Biocarbons Obtained from Fennel and Caraway Fruits as Adsorbents of Methyl Red Sodium Salt from Water System
Abstract
1. Introduction
2. Material and Method
2.1. Precursors
2.2. Direct Physical Activation
2.3. Biocarbons Characterization
2.4. Adsorption of Methyl Red Sodium Salt
3. Results
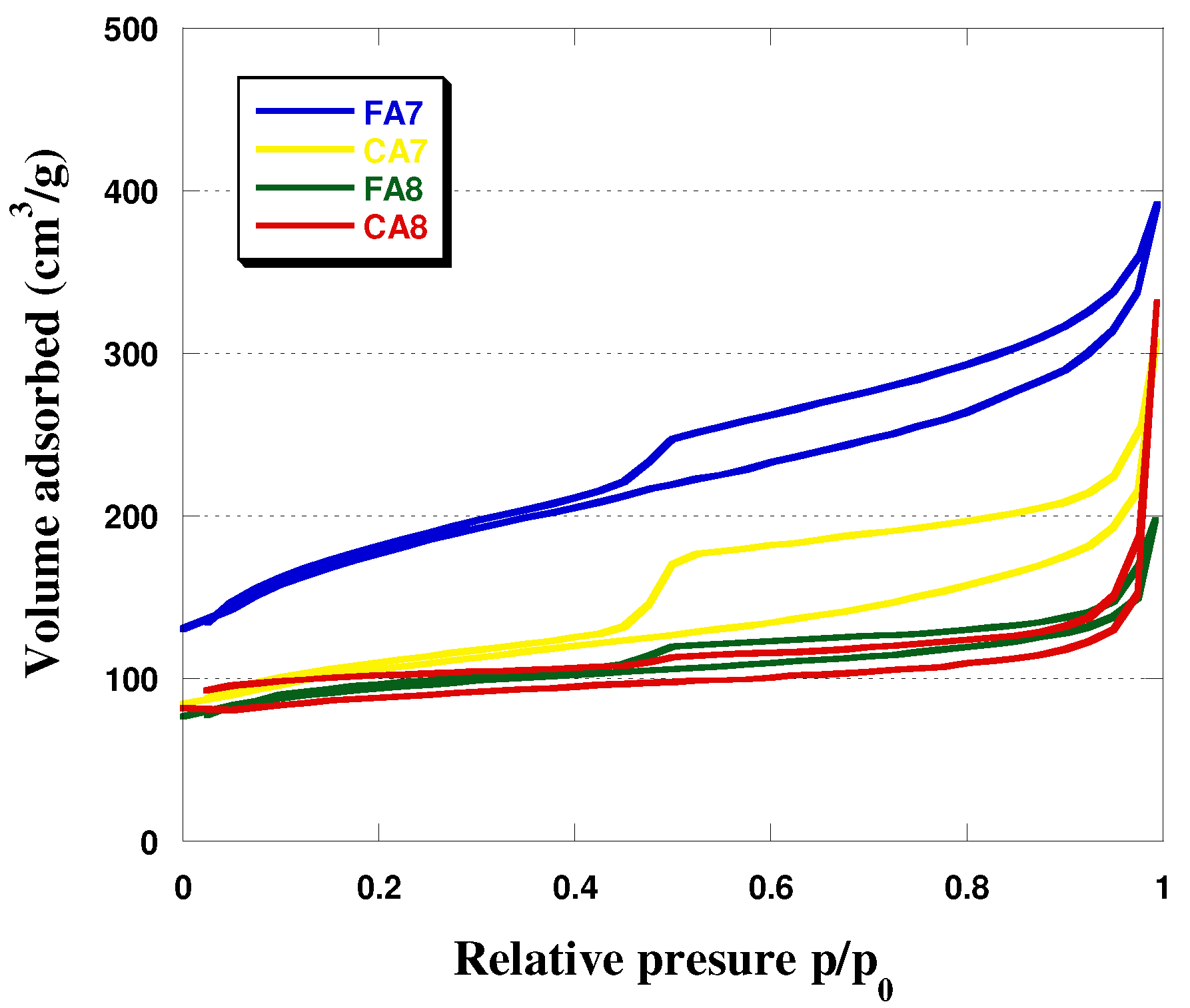
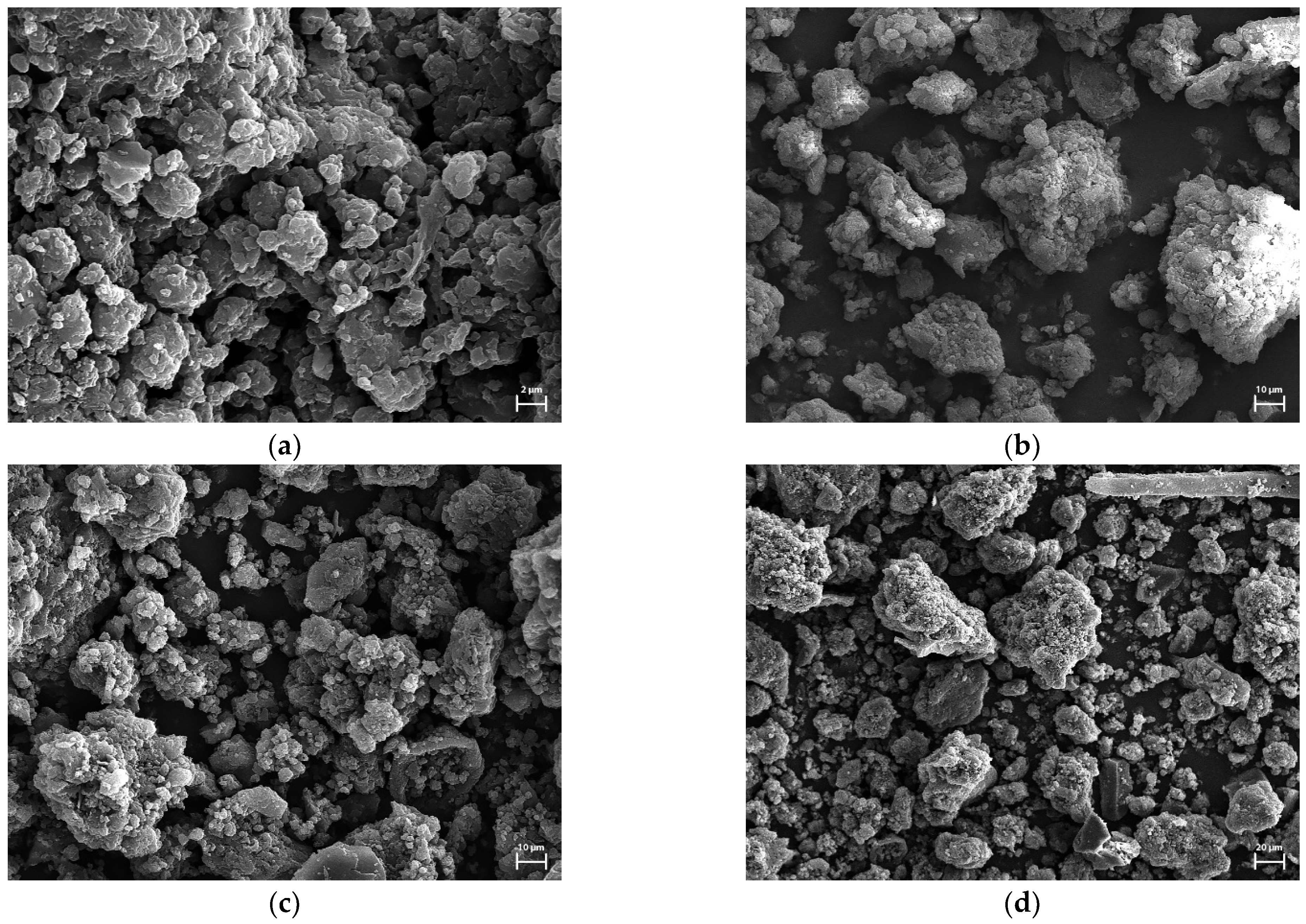
3.1. Characterization of Biocarbons
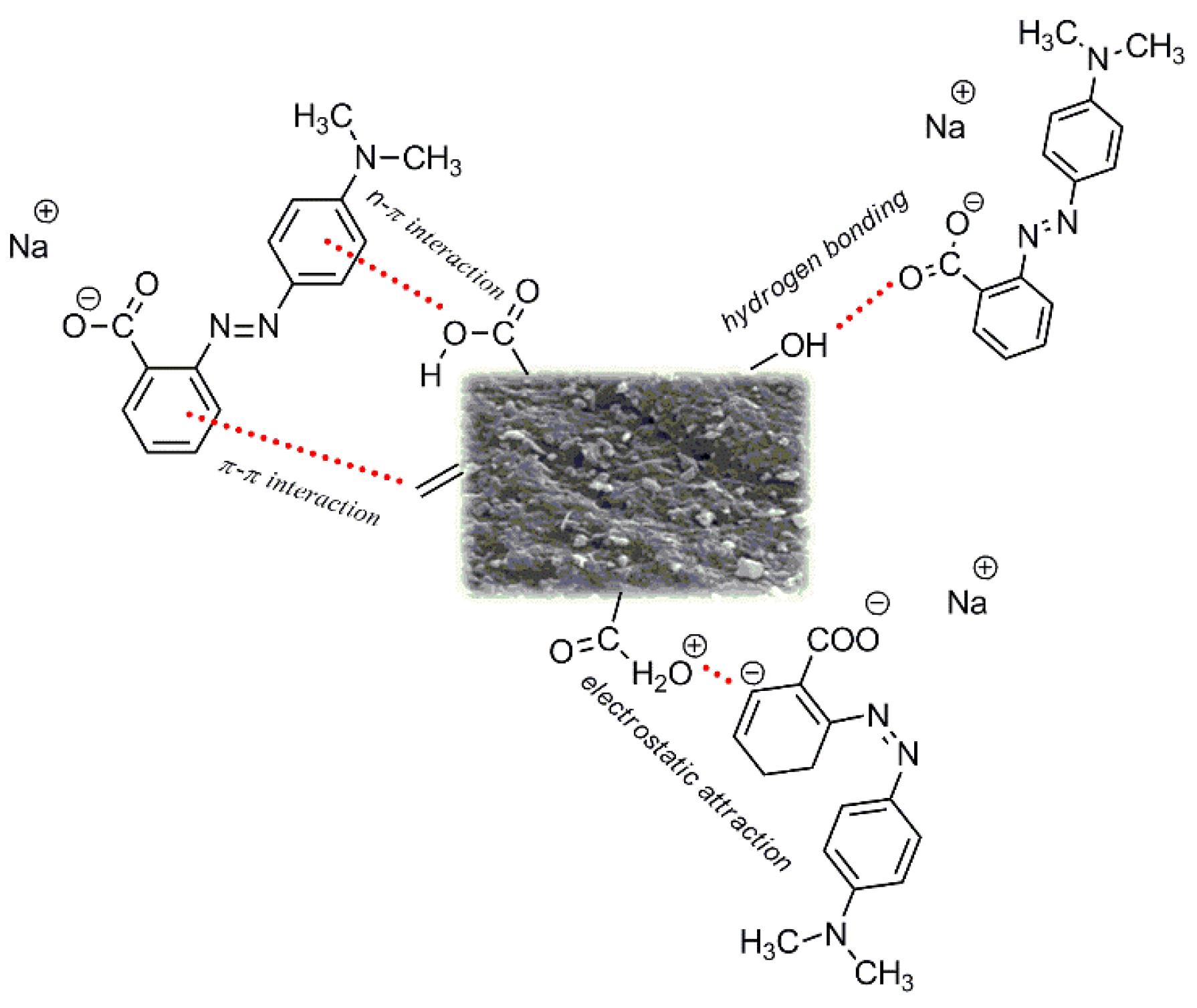
3.2. Adsorption Study
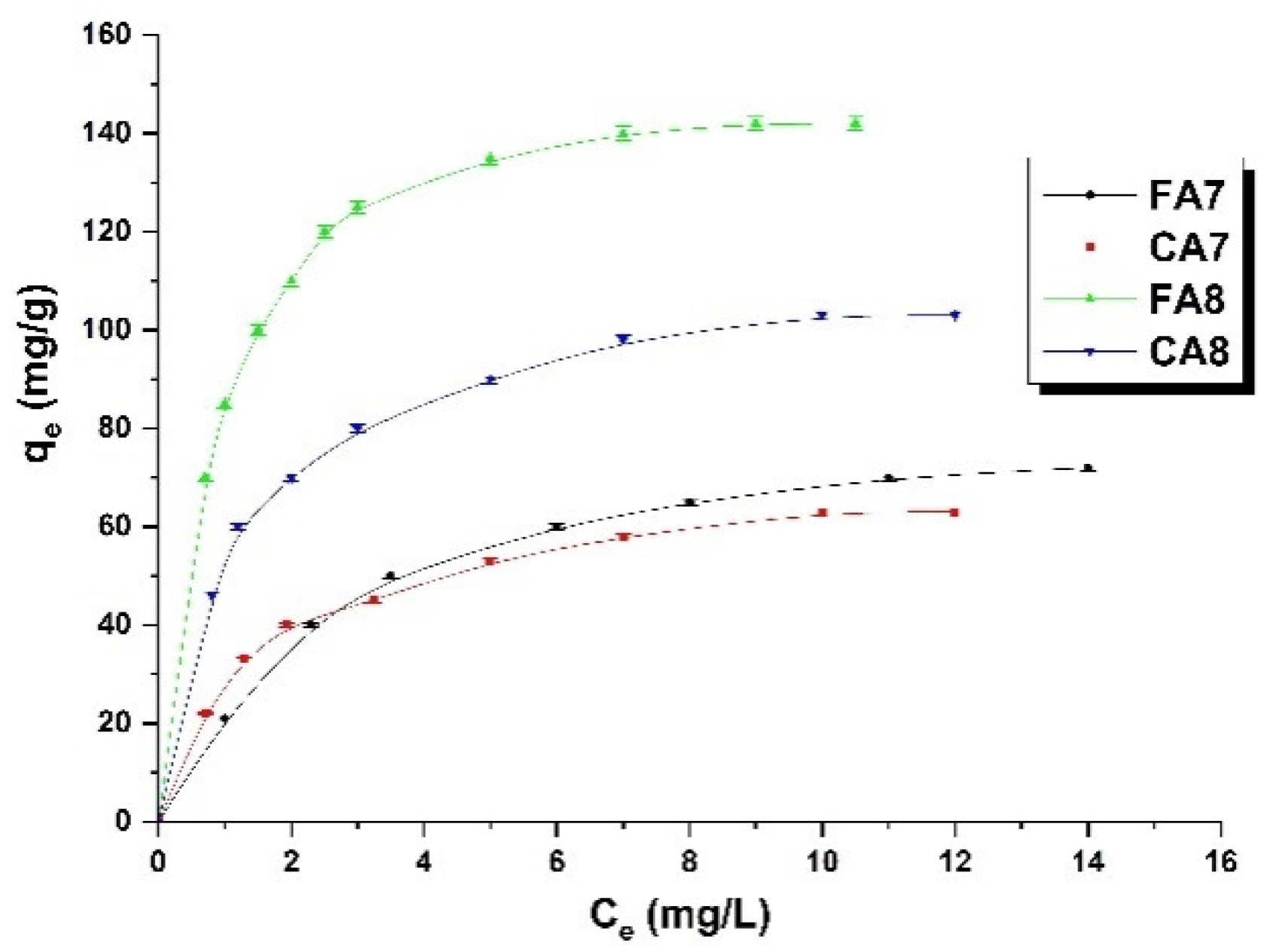
3.2.1. Adsorption Equilibrium
3.2.2. Adsorption Isotherms
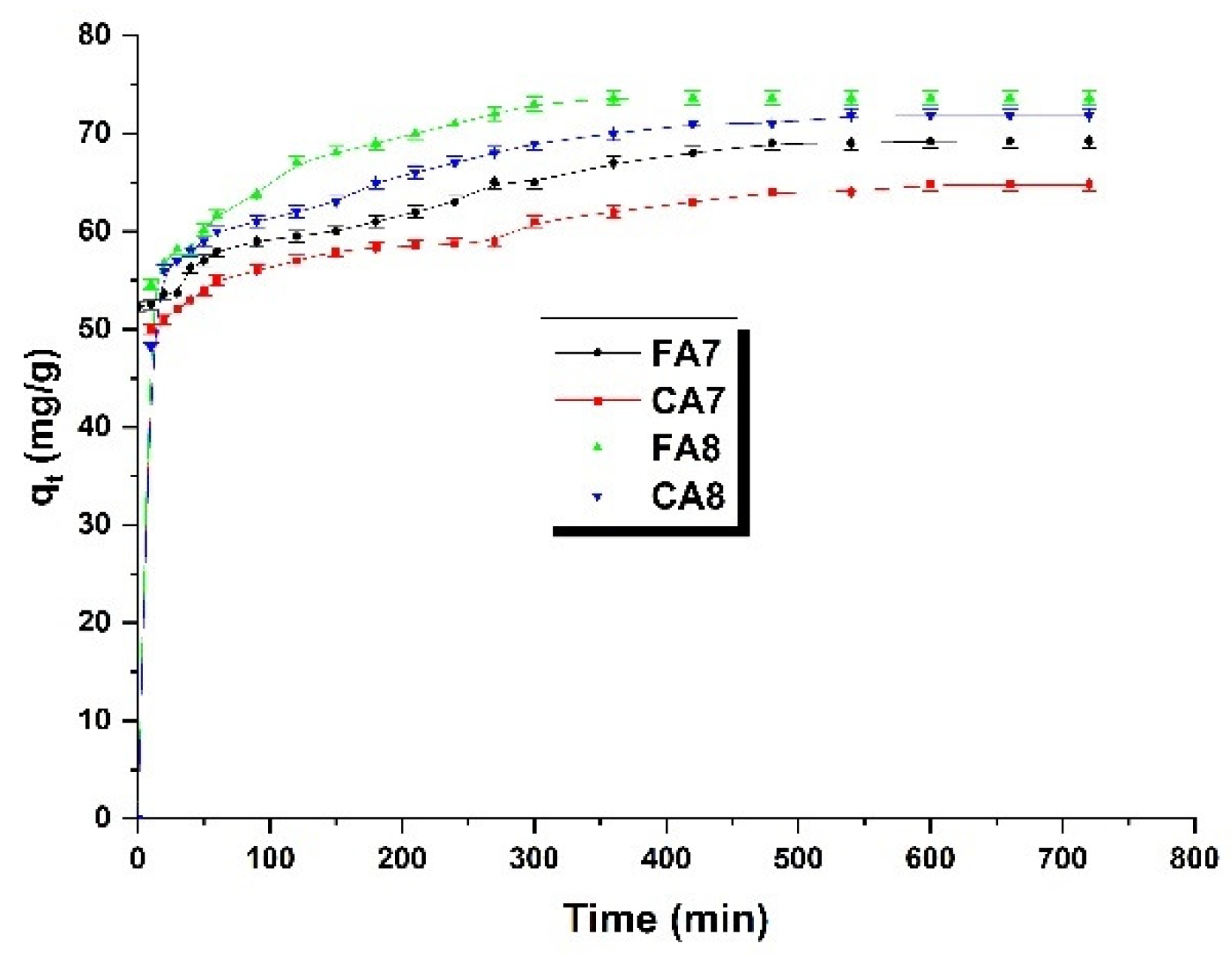
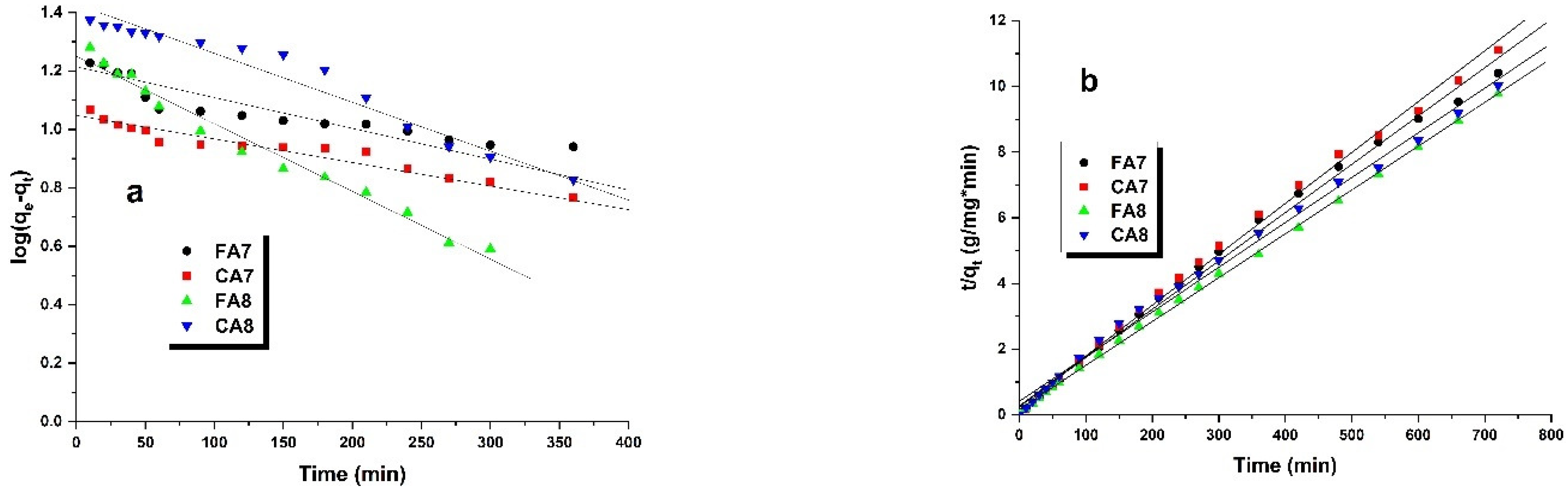
3.2.3. Adsorption Kinetics
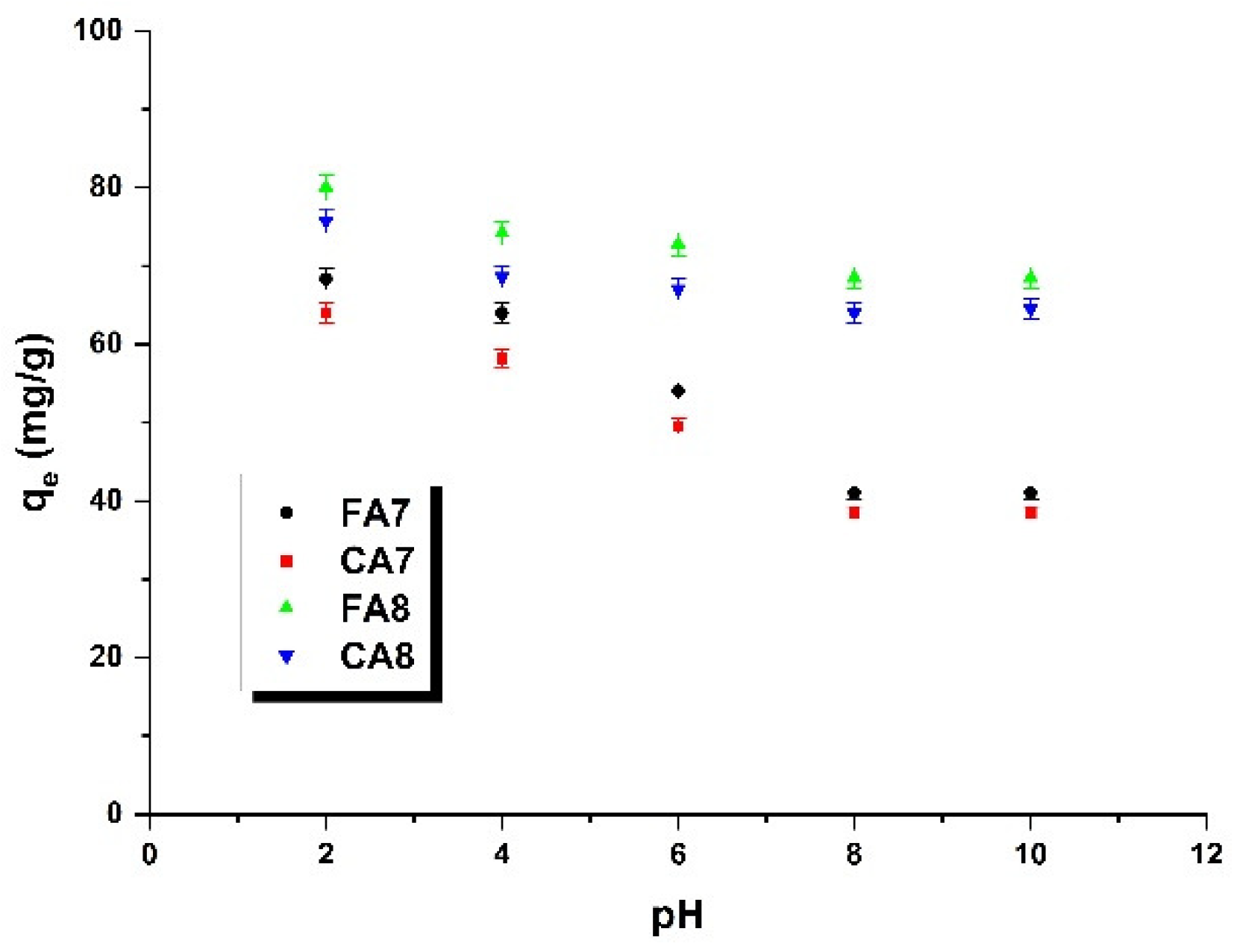
3.2.4. The Effect of pH
3.2.5. Thermodynamic Study
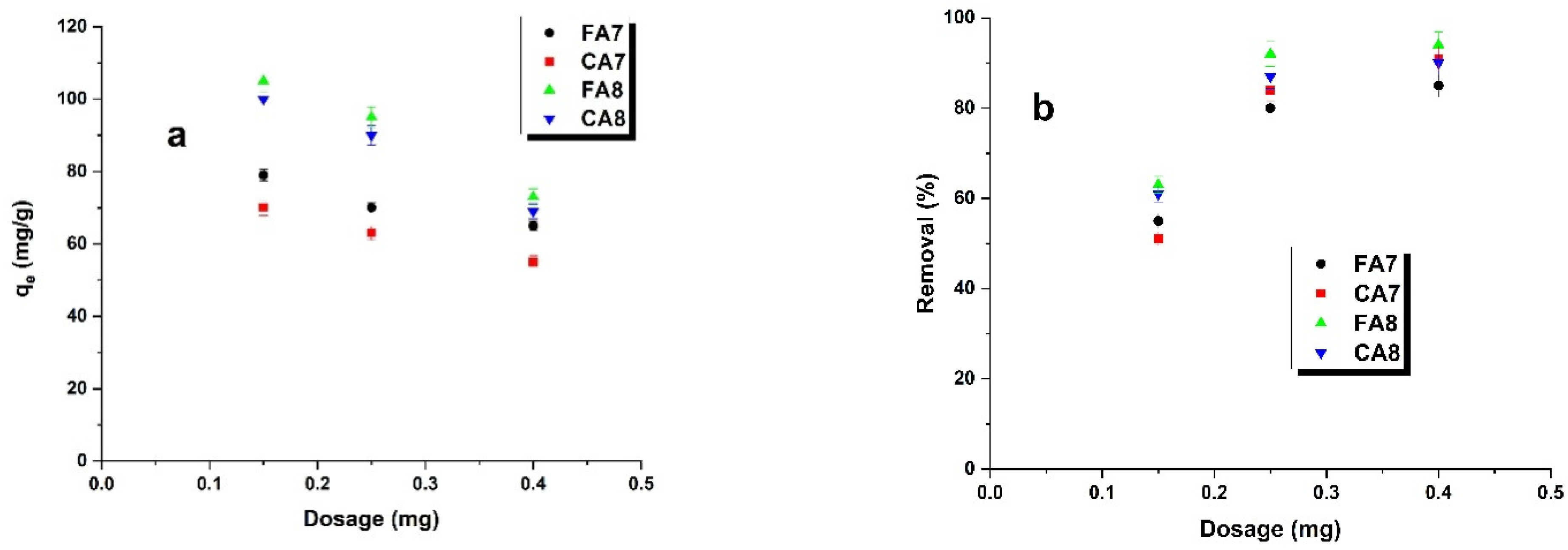
3.2.6. Effect of Adsorbent Amounts
3.2.7. Desorption
3.2.8. Adsorption Capacities of Selected Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piesse, M. Global Water Supply and Demand Trends Point towards Rising Water Insecurity. APO, 27 February 2020. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the projections of the world water development report. NPJ Clean Water 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Igliński, B.; Buczkowski, R.; Kujawski, W.; Cichosz, M.; Piechota, G. Geoenergy in Poland. Renew. Sust. Energ. Rev. 2012, 16, 2545–2557. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead toxicity and pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef] [PubMed]
- Aleksander-Kwaterczak, U.; Plenzler, D. Contamination of small urban watercourses on the example of a stream in Krakow (Poland). Environ. Earth Sci. 2019, 78, 530. [Google Scholar] [CrossRef]
- Caban, M.; Lis, E.; Kumirska, J.; Stepnowski, P. Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS (SIM) method based on Speedisk extraction disks and DIMETRIS derivatization. Sci. Total Environ. 2015, 538, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Nazar, W.; Niedoszytko, M. Air Pollution in Poland: A 2022 Narrative Review with Focus on Respiratory Diseases. Int. J. Environ. Res. Public Health 2022, 19, 895. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Wang, J.; Yan, L. Synthesis of lignin-poly (N-methylaniline)-reduced graphene oxide hydrogel for organic dye and lead ions removal. J. Bioresour. Bioprod. 2020, 5, 204–210. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of Granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Singh, P.; Mondal, K.; Sharma, A. Reusable electrospun mesoporous ZnO nanofiber mats for photocatalytic degradation of polycyclic aromatic hydrocarbon dyes in wastewater. J. Colloid Interf. Sci. 2013, 394, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; MAsiri, A.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Design 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Kamarehie, B.; Almasi, A.; Darvishmotevalli, M.; Salari, M.; Moradnia, M.; Karami, M.A. Removal of Rhodamine B from aqueous solution by stalk corn activated carbon: Adsorption and kinetic study. BiomassConv. Bioref. 2021, 1–10. [Google Scholar] [CrossRef]
- Khan, E.A.; Khan, T.A. Adsorption of methyl red on activated carbon derived from custard apple (Annonasquamosa) fruit shell: Equilibrium isotherm and kinetic studies. J. Mol. Liq. 2018, 249, 1195–1211. [Google Scholar] [CrossRef]
- Santhi, T.; Manonmani, S.; Smitha, T. Removal of methyl red from aqueous solution by activated carbon prepared from the Annonasqumosa seed by adsorption. Chem. Eng. Res. Bull. 2010, 14, 11–18. [Google Scholar] [CrossRef]
- Ioannou, Z.; Karasavvidis, C.; Dimirkou, A.; Antoniadis, V. Adsorption of methylene blue and methyl red dyes from aqueous solutions onto modified zeolites. Water Sci. Technol. 2013, 67, 1129–1136. [Google Scholar] [CrossRef]
- Ding, G.; Wang, B.; Chen, L.; Zhao, S. Simultaneous adsorption of methyl red and methylene blue onto biochar and an equilibrium modeling at high concentration. Chemosphere 2016, 163, 283–289. [Google Scholar] [CrossRef]
- Rajoriya, S.; Saharan, V.K.; Pundir, A.S.; Nigam, M.; Roy, K. Adsorption of methyl red dye from aqueous solution onto eggshell waste material: Kinetics, isotherms and thermodynamic studies. CRGC 2021, 4, 100180. [Google Scholar]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Pohl, A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Poll. 2020, 231, 1–17. [Google Scholar] [CrossRef]
- Perrich, J.R. Activated Carbon Adsorption for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Jiang, S.; Zhang, C.; He, S. High performance supercapacitors based on wood-derived thick carbon electrodes synthesized via green activation process. Inorg. Chem. Front. 2022.
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Dong, Y.; Ding, Y.; He, S. Nitrogen-doped carbon layer on cellulose derived free-standing carbon paper for high-rate supercapacitors. Appl. Surf. Sci. 2023, 608, 155144. [Google Scholar] [CrossRef]
- Bergna, D.; Hu, T.; Prokkola, H.; Romar, H.; Lassi, U. Effect of some process parameters on the main properties of activated carbon produced from peat in a lab-scale process. Waste Biomass Valori. 2020, 11, 2837–2848. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sust. Energ. Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Njewa, J.B.; Vunain, E.; Biswick, T. Synthesis and Characterization of Activated Carbons Prepared from Agro-Wastes by Chemical Activation. J. Chem. 2022, 2022, 9975444. [Google Scholar] [CrossRef]
- León, M.; Silva, J.; Carrasco, S.; Barrientos, N. Design, Cost Estimation and Sensitivity Analysis for a Production Process of Activated Carbon from Waste Nutshells by Physical Activation. Processes 2020, 8, 945. [Google Scholar] [CrossRef]
- Bazan, A.; Nowicki, P.; Pietrzak, R. The influence of activation procedure on the physicochemical and sorption properties of activated carbons prepared from pistachio nutshells for removal of NO2/H2S gases and dyes. J. Clean. Prod. 2017, 152, 211–222. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Nowicki, P.; Pietrzak, R. Characterization and application of bio-activated carbons prepared by direct activation of hay with the use of microwave radiation. Powder Technol. 2017, 319, 302–312. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Gralak-Podemska, B.; Nowicki, P.; Pietrzak, R. The use of microwave radiation for obtaining activated carbons from sawdust and their potential application in removal of NO2 and H2S. Chem. Eng. J. 2015, 269, 352–358. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Nowicki, P.; Pietrzak, R. Production of new activated bio-carbons by chemical activation of residue left after supercritical extraction of hops. Environ. Reserch. 2018, 161, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, P.; Gruszczyńska, K.; Urban, T.; Wiśniewska, T. Activated biocarbons obtained from post-fermentation residue as potential adsorbents of organic pollutants from the liquid phase. Physicochem. Probl. Miner. Process. 2022, 58, 146357. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Villabona-Ortíz, Á.; Figueroa-Lopez, K.J.; Ortega-Toro, R. Kinetics and adsorption equilibrium in the removal of azo-anionic dyes by modified cellulose. Sustainability 2022, 14, 3640. [Google Scholar] [CrossRef]
- Nagalakshmi, T.V.; Emmanuel, K.A.; Bhavani, P. Adsorption of disperse blue 14 onto activated carbon prepared from Jackfruit-PPI-I waste. Mater. Today Proc. 2019, 18, 2036–2051. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, H.; Miyamoto, N.; Kano, N.; Okawa, H. Adsorption of an anionic surfactant (sodium dodecyl sulfate) from an aqueous solution by modified cellulose with quaternary ammonium. Polymers 2022, 14, 1473. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Pietrzak, R. Adsorption of organic and inorganic pollutants on activated bio-carbons prepared by chemical activation of residues of supercritical extraction of raw plants. Chem. Eng. J. 2020, 393, 124785. [Google Scholar] [CrossRef]
- Sahnoun, S.; Boutahala, M. Adsorption removal of tartrazine by chitosan/polyaniline composite: Kinetics and equilibrium studies. Int. J. Biol. Macromol. 2018, 114, 1345–1353. [Google Scholar] [CrossRef]
- Gul, S.; Kanwal, M.; Qazi, R.A.; Gul, H.; Khattak, R.; Khan, M.S.; Khitab, F.; Krauklis, A.E. Efficient removal of methyl red dye by using bark of hopbush. Water 2022, 14, 2831. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H.J.; Liu, P.P.; Fang, W.; Geng, J.J. A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr (VI) in aqueous solutions. Int. J. Biol. Macromol. 2016, 87, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Tehrani-Bagha, A.R.; Nikkar, H.; Mahmoodi, N.M.; Markazi, M.; Menger, F.M. The sorption of cationic dyes onto kaolin: Kinetic, isotherm and thermodynamic studies. Desalination 2011, 266, 274–280. [Google Scholar] [CrossRef]
- Soldatkina, L.; Yanar, M. Equilibrium, Kinetic, and Thermodynamic Studies of Cationic Dyes Adsorption on Corn Stalks Modified by Citric Acid. Colloids Interfaces 2021, 5, 52. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef]
- Takkar, S.; Tyagi, B.; Kumar, N.; Kumari, T.; Iqbal, K.; Varma, A.; Thakur, I.S.; Mishra, A. Biodegradation of methyl red dye by a novel actinobacterium zhihengliuella sp. Istpl4: Kinetic studies, isotherm and biodegradation pathway. Environ. Technol. Innov. 2022, 26, 102348. [Google Scholar] [CrossRef]
- Chung, K.-T.; Fulk, G.; Andrews, A. Mutagenicity testing of some commonly used dyes. Appl. Environ. Microbiol. 1981, 42, 641–648. [Google Scholar] [CrossRef]
- Mas Har, M.R.S.; Sathasivam, K. The Removal of Methyl Red from Aqueous Solutions Using Banana Pseudostem Fibers. Am. J. Appl. Sci. 2009, 6, 1690–1700. [Google Scholar] [CrossRef]
- Ghaedi, M.; Kokhdan, S.N. Oxidized multiwalled carbon nanotubes for the removal of methyl red (MR): Kinetics and equilibrium study. Desalin. Water Treat. 2012, 49, 317–325. [Google Scholar] [CrossRef]




| Sample | Ash | C | H | N | O * |
|---|---|---|---|---|---|
| FA7 | 9.0 | 73.0 | 1.9 | 0.9 | 24.2 |
| CA7 | 7.8 | 82.2 | 1.2 | 0.8 | 15.8 |
| FA8 | 11.2 | 69.1 | 1.8 | 0.8 | 28.3 |
| CA8 | 8.5 | 72.0 | 1.1 | 0.8 | 26.1 |
| Biocarbons | Surface Area 1 (m2/g) | Total Pore Volume (cm3/g) | Micropore Area (m2/g) | Average Pore Diameter (nm) | Iodine Number (mg/g) |
|---|---|---|---|---|---|
| FA7 | 295 | 0.20 | 282 | 6.37 | 345 |
| CA7 | 233 | 0.51 | 222 | 7.65 | 303 |
| FA8 | 371 | 0.61 | 360 | 4.19 | 545 |
| CA8 | 330 | 0.48 | 223 | 3.68 | 489 |
| Biocarbons | Acidic Oxygen Functional Groups (mmol/g) | Basic Oxygen Functional Groups (mmol/g) | pH |
|---|---|---|---|
| FA7 | 0.25 ± 0.01 | 2.67 ± 0.02 | 9.56 ± 0.01 |
| CA7 | 0.19 ± 0.01 | 2.34 ± 0.02 | 9.33 ± 0.01 |
| FA8 | 0.05 ± 0.01 | 4.05 ± 0.03 | 10.46 ± 0.01 |
| CA8 | 0.09 ± 0.01 | 3.99 ± 0.03 | 10.00 ± 0.01 |
| Sample | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g(L/mg)1/n) | 1/n | R2 | AT (L/mg) | B | R2 | |
| FA7 | 71.43 | 0.008 | 0.900 | 16.48 | 0.547 | 0.996 | 3.07 | 16.81 | 0.918 |
| CA7 | 76.92 | 0.013 | 0.949 | 25.89 | 0.430 | 0.981 | 3.18 | 20.52 | 0.968 |
| FA8 | 142.86 | 0.025 | 0.976 | 78.16 | 0.277 | 0.993 | 4.53 | 39.15 | 0.972 |
| CA8 | 125.00 | 0.007 | 0.945 | 39.63 | 0.477 | 0.995 | 3.12 | 32.26 | 0.979 |
| Sample | qt(exp) (mg/g) | Pseudo-First-Order-Model | Pseudo-Second-Order-Model | ||||
|---|---|---|---|---|---|---|---|
| qe(cal) (mg/g) | k1(1/min) | R2 | qe(cal) (mg/g) | k2 (g mg−1×min−1) | R2 | ||
| FA7 | 69.20 | 16.37 | 0.0023 | 0.916 | 71.42 | 0.0006 | 0.994 |
| CA7 | 64.77 | 11.17 | 0.0002 | 0.963 | 66.67 | 0.0008 | 0.996 |
| FA8 | 73.65 | 17.83 | 0.0046 | 0.980 | 76.92 | 0.0009 | 0.999 |
| CA8 | 71.81 | 26.79 | 0.0023 | 0.976 | 74.63 | 0.0004 | 0.994 |
| Sample | Temperature (°C) | Gibbs Free Energy (kJ × mol−1) | Enthalpy (kJ × mol−1) | Entropy (J × mol−1 × K−1) |
|---|---|---|---|---|
| FA7 | 25 | −2.19 | 16.85 | 63.68 |
| 45 | −3.32 | |||
| 65 | −4.77 | |||
| CA7 | 25 | −1.90 | 17.35 | 65.05 |
| 45 | −2.98 | |||
| 65 | −4.52 | |||
| FA8 | 25 | −4.32 | 23.34 | 92.87 |
| 45 | −6.26 | |||
| 65 | −8.03 | |||
| CA8 | 25 | −5.09 | 25.18 | 100.77 |
| 45 | −7.07 | |||
| 65 | −9.16 |
| Adsorbent | Adsorption Capacity (mg/g) | Isotherm | Kinetics | Thermodynamics | References |
|---|---|---|---|---|---|
| Activated carbon from custard apple (Annona squamosa) fruit shell | 243.5 | Langmuir | pseudo-first-order | spontaneous, endothermic | [15] |
| Bark of the D. viscosa tree | 36.64 | Freundlich | - | - | [43] |
| Banana pseudostem fibers | 88.50 | pseudo-second-order | - | [50] | |
| Activated carbon prepared from Annona squamosa seeds | 27.68 | Langmuir | pseudo-second-order | - | [16] |
| Oxidized multiwalled carbon nanotubes | 108.69 | Langmuir | pseudo-first-order | spontaneous, endothermic | [51] |
| FA8 | 141 | Freundlich | pseudo-first-order | spontaneous, endothermic | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazan-Wozniak, A.; Paluch, D.; Wolski, R.; Cielecka-Piontek, J.; Nosal-Wiercińska, A.; Pietrzak, R. Biocarbons Obtained from Fennel and Caraway Fruits as Adsorbents of Methyl Red Sodium Salt from Water System. Materials 2022, 15, 8177. https://doi.org/10.3390/ma15228177
Bazan-Wozniak A, Paluch D, Wolski R, Cielecka-Piontek J, Nosal-Wiercińska A, Pietrzak R. Biocarbons Obtained from Fennel and Caraway Fruits as Adsorbents of Methyl Red Sodium Salt from Water System. Materials. 2022; 15(22):8177. https://doi.org/10.3390/ma15228177
Chicago/Turabian StyleBazan-Wozniak, Aleksandra, Dorota Paluch, Robert Wolski, Judyta Cielecka-Piontek, Agnieszka Nosal-Wiercińska, and Robert Pietrzak. 2022. "Biocarbons Obtained from Fennel and Caraway Fruits as Adsorbents of Methyl Red Sodium Salt from Water System" Materials 15, no. 22: 8177. https://doi.org/10.3390/ma15228177
APA StyleBazan-Wozniak, A., Paluch, D., Wolski, R., Cielecka-Piontek, J., Nosal-Wiercińska, A., & Pietrzak, R. (2022). Biocarbons Obtained from Fennel and Caraway Fruits as Adsorbents of Methyl Red Sodium Salt from Water System. Materials, 15(22), 8177. https://doi.org/10.3390/ma15228177








