Green Synthesis, Characterizations of Zinc Oxide Nanoparticles from Aqueous Leaf Extract of Tridax procumbens Linn. and Assessment of their Anti-Hyperglycemic Activity in Streptozoticin-Induced Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.1.1. Preparation of Plant Extract
2.1.2. Phytochemical Screening
2.2. Preparation of ZnO NPs from TPE
2.3. Physical Characterizations
2.3.1. UV-Visible Spectroscopy
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
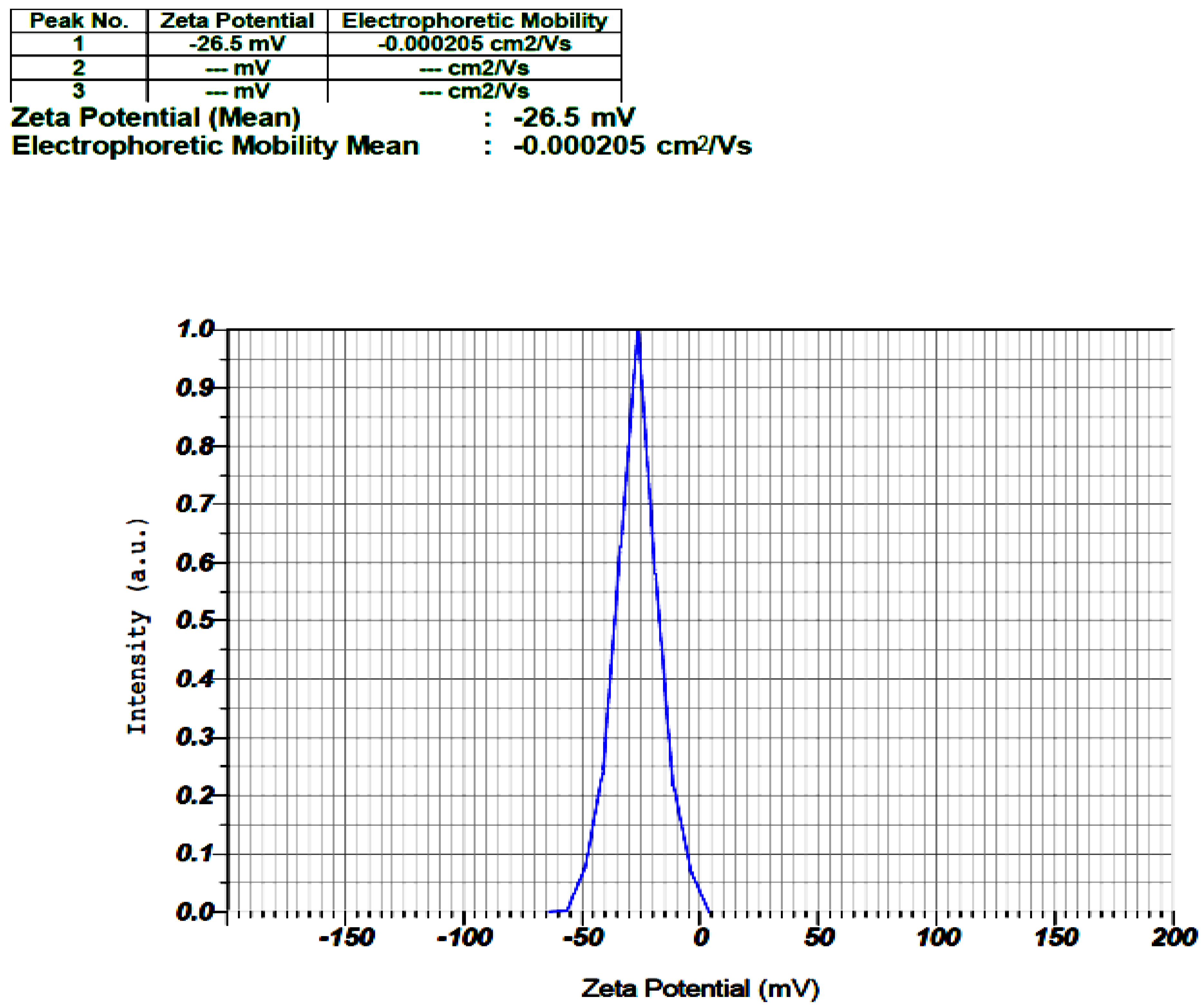
2.3.3. Zeta Potential (ZP)
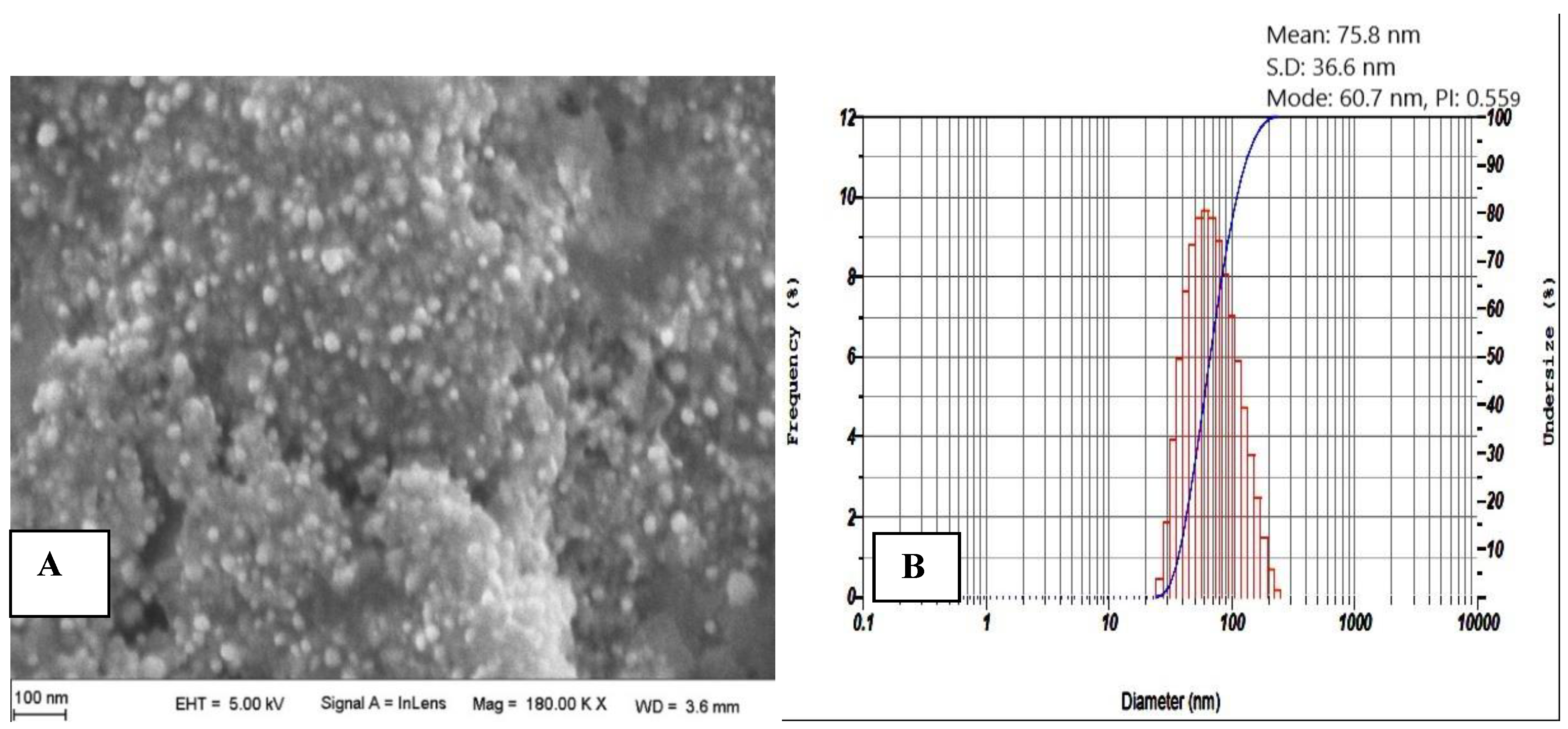
2.3.4. Scanning Electron Microscopy (SEM) Analysis
2.3.5. Particle Size Analysis
2.4. Animals
2.5. In-Vivo Antidiabetic Activity of TPE and TPE-Mediated ZnO NPs
2.5.1. Induction of Diabetes
2.5.2. Experimental Design
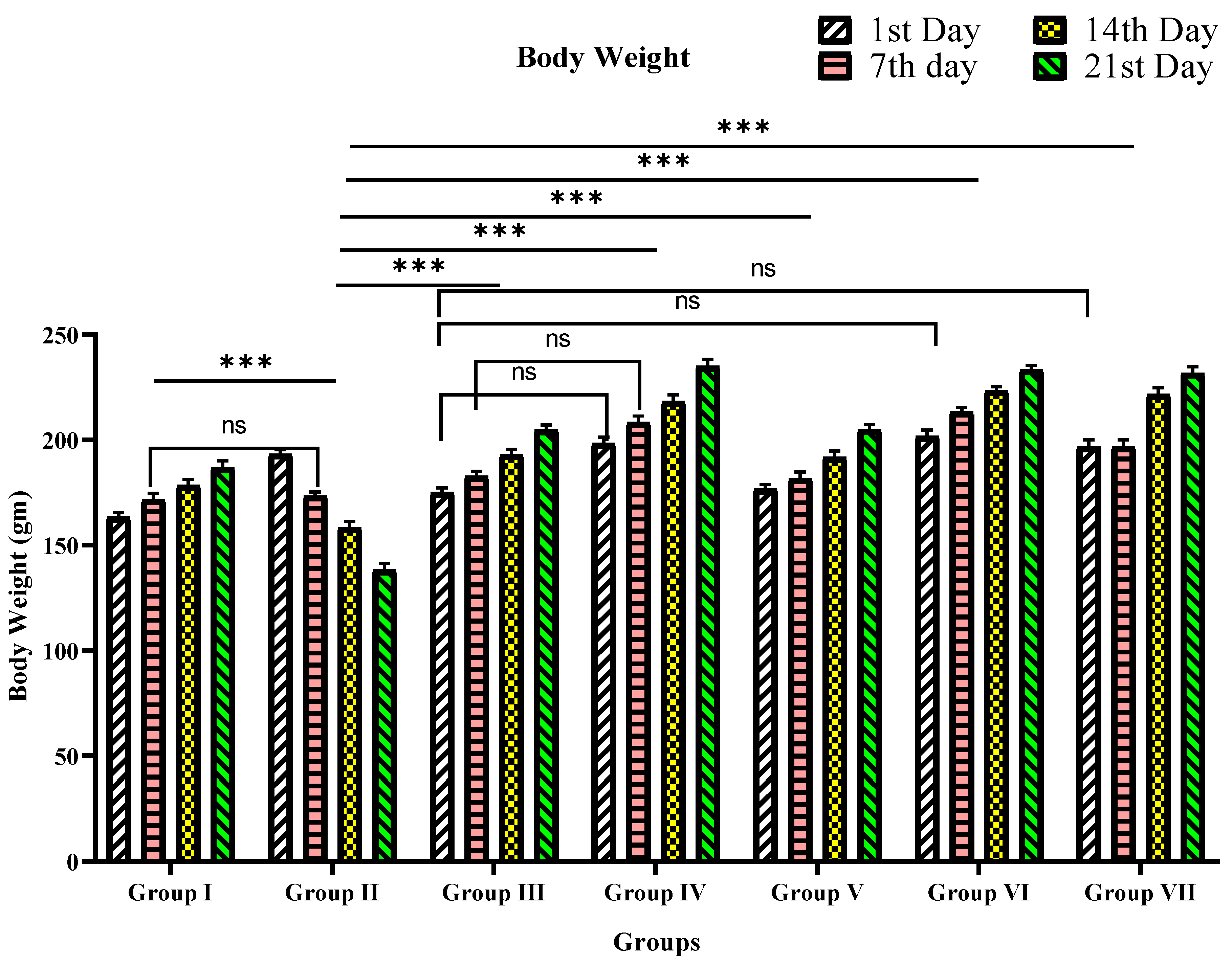
2.5.3. Body Weight (bw) Analysis
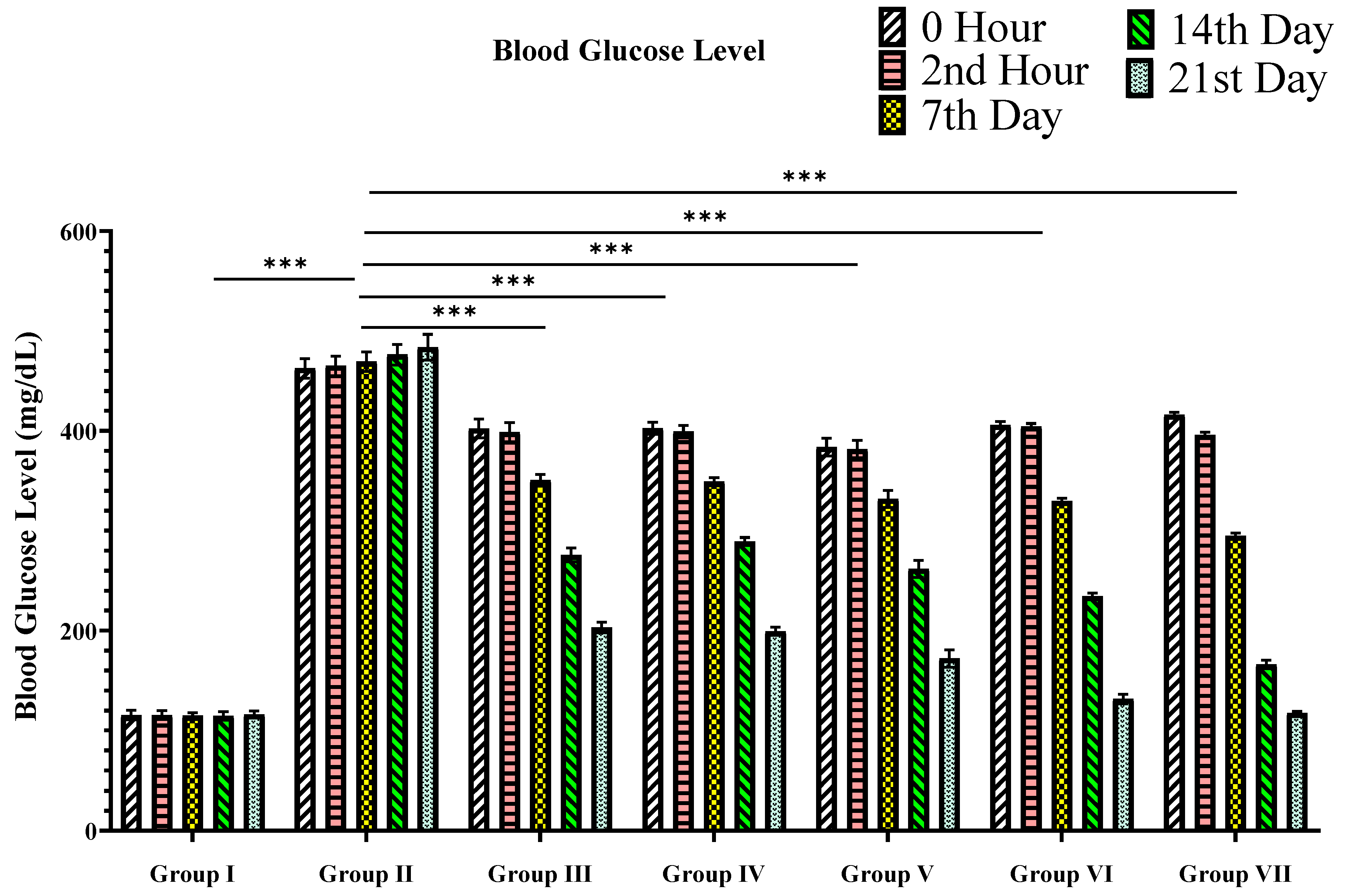
2.5.4. Blood Glucose Level Analysis
2.5.5. Biochemical Analysis
2.5.6. Histopathological Analysis
2.5.7. Statistical Analysis
3. Results and Discussion
3.1. Characterizations of TPE-Mediated ZnO NPs
3.1.1. UV-Visible Spectroscopy
3.1.2. FTIR
3.1.3. ZP
3.1.4. SEM and PS Analyses
3.2. Evaluation of the Anti-Diabetic Activity of TPE versus TPE-Mediated ZnO NPs
3.2.1. Blood Glucose Level
3.2.2. Body Weight
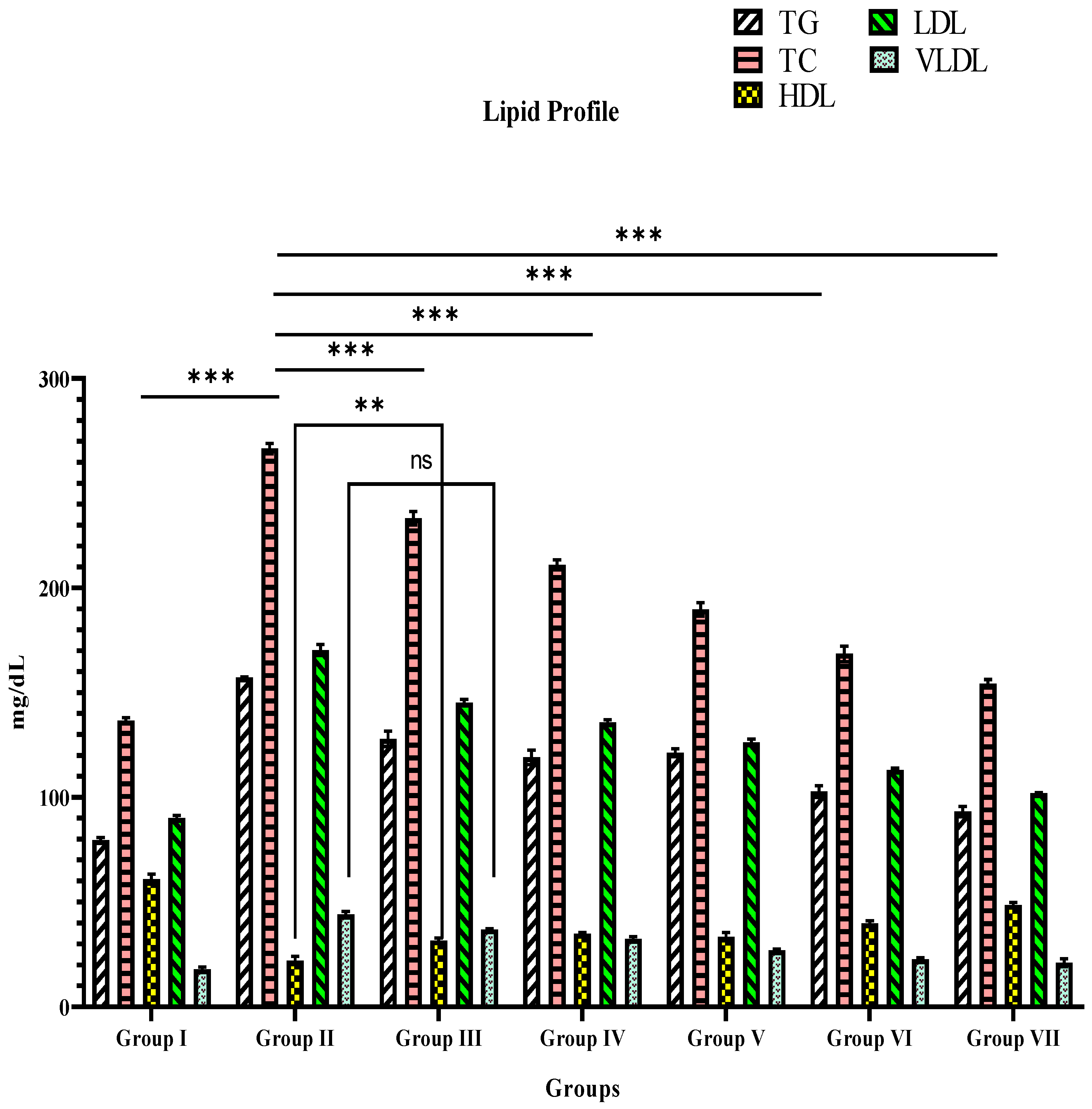
3.3. Blood Lipid Level
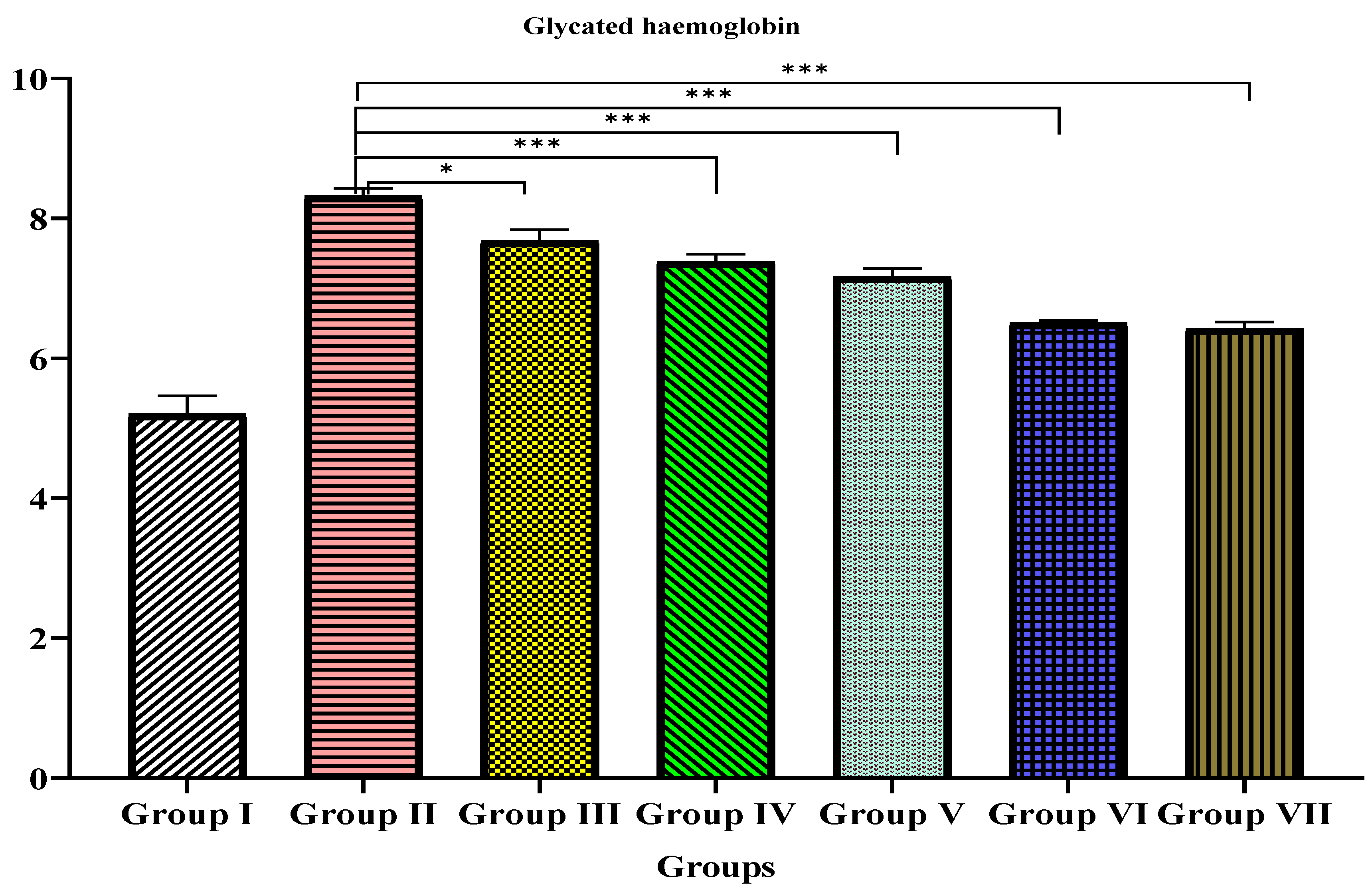
3.4. Glycated Hemoglobin
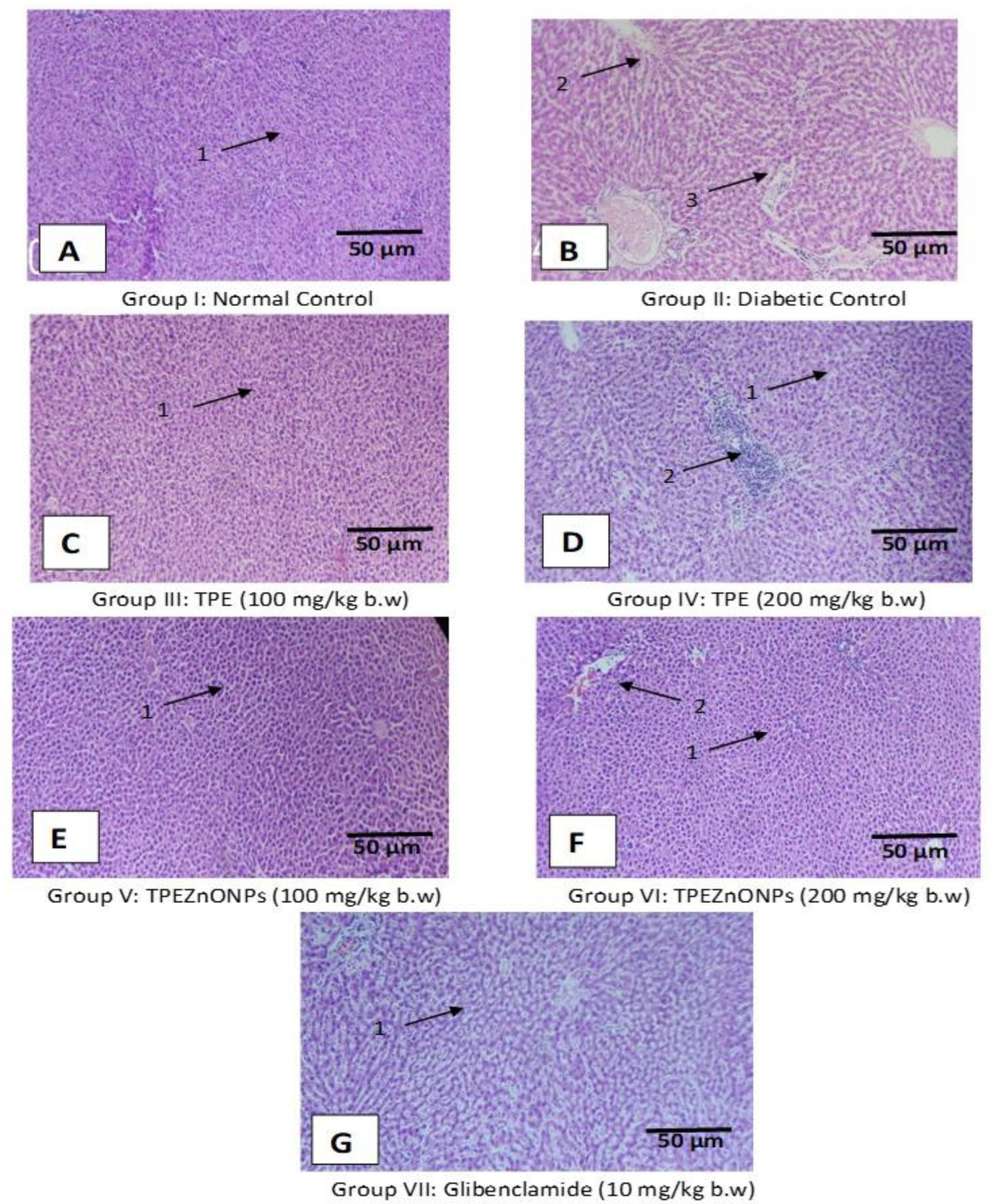
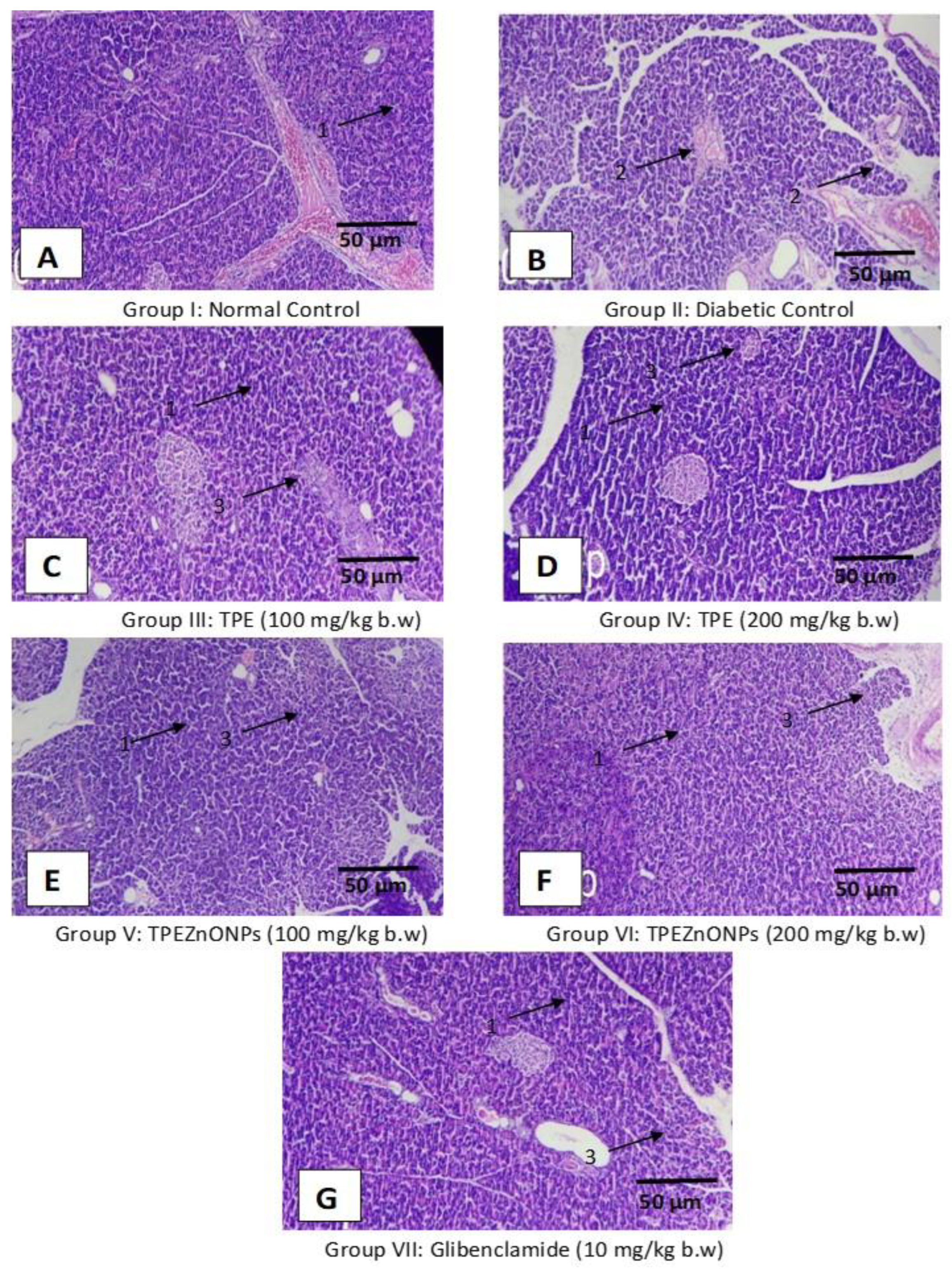
3.5. Histopathological Examination
3.5.1. Liver Cells
3.5.2. Pancreatic Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, S.A.; Rashid, M.M.; Uddin, M.G.; Robel, F.N.; Hossain, M.S.; Haque, M.A.; Jakaria, M. Biological efficacy of zinc oxide nanoparticles against diabetes: A preliminary study conducted in mice. Biosci. Rep. 2020, 40, BSR20193972. [Google Scholar] [CrossRef] [PubMed]
- Sengottaiyan, A.; Aravinthan, A.; Sudhakar, C.; Selvam, K.; Srinivasan, P.; Govarthanan, M.; Manoharan, K.; Selvankumar, T. Synthesis and characterization of Solanum nigrum-mediated silver nanoparticles and its protective effect on alloxan-induced diabetic rats. J. Nanostruct. Chem. 2016, 6, 41–48. [Google Scholar] [CrossRef]
- Pareek, H.; Sharma, S.; Khajja, B.S.; Jain, K.; Jain, G.C. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.). BMC Complement. Altern. Med. 2009, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Tang, K.S. The current and future perspectives of zinc oxide nanoparticles in the treatment of DM. Life Sci. 2019, 239, 117011. [Google Scholar] [CrossRef] [PubMed]
- Salles, B.C.C.; da Silva, M.A.; Taniguthi, L.; Ferreira, J.N.; da Rocha, C.Q.; Vilegas, W.; Dias, P.H.; Pennacchi, P.C.; Duarte, S.M.D.S.; Rodrigues, M.R.; et al. Passiflora edulis leaf extract: Evidence of antidiabetic and antiplatelet effects in rats. Biol. Pharm. Bull. 2020, 43, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, M.N.; Shah, G.M.; Menaa, F.; Khan, R.A.; Althobaiti, N.A.; Albalawi, A.E.; Alkreathy, H.M. Green Silver Nanoparticles Synthesized from Taverniera couneifolia Elicits Effective Anti-Diabetic Effect in Alloxan-Induced Diabetic Wistar Rats. Nanomaterials 2022, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Petchi, R.R.; Parasuraman, S.; Vijaya, C. Antidiabetic and antihyperlipidemic effects of an ethanolic extract of the whole plant of Tridax procumbens (Linn.) in streptozotocin-induced diabetic rats. J. Basic Clin. Pharm. 2013, 4, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Kokate, C.K. Practical Pharmacognosy, 4th ed.; Vallabh Prakasan: New Delhi, India, 1994; Volume 107. [Google Scholar]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Varatharajan, R.; Nyo, Y.H.; Renushia, R.; Raaginey, D.; Oh, A.N.; Akhtar, S.S.; Rupeshkumar, M.; Sundram, K.; Dhanaraj, S.A. Fenofibrate and dipyridamole treatments in low-doses either alone or in combination blunted the development of nephropathy in diabetic rats. Pharmacol. Res. 2014, 90, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Manik, S.; Gauttam, V.; Kalia, A. Anti-diabetic and antihyperlipidemic effect of allopolyherbal formulation in OGTT and STZ-induced diabetic rat model. Indian J. Exp. Biol. 2013, 51, 702–708. [Google Scholar] [PubMed]
- Prabhu, S.; Vinodhini, S.; Elanchezhiyan, C.; Rajeswari, D. Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J. Diabetes 2018, 10, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87. [Google Scholar] [PubMed]
- Madić, V.; Petrović, A.; Jušković, M.; Jugović, D.; Djordjević, L.; Stojanović, G.; Vasiljević, P. Polyherbal mixture ameliorates hyperglycemia, hyperlipidemia and histopathological changes of pancreas, kidney and liver in a rat model of type 1 diabetes. J. Ethnopharmacol. 2021, 265, 113210. [Google Scholar] [CrossRef] [PubMed]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S. Synthesis, characterization, and applications of metal nanoparticles. In Biomaterials and Bionanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 561–562. [Google Scholar]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Fatima Rana, N.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]



| Serial # | Phytochemical Compound Test | Result | |
|---|---|---|---|
| 1. | Carbohydrates | Molisch’s test | + |
| Fehling’s test | + | ||
| Barfoed’s test | + | ||
| 2. | Test of starch | + | |
| 3. | Proteins and amino acids | Million’s test | + |
| Biuret test | + | ||
| Ninhydrin test | + | ||
| 4. | Phenolic compounds and tannins | Ferric chloride test | + |
| Test with Lead acetate Solution | + | ||
| Gelatin test | + | ||
| 5. | Phytosterols | Salkowski test | + |
| Libermann–Burchards test | + | ||
| 6. | Test for fixed oils and fats | Spot test | + |
| Saponification | + | ||
| 7. | Test for alkaloids | Mayer’s test | + |
| Dragendroff’s test | + | ||
| Wagner’s test | + | ||
| Hager’s test | + | ||
| 8. | Test for glycosides | Legal’s test | + |
| Balget’s test | + | ||
| Borntrager’s test | + | ||
| Modified Borntrager’s test | + | ||
| 9. | Test for flavonoids | Ferric chloride test | + |
| Shinoda’s test | + | ||
| Fluorescence test | + | ||
| Reaction with alkali and acid | + | ||
| Zinc, HCl reduction test | + | ||
| Test for Flavonoids | + | ||
| 10. | Test for saponins | + | |
| 11. | Test for triterpenoids | - | |
| FTIR Assignment Number | Frequency (cm−1) | Standard Frequency (cm−1) Range | Bond | Functional Groups |
|---|---|---|---|---|
| 1 | 3377.72 | 3500–3200 | O-H stretch H-bonded | Alcohols, phenols |
| 3400–3250 | N-H stretch | Primary and secondary amines, amides | ||
| 2 | 2975.13 | 3000–2850 | C-H stretch | Alkanes |
| 3 | 1630.07 | 1650–1580 | N-H bend | Primary amines |
| 4 | 1520.41 | 1550–1475 | N-O asymmetric stretch | Nitro compounds |
| 5 | 1224.09 | 1320–1000 | C-O stretch | Alcohols, carboxylic acid, ester, ethers |
| 6 | 1138.42 | 1320–1000 | C-O stretch | Alcohols, carboxylic acid, ester, ethers |
| 7 | 1086.03 | 1250–1020 | C-N stretch | Aliphatic amines |
| 8 | 913.65 | 950–910 | O-H bend | Carboxylic acids |
| 9 | 642.60 | 690–515 | C-Br stretch | Alkyl halides |
| Groups | Blood Glucose Levels (mg/dL). Mean ± SEM Is Specified | ||||
|---|---|---|---|---|---|
| 1st Day | 7th Day | 14th Day | 21st Day | ||
| 0 h | 2nd h | ||||
| I | 115.5 ± 4.8 *** | 115.5 ± 4.5 *** | 114.8 ± 3.2 *** | 114.7 ± 4.2 *** | 116 ± 3.5 *** |
| II | 462.5 ± 9.7 | 465 ± 9.8 | 469.3 ± 9.8 | 476.3 ± 10.2 | 483.8 ± 12.8 |
| III | 402.5 ± 9.4 *** | 398.7 ± 9.7 *** | 350.5 ± 5.8 *** | 275.7 ± 6.9 *** | 203.3 ± 5.3 *** |
| IV | 402.7 ± 5.9 *** | 399.2 ± 6.2 *** | 349.3 ± 4.0 *** | 289.3 ± 4.0 *** | 199.3 ± 4.0 *** |
| V | 383.7 ± 8.9 *** | 381.8 ± 8.6 *** | 331.8 ± 8.6 *** | 261.8 ± 8.6 *** | 172 ± 8.7 *** |
| VI | 406 ± 3.4 *** | 404.5 ± 2.8 *** | 329.8 ± 2.7 *** | 234.5 ± 3.2 *** | 131.5 ± 4.8 *** |
| VII | 416.0 ± 2.7 *** | 396.0 ± 2.7 *** | 294.7 ± 3.2 *** | 166.3 ± 4.2 *** | 117.5 ± 1.9 *** |
| Group | Mean Body Weight (g) (Mean ± SEM) | |||
|---|---|---|---|---|
| 1st Day | 7th Day | 14th Day | 21st Day | |
| I | 163.3 ± 2.1 *** | 171.7 ± 3.0 ns | 178.3 ± 3.0 *** | 186.7 ± 3.3 *** |
| II | 193.3 ± 2.1 | 173.3 ± 2.1 | 158.3 ± 3.0 | 138.3 ± 3.0 |
| III | 175.0 ± 2.2 *** | 182.8 ± 2.3 ns | 193 ± 2.6 *** | 204.7 ± 2.4 *** |
| IV | 198.3 ± 3.0 ns | 208.3 ± 3.0 *** | 218.3 ± 0.0 *** | 235.0 ± 3.4 *** |
| V | 176.7 ± 2.1 *** | 181.7 ± 3.0 ns | 191.7 ± 3.0 *** | 205.0 ± 2.2 *** |
| VI | 201.7 ± 3.0 ns | 213.3 ± 2.8 *** | 223.3 ± 2.1 *** | 233.3 ± 2.1 *** |
| VII | 196.7 ± 3.3 ns | 196.7 ± 3.3 *** | 221.7 ± 3.0 *** | 231.7 ± 3.0 *** |
| Groups | Biochemical Parameters (mg/dL) | ||||
|---|---|---|---|---|---|
| TG | TC | HDL | LDL | VLDL | |
| I | 79.50 ± 1.3 *** | 136.50 ± 1.5 *** | 60.83 ± 2.4 *** | 90.0 ± 1.3 *** | 17.83 ± 1.1 *** |
| II | 157.0 ± 0.3 | 266.5 ± 2.4 | 22.1 ± 2.0 | 170.3 ± 2.6 | 44.00 ± 1.3 |
| III | 127.8 ± 3.6 *** | 233.3 ± 3.3 *** | 31.6 ± 1.1 ** | 145.2 ± 1.5 *** | 37.00 ± 0.3 ns |
| IV | 119 ± 3.0 *** | 211.0 ± 2.4 *** | 35.0 ± 0.6 *** | 135.8 ± 1.2 *** | 32.50 ± 1.1 *** |
| V | 121.2 ± 1.8 *** | 189.7 ± 3.1 *** | 33.5 ± 1.9 *** | 126.2 ± 1.5 *** | 27.00 ± 0.5 *** |
| VI | 102.7 ± 2.8 *** | 168.5 ± 3.7 *** | 39.8 ± 1.2 *** | 113.0 ± 0.8 *** | 22.67 ± 0.8 *** |
| VII | 93.17 ± 2.4 *** | 154.2 ± 1.9 *** | 48.5 ± 1.3 *** | 101.8 ± 0.30 *** | 21.17 ± 1.9 *** |
| Group | Glycated Haemoglobin (HbA1c)% |
|---|---|
| I | 5.2 ± 0.2 |
| II | 8.317 ± 0.1 *** |
| III | 7.68 ± 0.1 * |
| IV | 7.383 ± 0.1 *** |
| V | 7.162 ± 0.1 *** |
| VI | 6.505 ± 0.03 *** |
| VII | 6.421 ± 0.09 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.S.; Alqahtani, A.M.; Alqahtani, T.; Alamri, A.H.; Menaa, F.; Mani, R.K.; D. R., B.; Kavitha, K. Green Synthesis, Characterizations of Zinc Oxide Nanoparticles from Aqueous Leaf Extract of Tridax procumbens Linn. and Assessment of their Anti-Hyperglycemic Activity in Streptozoticin-Induced Diabetic Rats. Materials 2022, 15, 8202. https://doi.org/10.3390/ma15228202
Ahmed SS, Alqahtani AM, Alqahtani T, Alamri AH, Menaa F, Mani RK, D. R. B, Kavitha K. Green Synthesis, Characterizations of Zinc Oxide Nanoparticles from Aqueous Leaf Extract of Tridax procumbens Linn. and Assessment of their Anti-Hyperglycemic Activity in Streptozoticin-Induced Diabetic Rats. Materials. 2022; 15(22):8202. https://doi.org/10.3390/ma15228202
Chicago/Turabian StyleAhmed, Syed S., Ali M. Alqahtani, Taha Alqahtani, Ali H. Alamri, Farid Menaa, Rupesh Kumar Mani, Bharathi D. R., and Kunchu Kavitha. 2022. "Green Synthesis, Characterizations of Zinc Oxide Nanoparticles from Aqueous Leaf Extract of Tridax procumbens Linn. and Assessment of their Anti-Hyperglycemic Activity in Streptozoticin-Induced Diabetic Rats" Materials 15, no. 22: 8202. https://doi.org/10.3390/ma15228202
APA StyleAhmed, S. S., Alqahtani, A. M., Alqahtani, T., Alamri, A. H., Menaa, F., Mani, R. K., D. R., B., & Kavitha, K. (2022). Green Synthesis, Characterizations of Zinc Oxide Nanoparticles from Aqueous Leaf Extract of Tridax procumbens Linn. and Assessment of their Anti-Hyperglycemic Activity in Streptozoticin-Induced Diabetic Rats. Materials, 15(22), 8202. https://doi.org/10.3390/ma15228202






