Effect of Cathodic Protection Potential Fluctuations on the Corrosion of Low-Carbon Steels and Hydrogen Absorption by the Metal in Chloride Solutions with Nearly Neutral pH
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
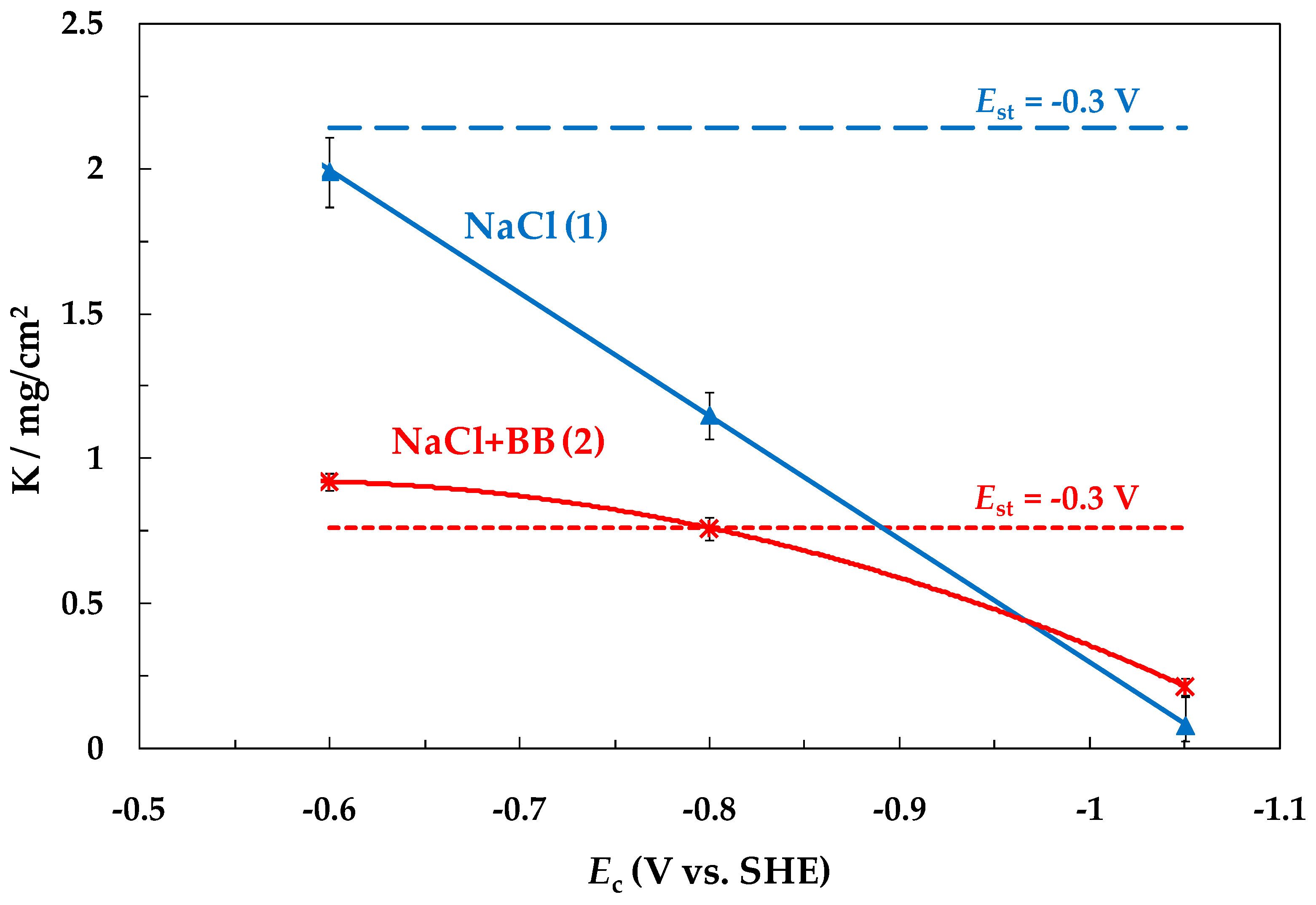
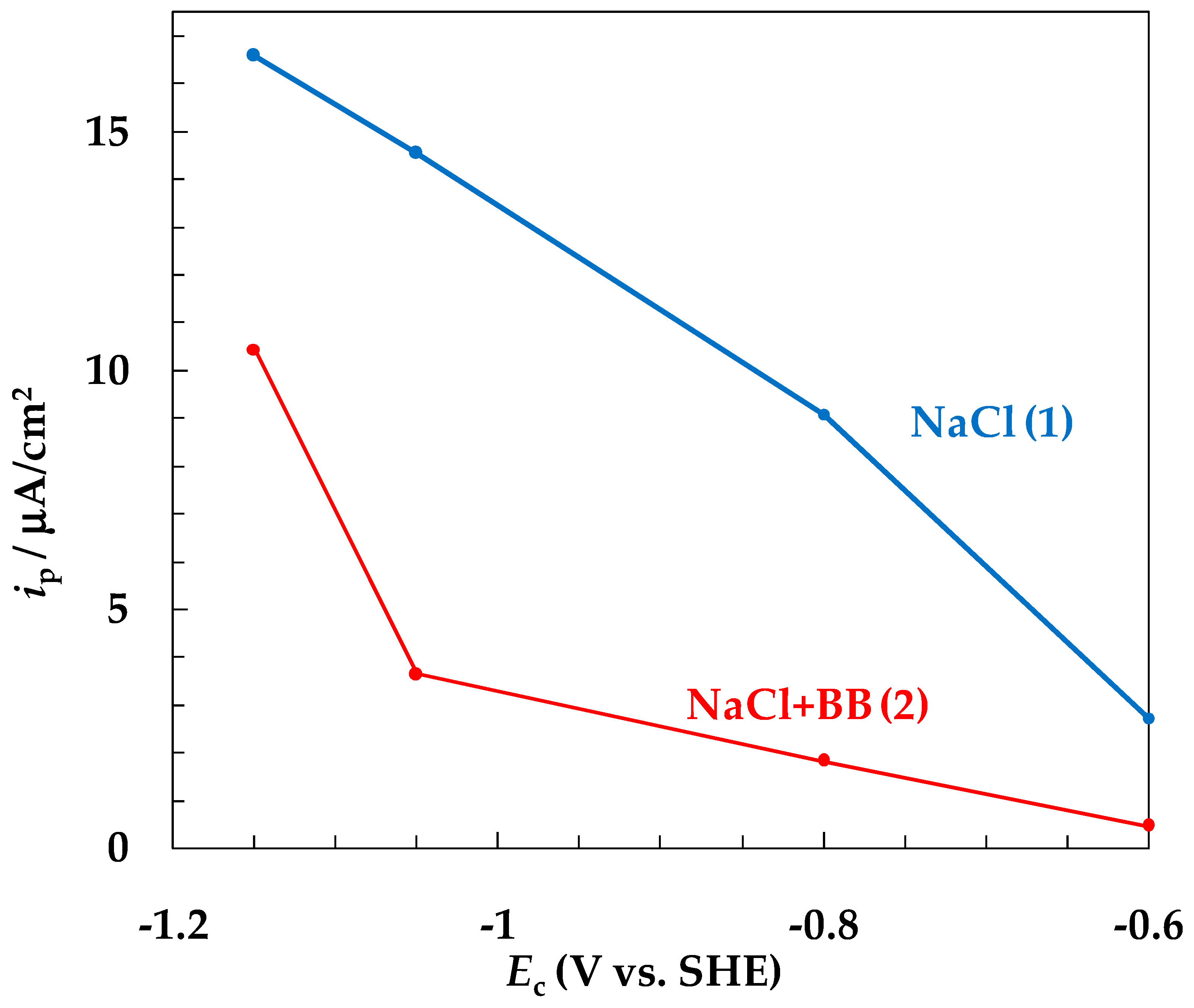
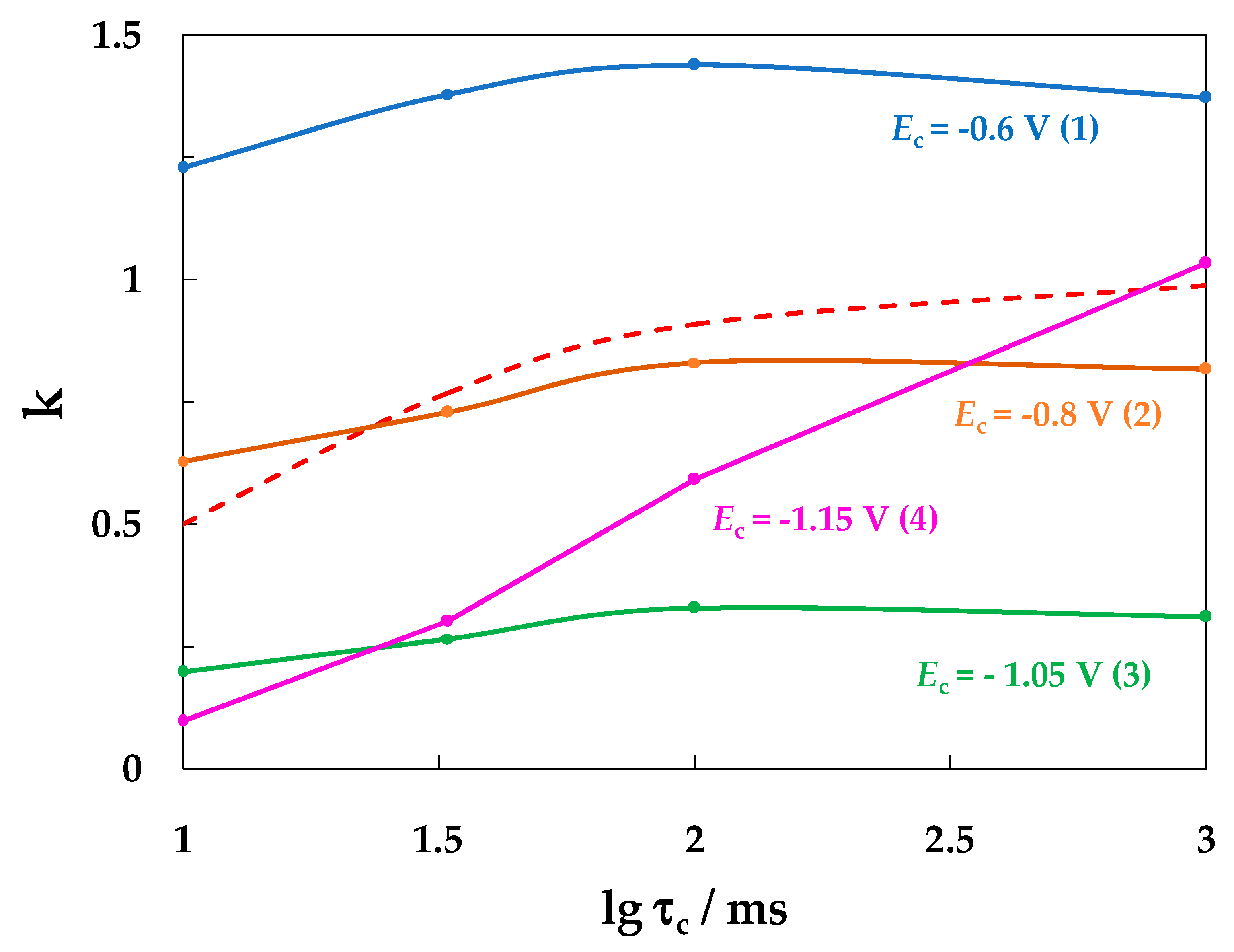
- With a decrease in the SACP cathodic half-period potential (Ec), the rate of general corrosion of carbon steel determined by the gravimetric method decreased both in buffered and unbuffered 3.5% NaCl solutions. A decrease in the rate of general corrosion under SACP correlated with an increase in the amount of absorbed hydrogen determined from the rate of hydrogen penetration through a steel membrane, which is consistent with the effect of anodic dissolution inhibition of iron at a constant potential and an increase in the degree of hydrogen coverage of the electrode surface [26,27,28,32,33].
- (2)
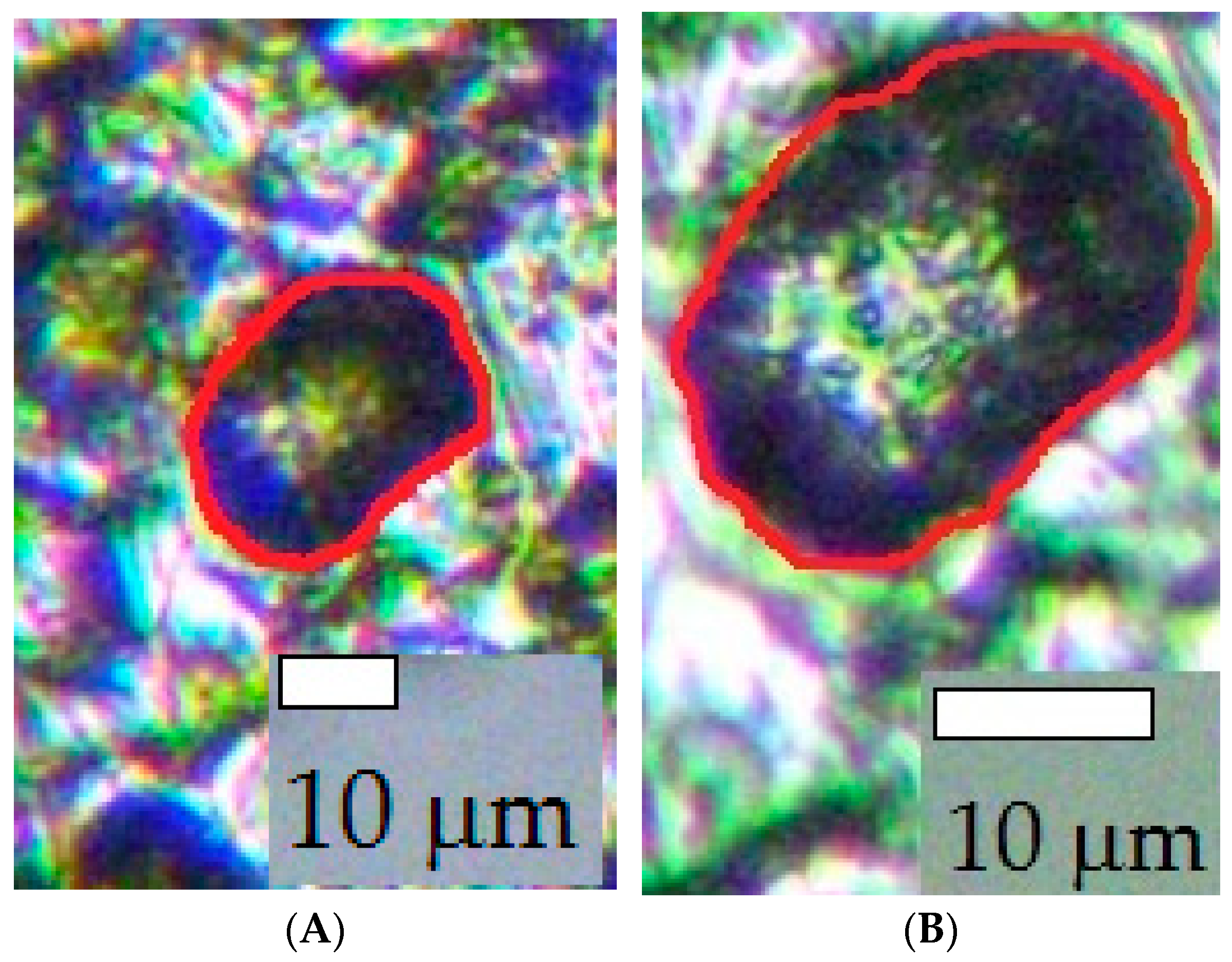
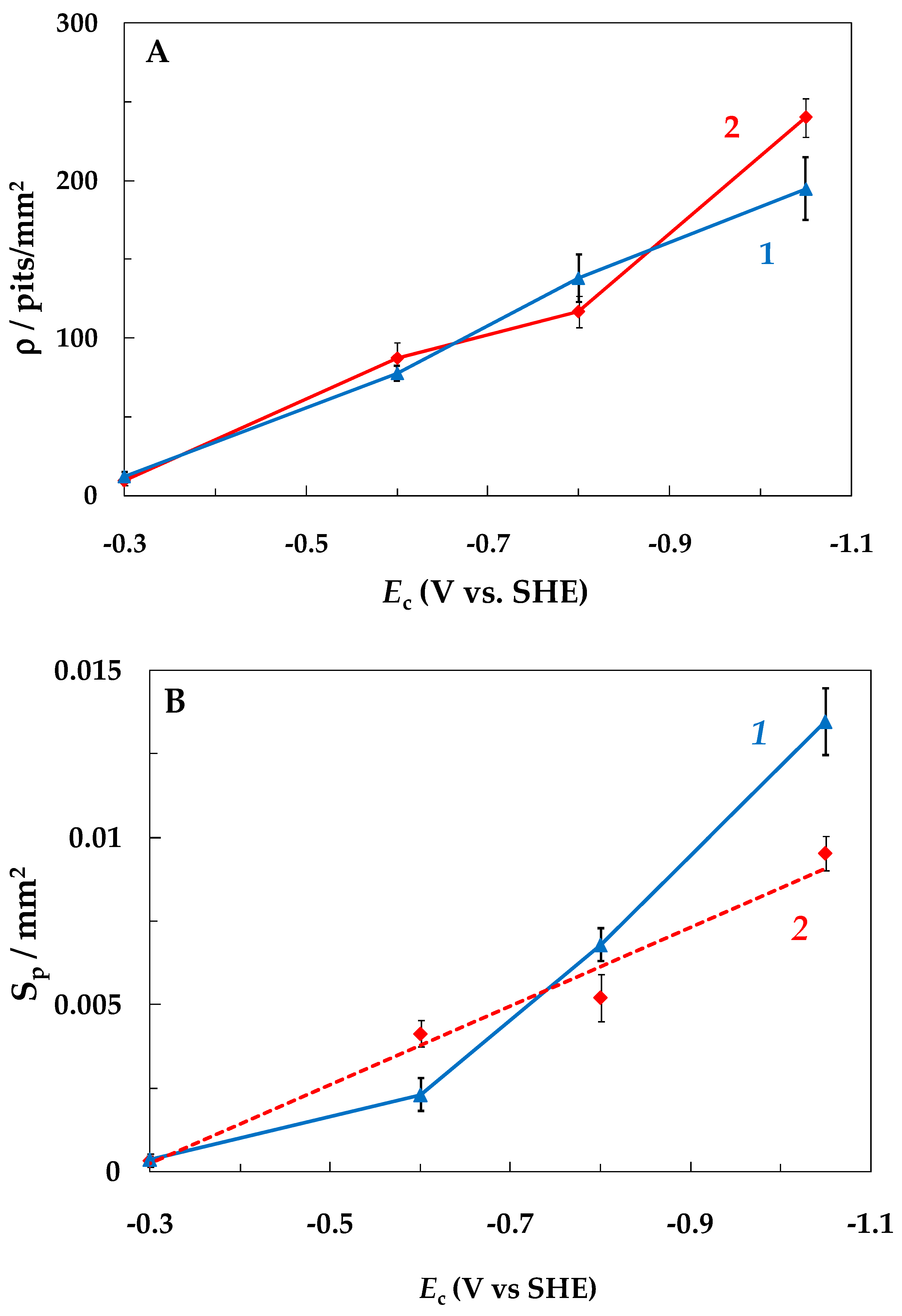
- With a decrease in Ec, the intensity of steel local corrosion, namely, the pit density and the total area of pits, increased both in buffered and unbuffered chloride solutions. Hence, an increase in the near-electrode solution pH is not the main cause of steel pitting corrosion under SACP.
- (3)
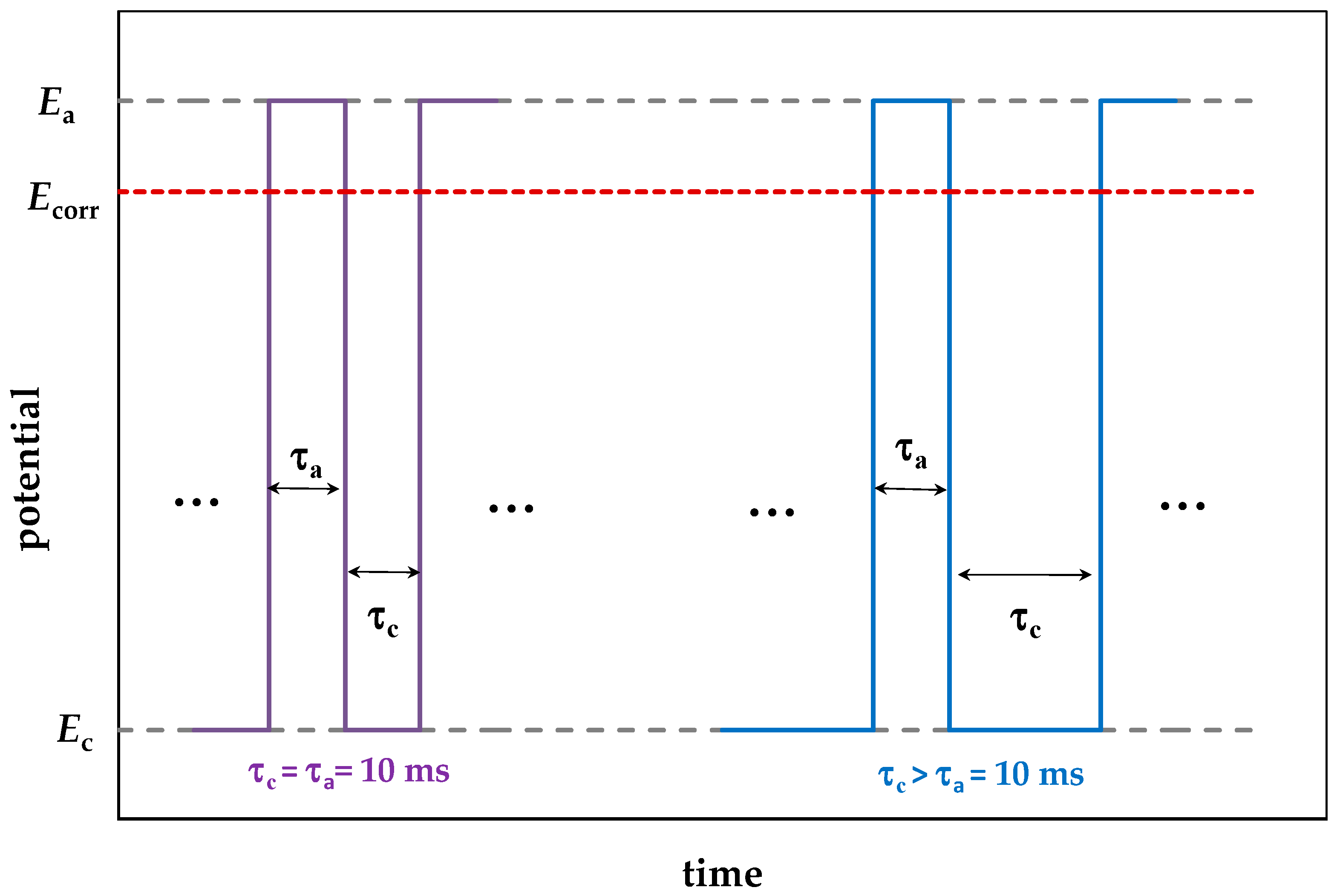
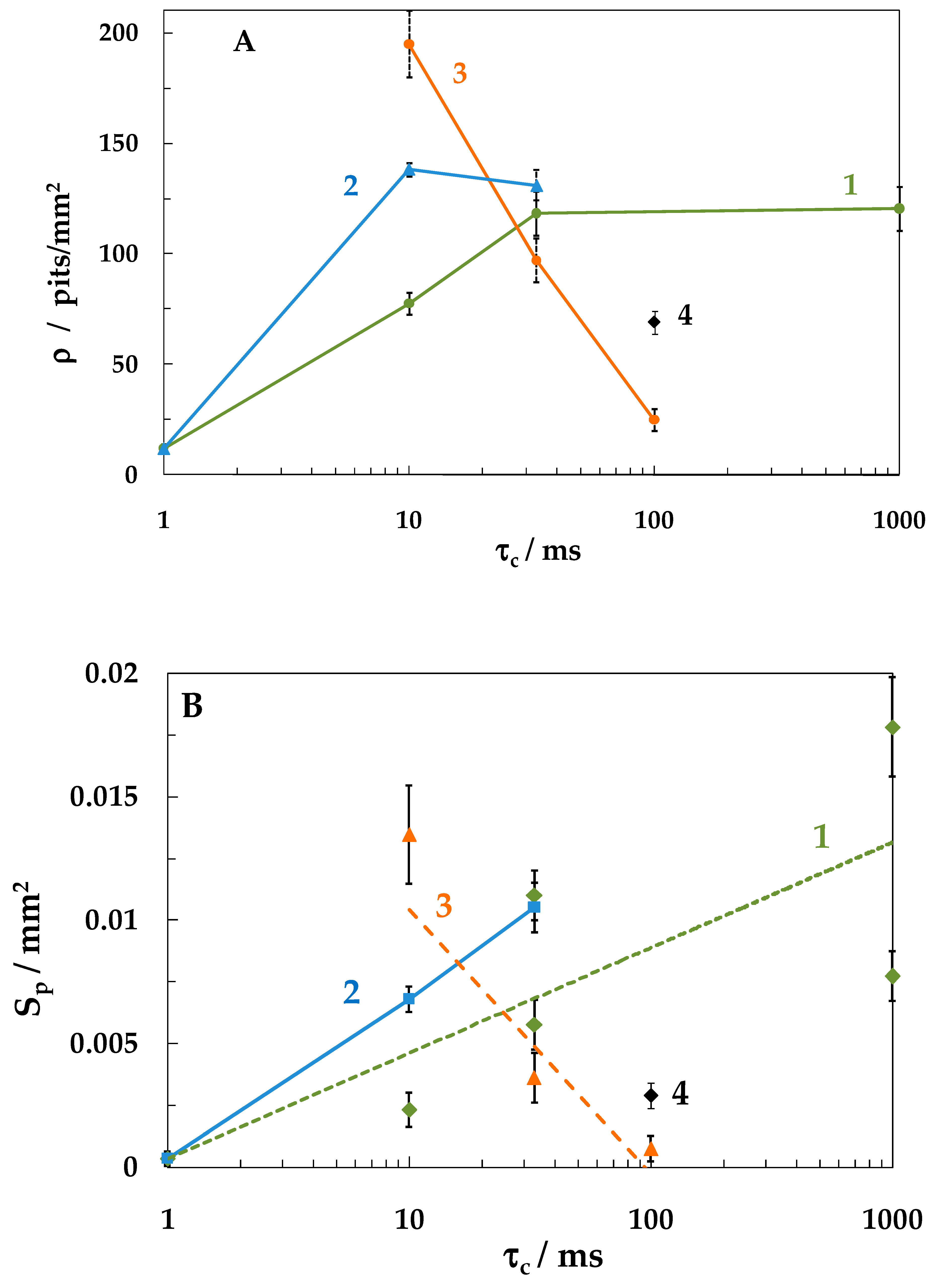
- An increase in the cathodic half-period duration increased the pit density and total area at less negative Ec values (−0.6 and −0.8 V), which correlated with an increase in the amount of hydrogen absorbed by the metal. At Ec = −1.05 V, the opposite effect was observed, which might be explained by deposition of iron oxide/hydroxide compounds on the metal. These compounds are insoluble in the near-surface electrolyte layer with a high pH. As a result, the layer of steel dissolution products blocks the centers of pit nucleation.
- (4)
- Based on the combination of data obtained, we conclude that the absorption of hydrogen by the metal determines the corrosive behavior of carbon steel under SACP in chloride solutions, i.e., it reduces the rate of general corrosion and increases the intensity of pitting corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AC Corrosion State-of-the-Art: Corrosion Rate, Mechanism and Mitigation Requirements, NACE, 2010, TG-35110. Available online: https://standards.globalspec.com/std/1243051/NACE%2035110 (accessed on 1 October 2022).
- Cheng, Y.F. Understand the AC induced pitting corrosion on pipelines in both high pH and neutral pH carbonate/bicarbonate solutions. Corros. Sci. 2014, 85, 304–310. [Google Scholar] [CrossRef]
- Marshakov, A.I.; Nenasheva, T.A.; Kasatkin, V.E.; Kasatkina, I.V. Effect of alternating current on dissolution rate of carbon steel in chloride electrolyte. II. Cathodic potentials. Prot. Met. Phys. Chem. Surf. 2018, 54, 1236–1245. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.L.; Wu, T.Q.; Yan, M.C.; Yu, C.K.; Sun, C. Effect of elastic stress and alternating current on corrosion of X80 pipeline steel in simulated soil solution. Fail. Anal. 2019, 100, 192–205. Available online: https://www.researchgate.net/publication/312318619 (accessed on 1 October 2022). [CrossRef]
- Shubina Helbert, V.; Nazarov, A.; Vucko, F.; Larché, N.; Thierry, D. Effect of Cathodic Polarisation Switch-Off on the Passivity and Stability to Crevice Corrosion of AISI 304L Stainless Steel. Materials 2021, 14, 2921. [Google Scholar] [CrossRef]
- Wan, H.; Song, D.; Liu, Z.; Du, C.; Zeng, Z.; Yang, X.; Li, X. Effect of alternating current on stress corrosion cracking behavior and mechanism of X80 pipeline steel in near-neutral solution. J. Nat. Gas. Sci. Eng. 2017, 38, 458–465. [Google Scholar] [CrossRef]
- Wu, W.; Pan, Y.; Liu, Z.; Du, C.; Li, X. Electrochemical and Stress Corrosion Mechanism of Submarine Pipeline in Simulated Seawater in Presence of Different Alternating Current Densities. Materials 2018, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Song, D.; Cai, Y.; Du, C. The AC corrosion and SCC mechanism of X80 pipeline steel in near neutral pH solution. Eng. Fail. Anal. 2020, 118, 104904. [Google Scholar] [CrossRef]
- Nenasheva, T.A.; Marshakov, A.I.; Ignatenko, V.E. The Influence of Alternating Current on Stress Corrosion Cracking of Grade X70 Pipe Steel. Prot. Met. Phys. Chem. Surf. 2020, 56, 1223–1231. [Google Scholar] [CrossRef]
- Li, Z.; Sun, B.; Liu, Q.; Yu, Y.; Liu, Z. Fundamentally understanding the effect of Non-stable cathodic potential on stress corrosion cracking of pipeline steel in Near-neutral pH solution. Constr. Build. Mater. 2021, 288, 123117. [Google Scholar] [CrossRef]
- ISO 18086; Corrosion of Metals and Alloys. Determination of AC Corrosion. Protection Criteria. ISO—International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/78148.html (accessed on 1 October 2022).
- Peabody, A.W. Peabody’s Control of Pipeline Corrosion, 2nd ed.; NACE International the Corrosion Society: Houston, TX, USA, 2001; pp. 226–231. Available online: https://www.cabdirect.org/cabdirect/abstract/20013090552 (accessed on 1 October 2022).
- Baeckmann, W.; Schwenk, W. Handbuch des kathodischen Korrosionsschutzes. In Theorie und Praxis der Elektrochemischen Schutzverfahren; Verlag Chemie: Weinheim, Germany, 1980; pp. 15–61. Available online: https://scholar.google.com/scholar_lookup?title=Handbuch+des+kathodischen+Korrosionsschutzes&author=Baeckmann,+W.&author=Schwenk,+W.&publication_year=1980&pages=15–61 (accessed on 1 October 2022).
- Huo, Y.; Tan, M.Y.; Forsyth, M. Visualizing dynamic passivation and localized corrosion processes occurring on buried steel surfaces under the effect of anodic transients. Electrochem. Commun. 2016, 66, 21–24. Available online: https://www.sciencedirect.com/science/article/pii/S1388248116300327?via%3Dihub (accessed on 1 October 2022). [CrossRef]
- Huo, Y.; Tan, M.Y.J. Measuring and understanding the critical duration and amplitude of anodic transients. Corros. Eng. Sci. Technol. 2017, 52, 65–72. [Google Scholar] [CrossRef]
- Huo, Y.; Tan, M.Y. Localized corrosion of cathodically protected pipeline steel under the effects of cyclic potential transients. Corros. Eng. Sci. Technol. 2018, 53, 348–354. [Google Scholar] [CrossRef]
- Gupta, R.K.; Tan, M.Y.; Esquivel, J.S.; Forsyth, M. Occurrence of anodic current and corrosion of steel in aqueous media under fluctuating cathodic protection potentials. Corrosion 2016, 72, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Li, X.G.; Du, C.W.; Cheng, Y.F. Local additional potential model for effect of strain rate on SCC of pipeline steel in an acidic soil solution. Corros. Sci. 2009, 51, 2863–2871. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Electrochemical state conversion model for occurrence of pitting corrosion on a cathodically polarized carbon steel in a near-neutral pH solution. Electrochim. Acta 2011, 56, 4167–4175. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Understand the occurrence of pitting corrosion of pipeline carbon steel under cathodic polarization. Electrochim. Acta 2012, 60, 259–263. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Cui, Z.Y.; Li, X.G.; Du, C.W.; Xing, Y.Y. Mechanistic aspect of stress corrosion cracking of X80 pipeline steel under non-stable cathodic polarization. Electrochem. Commun. 2014, 48, 127–129. [Google Scholar] [CrossRef]
- Dai, M.; Liu, J.; Huang, F.; Zhang, Y.; Cheng, Y.F. Effect of cathodic protection potential fluctuations on pitting corrosion of X100 pipeline steel in acidic soil environment. Corros. Sci. 2018, 143, 428–437. [Google Scholar] [CrossRef]
- Dai, M.; Liu, J.; Huang, F.; Hu, Q.; Cao, C.; Cheng, Y.F. Derivation of the mechanistic relationship of pit initiation on pipelines resulting from cathodic protection potential fluctuation. Corros. Sci. 2020, 163, 108226. [Google Scholar] [CrossRef]
- Rybkina, A.; Gladkikh, N.; Marshakov, A.; Petrunin, M.; Nazarov, A. Effect of Sign Alternating Cyclic Polarisation and Hydrogen Uptake on the Localised Corrosion of X70 Pipeline Steel in Near Neutral Solutions. Metals 2020, 10, 245. [Google Scholar] [CrossRef]
- Marshakov, A.I.; Nenasheva, T.A. The formation of corrosion defects upon cathodic polarization of X70 grade pipe steel. Prot. Met. Phys. Chem. Surf. 2015, 51, 1122–1132. [Google Scholar] [CrossRef]
- Marshakov, A.I.; Rybkina, A.A. Dissolution of iron and ionization of hydrogen in borate buffer under cyclic pulse polarization. Int. J. Electrochem. Sci. 2019, 14, 9468–9481. [Google Scholar] [CrossRef]
- Marshakov, A.I.; Rybkina, A.A.; Maleeva, M.A.; Rybkin, A.A. The effect of atomic hydrogen on the kinetics of iron passivation in neutral solution. Prot. Metals Phys. Chem. Surf. 2014, 3, 345–351. [Google Scholar] [CrossRef]
- Marshakov, A.I.; Rybkina, A.A.; Maksaeva, L.B.; Petrunin, M.A.; Nazarov, A.P. A study of the initial stages of iron passivation in neutral solutions using the quartz crystal resonatortechnique. Prot. Metals Phys. Chem. Surf. 2016, 5, 936–946. [Google Scholar] [CrossRef]
- Nenasheva, T.A.; Marshakov, A.I. Kinetics of Dissolution of Hydrogenated Carbon Steel in Electrolytes with pH Close to Neutral. Prot. Met. Phys. Chem. Surf. 2015, 51, 1018–1026. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stahurski, Z. The adsorption and diffusion of electrolytic hydrogen in palladium. Proc. Math. Phys. Eng. Sci. 1962, 270, 92–107. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Nenasheva, T.A.; Luchkin, A.Y.; Marshakov, A.I.; Kuznetsov, Y.I. Effect of Quaternary Ammonium Salts and 1,2,4-Triazole Derivatives on Hydrogen Absorption by Mild Steel in Hydrochloric Acid Solution. Materials 2022, 15, 6989. [Google Scholar] [CrossRef]
- Marshakov, A.I.; Rybkina, A.A.; Skuratnik, Y.B. Effect of absorbed hydrogen on the iron dissolution. Russ. J. Electrochem. 2000, 36, 1101–1107. [Google Scholar] [CrossRef]
- Rybkina, A.A.; Maleeva, M.A.; Marshakov, A.I. The effect of hydrogen sorbed by iron on anodic dissolution of metal in sulfate electrolytes. Prot. Metals. Phys. Chem. Surf. 2013, 49, 805–810. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The mechanism of hydrogen evolution on iron in acid solutions by determination of permeation rates. J. Electrochem. Soc. 1964, 3, 619–623. [Google Scholar] [CrossRef]
- Bockris, J.O.; McBreen, J.; Nanis, L. The hydrogen evolution kinetics and hydrogen entry into α-Iron. J. Electrochem. Soc. 1965, 112, 1025–1031. [Google Scholar] [CrossRef]
- Harrington, S.P.; Wang, F.; Devine, T.M. The structure and electronic properties of passive and prepassive films of iron in borate buffer. Electrochim. Acta 2010, 55, 4092–4102. Available online: https://www.sciencedirect.com/science/article/pii/S0013468609013735?via%3Dihub (accessed on 1 October 2022).
- Wroblova, H.; Brusic, V.; Bockris, J.O. Ellipsometric investigations of anodic film growth on iron in neutral solution. Prepassive film. J. Phys. Chem. 1971, 75, 2823–2829. [Google Scholar] [CrossRef]
- Lorenz, W.J.; Staikov, G.; Schindler, W.; Wiesbeck, W. The Role of Low-Dimensional Systems in Electrochemical Phase Formation and Dissolution Processes. J. Electrochem. Soc. 2002, 149, K47. [Google Scholar] [CrossRef]
- Marshakov, A.I.; Maksayeva, L.B.; Mikhailovskii, Y.N. Study of a discharge of hydronium ions and permeation of hydrogen in iron in conditions of the anodic polarization. Zashchita Metallov 1993, 29, 857–868. Available online: https://elibrary.ru/item.asp?id=12733161 (accessed on 1 October 2022).
- Cheng, Y.F. Fundamentals of hydrogen evolution reaction and its implications on near-neutral pH stress corrosion cracking of pipelines. Electrochim. Acta 2007, 52, 2661–2667. [Google Scholar] [CrossRef]


| C | Si | Mn | Ni | S | P | Cr | Cu | As | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.05 | 0.03 | 0.25 | 0.25 | 0.04 | 0.035 | 0.10 | 0.25 | 0.08 | balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshakov, A.I.; Rybkina, A.A. Effect of Cathodic Protection Potential Fluctuations on the Corrosion of Low-Carbon Steels and Hydrogen Absorption by the Metal in Chloride Solutions with Nearly Neutral pH. Materials 2022, 15, 8279. https://doi.org/10.3390/ma15238279
Marshakov AI, Rybkina AA. Effect of Cathodic Protection Potential Fluctuations on the Corrosion of Low-Carbon Steels and Hydrogen Absorption by the Metal in Chloride Solutions with Nearly Neutral pH. Materials. 2022; 15(23):8279. https://doi.org/10.3390/ma15238279
Chicago/Turabian StyleMarshakov, Andrey I., and Alevtina A. Rybkina. 2022. "Effect of Cathodic Protection Potential Fluctuations on the Corrosion of Low-Carbon Steels and Hydrogen Absorption by the Metal in Chloride Solutions with Nearly Neutral pH" Materials 15, no. 23: 8279. https://doi.org/10.3390/ma15238279
APA StyleMarshakov, A. I., & Rybkina, A. A. (2022). Effect of Cathodic Protection Potential Fluctuations on the Corrosion of Low-Carbon Steels and Hydrogen Absorption by the Metal in Chloride Solutions with Nearly Neutral pH. Materials, 15(23), 8279. https://doi.org/10.3390/ma15238279







