Adsorption of 2,4-D and MCPA Herbicides on Carbon Black Modified with Hydrogen Peroxide and Aminopropyltriethoxysilane
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Adsorbents Preparation and Characterization
2.3. Adsorption from Aqueous Solutions
3. Results and Discussion
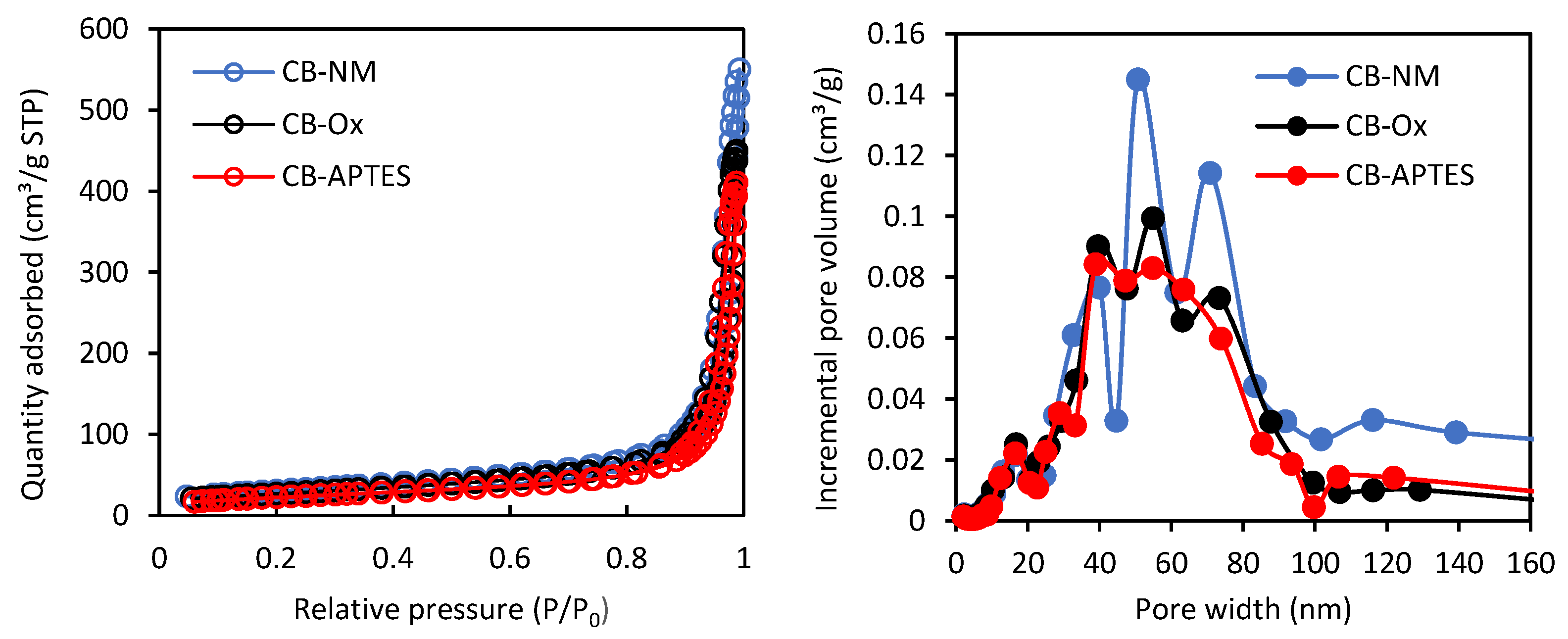
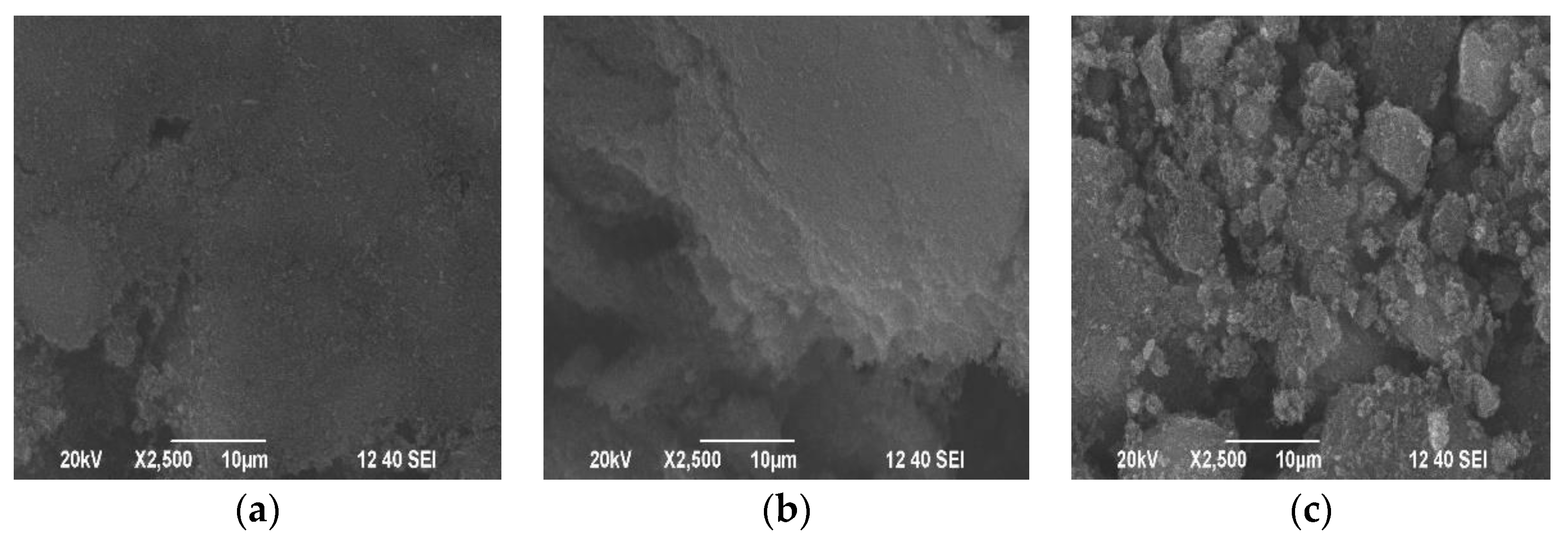
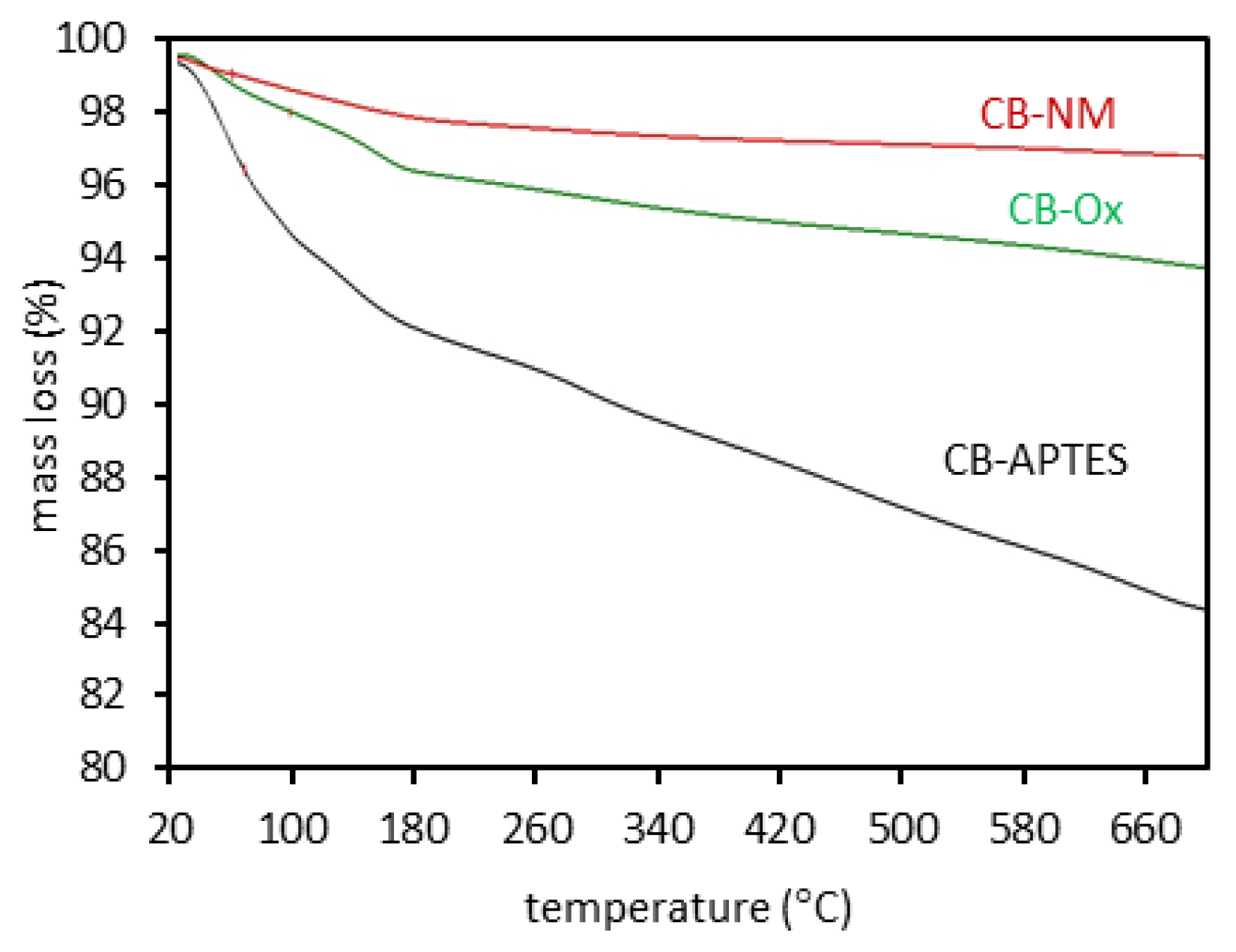
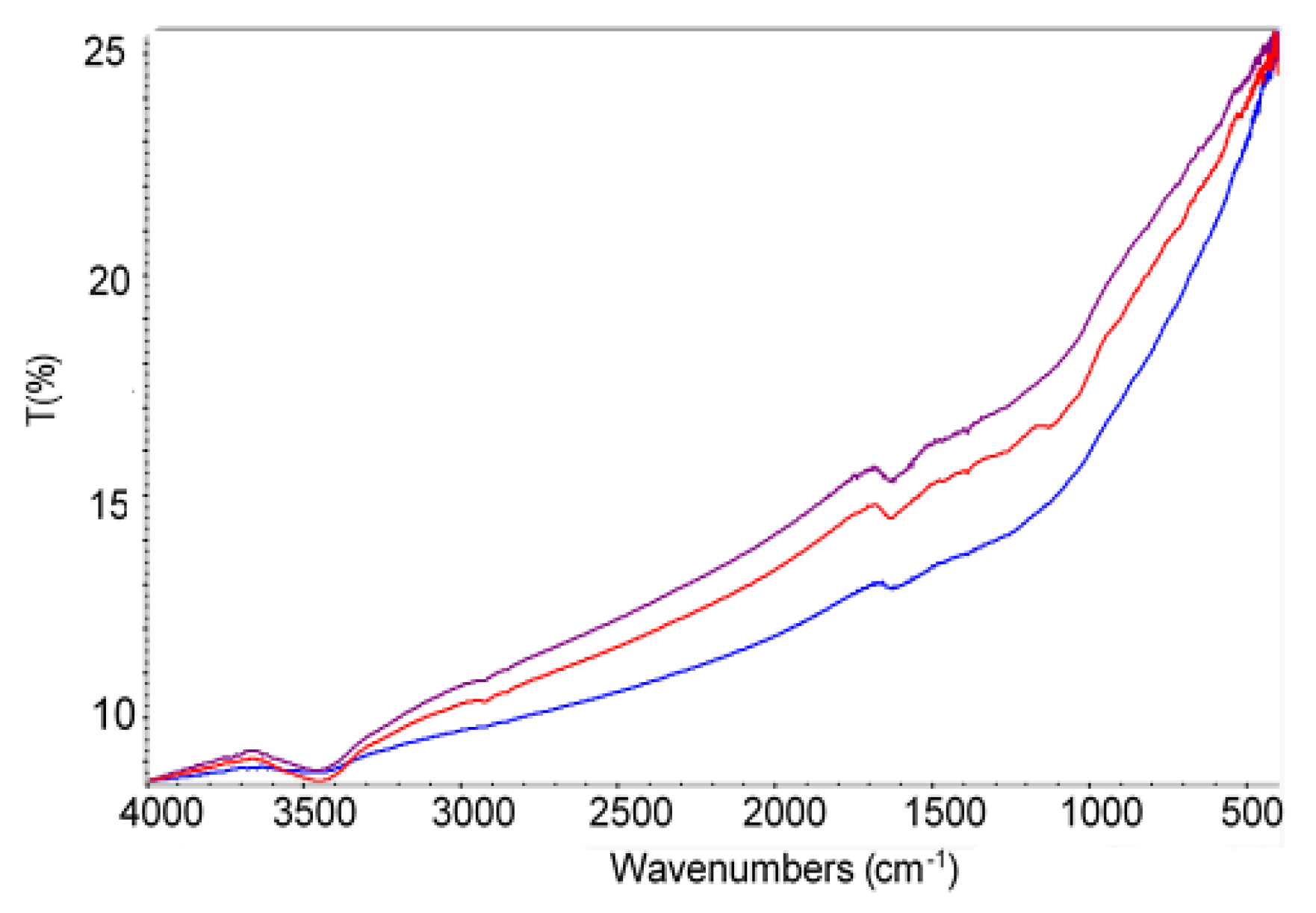
3.1. Adsorbents Characterization
3.2. Adsorption Study
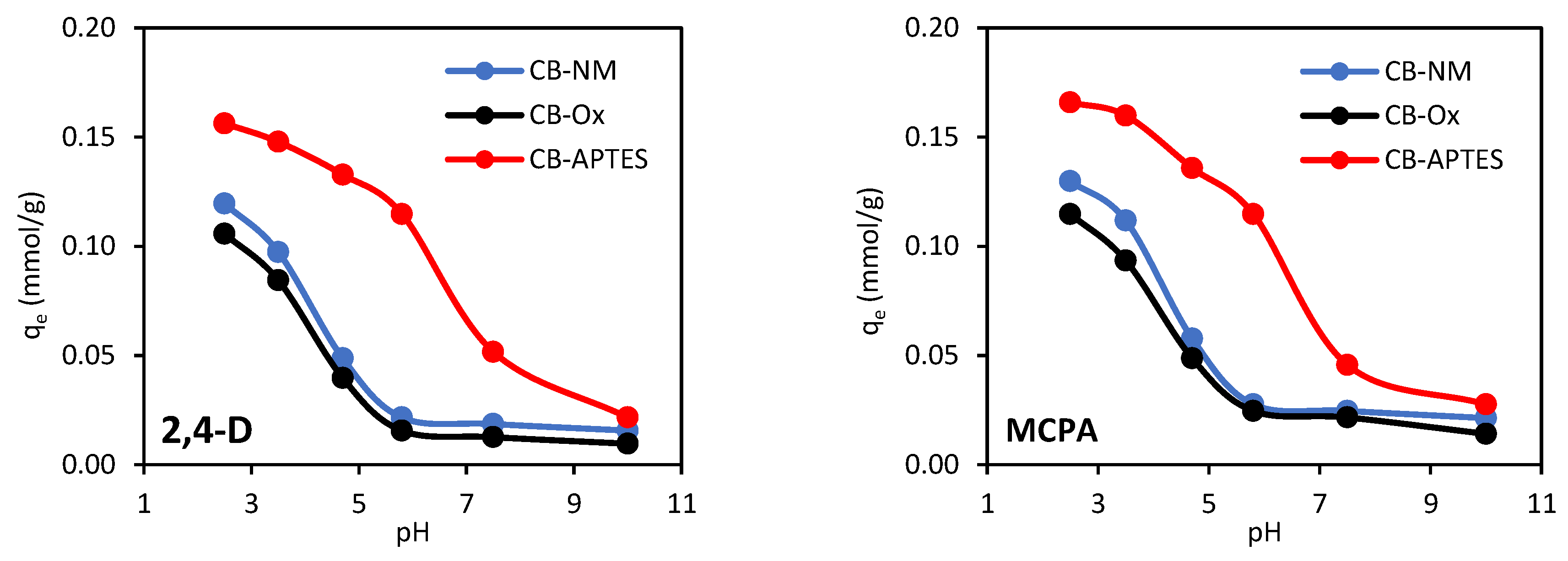
3.2.1. Effect of Solution pH
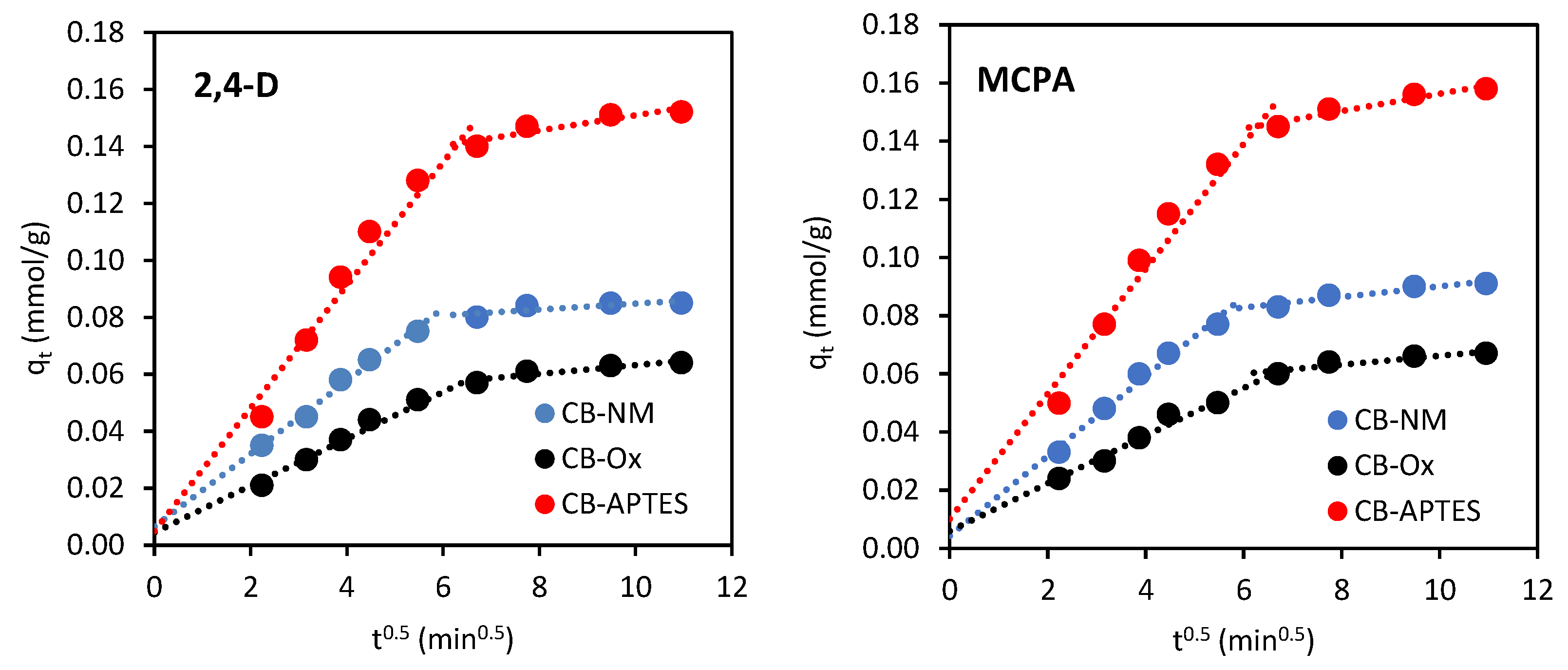
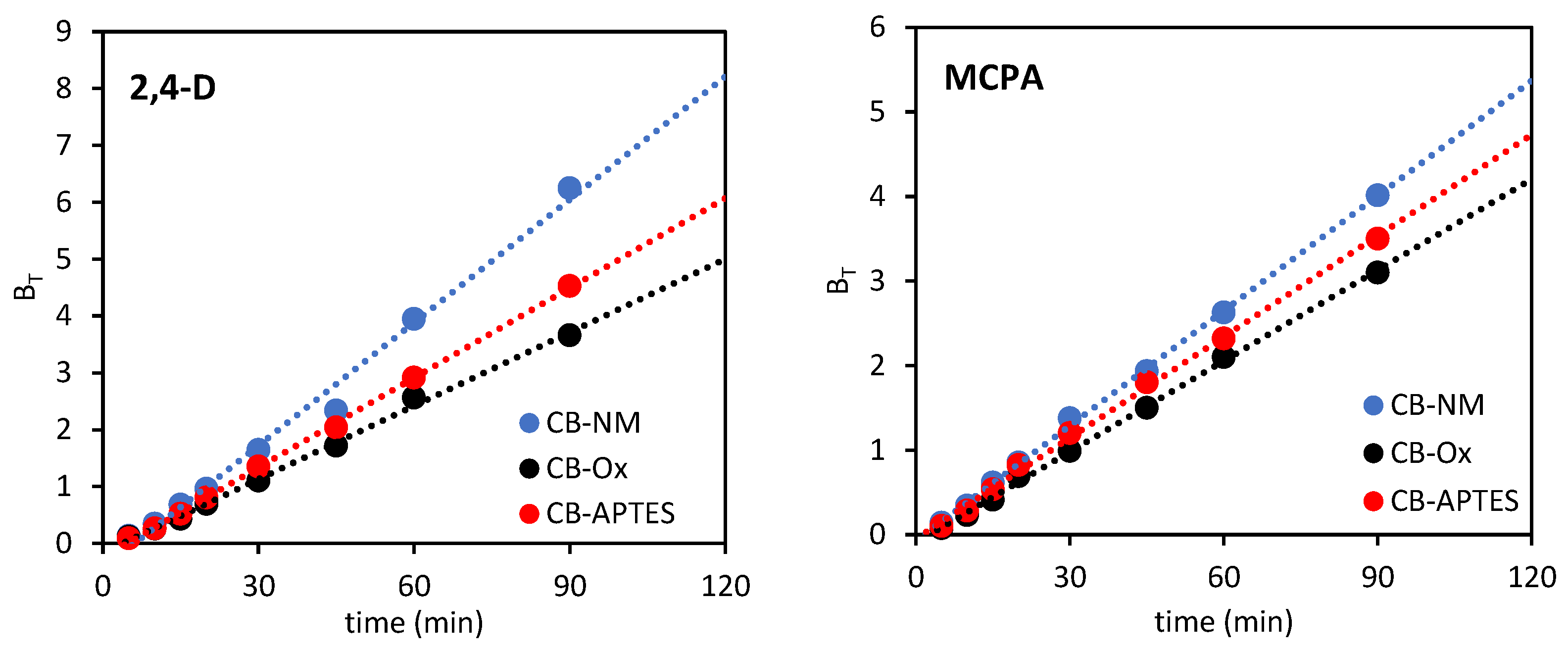
3.2.2. Adsorption Kinetics
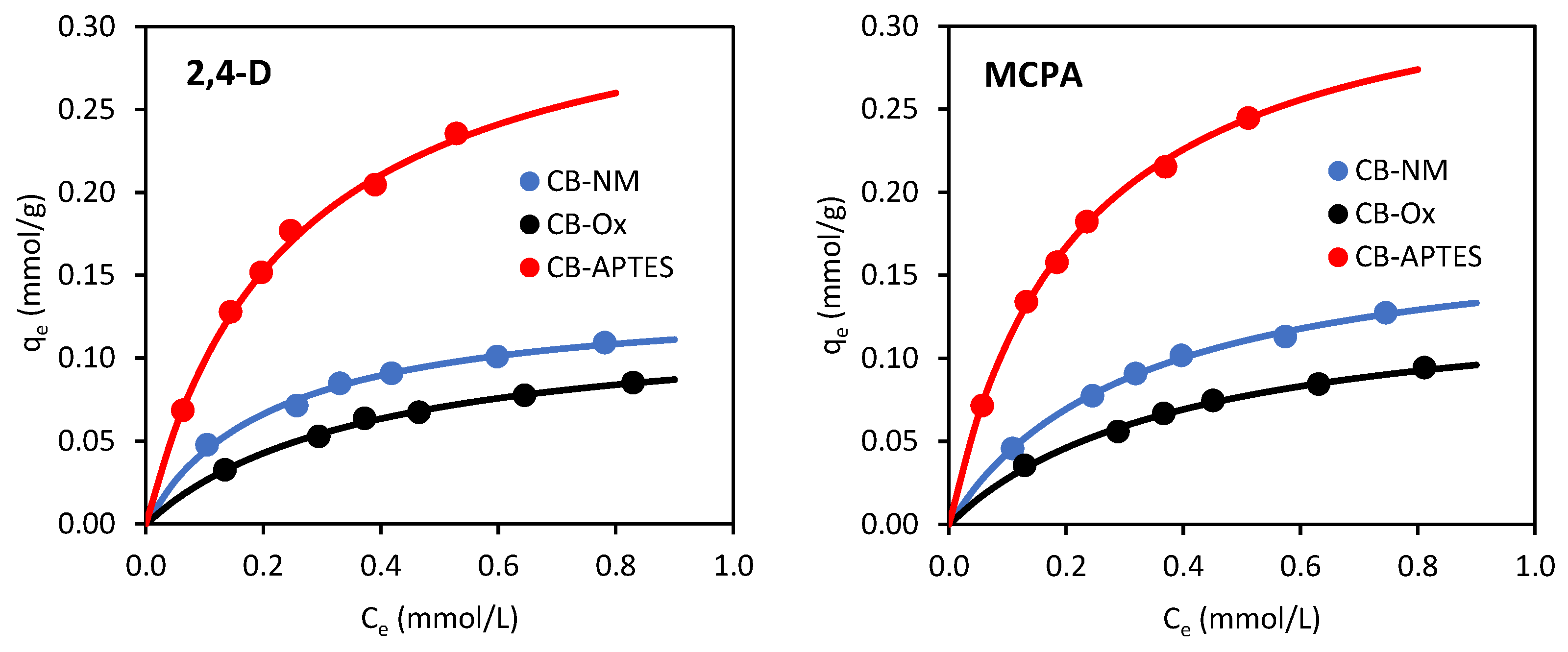
3.2.3. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Donnet, J.-B.; Bansal, R.C.; Wang, M.-J. Carbon Black. Science and Technology, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Singh, M.; Wal, R.L.V. Nanostructure quantification of carbon blacks. C J. Carbon Res. 2019, 5, 2. [Google Scholar] [CrossRef]
- Al-Hartomy, O.A.; Al-Solamy, F.; Al-Ghamdi, A.; Dishovsky, N.; Ivanov, M.; Mihaylov, M.; El-Tantawy, F. Influence of carbon black structure and specific surface area on the mechanical and dielectric properties of filled rubber composites. Int. J. Polym. Sci. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Al-Hartomy, O.A.; Al-Ghamdi, A.A.; Al-Solamy, F.R.; Dishovsky, N.; Mihaylov, M.; Ivanov, M.; El-Tantawy, F. A comparative study on the effect produced by fillers specifics on the dynamic mechanical and dielectric properties of natural rubber based composites. Res. Rev. Polym. 2012, 3, 93–101. [Google Scholar]
- Schröder, A.; Klüppel, M.; Schuster, R.H. Characterisation of surface activity of carbon black and its relation to polymer-filler interaction. Macromol. Mater. Eng. 2007, 292, 885–916. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon black reborn: Structure and chemistry for renewable energy harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef]
- Grossman, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2009, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Moszczyński, W.; Białek, A. Ecological production technology of phenoxyacetic herbicides MCPA and 2,4-D in the Highest World Standard. In Herbicides-Properties, Synthesis and Control of Weeds; Hasaneen, M.N., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 347–361. [Google Scholar] [CrossRef]
- von Stackelberg, K. A systematic review of carcinogenic outcomes and potential mechanisms from exposure to 2,4-D and MCPA in the environment. J. Toxicol. 2013, 2013, 1–53. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y.; Li, G. Characterization and evaluation of surface modified materials based on porous biochar and its adsorption properties for 2,4-dichlorophenoxyacetic acid. Chemosphere 2018, 210, 734–744. [Google Scholar] [CrossRef]
- Patiparn, P.; Takizawa, S. Effect of surface functional group on adsorption of organic pollutants on hexagonal mesoporous silicate. Water Sci. Technol. Water Supply 2006, 6, 17–25. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Application of nanoporous silicas as adsorbents for chlorinated aromatic compounds. A comparative study. Mater. Sci. Eng. C 2014, 41, 42–51. [Google Scholar] [CrossRef]
- Otalvara, J.O.; Avena, M.; Brigante, M. Adsorption of organic pollutants by amine functionalized mesoporous silica in aqueous solution. Effects of pH, ionic strength and some consequences of APTES stability. J. Environ. Chem. Eng. 2019, 7, 103325. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A. Removal of 2,4-D herbicide from aqueous solution by aminosilane-grafted mesoporous carbons. Adsorption 2019, 25, 345–355. [Google Scholar] [CrossRef]
- Mohammadi, F.; Esrafili, A.; Kermani, M.; Behbahani, M. Application of modified magnetic nanoparticles with amine groups as an efficient solid sorbent for simultaneous removal of 2,4-dichlorophenoxyacetic acid and 2-methyl-4-chlorophenoxyacetic acid from aqueous solution: Optimization and modeling. J. Iran. Chem. Soc. 2018, 15, 421–429. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Białek, A.; Świątkowski, A. Effect of activated carbon surface chemistry on adsorption of phenoxy carboxylic acid herbicides from aqueous solutions. Desalin. Water Treat. 2020, 186, 450–459. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Szala, M.; Świątkowski, A. Adsorption of 2,4-dichlorophenol and 2,4-dichlorophenoxyacetic acid from aqueous solution on carbonaceous materials obtained by combustion synthesis. J. Taiwan Inst. Chem. Eng. 2016, 63, 371–378. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; Taylor and Francis, CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Abdel daiem, M.M.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Ocampo-Pérez, R. Single, competitive, and dynamic adsorption on activated carbon of compounds used as plasticizers and herbicides. Sci. Total Environ. 2015, 537, 335–342. [Google Scholar] [CrossRef]
- Doczekalska, B.; Kuśmierek, K.; Świątkowski, A.; Bartkowiak, M. Adsorption of 2,4-dichlorophenoxyacetic acid and 4-chloro-2-metylphenoxyacetic acid onto activated carbons derived from various lignocellulosic materials. J. Environ. Sci. Health B 2018, 53, 290–297. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Dąbek, L.; Świątkowski, A. Removal of phenoxy herbicides from aqueous solutions using lignite as a low-cost adsorbent. Desalin. Water Treat. 2022, 260, 111–118. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 1–19. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Role of surface chemistry in adsorption of phenol on activated carbons. J. Colloid Interf. Sci. 2003, 264, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Derylo-Marczewska, A.; Sternik, D.; Swiatkowski, A.; Kusmierek, K.; Gac, W.; Buczek, B. Adsorption of phenol from aqueous and cyclohexane solutions on activated carbons with differentiated surface chemistry. Thermochim. Acta 2022, 715, 179299. [Google Scholar] [CrossRef]
- Ocampo-Pérez, R. Abdel daiem, M.M.; Rivera-Utrilla, J.; Méndez-Díaz, J.D.; Sánchez-Polo, M. Modeling adsorption rate of organic micropollutants present in landfill leachates onto granular activated carbon. J. Colloid Interface Sci. 2012, 385, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, W.; Kusmierek, K.; Swiatkowski, A. Sorption equilibrium prediction of competitive adsorption of herbicides 2,4-D and MCPA from aqueous solution on activated carbon using ANN. Adsorption 2014, 20, 899–904. [Google Scholar] [CrossRef]
- Cardoso, L.P.; Valim, J.B. Study of acids herbicides removal by calcined Mg–Al–CO3–LDH. J. Phys. Chem. Solids 2006, 67, 987–993. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Swiatkowski, A.; Tarasiuk, B. Adsorption of selected herbicides from aqueous solutions on activated carbon. J. Therm. Anal. Calorim. 2010, 101, 785–794. [Google Scholar] [CrossRef]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U., Jr.; Mohan, D.; Mlsna, T. Phenoxy herbicide removal from aqueous solutions using fast pyrolysis switchgrass biochar. Chemosphere 2017, 174, 49–57. [Google Scholar] [CrossRef]
- Nyazi, K.; Bacaoui, A.; Yaacoubi, A.; Darmstadt, H.; Adnot, A.; Roy, C. Influence of carbon black surface chemistry on the adsorption of model herbicides from aqueous solution. Carbon 2005, 43, 2218–2221. [Google Scholar] [CrossRef]
- Khoshnood, M.; Azizian, S. Adsorption of 2,4-dichlorophenoxyacetic acid pesticide by graphitic carbon nanostructures prepared from biomasses. J. Ind. Eng. Chem. 2012, 18, 1796–1800. [Google Scholar] [CrossRef]
- Bahrami, M.; Amiri, M.J.; Beigzadeh, B. Adsorption of 2,4-dichlorophenoxyacetic acid using rice husk biochar, granular activated carbon, and multi-walled carbon nanotubes in a fixed bed column system. Water Sci. Technol. 2018, 78, 1812–1821. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Skrzypczyńska, K.; Dąbek, L. Adsorptive and electrochemical properties of carbon nanotubes, activated carbon, and graphene oxide with relatively similar specific surface area. Materials 2021, 14, 496. [Google Scholar] [CrossRef] [PubMed]

| Herbicide | 2,4-D | MCPA |
|---|---|---|
| CAS No. | 94-75-7 | 94-74-6 |
| Molecular formula |  |  |
| Molecular weight (g/mol) | 221.04 | 200.62 |
| Solubility in water (g/L) | 0.89 | 0.82 |
| pKa | 2.98 | 3.14 |
| Carbon Black | SBET (m2/g) | C Constant | Vmi (cm3/g) | Vme (cm3/g) | Vt (cm3/g) |
|---|---|---|---|---|---|
| CB-NM | 108 | 145 | 0.0503 | 0.0414 | 0.464 |
| CB-Ox | 95 | 155 | 0.0428 | 0.0398 | 0.441 |
| CB-APTES | 82 | 74 | 0.0372 | 0.0376 | 0.413 |
| Carbon Black | C | O | Si | S | N |
|---|---|---|---|---|---|
| (wt.%) | |||||
| CB-NM | 95.6 | 4.1 | - | 0.3 | - |
| CB-Ox | 92.8 | 6.9 | - | 0.3 | - |
| CB-APTES | 94.6 | 3.7 | 1.1 | 0.2 | 0.4 |
| Adsorbate/Kinetic Model | Adsorbent | ||
|---|---|---|---|
| 2,4-D | CB-NM | CB-Ox | CB-APTES |
| qEXP (mmol/g) | 0.085 | 0.064 | 0.152 |
| Pseudo-first-order | |||
| k1 (1/min) | 0.0734 | 0.0458 | 0.0551 |
| qeCAL (mmol/g) | 0.087 | 0.054 | 0.137 |
| R2 | 0.990 | 0.975 | 0.988 |
| Δq (%) | 8.91 | 15.93 | 11.12 |
| Pseudo-second-order | |||
| k2 (g/mmol∙min) | 1.236 | 1.021 | 0.508 |
| qCAL (mmol/g) | 0.092 | 0.071 | 0.169 |
| R2 | 0.998 | 0.998 | 0.999 |
| Δq (%) | 3.53 | 3.71 | 2.86 |
| MCPA | CB-NM | CB-Ox | CB-APTES |
| qEXP (mmol/g) | 0.091 | 0.067 | 0.158 |
| Pseudo-first-order | |||
| k1 (1/min) | 0.0484 | 0.0469 | 0.0479 |
| qCAL (mmol/g) | 0.071 | 0.059 | 0.127 |
| R2 | 0.982 | 0.990 | 0.985 |
| Δq (%) | |||
| Pseudo-second-order | |||
| k2 (g/mmol∙min) | 1.0587 | 0.9471 | 0.5197 |
| qCAL (mmol/g) | 0.098 | 0.074 | 0.170 |
| R2 | 0.999 | 0.999 | 0.998 |
| Δq (%) | 1.92 | 2.33 | 4.01 |
| Adsorbate/Isotherm | Adsorbent | ||
|---|---|---|---|
| 2,4-D | CB-NM | CB-Ox | CB-APTES |
| Freundlich | |||
| KF ((mmol/g)(L/mmol)1/n) | 0.127 | 0.099 | 0.365 |
| 1/n | 0.418 | 0.532 | 0.574 |
| R2 | 0.962 | 0.978 | 0.971 |
| Δq (%) | 17.51 | 14.55 | 15.77 |
| Langmuir | |||
| qm (mmol/g) | 0.138 | 0.124 | 0.340 |
| KL (L/mmol) | 4.621 | 2.622 | 4.061 |
| R2 | 0.996 | 0.997 | 0.996 |
| Δq (%) | 4.32 | 3.87 | 4.88 |
| Temkin | |||
| bT (kJ/mol) | 43.03 | 22.34 | 37.07 |
| AT (L/g) | 79.61 | 85.08 | 34.74 |
| R2 | 0.993 | 0.990 | 0.985 |
| Δq (%) | 8.69 | 9.98 | 12.69 |
| MCPA | CB-NM | CB-Ox | CB-APTES |
| Freundlich | |||
| KF ((mmol/g)(L/mmol)1/n) | 0.157 | 0.110 | 0.383 |
| 1/n | 0.531 | 0.534 | 0.556 |
| R2 | 0.977 | 0.989 | 0.951 |
| Δq (%) | 13.84 | 10.01 | 19.62 |
| Langmuir | |||
| qm (mmol/g) | 0.181 | 0.139 | 0.348 |
| KL (L/mmol) | 3.108 | 2.478 | 4.615 |
| R2 | 0.997 | 0.996 | 0.999 |
| Δq (%) | 3.51 | 4.55 | 2.39 |
| Temkin | |||
| bT (kJ/mol) | 26.67 | 21.77 | 42.09 |
| AT (L/g) | 58.67 | 76.65 | 31.42 |
| R2 | 0.985 | 0.991 | 0.991 |
| Δq (%) | 11.99 | 9.69 | 9.87 |
| Adsorbent | SBET (m2/g) | Adsorption Capacity (qm), mmol/g | Ref. | |
|---|---|---|---|---|
| 2,4D | MCPA | |||
| CB-NM | 108 | 0.138 | 0.181 | this paper |
| CB-Ox | 95 | 0.124 | 0.139 | this paper |
| CB-APTES | 82 | 0.340 | 0.348 | this paper |
| Carbopack B carbon black | 97 | 0.309 | - | [17] |
| Vulcan XC 72 carbon black | 227 | 0.325 | - | [17] |
| SRB N762 carbon black | 24.3 | 0.030 | 0.029 | [32] |
| CA N660 carbon black | 36.0 | 0.096 | 0.094 | [32] |
| CO N539 carbon black | 39.4 | 0.110 | 0.115 | [32] |
| CA N375 carbon black | 90.4 | 0.238 | 0.224 | [32] |
| CO N375 carbon black | 94.6 | 0.247 | 0.240 | [32] |
| CA N115 carbon black | 137 | 0.188 | 0.185 | [32] |
| SRB N762 carbon black | 24.3 | 0.030 | 0.029 | [32] |
| carbon materials from cotton | 27.4 | 0.149 | - | [33] |
| carbon materials from filter paper | 182 | 0.348 | - | [33] |
| multiwalled carbon nanotubes | 200 | 0.099 | - | [34] |
| single-walled carbon nanotubes | 597 | 2.001 | - | [35] |
| reduced graphene oxide | 512 | 1.222 | - | [35] |
| biochar | 523 | 0.039 | - | [10] |
| K2CO3 activated biochar (KBC) | 680 | 0.090 | - | [10] |
| oxidated biochar (OKBC) | 289 | 0.103 | - | [10] |
| aminated biochar (NKBC) | 691 | 0.058 | - | [10] |
| aminosilane grafted carbons CKIT-6 | 834 | 0.497 | - | [14] |
| Sorbo Norit activated carbon | 1225 | 1.490 | 2.080 | [19] |
| Ceca AC40 activated carbon | 1201 | 1.560 | 2.599 | [19] |
| Norit R3-ex activated carbon | 1390 | - | 1.896 | [16] |
| HNO3 treated R3-ex | 1296 | - | 1.376 | [16] |
| NH3 treated R3-ex | 1212 | - | 2.703 | [16] |
| activated carbon from willow | 1280 | 2.310 | 2.413 | [20] |
| activated carbon from miscanthus | 1420 | 2.577 | 2.677 | [20] |
| activated carbon from flax shives | 1587 | 2.682 | 2.725 | [20] |
| activated carbon from hemp shives | 1324 | 2.446 | 2.460 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legocka, I.; Kuśmierek, K.; Świątkowski, A.; Wierzbicka, E. Adsorption of 2,4-D and MCPA Herbicides on Carbon Black Modified with Hydrogen Peroxide and Aminopropyltriethoxysilane. Materials 2022, 15, 8433. https://doi.org/10.3390/ma15238433
Legocka I, Kuśmierek K, Świątkowski A, Wierzbicka E. Adsorption of 2,4-D and MCPA Herbicides on Carbon Black Modified with Hydrogen Peroxide and Aminopropyltriethoxysilane. Materials. 2022; 15(23):8433. https://doi.org/10.3390/ma15238433
Chicago/Turabian StyleLegocka, Izabella, Krzysztof Kuśmierek, Andrzej Świątkowski, and Ewa Wierzbicka. 2022. "Adsorption of 2,4-D and MCPA Herbicides on Carbon Black Modified with Hydrogen Peroxide and Aminopropyltriethoxysilane" Materials 15, no. 23: 8433. https://doi.org/10.3390/ma15238433
APA StyleLegocka, I., Kuśmierek, K., Świątkowski, A., & Wierzbicka, E. (2022). Adsorption of 2,4-D and MCPA Herbicides on Carbon Black Modified with Hydrogen Peroxide and Aminopropyltriethoxysilane. Materials, 15(23), 8433. https://doi.org/10.3390/ma15238433








