Attempt to Optimize the Corrosion Resistance of HRB400 Steel Rebar with Cr and RE
Abstract
1. Introduction
2. Experimental
2.1. Materials and Microstructure Observation
2.2. Immersion Test
2.3. Electrochemical Measurements
2.4. Dry/Wet Cyclic Tests
3. Results and Discussion
3.1. Microstructure Observation
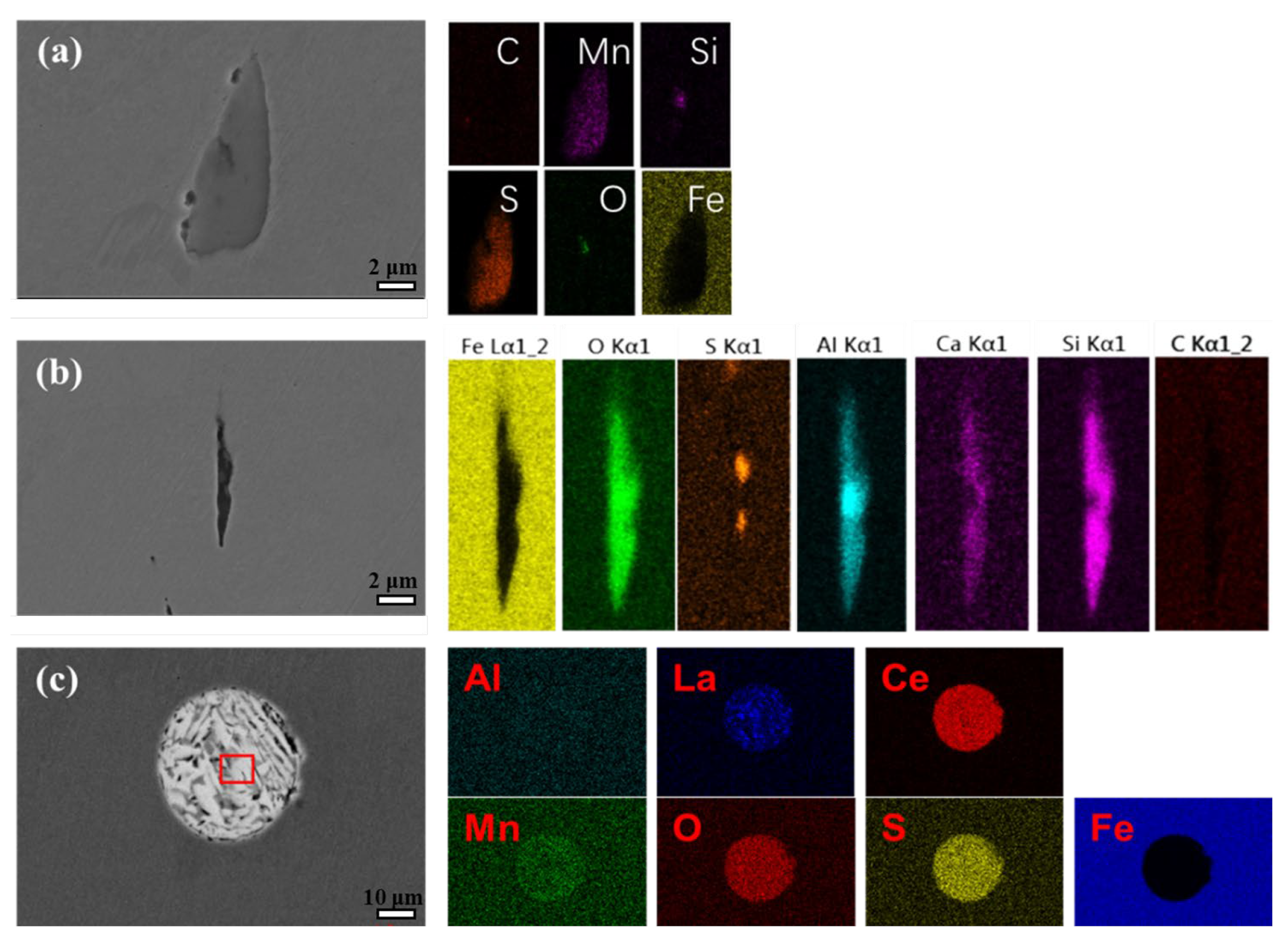
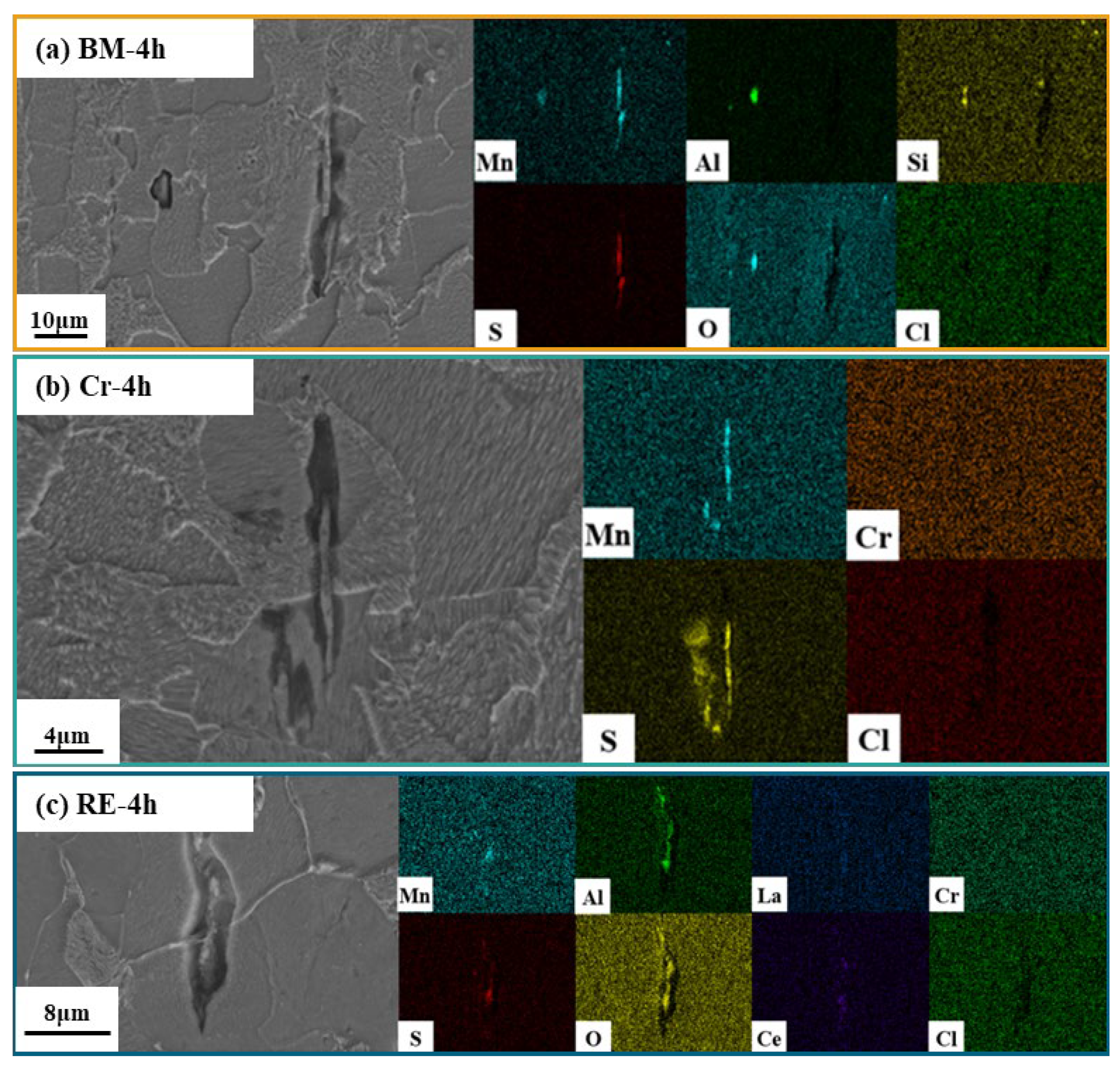
3.2. Inclusion Observation
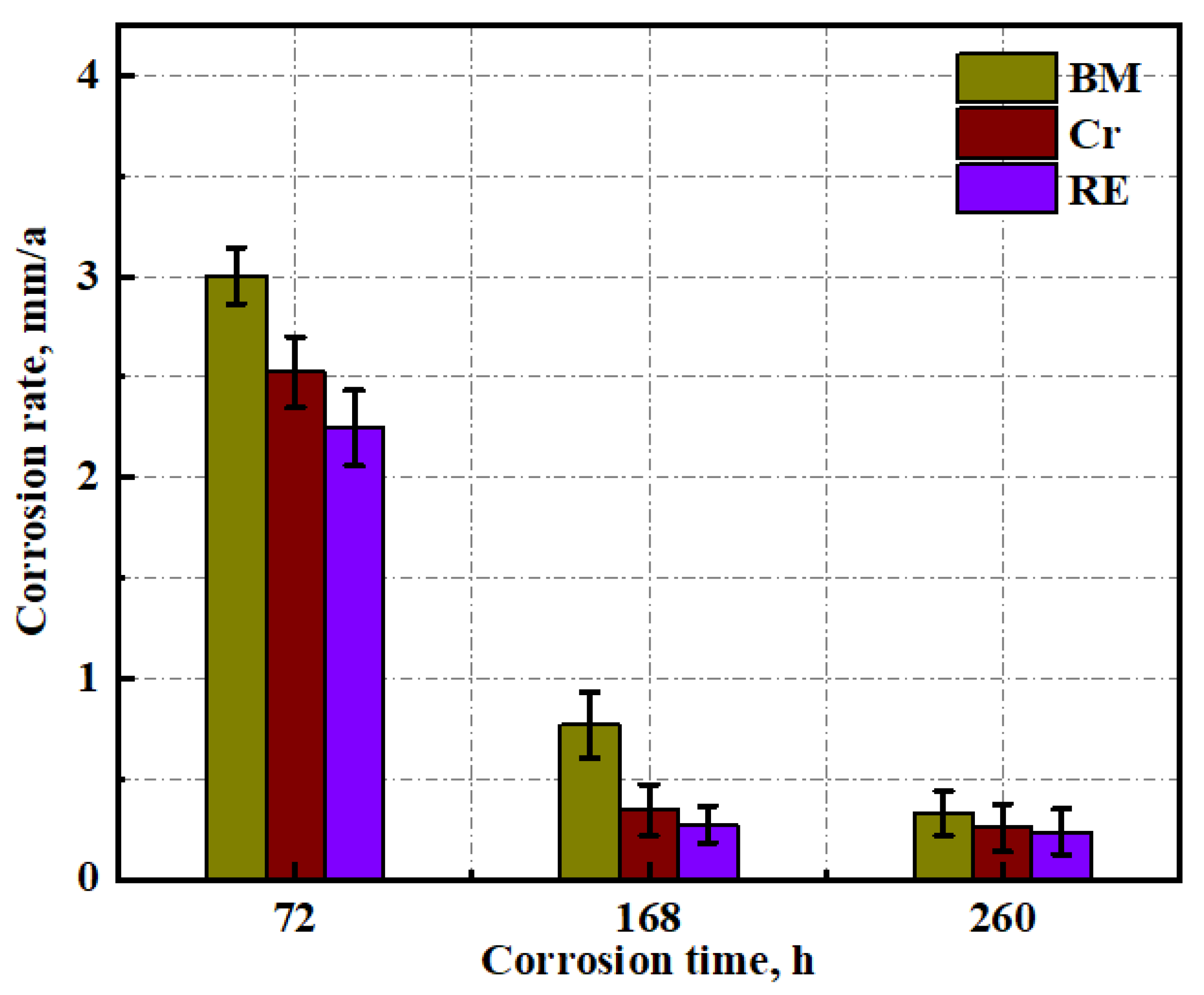
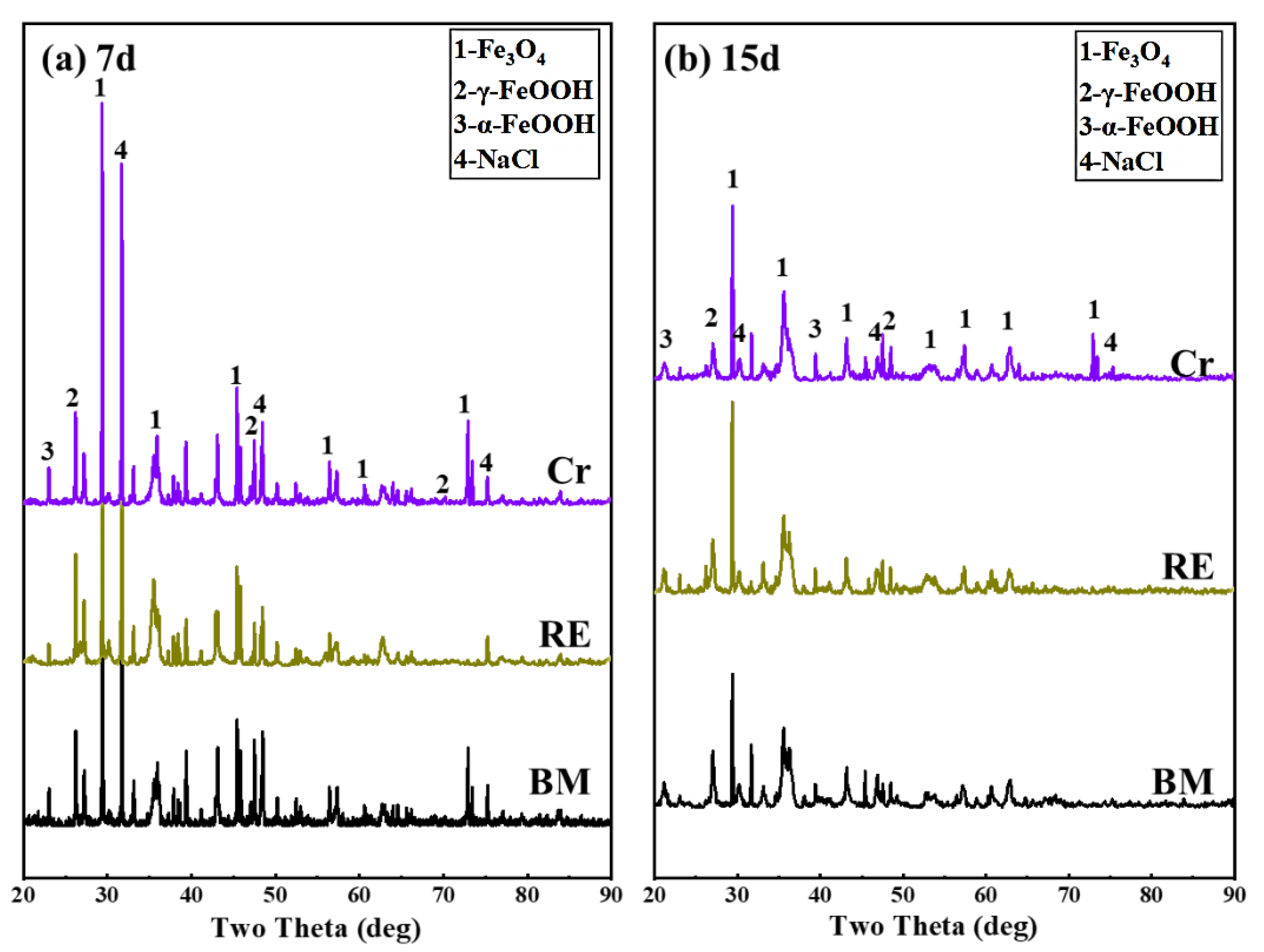
3.3. Immersion Test Results
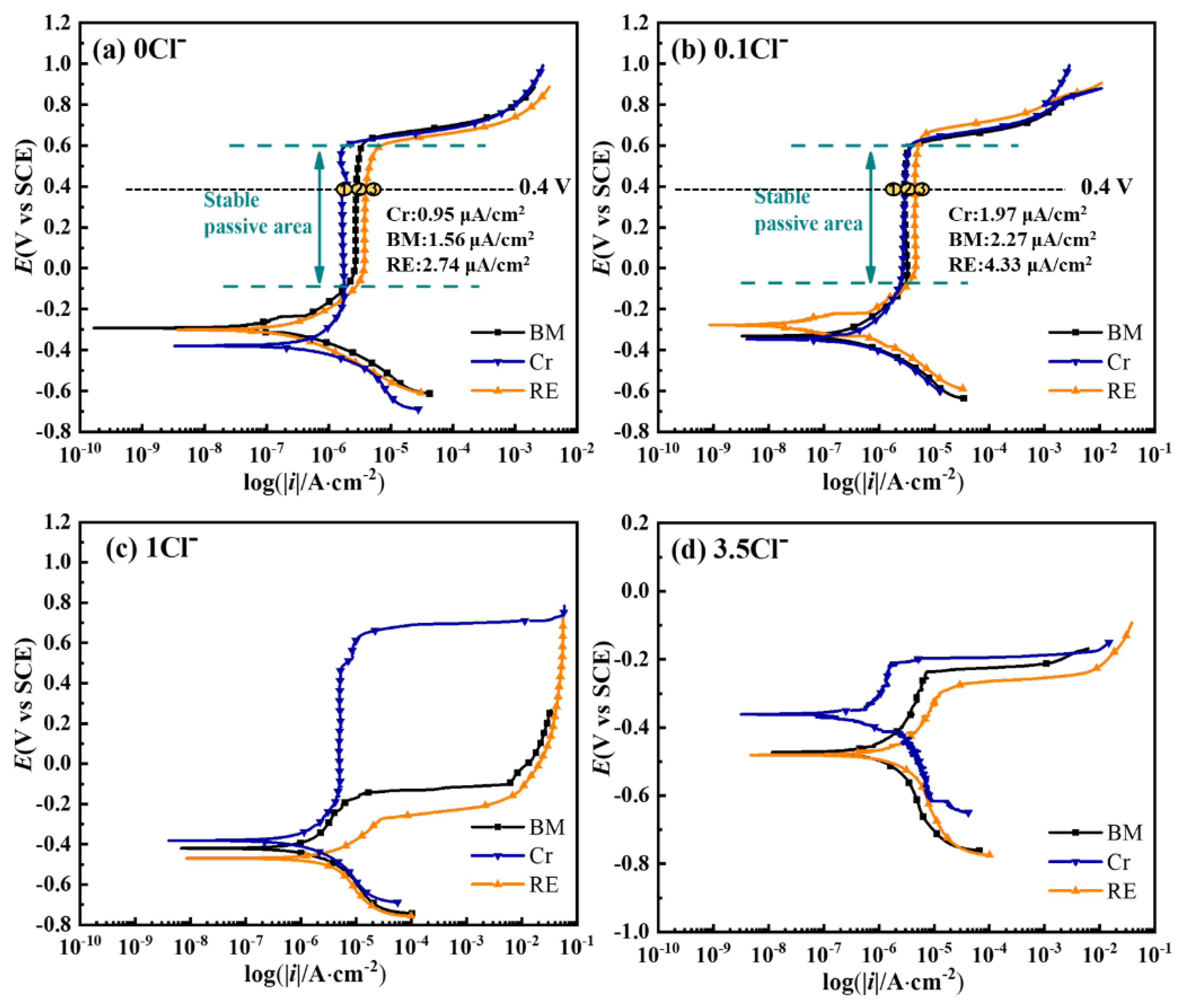
3.4. Electrochemical Results and XPS Analysis of the Passive Film
3.5. Corrosion Protectiveness of the Rust Layer after the Passive Breakdown Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Jiang, L.H.; Xu, J.; Xiong, C.S.; Song, Z.J. Influence of carbonation on chloride-induced reinforcement corrosion in simulated concrete pore solutions. Constr. Build. Mater. 2014, 56, 16–20. [Google Scholar] [CrossRef]
- Torkamni, H.; Sh, R.; Garcia-Mateo, C.; Rassizadehghani, J.; Palizdar, Y.; San Martín, D. Evolution of pearlite microstructure in low-carbon cast microalloyed due to the addition of La and Ce. Metall. Mater. Trans. A 2018, 49, 4495–4508. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, M.; Jia, Z.; Weng, Y.; Xiang, K.; Wu, X.; Liu, Q. On the formation of anti-phase boundaries and interphase boundaries in Al3Zr precipitates of Al-Cu-Zr alloy studied at atomic scale. J. Alloys Compd. 2021, 887, 161442. [Google Scholar] [CrossRef]
- Li, Z.; Xue, W.; Chen, Y.; Yu, W.; Xiao, K. Microstructure and Grain Boundary Corrosion Mechanism of Pearlitic Material. J. Mater. Eng. Perform. 2021, 31, 483–494. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Revilla, R.I.; Sun, T.; Zhao, J.; Zhang, D.; Yang, S.; Liu, Z.; Cheng, X.; Terryn, H.; et al. Towards a better understanding of localised corrosion induced by typical non-metallic inclusions in low-alloy steels. Corros. Sci. 2021, 179, 109150. [Google Scholar] [CrossRef]
- Schnatterer, C.; Zander, D. Influence of the grain boundary chemistry on the intergranular corrosion mechanisms of a high-strength Al-Mg-Si alloy. Surf. Interface Anal. 2016, 48, 750–754. [Google Scholar] [CrossRef]
- Wu, H.; Lei, H.; Chen, Y.F.; Qiao, J. Comparison on corrosion behaviour and mechanical properties of structural steel exposed between urban industrial atmosphere and laboratory simulated environment. Constr. Build. Mater. 2019, 211, 228–243. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, K.; Li, Q. Comparison of the rusting behaviors of S450EW weathering steel under continuous spray and wet/dry cycling. Constr. Build. Mater. 2021, 309, 125211. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, D.; Liao, B.; Zhao, T.; Fan, Y. A study on the dual-phase treatment of weathering steel 09CuPCrNi. Mater. Lett. 2004, 58, 1524–1529. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, J.J.; Qiao, H.H. Effect of Copper Tailing Content on Corrosion Resistance of Steel Reinforcement in a Salt Lake Environment. Materials 2019, 12, 3069. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, Z.; Cheng, X.; Li, X.; Liu, B. Atmospheric Corrosion Behavior and Mechanism of a Ni-Advanced Weathering Steel in Simulated Tropical Marine Environment. J. Mater. Eng. Perform. 2017, 26, 6075–6086. [Google Scholar] [CrossRef]
- Xiao, K.; Gao, X.; Yan, L.; Yi, P.; Zhang, D.; Dong, C.; Wu, J.; Li, X. Atmospheric corrosion factors of printed circuit boards in a dry-heat desert environment: Salty dust and diurnal temperature difference. Chem. Eng. J. 2018, 336, 92–101. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, S.; Liu, Z.; Du, C.; Li, X. Effect of cathodic polarisation on stress corrosion cracking behaviour of a Ni(Fe, Al)-maraging steel in artificial seawater. Corros. Sci. 2021, 179, 109176. [Google Scholar] [CrossRef]
- Avci, R.; Davis, B.H.; Wolfenden, M.L.; Beech, I.B.; Lucas, K.; Paul, D. Mechanism of MnS-mediated pit initiation and propagation in carbon steel in an anaerobic sulfidogenic media. Corros. Sci. 2013, 76, 267–274. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X.; Dai, Z.; Liu, R.; Li, Z.; Cui, L.; Chen, M.; Ke, L. Title Synergistic Effect of Al2O3 Inclusion and Pearlite on the Localized Corrosion Evolution Process of Carbon Steel in Marine Environment. Materials 2018, 11, 2277. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Liu, Z.; Zhang, D.; Li, X.; Terryn, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, H.; Li, X.; Che, Z.; Yang, S.; Du, C. Initiation Mechanism of Localized Corrosion Induced by Al2O3-MnS Composite Inclusion in Low-Alloy Structural Steel. Metals 2022, 12, 587. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Man, C.; Xiao, K.; Li, X. Insight into the mechanism of alloying elements (Sn, Be) effect on copper corrosion during long-term degradation in harsh marine environment. Appl. Surf. Sci. 2018, 455, 543–553. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.Q.; Li, X.G.; Jin, Z.; Liu, H.X. Corrosion behavior of Cr modified HRB400 steel rebar in simulated concrete pore solution. Constr. Build. Mater. 2015, 93, 884–890. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Chen, L.; Dong, B.; Yang, W.; Fan, Y.; Zhao, Y. On how the corrosion behavior and the functions of Cu, Ni and Mo of the weathering steel in environments with different NaCl concentrations. Corros. Sci. 2021, 192, 109851. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Dong, B.; Wu, Y.; Fan, Y.; Zhao, Y.; Chen, L. Investigation on the nickel content design and the corrosion resistance of low alloy steel welded joint in tropical marine atmosphere environment. Mater. Corros. 2021, 72, 1620–1634. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, X.; Li, Y.; Lv, X. The function of Cr on the rust formed on weathering steel performed in a simulated tropical marine atmosphere environment. Constr. Build. Mater. 2021, 277, 122298. [Google Scholar] [CrossRef]
- Zhu, T.; Huang, F.; Liu, J.; Hu, Q.; Li, W. Effects of inclusion on corrosion resistance of weathering steel in simulated industrial atmosphere. Anti-Corros. Method Mater. 2016, 63, 490–498. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Zhang, T.; Liu, Z.; Liu, C. Investigation on the SCC behavior of ZL101A aluminum alloy in simulated marine environments. Surf. Topogr. Metrol. Prop. 2021, 9, 45001. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Z.; Li, Q.; Zhang, T.; Liu, C. Effect of Second Phase on the Pitting Corrosion of ZL101A Aluminum Alloy in Thin Electrolyte Layer Environment Containing Cl. Arab. J. Sci. Eng. 2022, 47, 13857–13872. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Qi, Y.; Chen, L.; Chen, C. Mechanism of (Mg,Al,Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Eckermann, F.; Suter, T.; Uggowitzer, P.J.; Afseth, A.; Schmutz, P. Investigation of the exfoliation-like attack mechanism in relation to Al–Mg–Si alloy microstructure. Corros. Sci. 2008, 50, 2085–2093. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, B.; Wang, J.; Wang, G.; Han, E.; Ke, W. Anisotropic 3D growth of corrosion pits initiated at MnS inclusions for A537 steel during corrosion fatigue. Corros. Sci. 2010, 52, 2867–2877. [Google Scholar] [CrossRef]
- Tumidajski, P.J.; Chan, G.W. Effect of sulphate and carbon dioxide on chloride diffusivity. Cem. Concr. Res. 1996, 26, 551–556. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, X.; Zhao, J.; Fan, Y.; Li, X. A study of rust layer of low alloy structural steel containing 0.1% Sb in atmospheric environment of the Yellow Sea in China. Corros. Sci. 2021, 188, 109549. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.; Cheng, X.; Li, X. Comparative effect of (1 1 1) and (1 1 0) crystallographic orientation on the passive behavior of low alloy steels in bicarbonate solution. Appl. Surf. Sci. 2021, 561, 150066. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.H.; Yang, X.G.; Wang, C.; Guo, W.J. Pitting corrosion induced current oscillations during electrodissolution of Al in HClO4 solutions. J. Electroanal. Chem. 2004, 572, 41–49. [Google Scholar] [CrossRef]
- Esfahani, Z.; Rahimi, E.; Sarvghad, M.; Rafsanjani-Abbasi, A.; Davoodi, A. Correlation between the histogram and power spectral density analysis of AFM and SKPFM images in an AA7023/AA5083 FSW joint. J. Alloys Compd. 2018, 744, 174–181. [Google Scholar] [CrossRef]
- Chen, X.; Dong, J.; Han, E.; Ke, W. Effect of Ni on the ion-selectivity of rust layer on low alloy steel. Mater. Lett. 2007, 61, 4050–4053. [Google Scholar] [CrossRef]
- Choi, Y.; Shim, J.; Kim, J. Effects of Cr, Cu, Ni and Ca on the corrosion behavior of low carbon steel in synthetic tap water. J. Alloys Compd. 2005, 391, 162–169. [Google Scholar] [CrossRef]
- Dan, W.; Shujian, Z.; Qingdong, Z. The influence of Cr on the microstructure and electrochemical behavior of high strength low-alloy steel. Mater. Res. Express. 2020, 7, 66522. [Google Scholar] [CrossRef]
- Lv, W.; Yan, L.; Pang, X.; Qiao, L.; Su, Y.; Salvatori, I.; Gao, K.; Yang, H. Synergistic effect of Cu and Cr on pitting behavior induced by MnS inclusions in low alloy steels. J. Alloys Compd. 2021, 864, 158133. [Google Scholar] [CrossRef]
- Sun, M.; Du, C.; Liu, Z.; Liu, C.; Li, X.; Wu, Y. Fundamental understanding on the effect of Cr on corrosion resistance of weathering steel in simulated tropical marine atmosphere. Corros. Sci. 2021, 186, 109427. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Dong, B.; Mao, R.; Sun, Y.; Chen, L. Corrosion of Cu-doped Ni-Mo low-alloy steel in a severe marine environment. J. Phys. Chem. Solids. 2022, 163, 110584. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Cano, H.; Díaz, I.; de la Fuente, D.; Chico, B.; Morcillo, M. Effect of Cu, Cr and Ni alloying elements on mechanical properties and atmospheric corrosion resistance of weathering steels in marine atmospheres of different aggressivities. Mater. Corros. 2018, 69, 8–19. [Google Scholar] [CrossRef]




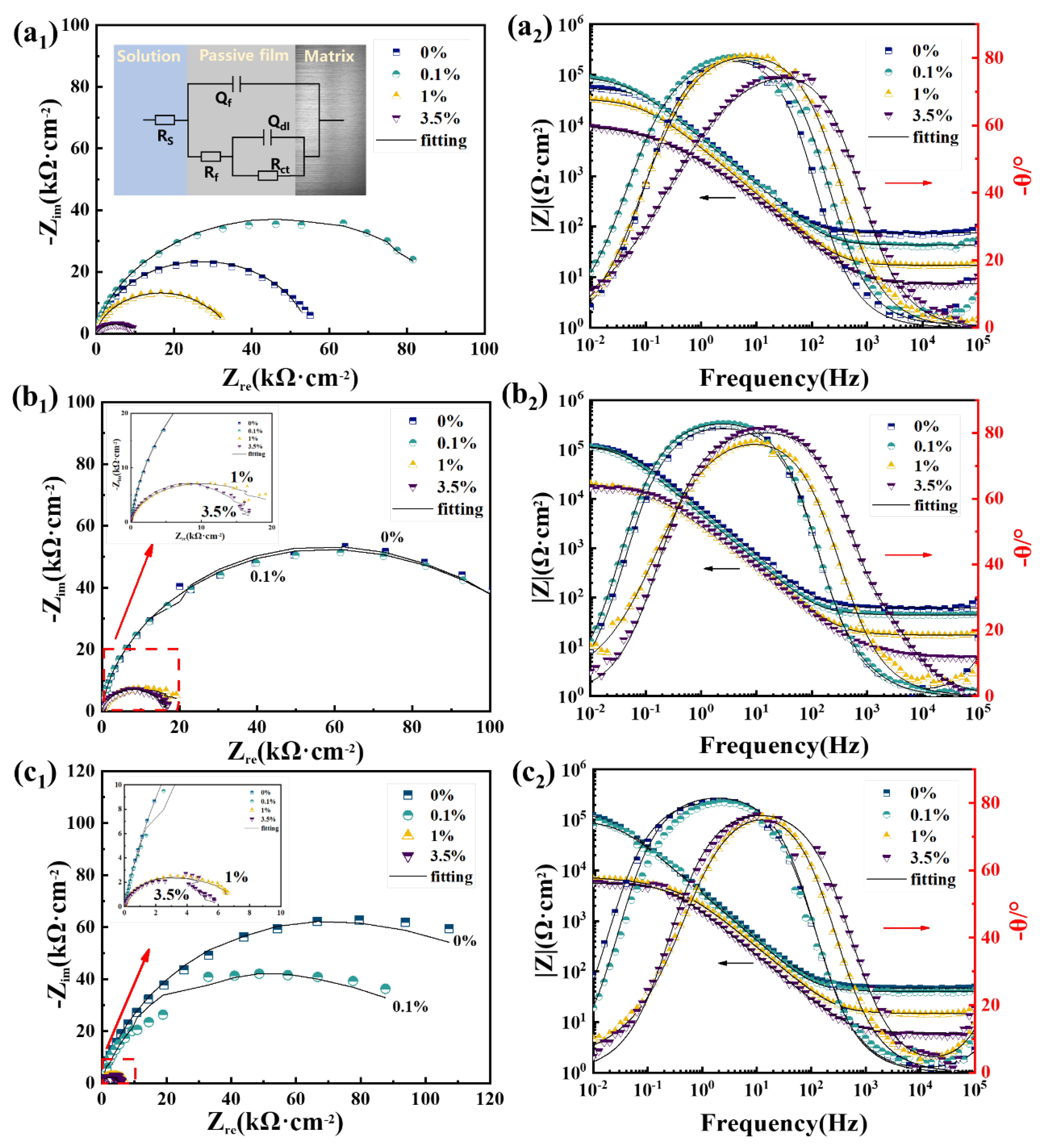



| Steels | Solution | Rct, kΩ·cm2 | Qdl | Rf, kΩ·cm2 | Qf | ||
|---|---|---|---|---|---|---|---|
| Y0 Ω−1·cm2·sn | n | Y0 Ω−1·cm2·sn | n | ||||
| BM | 0 Cl | 61.50 | 1.26 × 10−5 | 0.78 | 72.39 | 1.82 × 10−5 | 0.84 |
| 0.1 Cl | 26.08 | 8.45 × 10−6 | 0.61 | 33.17 | 2.76 × 10−5 | 0.94 | |
| 1Cl | 20.31 | 4.91 × 10−5 | 0.92 | 23.56 | 1.22 × 10−4 | 0.74 | |
| 3.5 Cl | 14.59 | 5.13 × 10−5 | 0.93 | 18.23 | 9.38 × 10−5 | 0.78 | |
| Cr | 0 Cl | 77.80 | 5.30 × 10−6 | 0.62 | 88.31 | 2.89 × 10−5 | 0.93 |
| 0.1 Cl | 35.21 | 1.64 × 10−5 | 0.88 | 43.74 | 2.03 × 10−5 | 0.89 | |
| 1 Cl | 28.56 | 5.95 × 10−5 | 0.91 | 29.67 | 6.09 × 10−5 | 0.87 | |
| 3.5 Cl | 11.73 | 4.56 × 10−5 | 0.88 | 21.53 | 1.11 × 10−5 | 0.98 | |
| RE | 0 Cl | 46.73 | 3.69 × 10−8 | 1.00 | 55.78 | 4.92 × 10−5 | 0.91 |
| 0.1 Cl | 25.33 | 5.18 × 10−5 | 0.93 | 32.46 | 8.64 × 10−6 | 0.75 | |
| 1 Cl | 20.72 | 7.55 × 10−5 | 0.91 | 26.19 | 2.32 × 10−4 | 0.79 | |
| 3.5 Cl | 6.07 | 5.74 × 10−8 | 0.92 | 10.50 | 1.20 × 10−4 | 0.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, N.; Liu, C.; Li, J.; Zhang, T.; Cheng, X.; Yang, S. Attempt to Optimize the Corrosion Resistance of HRB400 Steel Rebar with Cr and RE. Materials 2022, 15, 8269. https://doi.org/10.3390/ma15228269
Liu T, Li N, Liu C, Li J, Zhang T, Cheng X, Yang S. Attempt to Optimize the Corrosion Resistance of HRB400 Steel Rebar with Cr and RE. Materials. 2022; 15(22):8269. https://doi.org/10.3390/ma15228269
Chicago/Turabian StyleLiu, Tao, Nannan Li, Chao Liu, Jingshe Li, Tianyi Zhang, Xuequn Cheng, and Shufeng Yang. 2022. "Attempt to Optimize the Corrosion Resistance of HRB400 Steel Rebar with Cr and RE" Materials 15, no. 22: 8269. https://doi.org/10.3390/ma15228269
APA StyleLiu, T., Li, N., Liu, C., Li, J., Zhang, T., Cheng, X., & Yang, S. (2022). Attempt to Optimize the Corrosion Resistance of HRB400 Steel Rebar with Cr and RE. Materials, 15(22), 8269. https://doi.org/10.3390/ma15228269






