, Y.; Mishra, V.; Chaparro, C.; Vlasko-Vlasov, V.K.; Koshelev, A.E.; Welp, U.; Crabtree, W.; Zhu, S.; Zhigadlo, N.D.; et al. Huge critical current density and tailored superconducting anisotropy in SmFeAsO0.8F0.15 by low-density columnar-defect incorporation. Nat. Commun. 2013, 4, 2655. [Google Scholar] [CrossRef]
- Kidszun, M.; Haindl, S.; Thersleff, T.; Hänisch, J.; Kauffmann, A.; Iida, K.; Freudenberger, J.; Schultz, L.; Holzapfel, B. Critical current scaling and anisotropy in oxypnictide superconductors. Phys. Rev. Lett. 2011, 106, 137001. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Q.; Hu, K.; Gao, B.; Li, W.; Mu, G.; Xie, X. Strong anisotropy effect in an iron-based superconductor CaFe0.882Co0.118AsF. Supercond. Sci. Technol. 2017, 30, 074003. [Google Scholar] [CrossRef]
- Wang, X.C.; Liu, U.Q.; Lv, V.Y.; Gao, W.B.; Yang, L.X.; Yu, R.C.; Li, F.Y.; Jin, C.Q. The superconductivity at 18 K in LiFeAs system. Solid State Commun. 2008, 148, 538–540. [Google Scholar] [CrossRef]
- Iyo, A.; Kawashima, K.; Kinjo, T.; Nishio, T.; Ishida, S.; Fujihisa, H.; Gotoh, Y.; Kihou, K.; Yoshida, Y. New-structure-type Fe-based superconductors: CaAFe4As4 (A = K, Rb, Cs) and SrAFe4As4 (A = Rb, Cs). J. Am. Chem. Soc. 2016, 138, 3410–3415. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.R. Superconductivity in iron compounds. Rev. Mod. Phys. 2011, 83, 1589. [Google Scholar] [CrossRef]
- Hosono, H.; Yamamoto, A.; Hiramatsu, H.; Ma, Y. Recent advances in iron-based superconductors toward applications. Mater. Today 2018, 21, 278–302. [Google Scholar] [CrossRef]
- Nakajima, M.; Ishida, S.; Tanaka, T.; Kihou, K.; Tomioka, Y.; Saito, T.; Lee, C.H.; Fukazawa, H.; Kohori, Y.; Kakeshita, T.; et al. Normal-state charge dynamics in doped BaFe2As2: Roles of doping and necessary ingredients for superconductivity. Sci. Rep. 2014, 4, 5873. [Google Scholar] [CrossRef]
- Luo, H.Q.; Cheng, P.; Wang, Z.S.; Yang, H.; Jia, Y.; Fang, L.; Ren, C.; Shan, L.; Wen, H.H. Normal state transport properties in single crystals of Ba1−xKxFe2As2 and NdFeAsO1−xFx. Phys. C Supercond. 2019, 469, 477–484. [Google Scholar] [CrossRef]
- Luo, H.; Yamani, Z.; Chen, Y.; Lu, X.; Wang, M.; Li, S.; Maier, T.A.; Danilkin, S.; Adroja, D.T.; Dai, P. Electron doping evolution of the anisotropic spin excitations in BaFe2−xNixAs2. Phys. Rev. B 2012, 86, 024508. [Google Scholar] [CrossRef]
- Xu, Z.; Tao, Q.; Li, L.; Shen, J.; Lin, X.; Cao, G. Ni doping effect and phase diagram of Ni-doped BaFe2−xNixAs2. Phys. C Supercond. Appl. 2010, 470, S447–S448. [Google Scholar] [CrossRef]
- Analytis, J.G.; Kuo, H.H.; McDonald, R.D.; Wartenb, M.; Hussey, N.E.; Fisher, I.R. Transport near a quantum critical point in BaFe2(As1−xPx)2. Nat. Phys. 2014, 10, 194–197. [Google Scholar] [CrossRef]
- Eom, M.J.; Na, S.W.; Hoch, C.; Kremer, R.K.; Kim, J.S. Evolution of transport properties of BaFe2−xRuxAs2 in a wide range of isovalent Ru substitution. Phys. Rev. B 2012, 85, 024536. [Google Scholar] [CrossRef]
- Vlasenko, V.A.; Pervakov, K.S.; Eltsev, Y.F.; Berbentsev, V.D.; Tsapleva, A.S.; Lukyanov, P.A.; Abdyukhanov, I.M.; Pudalov, V.M. Critical current and microstructure of FeSe wires and tapes prepared by PIT method. IEEE Trans. Appl. Supercond. 2019, 29, 6900505. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y. Progress in the development of the 122-type IBS wires. Superconductivity 2022, 2, 100010. [Google Scholar] [CrossRef]
- Zhang, X.; Oguro, H.; Yao, C.; Dong, C.; Xu, Z.; Wang, D.; Awaji, S.; Watanabe, K.; Ma, Y. Superconducting properties of 100-m class Sr0.6K0.4Fe2As2 tape and pancake coils. IEEE Trans. Appl. Supercond. 2017, 27, 7300705. [Google Scholar] [CrossRef]
- Qian, X.; Jiang, S.; Ding, H.; Huang, P.; Pang, Y.; Jiang, D.; Zhang, X.; Ma, Y.; Chen, W. Development of the iron-based superconducting coils for high magnetic field application. Phys. C Supercond. Appl. 2021, 584, 1353855. [Google Scholar] [CrossRef]
- Qian, X.; Jiang, S.; Ding, H.; Huang, P.; Zou, G.; Jiang, D.; Zhang, X.; Ma, Y.; Chen, W. Performance testing of the iron-based superconductor inserted coils under high magnetic field. Phys. C Supercond. Appl. 2021, 580, 1353787. [Google Scholar] [CrossRef]
- Richter, S.; Kurth, F.; Iida, K.; Pervakov, K.; Pukenas, A.; Tarantini, C.; Jaroszynski, J.; Hänisch, J.; Grinenko, V.; Skrotzki, W.; et al. Superconducting properties of Ba (Fe1–xNix)2As2 thin films in high magnetic fields. Appl. Phys. Lett. 2017, 110, 022601. [Google Scholar] [CrossRef]
- Grünewald, L.; Langer, M.; Meyer, S.; Nerz, D.; Hänisch, J.; Holzapfel, B.; Gerthsen, D. Structural and chemical properties of superconducting Co-doped BaFe2As2 thin films grown on CaF2. Supercond. Sci. Technol. 2021, 34, 035005. [Google Scholar] [CrossRef]
- Sato, H.; Hiramatsu, H.; Kamiya, T.; Hosono, H. Enhanced critical-current in P-doped BaFe2As2 thin films on metal substrates arising from poorly aligned grain boundaries. Sci. Rep. 2016, 6, 36828. [Google Scholar] [CrossRef]
- Qin, D.; Iida, K.; Hatano, T.; Saito, H.; Ma, Y.; Wang, C.; Hata, S.; Naito, M.; Yamamoto, A. Realization of epitaxial thin films of the superconductor K-doped BaFe2As2. Phys. Rev. Mater. 2021, 5, 014801. [Google Scholar] [CrossRef]
- Hanzawa, K.; Matsumoto, J.; Iimura, S.; Kohama, Y.; Hiramatsu, H.; Hosono, H. High upper critical field (120 T) with small anisotropy of highly hydrogen-substituted SmFeAsO epitaxial film. Phys. Rev. Mater. 2022, 6, L111801. [Google Scholar] [CrossRef]
- Weiss, J.D.; Jiang, J.; Polyanskii, A.A.; Hellstrom, E.E. Mechanochemical synthesis of pnictide compounds and superconducting Ba0.6K0.4Fe2As2 bulks with high critical current density. Supercond. Sci. Technol. 2013, 26, 074003. [Google Scholar] [CrossRef]
- Shi, S.L.; Fang, A.H.; Xie, X.M.; Huang, F.Q.; Jiang, M.H. MgF2 Doping of SmFeAsO Superconductors Prepared by Mechanical Alloying and Rapid Annealing. Chem. Mater. 2011, 23, 3039–3044. [Google Scholar] [CrossRef]
- Yang, C.; Xia, L.; Sun, X.; Zheng, P.; Yu, Z.; Chen, Y.; Zhang, Y.; Pan, X.; Chen, C.; Yan, G.; et al. Effects of Ti doping on properties of Nb3Al superconductor fabricated by high-energy ball milling. Ceram. Int. 2019, 45, 15681–15688. [Google Scholar] [CrossRef]
- Kurama, H.; Erkuş, Ş.; Gaşan, H. The effect of process control agent usage on the structural properties of MgB2 synthesized by high energy ball mill. Ceram. Int. 2017, 43, S391–S396. [Google Scholar] [CrossRef]
- Tessier, P.; Trudeau, M.L.; Ström-Olsen, J.O.; Schulz, R. Structural transformations and metastable phases produced by mechanical deformations in the Bi–Sr–Ca–Cu–O superconducting system. J. Mater. Res. 1993, 8, 1258–1267. [Google Scholar] [CrossRef]
- Estemirova, S.K.; Mitrofanov, V.Y. The double superconducting transition in DyBa2Cu3O6+ δ. Ceram. Int. 2016, 42, 16127–16131. [Google Scholar] [CrossRef]
- Ammar, H.R.; Sivasankaran, S.; Alaboodi, A.S. Investigation of the microstructure and compressibility of biodegradable Fe-Mn-Cu/W/Co nanostructured alloy powders synthesized by mechanical alloying. Materials 2021, 14, 3088. [Google Scholar] [CrossRef]
- Lesz, S.; Hrapkowicz, B.; Karolus, M.; Gołombek, K. Characteristics of the Mg-Zn-Ca-Gd alloy after mechanical alloying. Materials 2021, 14, 226. [Google Scholar] [CrossRef]
- Ulbrich, K.F.; Campos, C.E.M. Nanosized tetragonal β-FeSe phase obtained by mechanical alloying: Structural, microstructural, magnetic and electrical characterization. RSC Adv. 2018, 8, 8190–8198. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A novel technique to synthesize advanced materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef] [PubMed]
- Oanh, N.T.H.; Binh, D.N.; Dang Duc, D.; Hoang Thi Ngoc, Q.; Viet, N.H. Effect of Transition Elements on the Thermal Stability of Glassy Alloys 82Al–16Fe–2TM (TM: Ti, Ni, Cu) Prepared by Mechanical Alloying. Materials 2021, 14, 3978. [Google Scholar] [CrossRef] [PubMed]
- Pervakov, K.S.; Vlasenko, V.A. Synthesis of electron-and hole-doped bulk BaFe2As2 superconductors by mechanical alloying. Ceram. Int. 2020, 46, 8625–8630. [Google Scholar] [CrossRef]
- Pervakov, K.S.; Kulikova, L.F.; Tsvetkov, A.Y.; Vlasenko, V.A. Novel Iron-Based Superconductor Ca0.5Sm0.5FeAsF. Bull. Lebedev Phys. Inst. 2022, 49, 242–246. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist.-Cryst. Mater. 2022, 229, 345–352. [Google Scholar] [CrossRef]
- Maltsev, E.I.; Pervakov, K.S.; Vlasenko, V.A. Synthesis of iron-based superconductor Ba0.6K0.4Fe2As2 by mechanical alloying. Bull. Lebedev Phys. Inst. 2019, 46, 248–250. [Google Scholar] [CrossRef]
- Häßler, W.; Hermann, H.; Herrmann, M.; Rodig, C.; Aubele, A.; Schmolinga, L.; Holzapfel, B. Influence of the milling energy transferred to the precursor powder on the microstructure and the superconducting properties of MgB2 wires. Supercond. Sci. Technol. 2012, 26, 025005. [Google Scholar] [CrossRef]
- Tokuta, S.; Shimada, Y.; Yamamoto, A. Evolution of intergranular microstructure and critical current properties of polycrystalline Co-doped BaFe2As2 through high-energy milling. Supercond. Sci. Technol. 2020, 33, 094010. [Google Scholar] [CrossRef]
- Tokuta, S.; Yamamoto, A. Enhanced upper critical field in Co-doped Ba122 superconductors by lattice defect tuning. APL Mater. 2019, 7, 111107. [Google Scholar] [CrossRef]
- Cortes Gil, R.; Parker, D.R.; Pitcher, M.J.; Hadermann, J.; Clarke, S.J. Indifference of superconductivity and magnetism to size-mismatched cations in the layered iron arsenides Ba1−xNaxFe2As2. Chem. Mater. 2010, 22, 4304. [Google Scholar] [CrossRef]
- Taddei, K.M.; Allred, J.M.; Bugaris, D.E.; Lapidus, S.; Krogstad, M.J.; Stadel, R.; Claus, H.; Chung, D.Y.; Kanatzidis, M.G.; Rosenkranz, S.; et al. Detailed magnetic and structural analysis mapping a robust magnetic C4 dome in Sr1–xNaxFe2As2. Phys. Rev. B Condens. Matter. Mater. Phys. 2016, 93, 134510. [Google Scholar] [CrossRef]

| Chemical Formula | Composition by EDS | Tc, K | Unit Cell Parameter | ||
|---|---|---|---|---|---|
| R(T) | χ’(T) | a, Å | c, Å | ||
| BaFe1.9Ni0.1As2 [43] | Ba1.022Fe1.907Ni0.093As2.003 | 21 K | 18.5 K | 3.9586(2) | 12.9820(7) |
| BaFe1.92 Ni0.08As2 [43] Ba0.6K0.4Fe2As2 [43] Ba0.7K0.3Fe2As2 [43] Ba0.6Na0.4Fe2As2 * Sr0.5Na0.5Fe2As2 * Ca0.5Sm0.5FeAsF [44] | Ba1.106Fe1.921Ni0.079As1.776 Ba0.729K0.271Fe1.92As1.92 Ba0.734K0.266Fe2.15As1.99 Ba0.73Na0.27Fe2.14As1.87 -- Ca0.56Sm0.44Fe0.9As0.97F1.25 | - 37.5 K - 33.5 K 34 K 54 K | 15 K 36 K 25 K 33 K 33 K 53 K | - 3.9405(7) - 3.9476(6) 3.8806(2) - | - 13.195(3) - 13.057(2) 12.5491(8) - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasenko, V.A.; Degtyarenko, A.Y.; Shilov, A.I.; Tsvetkov, A.Y.; Kulikova, L.F.; Medvedev, A.S.; Pervakov, K.S. Phase Formation of Iron-Based Superconductors during Mechanical Alloying. Materials 2022, 15, 8438. https://doi.org/10.3390/ma15238438
Vlasenko VA, Degtyarenko AY, Shilov AI, Tsvetkov AY, Kulikova LF, Medvedev AS, Pervakov KS. Phase Formation of Iron-Based Superconductors during Mechanical Alloying. Materials. 2022; 15(23):8438. https://doi.org/10.3390/ma15238438
Chicago/Turabian StyleVlasenko, Vladimir A., Alena Yu. Degtyarenko, Andrei I. Shilov, Alexey Yu. Tsvetkov, Lyudmila F. Kulikova, Alexey S. Medvedev, and Kirill S. Pervakov. 2022. "Phase Formation of Iron-Based Superconductors during Mechanical Alloying" Materials 15, no. 23: 8438. https://doi.org/10.3390/ma15238438
APA StyleVlasenko, V. A., Degtyarenko, A. Y., Shilov, A. I., Tsvetkov, A. Y., Kulikova, L. F., Medvedev, A. S., & Pervakov, K. S. (2022). Phase Formation of Iron-Based Superconductors during Mechanical Alloying. Materials, 15(23), 8438. https://doi.org/10.3390/ma15238438






