Comparison and Analysis of Diffusion Models: Growth Kinetics of Diiron Boride Layers on ASTM A283 Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Boriding Treatment
2.2. Description Tools
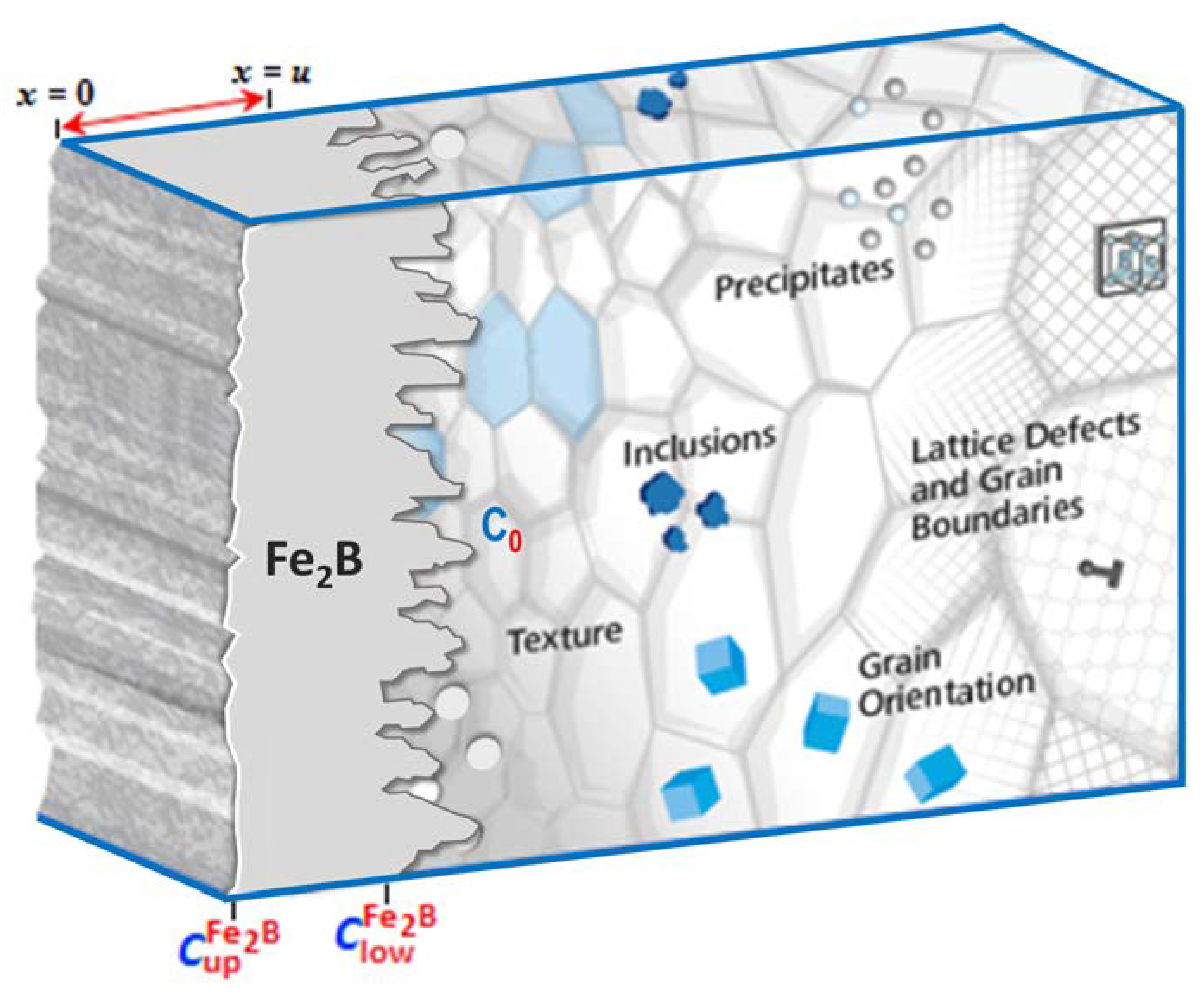
2.3. First Approach: Steady-State Diffusion Model
- (i)
- Once the threshold value of the boron concentration () at the surface is reached, the formation of layers in flat fronts begins;
- (ii)
- An initial Fe2B layer () is formed after a given incubation period ();
- (iii)
- The boride layer grows as a consequence of the perpendicular diffusion of boron atoms on the substrate surface;
- (iv)
- Fe2B layer formation occurs under thermodynamic equilibrium conditions;
- (v)
- The growth kinetics are regulated by the diffusivity of boron atoms in the composition of the boride layer;
- (vi)
- The flow of boron atoms is one-dimensional;
- (vii)
- The boron concentration at the surface and growth interface remains constant in the Fe2B layer during the process;
- (viii)
- The Fe2B layer is narrow in comparison with the thickness of the sample;
- (ix)
- The temperature at each point of the sample is identical during the whole process;
- (x)
- The chemical potential does not vary with time.
2.4. Second Approach: The Integral Diffusion Model
3. Results
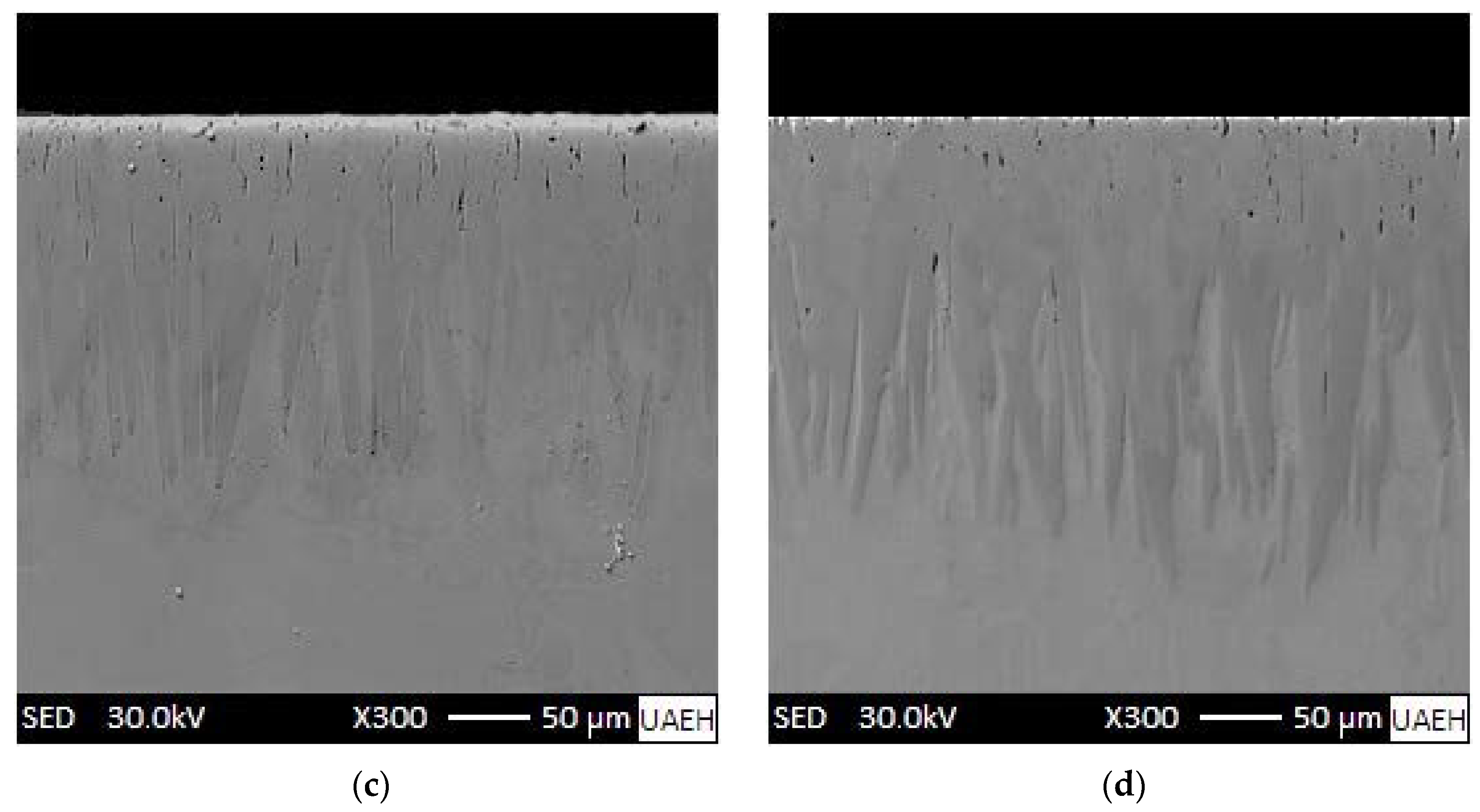
3.1. SEM Examinations of the Cross-Sectional Views of Borided Coatings
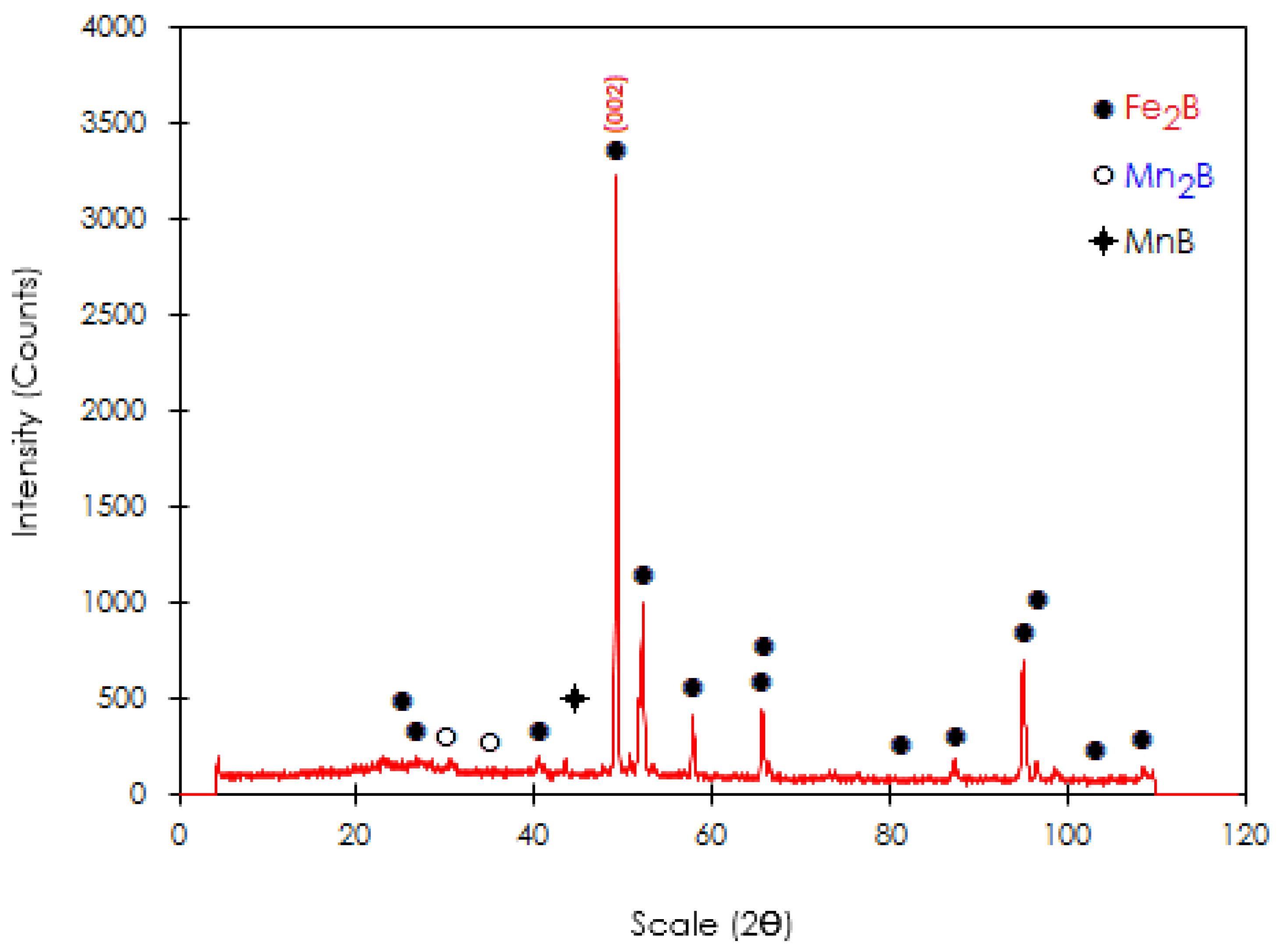
3.2. Peak Profile Analysis in X-ray Diffraction
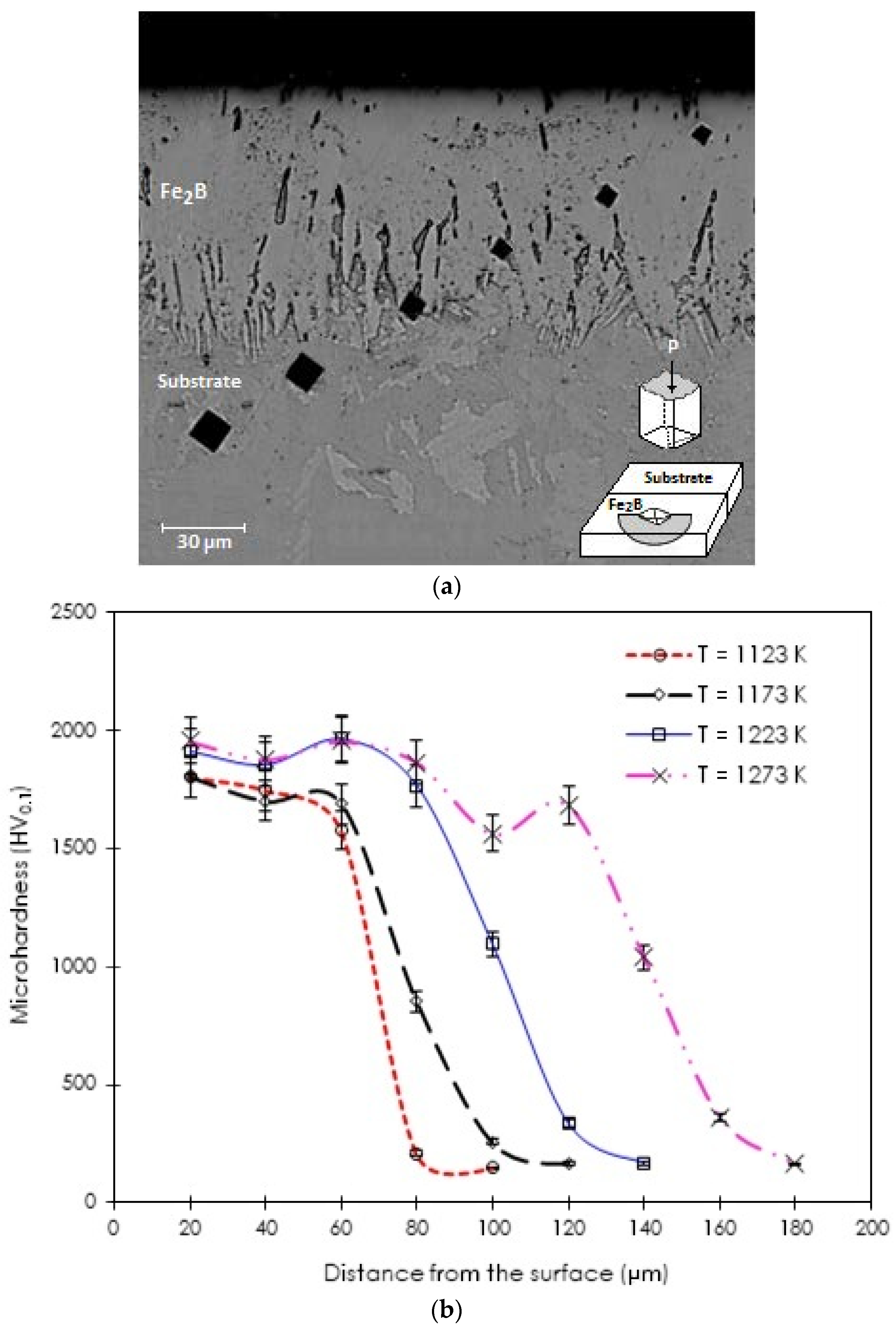
3.3. Microhardness Vickers Profile
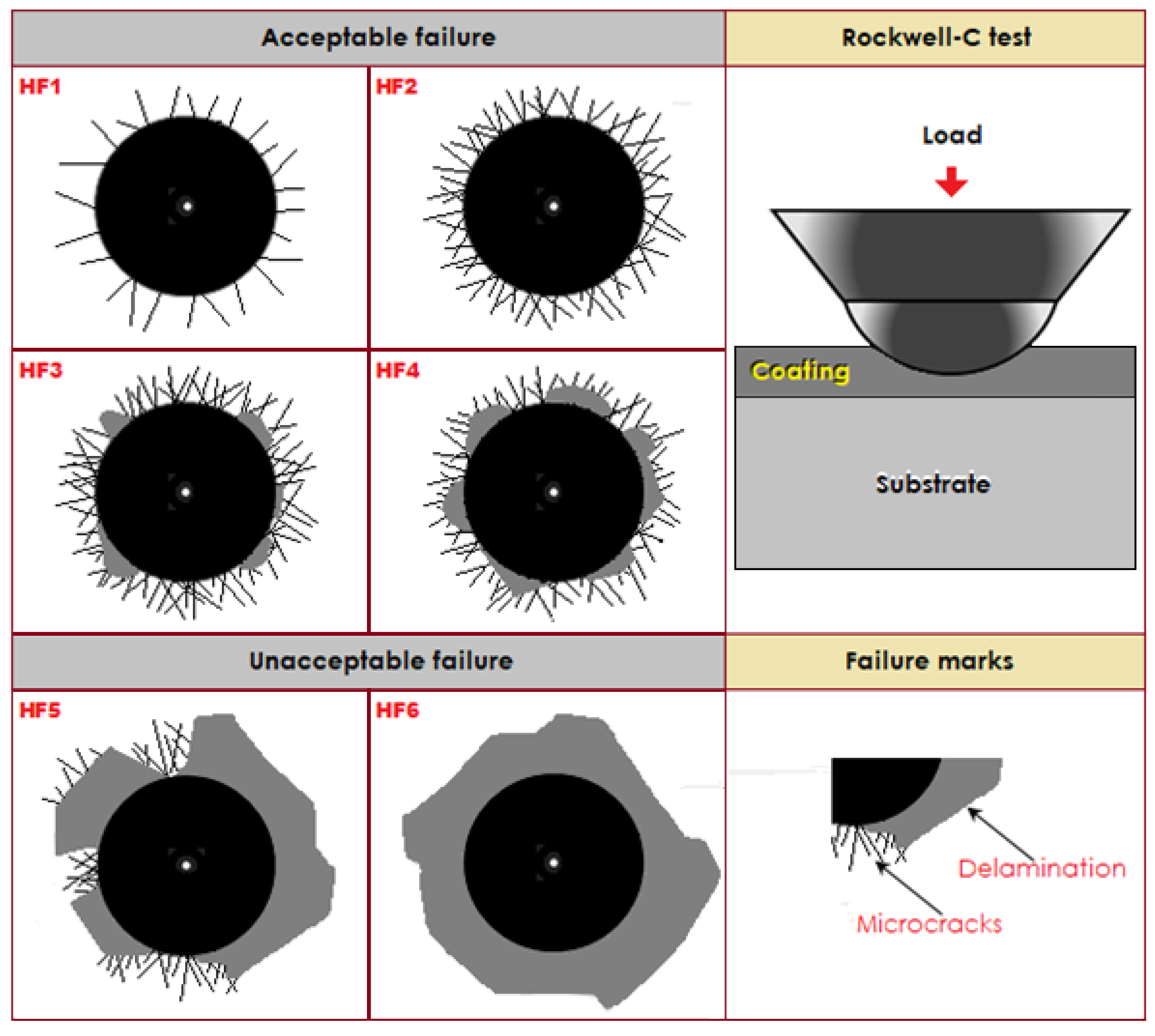
3.4. Rockwell-C Cohesion Indentation Tests
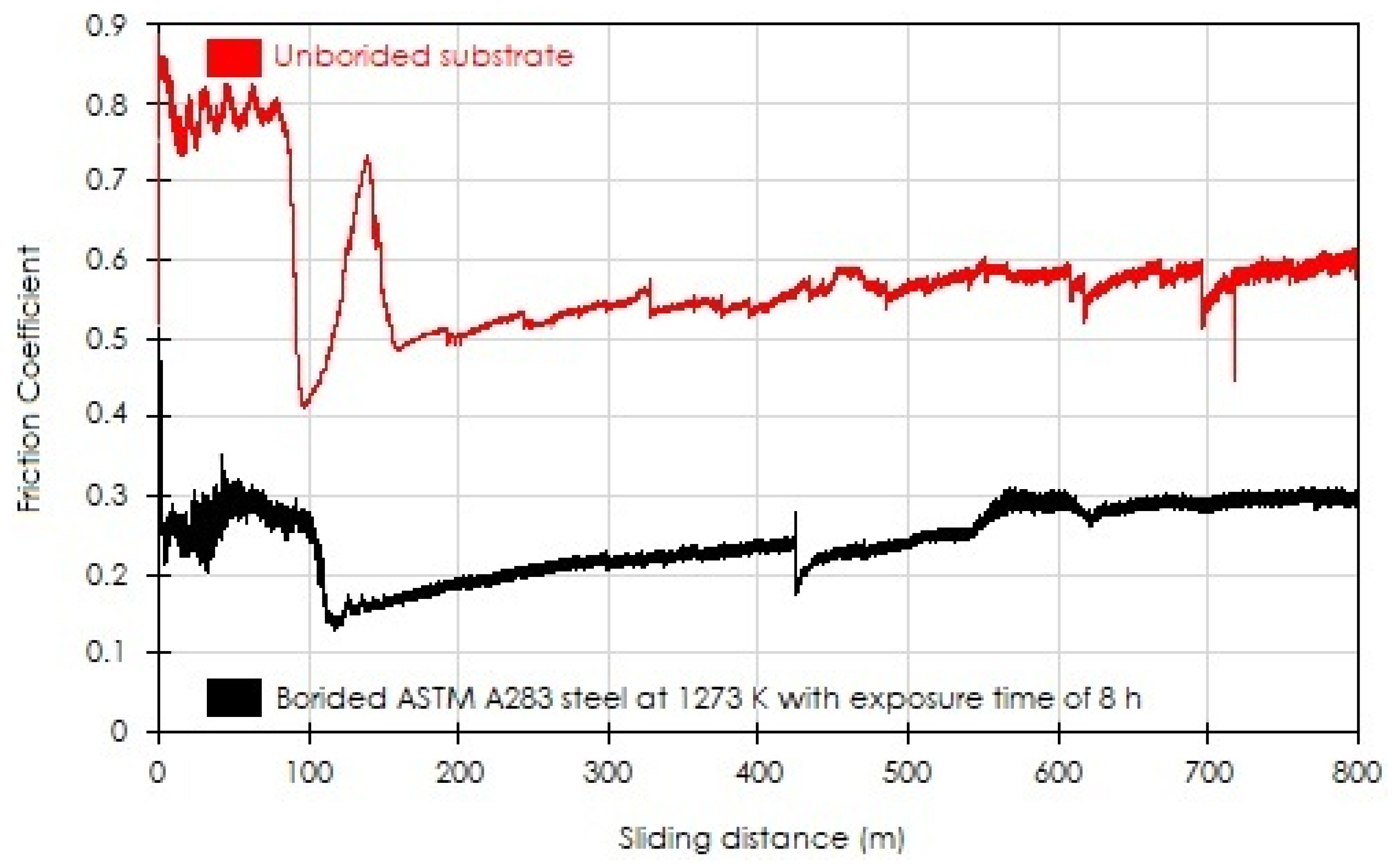
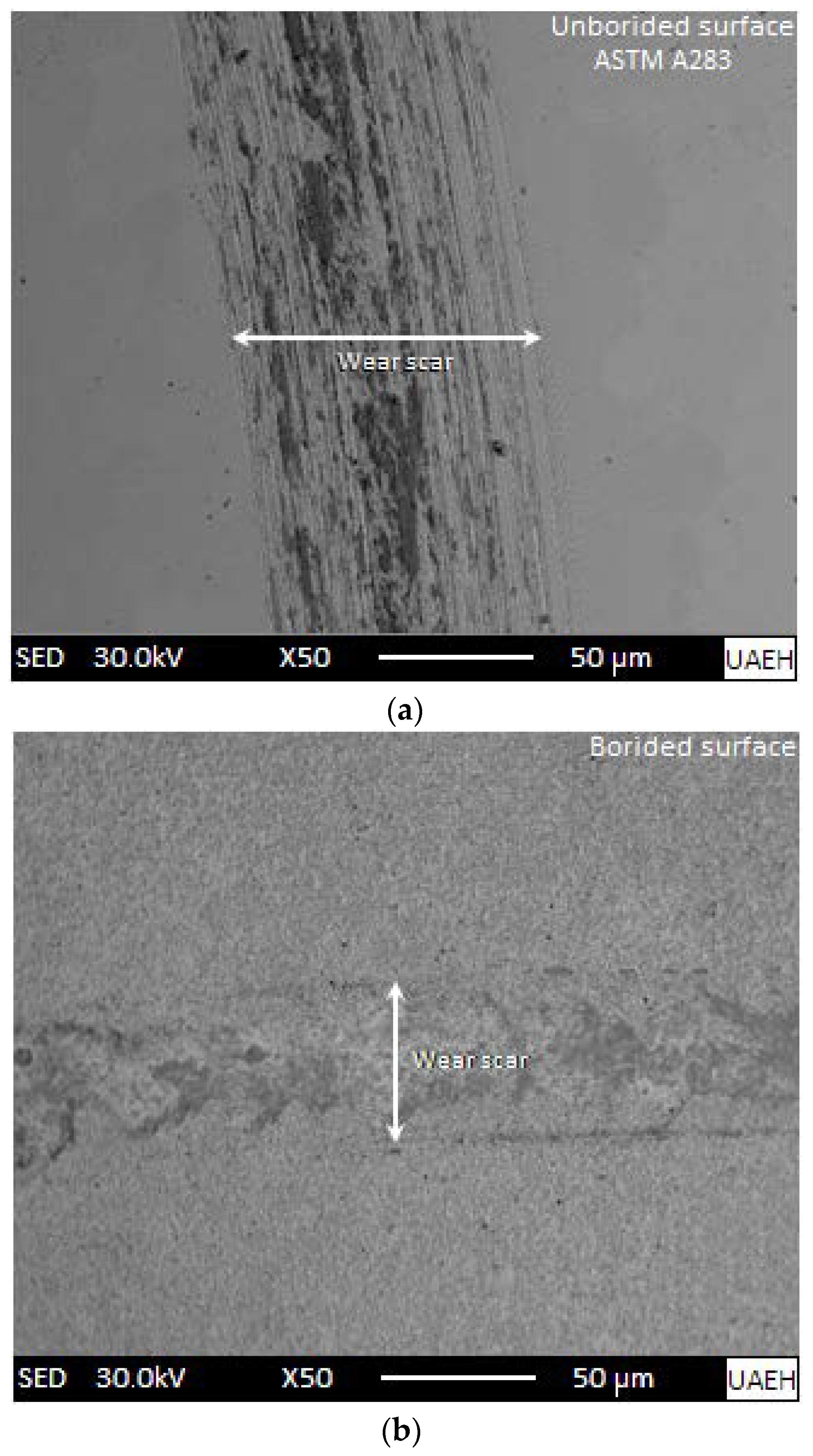
3.5. Pin-on-Disc Tests
3.6. Assessing the Minimum Boron Energy Value in Diiron Boride for ASTM A283 Steel
4. Discussion
Analysis of Mathematical Diffusion Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sepsi, M.; Szobota, P.; Mertinger, V. Quasi-Non-destructive Characterization of Carburized Case Depth by an Application of Centerless X-ray Diffractometers. J. Mater. Eng. Perform. 2022, 31, 4668–4678. [Google Scholar] [CrossRef]
- Koga, N.; Tanahara, K.; Umezawa, O. Deformation Structure Around a Crack in γ′-Fe4N Layer of Nitrided Extra-Low-Carbon Steel Subjected to Cyclic Tensile Test. Met. Mater. Trans. A 2022, 53, 1150–1155. [Google Scholar] [CrossRef]
- Belaid, M.; Fares, M.L.; Assalla, O.; Boukari, F. Surface characterization of a modified cold work tool steel treated by powder-pack boronizing. Mater. Und Werkst. 2022, 53, 15–38. [Google Scholar] [CrossRef]
- Kulka, M. Trends in thermochemical techniques of boriding. In Current Trends in Boriding, 1st ed.; Springer: Cham, Switzerland, 2019; pp. 1–281. [Google Scholar]
- Campos, I.; Oseguera, J.; Figueroa, U.; García, J.A.; Bautista, O.; Kelemenis, G. Kinetic study of boron diffusion in the paste-boriding process. Mater. Sci. Eng. A 2003, 352, 261–265. [Google Scholar] [CrossRef]
- Türkmen, İ.; Yalamaç, E. Effect of Alternative Boronizing Mixtures on Boride Layer and Tribological Behaviour of Boronized SAE 1020 Steel. Met. Mater. Int. 2022, 28, 1114–1128. [Google Scholar] [CrossRef]
- Smol’nikov, E.A.; Sarmanova, L.M. Study of the possibility of liquid boriding of high-speed steels. Met. Sci. Heat Treat. 1982, 24, 785–788. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Piasecki, A. Nanomechanical characterization and fracture toughness of FeB and Fe2B iron borides produced by gas boriding of Armco iron. Surf. Coat. Technol. 2017, 325, 515–532. [Google Scholar] [CrossRef]
- Gunes, I.; Ulker, S.; Taktak, S. Kinetics of plasma paste boronized AISI 8620 steel in borax paste mixtures. Prot. Met. Phys. Chem. Surf. 2013, 49, 567–573. [Google Scholar] [CrossRef]
- Sikorski, K.; Wierzchoń, T.; Bieliński, P. X-ray microanalysis and properties of multicomponent plasma-borided layers on steels. J. Mater. Sci. 1998, 33, 811–815. [Google Scholar] [CrossRef]
- Jain, V.; Sundararajan, G. Influence of the pack thickness of the boronizing mixture on the boriding of steel. Surf. Coat. Technol. 2002, 149, 21–26. [Google Scholar] [CrossRef]
- Türkmen, İ.; Yalamaç, E.; Keddam, M. Investigation of tribological behaviour and diffusion model of Fe2B layer formed by pack-boriding on SAE 1020 steel. Surf. Coat. Technol. 2019, 377, 124888. [Google Scholar] [CrossRef]
- Morgado-González, I.; Ortiz-Dominguez, M.; Keddam, M. Characterization of Fe2B layers on ASTM A1011 steel and modeling of boron diffusion. Mater. Test. 2022, 64, 55–66. [Google Scholar] [CrossRef]
- Campos, I.; Islas, M.; González, E.; Ponce, P.; Ramírez, G. Use of fuzzy logic for modeling the growth of Fe2B boride layers during boronizing. Surf. Coat. Technol. 2006, 201, 2717–2723. [Google Scholar] [CrossRef]
- López Perrusquia, N.; Antonio Doñu Ruiz, M.; Vargas Oliva, E.Y.; Cortez Suarez, V. Diffusion of Hard Coatings on Ductile Cast Iron. MRS Proc. 2012, 1481, 105–112. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Campos-Silva, I.; Hernández-Sánchez, E.; Nava-Sánchez, J.L.; Martínez-Trinidad, J.; Jiménez-Reyes, M.Y.; Damián-Mejía, O. Estimation of Fe2B growth on low-carbon steel based on two diffusion models. Int. J. Mater. Res. 2011, 102, 429–434. [Google Scholar] [CrossRef]
- Abdellah, Z.N.; Keddam, M.; Jurči, P. Simulation of boronizing kinetics of ASTM A36 steel with the alternative kinetic model and the integral method. Koroze A Ochr. Mater. 2021, 65, 33–39. [Google Scholar] [CrossRef]
- Raden, D.R.; Tomohiro, T.; Kisaragi, Y.; Yoshihiro, T. The Effects of Structure Orientation on the Growth of Fe2B Boride by Multi-Phase-Field Simulation. Mater. Trans. 2010, 51, 62–67. [Google Scholar]
- Campos-Silva, I.; Ortiz-Domínguez, M.; VillaVelázquez, C.; Escobar, R.; López, N. Growth kinetics of boride layers: A modified approach, Defect Diffus. Forum 2007, 272, 79–86. [Google Scholar]
- Milinovi’c, A.; Stojši´c, J.; Kladari´c, I.; Matijevi´c, B. Evaluation of Boride Layers on C70W2 Steel Using a New Approach to Characterization of Boride Layers. Materials 2022, 15, 3891. [Google Scholar] [CrossRef]
- VDI 3198; Verein Deutscher Ingenieure Normen. VDI-Verlag Düsseldorf: Düsseldorf, Germany, 1991.
- Ortiz-Domínguez, M.; Gómez-Vargas, O.A.; Keddam, M.; Arenas-Flores, A.; García-Serrano, J. Kinetics of boron diffusion and characterization of Fe2B layers on AISI 9840 steel. Prot. Met. Phys. Chem. Surf. 2017, 53, 534–547. [Google Scholar] [CrossRef]
- Ortiz-Dominguez, M.; Gomez-Vargas, O.A.; Ares de Parga, G.; Torres-Santiago, G.; Velazquez-Mancilla, R.; Caste-llanos-Escamilla, V.A.; Mendoza-Camargo, J.; Trujillo-Sanchez, R. Modeling of the Growth Kinetics of Boride Layers in Powder-Pack Borided ASTM A36 Steel Based on Two Different Approaches. Adv. Mater. Sci. Eng. 2019, 2019, 5985617. [Google Scholar] [CrossRef]
- Brakman, C.M.; Gommers, A.W.J.; Mittemeijer, E.J. Boriding of Fe and Fe–C, Fe–Cr, and Fe–Ni alloys; Boride-layer growth kinetics. J. Mater. Res. 1989, 4, 1354–1370. [Google Scholar] [CrossRef]
- Okamoto, H. B-Fe (boron-iron). J. Phase Equilibria Diffus. 2004, 25, 297–298. [Google Scholar] [CrossRef]
- Krukovich, M.G.; Prusakov, B.A.; Sizov, I.G. The components and phases of systems “Boron-Iron” and “Boron-Carbon-Iron”. In: Plasticity of Boronized Layers. Springer Ser. Mater. Sci. 2016, 237, 13–21. [Google Scholar]
- Yu, L.G.; Chen, X.J.; Khor, K.A.; Sundararajan, G. FeB/Fe2B phase transformation during SPS pack-boriding: Boride layer growth kinetics. Acta Mater. 2005, 53, 2361–2368. [Google Scholar] [CrossRef]
- Ninham, A.J.; Hutchings, I.M. On the morphology of thermochemically produced Fe2B/Fe interfaces. J. Vac. Sci. Technol. A 1986, 4, 2827–2831. [Google Scholar] [CrossRef]
- Rodríguez -Castro, G.; Campos-Silva, I.; Martínez-Trinidad, J.; Figueroa-López, U.; Arzate-Vázquez, I.; Hernández-Sánchez, E.; Hernández-Sánchez, J. Mechanical behavior of AISI 1045 steels subjected to powder-pack boriding. Kov. Mater. 2012, 50, 357–364. [Google Scholar] [CrossRef][Green Version]
- Palombarini, G.; Carbucicchio, M. On the morphology of thermochemically produced Fe2B/Fe interfaces. J. Mater. Sci. Lett. 1984, 3, 791–794. [Google Scholar] [CrossRef]
- Martini, C.; Palombarini, G.; Carbucicchio, M. Mechanism of thermochemical growth of iron borides on iron. J. Mater. Sci. 2004, 39, 933–937. [Google Scholar] [CrossRef]
- VillaVelázquez-Mendoza, C.I.; Rodríguez-Mendoza, J.L.; Ibarra-Galván, V.; Hodgkins, R.P.; López-Valdivieso, A.; Serrato-Palacios, L.L.; Leal-Cruz, A.L.; Ibarra-Junquera, V. Effect of substrate roughness, time and temperature on the processing of iron boride coatings: Experimental and statistical approaches. Int. J. Surf. Sci. Eng. 2014, 8, 713–791. [Google Scholar]
- Zhong, J.; Qin, W.; Wang, X.; Medvedovski, E.; Szpunar, J.A.; Guan, K. Mechanism of Texture Formation in Iron Boride Coatings on Low-Carbon Steel. Met. Mater. Trans. A 2019, 50, 58–62. [Google Scholar] [CrossRef]
- Eroglu, M. Boride coatings on steel using shielded metal arc welding electrode: Microstructure and hardness. Surf. Coat. Technol. 2009, 203, 2229–2235. [Google Scholar] [CrossRef]
- Lin, L.; Han, K. Optimization of surface properties by flame spray coating and boriding. Surf. Coat. Technol. 1998, 106, 100–105. [Google Scholar] [CrossRef]
- Elias-Espinosa, M.; Ortiz-Domínguez, M.; Keddam, M.; Flores-Rentería, M.A.; Damián-Mejía, O.; Zuno-Silva, J.; Hernández-Ávila, E.; Cardoso-Legorreta, E.; Arenas-Flores, A. Growth Kinetics of the Fe2B Layers and Adhesion on Armco Iron Substrate. J. Mater. Eng. Perform. 2014, 23, 2943–2952. [Google Scholar] [CrossRef]
- Kayali, Y.; Kara, R. Investigation of Wear Behavior and Diffusion Kinetic Values of Boronized Hardox-450 Steel. Prot. Met. Phys. Chem. Surf. 2021, 57, 1025–1033. [Google Scholar] [CrossRef]
- Keddam, M.; Ortiz-Domínguez, M.; Cruz-Avilés, A.; Morgado-González, I.; Gómez-Vargas, O.A.; Cardoso-Legorreta, E.; Zuno-Silva, J. Kinetic investigation, metallurgical and tribological properties of diiron boride layers on ASTM A572 steel. Met. Sci. Heat Treat. 2022, accepted. [Google Scholar]
- Ferraro, G. The Rise and Development of the Theory of Series up to the Early 1820s, 1st ed.; Springer: New York, NY, USA, 2008; pp. 1–392. [Google Scholar]
- Fang, H.M.; Zhang, G.S.; Xia, L.S. Properties and Growth Kinetics of the Boride Layer of a Boriding-Strengthened Fe-Based Powder Metallurgical Material. Strength Mater. 2021, 53, 65–72. [Google Scholar] [CrossRef]
- Kartal, G.; Eryilmaz, O.L.; Krumdick, G.; Erdemir, A.; Timur, S. Kinetics of electrochemical boriding of low carbon steel. Appl. Surf. Sci. 2011, 257, 6928–6934. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U.; Bindal, C. An approach to kinetic study of borided steels. Surf. Coat. Technol. 2005, 191, 274–285. [Google Scholar] [CrossRef]
- Arslan, M.; Coskun, O.K.; Karimzadehkhoei, M.; Sireli, G.K.; Timur, S. Evaluation of pulse current integrated CRTD-Bor for boron diffusion in low carbon steel. Mater. Lett. 2022, 308, 131299. [Google Scholar] [CrossRef]
- Milinović, A.; Marušić, V.; Samardžić, I. Research into boride layers growth kinetics on C45 carbon steel. Metalurgija 2016, 55, 671–674. [Google Scholar]
- Su, Z.G.; Lv, X.X.; An, J.; Yang, Y.L.; Sun, S.J. Role of RE Element Nd on Boronizing Kinetics of Steels. J. Mater. Eng. Perform. 2012, 21, 1337–1345. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Moren, A.E. Diffusion modeling of the carburization process. Met. Mater. Trans. A 1978, 9, 1515–1525. [Google Scholar] [CrossRef]
- Cavaliere, P.; Zavarise, G.; Perillo, M. Modeling of the carburizing and nitriding processes. Comput. Mater. Sci. 2009, 46, 26–35. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M. Analysis of the growth kinetics of Fe2B layers by the integral method. J. Min. Metall. Sect. B-Metall. 2018, 54, 361–367. [Google Scholar] [CrossRef]
- Keddam, M.; Ortiz-Dominguez, M.; Elias-Espinosa, M.; Arenas-Flores, A.; Zuno-Silva, J.; Zamarripa-Zepeda, D.; Gómez-Vargas, O.A. Kinetic Investigation and Wear Properties of Fe2B Layers on AISI 12L14 Steel. Met. Mater. Trans. A 2018, 49, 1895–1907. [Google Scholar] [CrossRef]
- Keddam, M.; Elias-Espinosa, M.; Ortiz-Domínguez, M.; Simón-Marmolejo, I.; Zuno-Silva, J. Pack-boriding of AISI P20 steel: Estimation of boron diffusion coefficients in the Fe2B layers and tribological behaviour. Int. J. Surf. Sci. Eng. 2018, 11, 563–585. [Google Scholar] [CrossRef]
- Zuno-Silva, J.; Keddam, M.; Ortiz-Domínguez, M.; Elias-Espinosa, M.; Arenas-Flores, A.; Cervantes-Sodi, F.; Reyes-Retana, J.A. Growth kinetics of Fe2B layers formed on the AISI 4150 steel by different approaches. Indian J. Eng. Mater. Sci. 2020, 27, 277–287. [Google Scholar]
- Zuno-Silva, J.; Keddam, M.; Ortiz-Domínguez, M.; Elias-Espinosa, M.; Cervantes-Sodi, F.; Oseguera-Peña, J.; Fernández-De Dios, L.D.; Gómez-Vargas, O.A. Kinetics of Formation of Fe2B Layers on AISI S1 Steel. Mater. Res. 2018, 21, e20180173. [Google Scholar] [CrossRef]







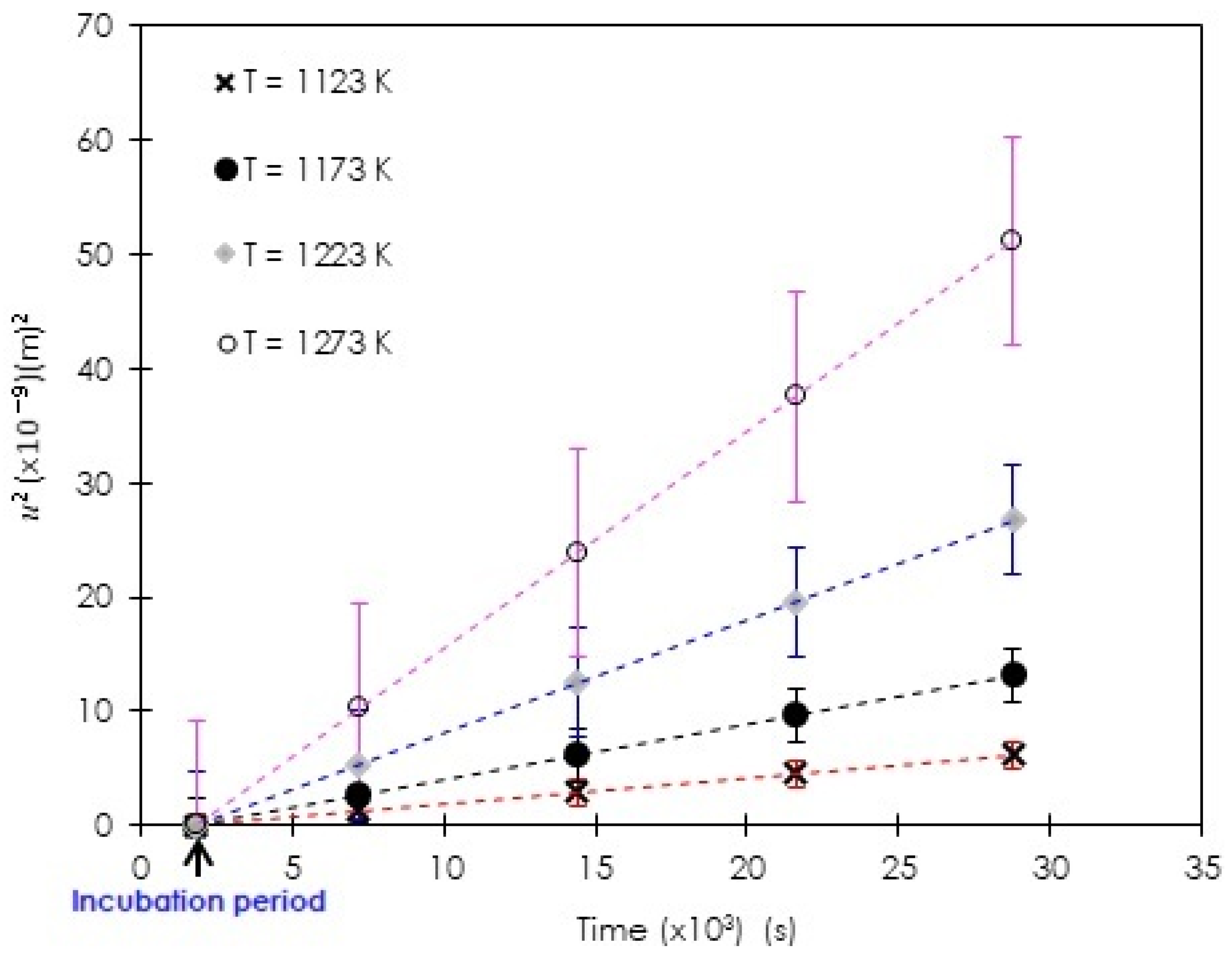
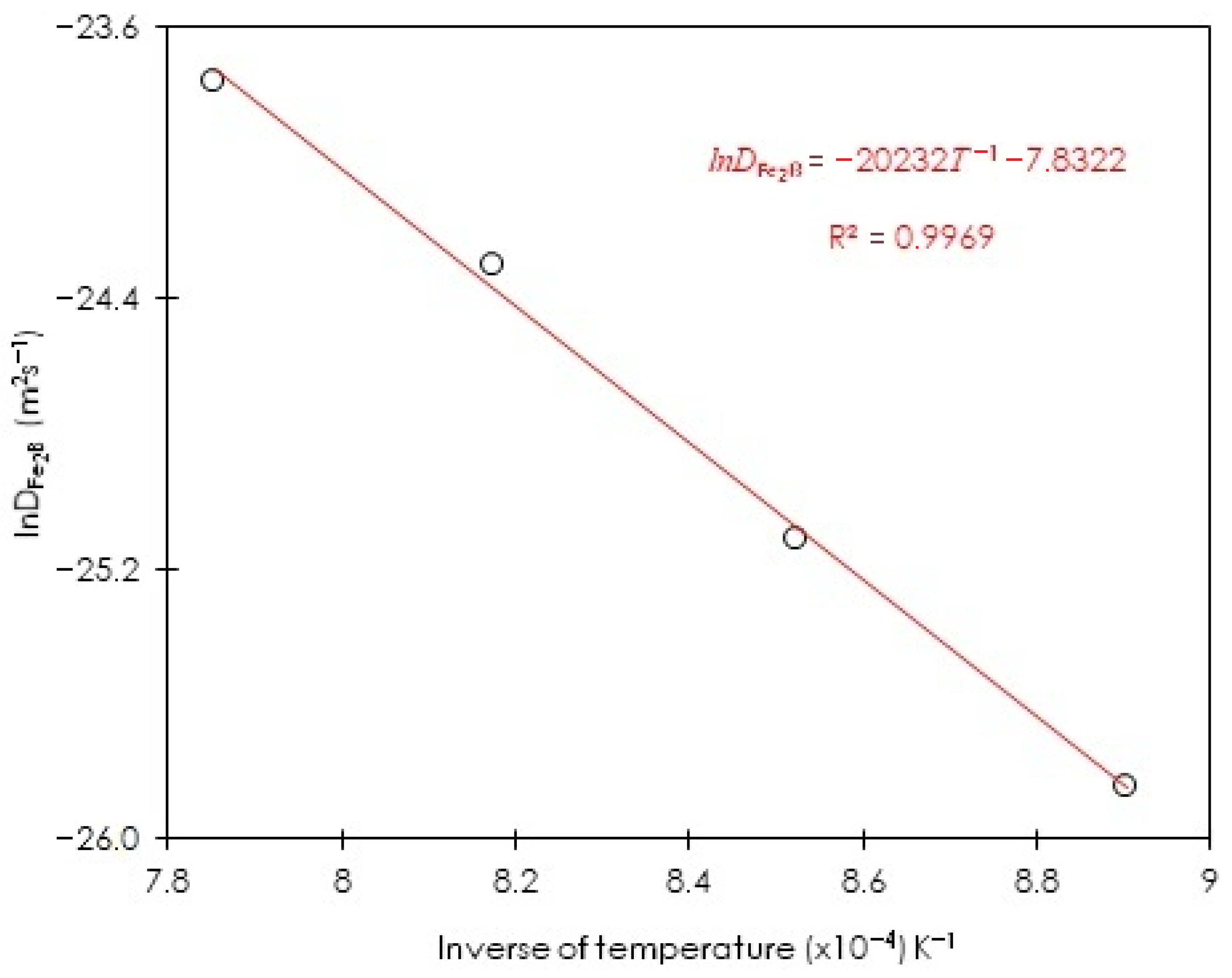
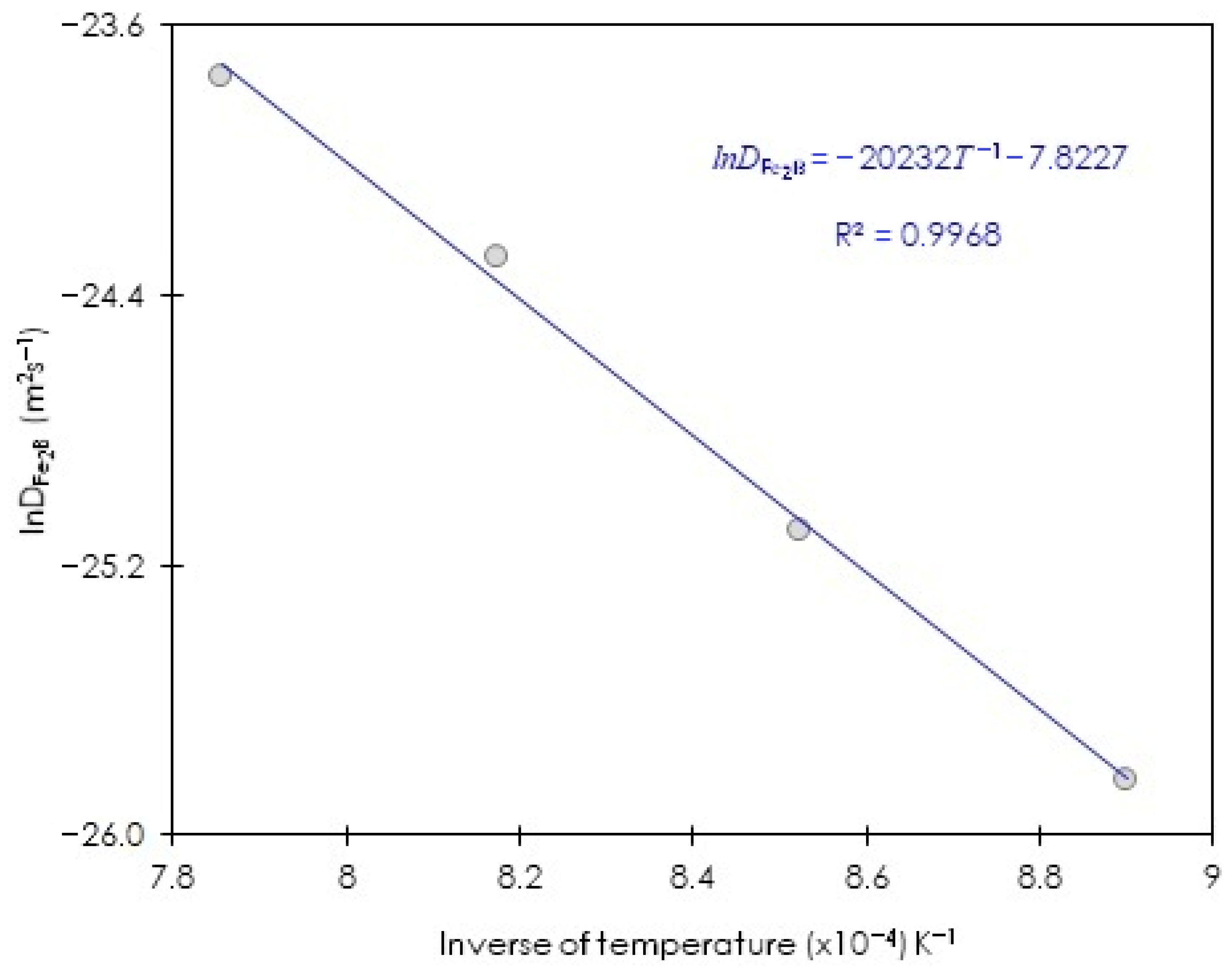
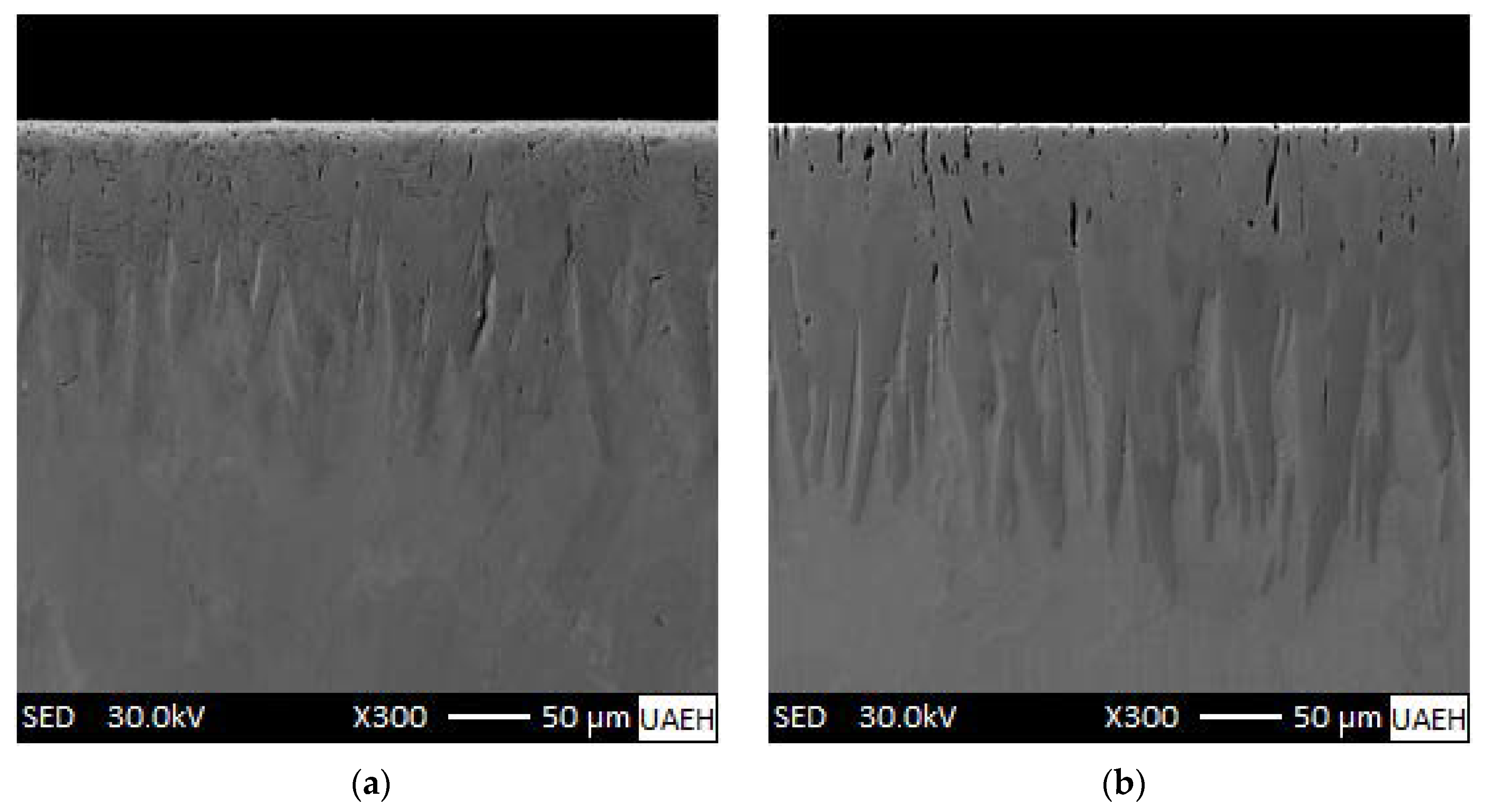
| Boriding Temperature T (K) | Incubation Period (s) | |
|---|---|---|
| 1123 | 2.3 × 10−13 | 1798 |
| 1173 | 4.7 × 10−13 | 1796 |
| 1223 | 1.1 × 10−12 | 1796 |
| 1273 | 1.8 × 10−12 | 1795 |
| Type of Steel | Boriding Process | Activation Energy (kJmol−1) | Temperature Range of Investigation (K) | Approach for Calculation | References |
|---|---|---|---|---|---|
| AISI 8620 | Plasma-paste boriding | (FeB + Fe2B) 99–108 | 973–1073 | Parabolic growth law | [9] |
| ASTM A1011 | Powder | (Fe2B) 159 | 1123–1273 | Mean diffusion coefficient method | [13] |
| Hardox–450 | Powder | (Fe2B) 158 | 1123–1223 | Parabolic relation | [37] |
| PM Iron alloy at 3 wt.%C | Powder | (FeB + Fe2B) 164 | 1123–1223 | Parabolic relation | [40] |
| AISI 1018 | Electrochemical boriding | (FeB + Fe2B) 173 ± 8 | 1123–1273 | Parabolic relation | [41] |
| AISI 5140 | Salt bath | (FeB + Fe2B) 223 | 1123–1273 | Parabolic relation | [42] |
| Low carbon steel | Pulse current integrated CRTD-Bor | Dominant Fe2B 38 | 1123–1323 | Parabolic relation | [43] |
| C45 steel | Powder | (FeB + Fe2B) 199 | 1143–1243 | Parabolic relation | [44] |
| AISI 1045 | Powder | (FeB + Fe2B) 198–137 with wt.% Nd2O3 | 1053–1213 | Parabolic relation | [45] |
| ASTM A283 | Powder | (Fe2B) 168 | 1123–1273 | Steady-state diffusion model and Integral method | This work |
| Boronizing Conditions T (K) | Experimental Values of Fe2B Layers’ Thicknesses (μm) | Calculated Fe2B Layers’ Thicknesses (μm) |
|---|---|---|
| 1223 K for 9 h | 181 ± 27 | 175 |
| 1273 K for 9 h | 241 ± 38 | 246 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Domínguez, M.; Gómez-Vargas, O.A.; Bárcenas-Castañeda, M.; Castellanos-Escamilla, V.A. Comparison and Analysis of Diffusion Models: Growth Kinetics of Diiron Boride Layers on ASTM A283 Steel. Materials 2022, 15, 8420. https://doi.org/10.3390/ma15238420
Ortiz-Domínguez M, Gómez-Vargas OA, Bárcenas-Castañeda M, Castellanos-Escamilla VA. Comparison and Analysis of Diffusion Models: Growth Kinetics of Diiron Boride Layers on ASTM A283 Steel. Materials. 2022; 15(23):8420. https://doi.org/10.3390/ma15238420
Chicago/Turabian StyleOrtiz-Domínguez, Martín, Oscar Armando Gómez-Vargas, Mariana Bárcenas-Castañeda, and Víctor Augusto Castellanos-Escamilla. 2022. "Comparison and Analysis of Diffusion Models: Growth Kinetics of Diiron Boride Layers on ASTM A283 Steel" Materials 15, no. 23: 8420. https://doi.org/10.3390/ma15238420
APA StyleOrtiz-Domínguez, M., Gómez-Vargas, O. A., Bárcenas-Castañeda, M., & Castellanos-Escamilla, V. A. (2022). Comparison and Analysis of Diffusion Models: Growth Kinetics of Diiron Boride Layers on ASTM A283 Steel. Materials, 15(23), 8420. https://doi.org/10.3390/ma15238420






