Mechanical Properties and Corrosion Behavior of Silica Nanoparticle Reinforced Magnesium Nanocomposite for Bio-Implant Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
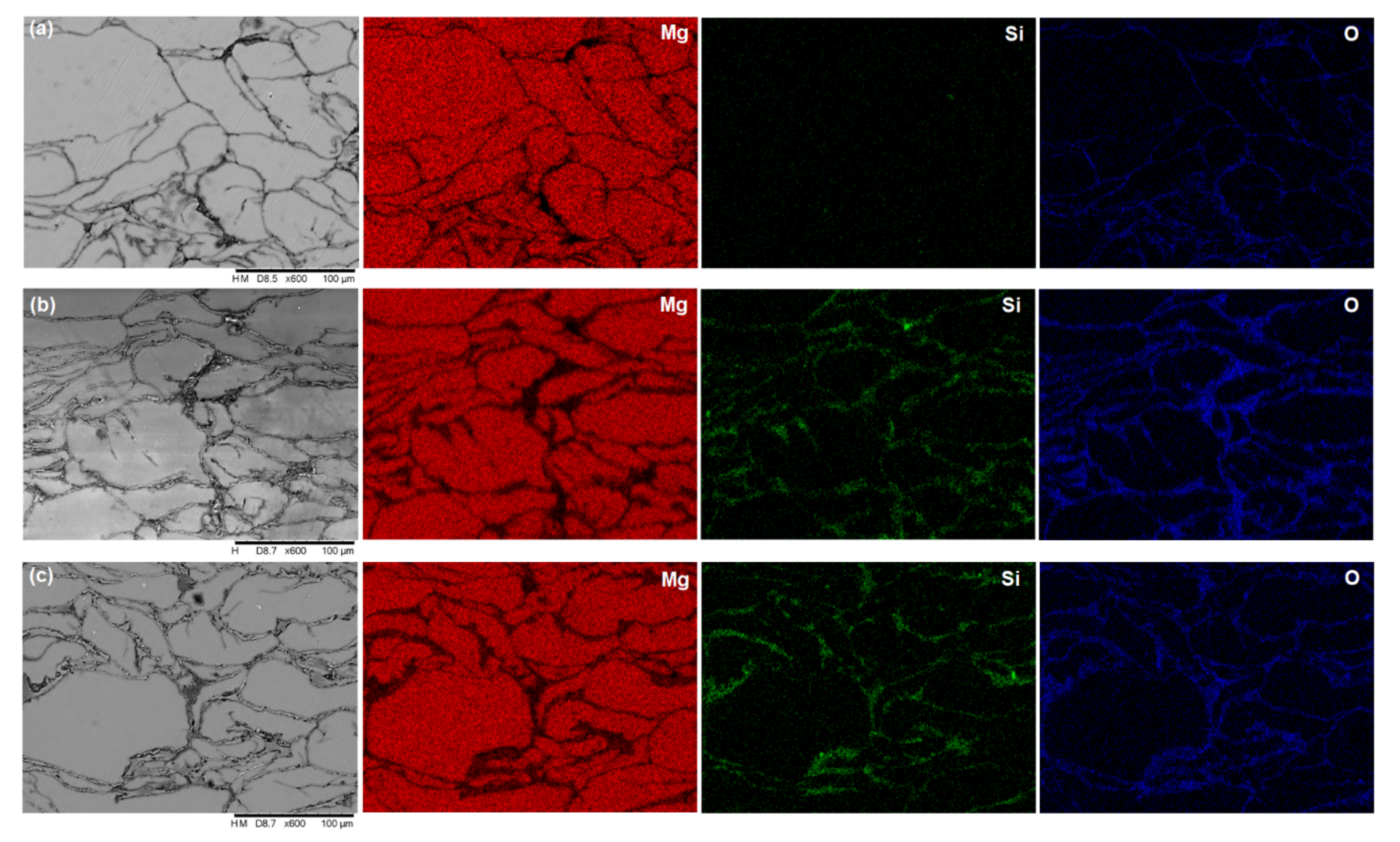
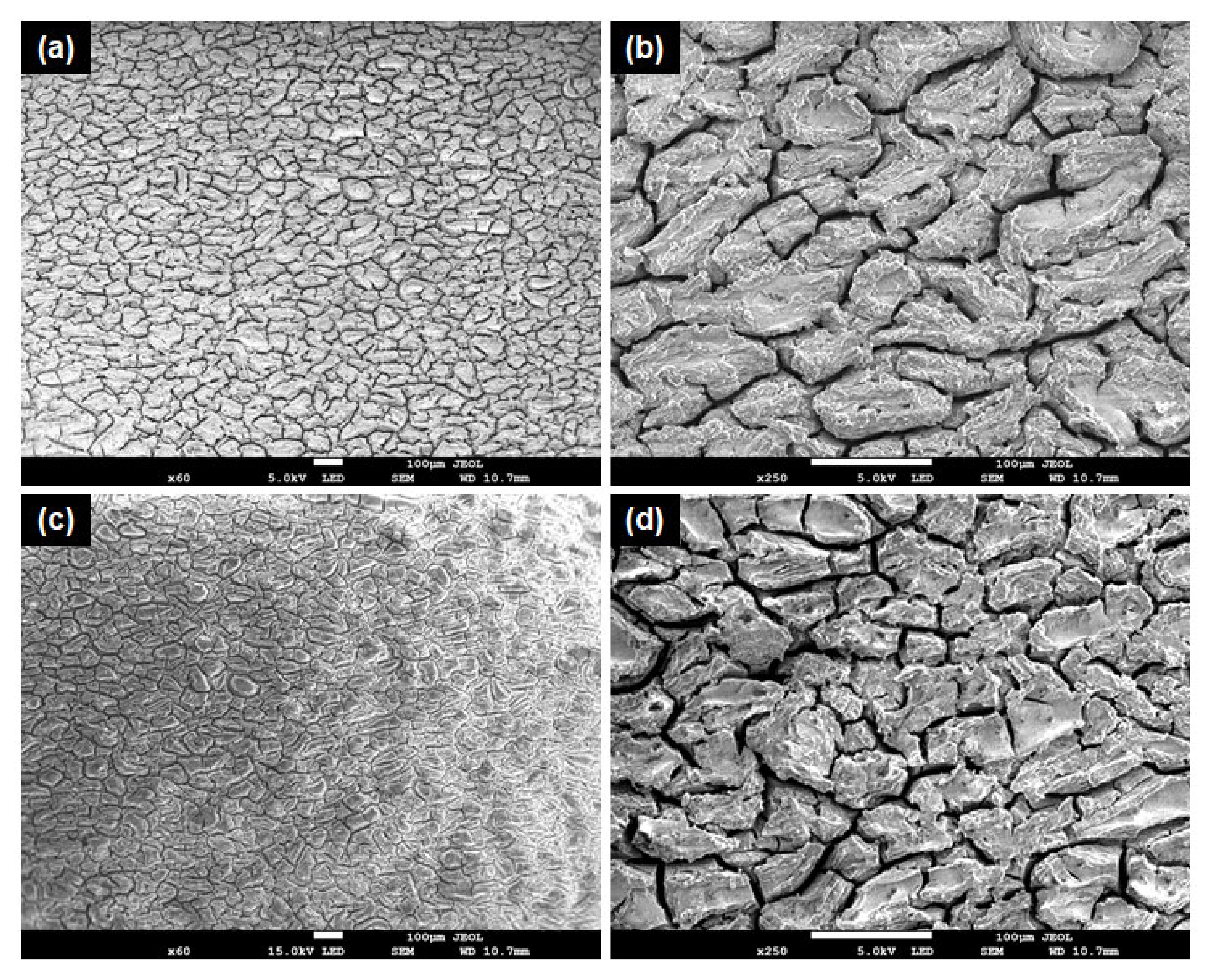
3.1. Microstructural Characterization
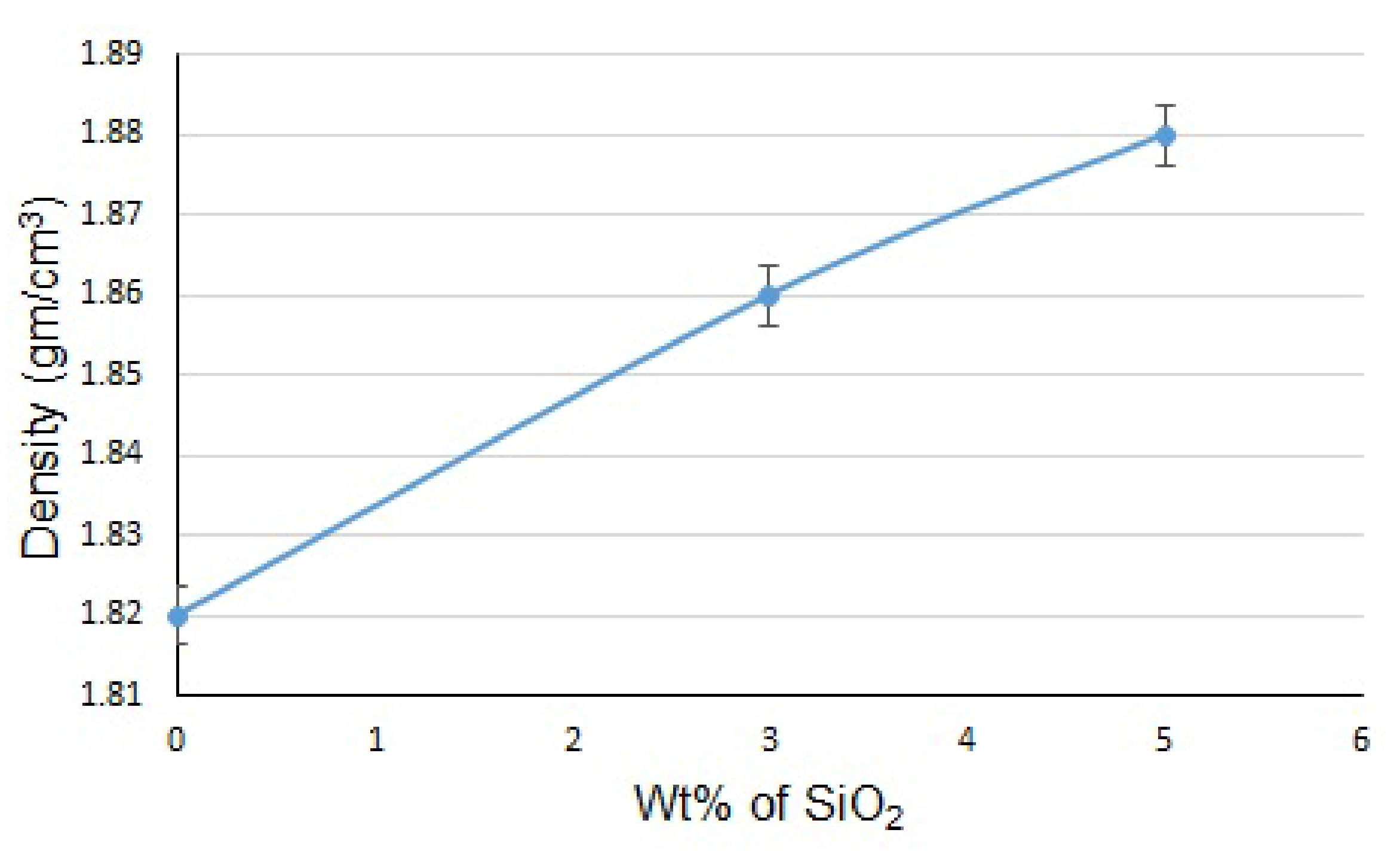
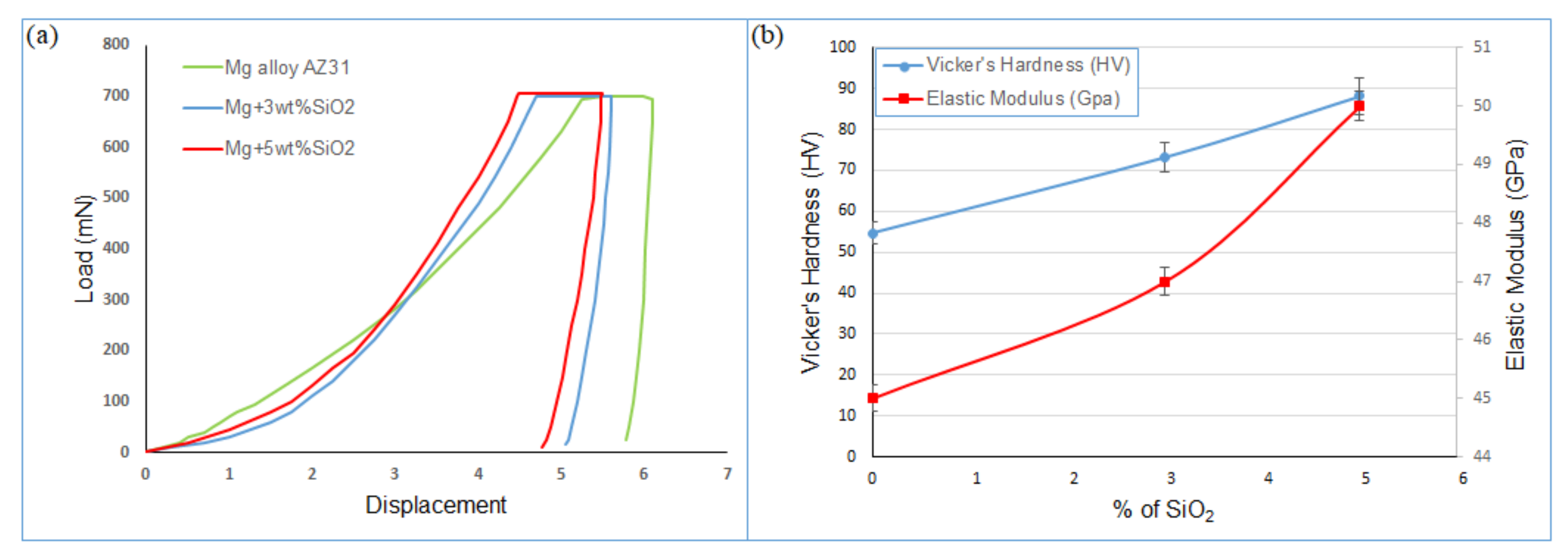
3.2. Mechanical Characterization
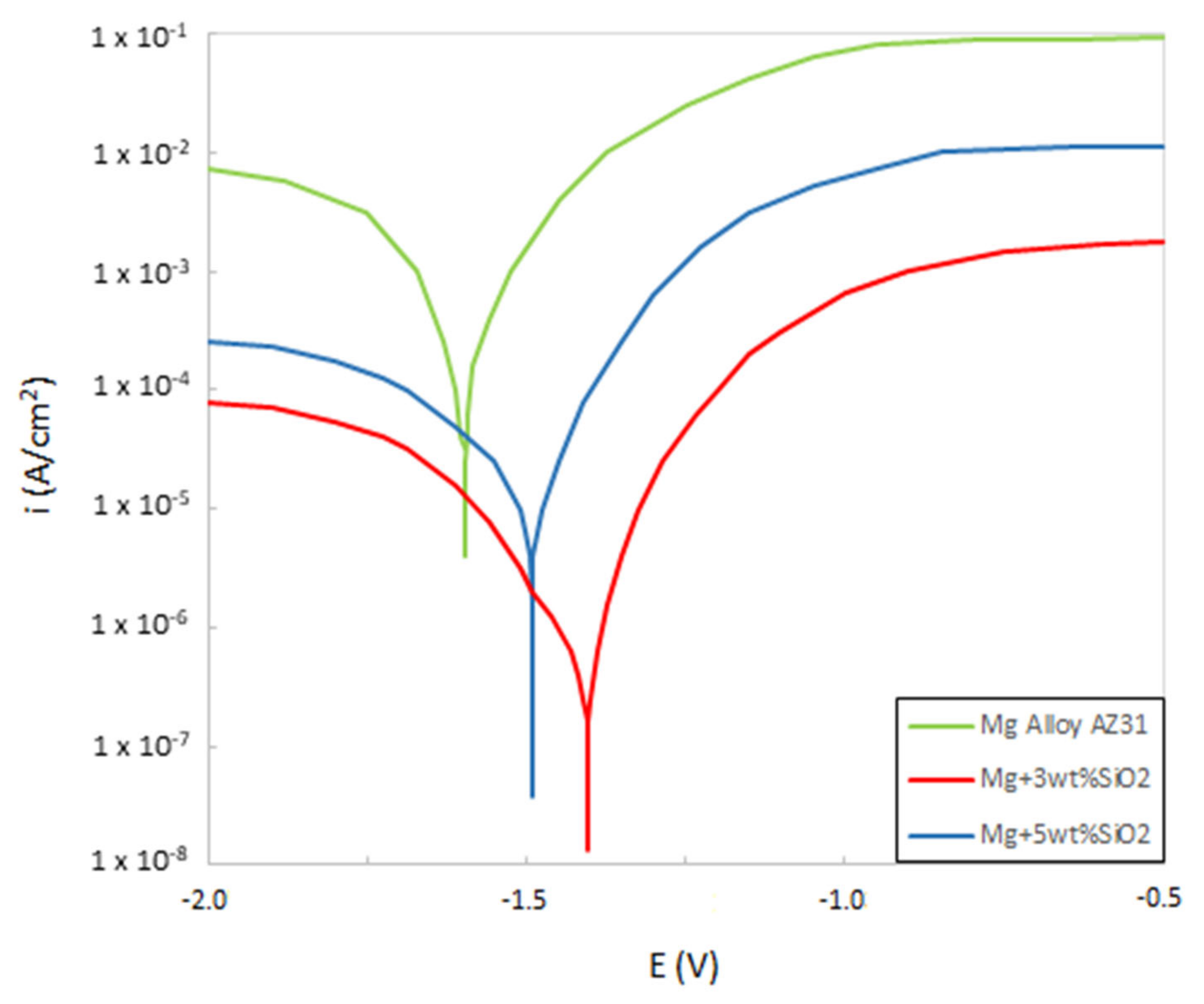
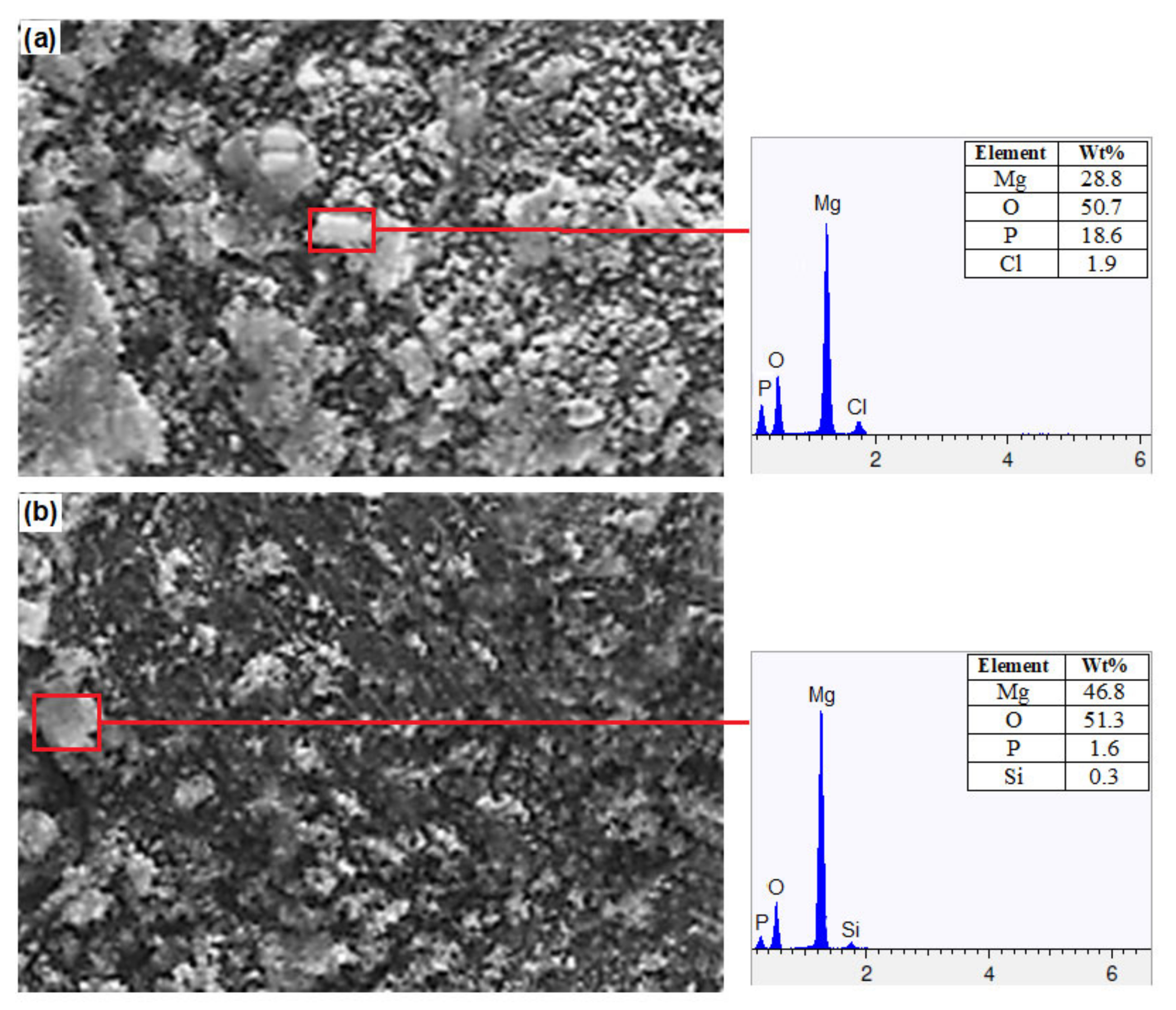
3.3. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Mag. Alloy. 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Panemangalore, D.B.; Rajashekhara, S.; Tingaud, D.; Touzin, M.; Ji, G. Biocompatible silica-based magnesium composites. J. Alloys Compd. 2019, 772, 49–57. [Google Scholar] [CrossRef]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials 2009, 30, 1512–1523. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, C.; Huo, K.; Tang, G.; Tian, X. Corrosion behavior of ZrN/Zr coated biomedical AZ91 magnesium alloy. Surf. Coat. Technol. 2009, 203, 2554–2557. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, C.; Huo, K.; Tang, G.; Tian, X.; Chu, P.K. Influence of heat treatment on degradation behavior of bio-degradable die-cast AZ63 magnesium alloy in simulated body fluid. Mater. Sci. Eng. A 2007, 456, 350–357. [Google Scholar]
- Al-Maamari, A.E.A.; Iqbal, A.A.; Nuruzzaman, D.M. Wear and mechanical characterization of Mg–Gr self-lubricating composite fabricated by mechanical alloying. J. Magnes. Alloy. 2019, 7, 283–290. [Google Scholar] [CrossRef]
- Gu, X.-N.; Zheng, Y.-F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. A 2009, 90, 882–893. [Google Scholar] [CrossRef]
- Song, G. Recent progress in corrosion and protection of magnesium alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Torabi, H.; Hoseini, M.; Sadrkhah, M.; Faraji, G.; Masoumi, A. Microstructure, mechanical properties and bio-corrosion prop-erties of Mg-HA bionanocomposites fabricated by a novel severe plastic deformation process. Ceram. Int. 2020, 46, 2836–2844. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. 3D printed hydroxyapatite composite scaffolds with enhanced mechanical properties. Ceram. Int. 2019, 45, 10991–10996. [Google Scholar] [CrossRef]
- Vassal, M.F.; Nunes-Pereira, J.; Miguel, S.P.; Correia, I.J.; Silva, A.P. Microstructural, mechanical and biological properties of hydroxyapatite- CaZrO3 biocomposites. Ceram. Int. 2019, 45, 8195–8203. [Google Scholar] [CrossRef]
- Fan, X. Preparation and performance of hydroxyapatite/Ti porous biocomposite scaffolds. Ceram. Int. 2019, 45, 16466–16469. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, Z.X.; Zhang, J.Y.; Wang, F.; Li, Y.Y. Influence of pre-solution treatment on microstructure and mechanical properties of Mg–Gd–Nd–Zn–Zr alloy processed by ECAP. Adv. Eng. Mater. 2015, 18, 833–838. [Google Scholar] [CrossRef]
- Salernitano, E.; Migliaresi, C. Composite materials for biomedical applications: A review. J. Appl. Biomater. Biomech. 2003, 1, 3–18. [Google Scholar]
- Kang, M.-H.; Jang, T.-S.; Kim, S.W.; Park, H.-S.; Song, J.; Kim, H.-E.; Jung, K.-H.; Jung, H.-D. MgF2-coated porous magnesium/alumina scaffolds with improved strength, corrosion resistance, and biological performance for biomedical applications. Mater. Sci. Eng. C 2016, 62, 634–642. [Google Scholar] [CrossRef]
- Amaravathy, P.; Sathyanarayanan, S.; Sowndarya, S.; Rajendran, N. Bioactive HA/TiO2 coating on magnesium alloy for bio-medical applications. Ceram. Int. 2014, 40, 6617–6630. [Google Scholar] [CrossRef]
- Amiri, H.; Mohammadi, I.; Afshar, A. Electrophoretic deposition of nano-zirconia coating on AZ91D magnesium alloy for bio-corrosion control purposes. Surf. Coat. Technol. 2017, 311, 182–190. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Saud, S.N.; Yaghoubidoust, F.; Akbari, E. Structure, corrosion behavior, and antibacterial properties of nano-silica/graphene oxide coating on biodegradable magnesium alloy for biomedical ap-plications. Vacuum 2016, 131, 106–110. [Google Scholar] [CrossRef]
- Ferreira, P.C.; Piai, K.D.A.; Takayanagui, A.M.M.; Segura-Muñoz, S.I. Aluminum as a risk factor for Alzheimer’s disease. Rev. Latino-Am. Enferm. 2008, 16, 151–157. [Google Scholar] [CrossRef]
- Beck, G.R., Jr.; Ha, S.-W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.-K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Yildirimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Draenert, F.G.; Albert, O.; Schr€oder, H.C.; Mail€ander, V.; Mitov, G.; Müller, W.E.G. Bioactive and biode-gradable silica biomaterial for bone regeneration. Bone 2014, 67, 292–304. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Wang, L.; Qin, X.; Xiong, W.; Zhu, X. Fabrication and mechanical properties of bulk nanocrystalline intermetallic Mg2Si. Mater. Sci. Eng. A 2007, 459, 216–222. [Google Scholar] [CrossRef]
- Kondoh, K.; Oginuma, H.; Kimura, A.; Matsukawa, S.; Aizawa, T. In-situ Synthesis of Mg2Si Intermetallics via Powder Metallurgy Process. Mater. Trans. 2003, 44, 981–985. [Google Scholar] [CrossRef]
- Lu, L.; Lai, M.O.; Hoe, M.L. Formation of nanocrystalline Mg2Si and Mg2Si dispersion strengthened Mg-Al alloy by mechanical alloying. Nanostruct. Mater. 1998, 10, 551–563. [Google Scholar] [CrossRef]
- Sun, B.; Li, S.; Imai, H.; Umeda, J.; Kondoh, K. Kinetic Analysis of Solid-State Formation of Mg2Si by Powder Metallurgy Process. J. High Temp. Soc. 2011, 37, 321–325. [Google Scholar] [CrossRef][Green Version]
- Olszówka-Myalska, A.; McDonald, S.A.; Withers, P.J.; Myalska, H.; Moskal, G. Microstructure of in-situ Mg metal matrix composites based on silica nanoparticles. Solid State Phenom. 2012, 191, 189–198. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S. The role of Mg2Si on the corrosion behavior of wrought Mg-Zn-Mn alloy. Intermetallics 2008, 16, 860–867. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S. The role of Si and Ca on new wrought Mg-Zn-Mn based alloy. Mater. Sci. Eng. A 2007, 447, 35–43. [Google Scholar] [CrossRef]
- Gupta, M.; Meenashisundaram, G.K. Insight into Designing Biocompatible Magnesium Alloys and Composites; Springer: Singapore, 2015. [Google Scholar]


| Al | Zn | Mn | Cu | Mg |
|---|---|---|---|---|
| 2.83 | 0.8 | 0.37 | 0.002 | Balance |
| Parameter | Mg Alloy AZ31 | SiO2 |
|---|---|---|
| Density (g/cm3) | 1.8 | 2.6 |
| Tensile Strength (MPa) | 290 | 135 |
| Young’s Modulus (GPa) | 45 | 70 |
| Poisson’s ratio | 0.35 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.A.; Ismail, N.B. Mechanical Properties and Corrosion Behavior of Silica Nanoparticle Reinforced Magnesium Nanocomposite for Bio-Implant Application. Materials 2022, 15, 8164. https://doi.org/10.3390/ma15228164
Iqbal AA, Ismail NB. Mechanical Properties and Corrosion Behavior of Silica Nanoparticle Reinforced Magnesium Nanocomposite for Bio-Implant Application. Materials. 2022; 15(22):8164. https://doi.org/10.3390/ma15228164
Chicago/Turabian StyleIqbal, AKM Asif, and Norfatihah Binti Ismail. 2022. "Mechanical Properties and Corrosion Behavior of Silica Nanoparticle Reinforced Magnesium Nanocomposite for Bio-Implant Application" Materials 15, no. 22: 8164. https://doi.org/10.3390/ma15228164
APA StyleIqbal, A. A., & Ismail, N. B. (2022). Mechanical Properties and Corrosion Behavior of Silica Nanoparticle Reinforced Magnesium Nanocomposite for Bio-Implant Application. Materials, 15(22), 8164. https://doi.org/10.3390/ma15228164





