Polymeric Orthosis with Electromagnetic Stimulator Controlled by Mobile Application for Bone Fracture Healing: Evaluation of Design Concepts for Medical Use
Abstract
1. Introduction
2. Materials and Methods
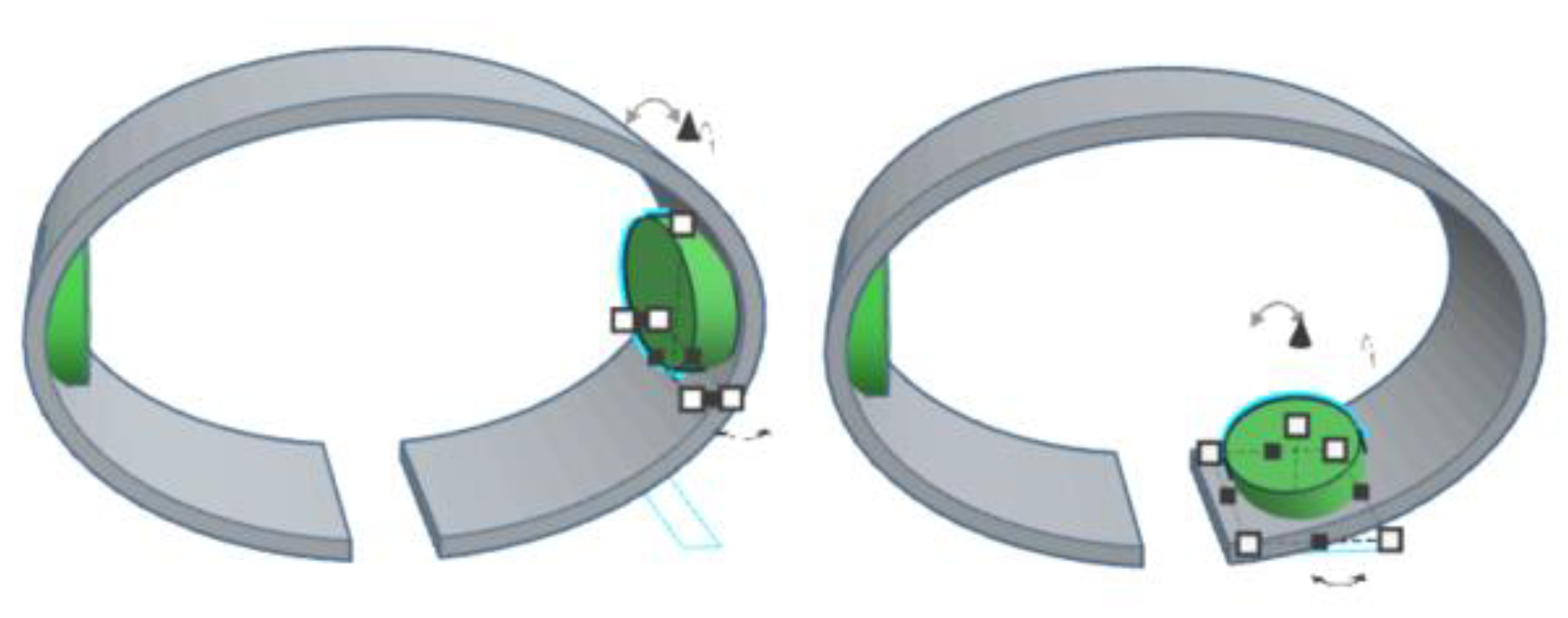
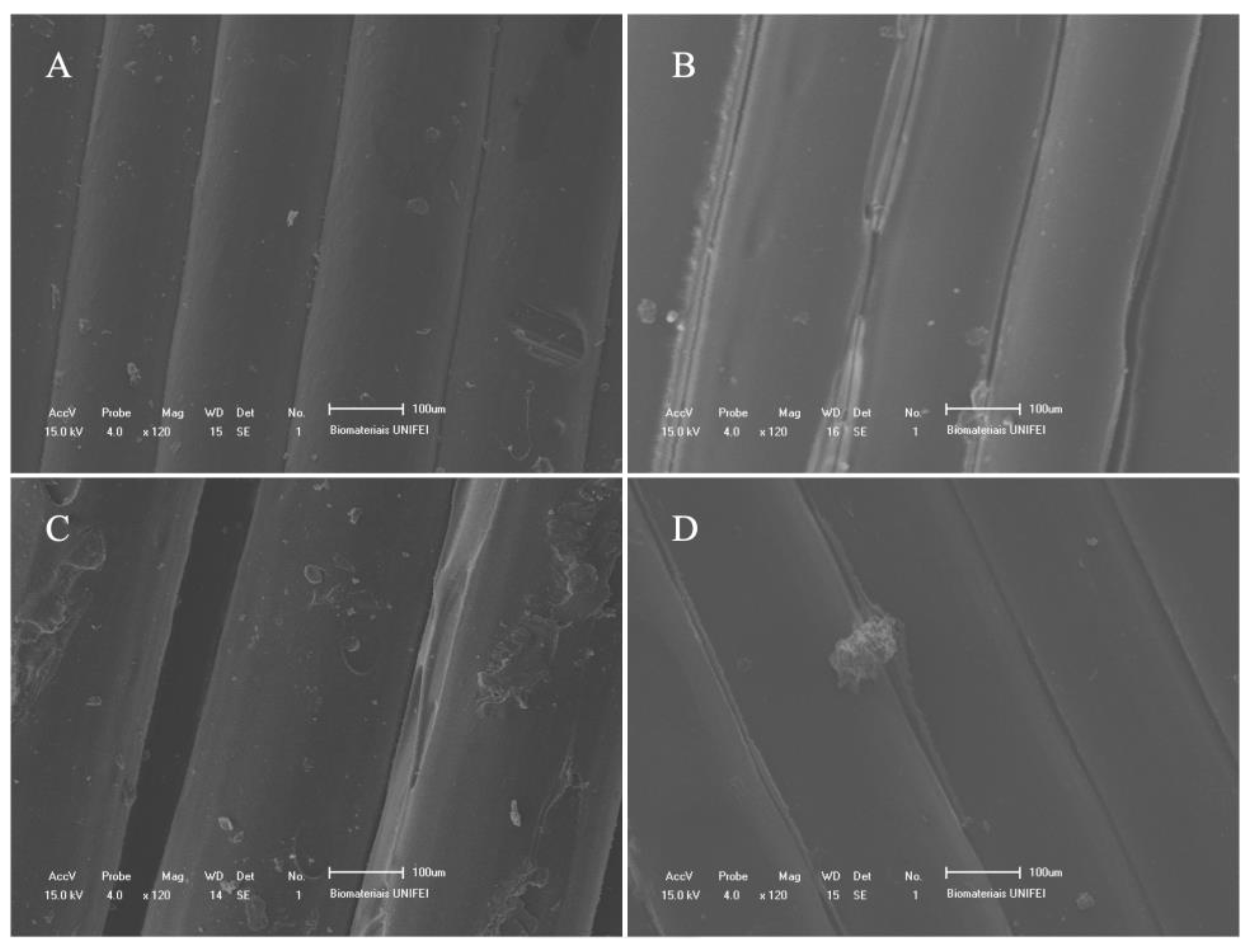
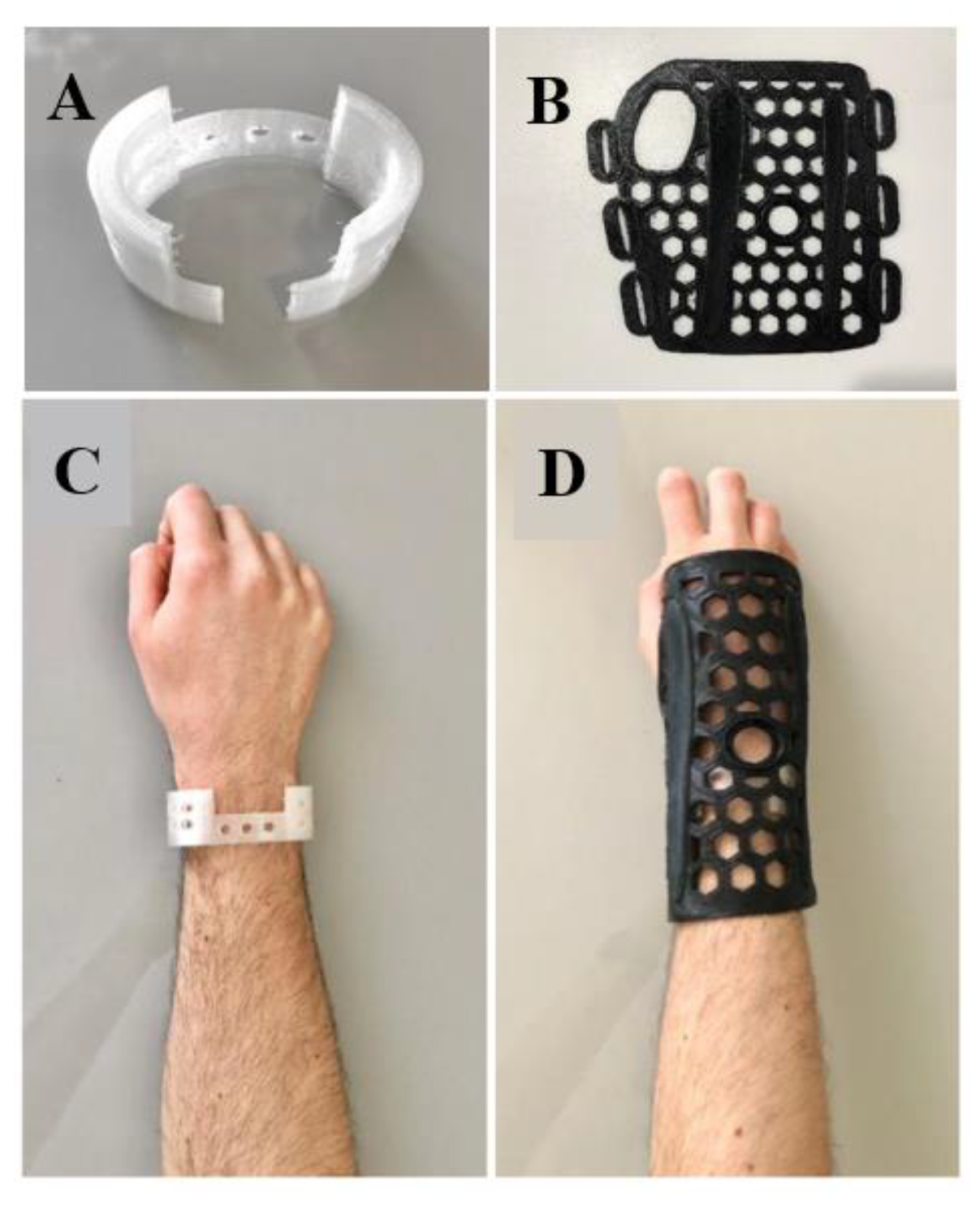
2.1. Orthosis Development
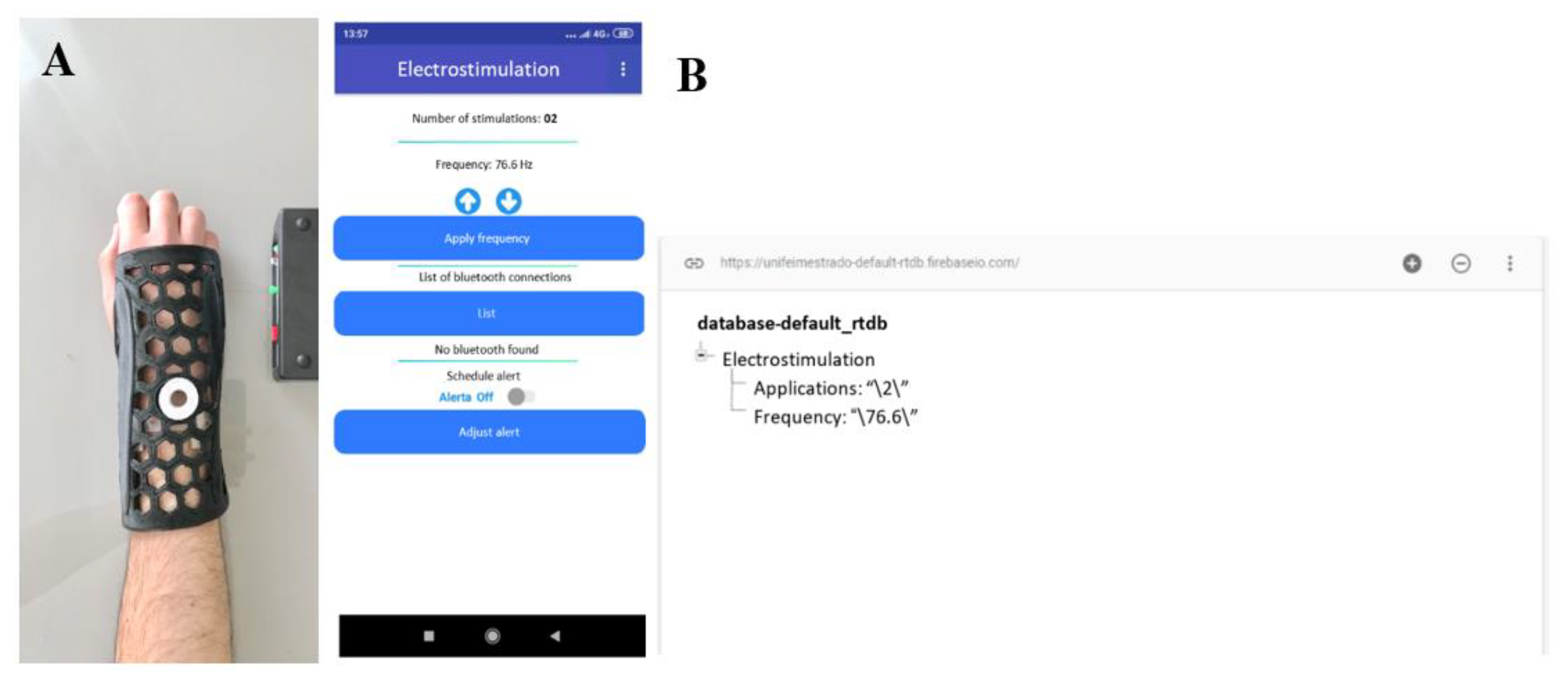
2.2. CMF Stimulator Development
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




| Questions | Grade |
|---|---|
| The use of orthopedic plaster in limb immobilization has some disadvantages. One of them is: overheating and moisture retention in the region where the plaster will be placed, by sweat or water. How much do you agree with this statement? | 3.0 ± 0.00 |
| The use of orthopedic plaster in limb immobilization has some disadvantages. One of them is: difficulty in visualizing and monitoring the progression of treatment. How much do you agree with this statement? | 2.29 ± 0.76 |
| The use of orthopedic plaster in limb immobilization has some disadvantages. One of them is: reduction of the patient’s daily functions not directly related to the fracture. For example, bath time is difficult. How much do you agree with this statement? | 2.71 ± 0.49 |
| The use of orthopedic plaster in limb immobilization has some disadvantages. One of them is: resistance to treatment by the patient, mainly due to aesthetic aspects. How much do you agree with this statement? | 2.0 ± 0.63 |
| The use of orthopedic plaster in limb immobilization has some disadvantages. One of them is: prolonged recovery time, as it is an inert material, which only immobilizes, but does not act directly to accelerate the recovery process. How much do you agree with this statement? | 2.43 ± 0.79 |
| Questions | Grade |
|---|---|
| Traditional 3D-printed orthoses for immobilization of a fractured limb have the disadvantage of high patient mobility. That is, the region is not completely immobilized, giving degrees of freedom to the patient’s movement. How much do you agree with this statement? | 2.0 ± 0.82 |
| Traditional 3D-printed orthoses for immobilization of a fractured limb have the disadvantage of the physician’s difficulty in defining the fixation points. That is, the orthosis cannot stabilize the fractured region like an orthopedic cast. How much do you agree with this statement? | 1.71 ± 1.11 |
| Traditional 3D-printed orthoses for immobilization of a fractured limb have the disadvantage of being easy for the patient to remove, impairing the outcome of the treatment. How much do you agree with this statement? | 1.71 ± 0.82 |
| Questions | Grade |
|---|---|
| In the project concept described in the documentation provided, the use of a 3D-printed malleable mesh was proposed to be positioned over the fracture region, that is, over the points of interest for orthopedic fixation and stabilization. A second immobilization mesh will be placed over the first, in order to prevent patient movement. This concept solves the problem seen in printed orthoses of not having well-defined fixation points and allowing degrees of freedom for patient movement. How much do you agree with this statement? | 2.43 ± 0.79 |
| In the project concept described in the documentation delivered, it was proposed to use holes in the meshes to allow ventilation of the user’s skin. Also, the materials used in printing are biocompatible. These aspects of the concept prevent bacteria buildup, odor and skin irritation. How much do you agree with this statement? | 2.86 ± 0.38 |
| In the design concept described in the documentation provided, it was proposed that the immobilization mesh should be heated to around 55 °C to become malleable. Using the fixation mesh prior to modeling the immobilization mesh over the patient’s arm would help to prevent the heated mesh from having direct contact with the patient’s skin. This concept allows the mesh to be molded according to the patient’s anatomy, as well as avoiding burns. How much do you agree with this statement? | 2.8 ± 0.45 |
| In the project concept described in the documentation delivered, it was proposed that the immobilization mesh has dimensions pre-formatted to the patient’s arm. Once heated, the healthcare professional will have around 20 seconds to model it on the patient’s arm or until the mesh temperature drops below 50°C. This time is understood as sufficient for modeling, since the printed immobilization mesh already has dimensions close to those of the patient’s anthropometry. How much do you agree with this statement? | 2.5 ± 0.50 |
| In general, the use of electrostimulators, through the application of magnetic and/or electromagnetic fields on the fractured region, are beneficial for the treatment. They allow, when used for a while and with adequate intensity and frequency specifications, to accelerate bone reintegration and, consequently, the patient’s recovery. In other words, it helps to reduce the treatment time. How much do you agree with this statement? | 3.0 ± 0.00 |
| In the project concept described in the delivered documentation, a combined magnetic field (CMF) electrostimulator was proposed, which, according to the scientific literature, needs applications of 30 min/day to assist in accelerating fracture recovery. The use of CMF is known to be conducive to bone fracture recovery. How much do you agree with this statement? | 2.5 ± 0.55 |
| In the project concept described in the documentation delivered, a form of light and sound alerts was proposed so that the patient does not forget the moment of electrostimulation. The system also counts how many times the patient has performed electrostimulation, and the count only occurs when the control system is coupled to the electrostimulator. These features of the concept avoid the problem of the patient forgetting to perform the daily electrostimulation or mistakenly reporting to the health professional that he performed all the sessions, when he did not actually perform them. How much do you agree with this statement? | 2.83 ± 0.41 |
| In the project concept described in the delivered documentation, a combined magnetic field (CMF) electrostimulator was proposed that does not require metallic electrodes to be placed directly on the patient’s skin. Nor does it require direct electrical current to be applied to the patient’s body during electrostimulation, as only the magnetic/electromagnetic field is applied. This aspect of the concept avoids problems of electric shock and electric current burn to the patient. How much do you agree with this statement? | 3.0 ± 0.00 |
| In the project concept described in the documentation delivered, it was proposed that the immobilization mesh be attached to the patient’s arm by tissue strips. One possibility is that the strips can be attached to a portable electronic system, which will notify you when they are taken out or opened. This possibility would be a valid resource for monitoring the health professional, who would know that the patient did not use the mesh all the time. Thus, he could instruct the patient about the problems arising from the removal of the mesh for his treatment. How much do you agree with this statement? | 2.86 ± 0.38 |
References
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, P.A.B.; Rocha, A.D.J.; Fonseca, R.J.D.S.; Teixeira, T.R.G.; Mattos, E.D.S.R.; Guedes, A. Femoral fractures in the elderly in Brasil-incidence, lethality, and costs (2008–2018). Rev. Assoc. Médica Bras. 2020, 66, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.R.; Anokye, N.K.; Vlachopoulos, D.; Barbieri, F.A.; Turi-Lynch, B.C.; Codogno, J.S.; Agostinete, R.R.; Fernandes, R.A. Impact of sports participation on incidence of bone traumatic fractures and health-care costs among adolescents: Growth study. Physician Sportsmed. 2019, 48, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, S.M.; Balogh, Z.J. The Global Burden of Surgical Management of Osteoporotic Fractures. World J. Surg. 2020, 44, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.G.; Bae, G.; Kwon, H.-Y.; Yang, H. Aging and direct medical costs of osteoporotic fractures. J. Bone Miner. Metab. 2021, 39, 589–597. [Google Scholar] [CrossRef]
- Schlégl, T.; Told, R.; Kardos, K.; Szőke, A.; Ujfalusi, Z.; Maróti, P. Evaluation and Comparison of Traditional Plaster and Fiberglass Casts with 3D-Printed PLA and PLA–CaCO3 Composite Splints for Bone-Fracture Management. Polymers 2022, 14, 3571. [Google Scholar] [CrossRef]
- Prakash, C.; Singh, S. On the characterization of functionally graded biomaterial primed through a novel plaster mold casting process. Mater. Sci. Eng. C 2020, 110, 110654. [Google Scholar] [CrossRef]
- Szostakowski, B.; Smitham, P.; Khan, W. Plaster of Paris–Short History of Casting and Injured Limb Immobilzation. Open Orthop. J. 2017, 11, 291–296. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Schofield, K.A. Utilization of 3D printed orthoses for musculoskeletal conditions of the upper extremity: A systematic review. J. Hand Ther. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Lu, P.; Liao, Z.; Zeng, Q.; Chen, H.; Huang, W.; Liu, Z.; Chen, Y.; Zhong, J.; Huang, G. Customized Three-Dimensional-Printed Orthopedic Close Contact Casts for the Treatment of Stable Ankle Fractures: Finite element analysis and a pilot study. ACS Omega 2021, 6, 3418–3426. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, G.; Yu, L.; Wang, Y.; Fang, Y.; Shen, Y.; Huang, X.; Qiao, L.; Yang, J.; Zhang, Y.; et al. Effects of a 3D-printed orthosis compared to a low-temperature thermoplastic plate orthosis on wrist flexor spasticity in chronic hemiparetic stroke patients: A randomized controlled trial. Clin. Rehabil. 2019, 34, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Katt, B.; Imbergamo, C.; Seigerman, D.; Rivlin, M.; Beredjiklian, P.K. The use of 3D printed customized casts in children with upper extremity fractures: A report of two cases. Arch. Bone Jt. Surg. 2020, 9, 126. [Google Scholar]
- Palanisamy, P.; Alam, M.; Li, S.; Chow, S.K.H.; Zheng, Y. Low-Intensity Pulsed Ultrasound Stimulation for Bone Fractures Healing. J. Ultrasound Med. 2021, 41, 547–563. [Google Scholar] [CrossRef] [PubMed]
- e Silva, E.P.; Huang, B.; Helaehil, J.V.; Nalesso, P.R.L.; Bagne, L.; de Oliveira, M.A.; Albiazetti, G.C.C.; Aldalbahi, A.; El-Newehy, M.; Santamaria, M., Jr.; et al. In vivo study of conductive 3D printed PCL/MWCNTs scaffolds with electrical stimulation for bone tissue engineering. Bio-Des. Manuf. 2021, 4, 190–202. [Google Scholar] [CrossRef]
- Cai, J.; Shao, X.; Yang, Q.; Yang, Y.; Yan, Z.; Luo, E.; Feng, X.; Jing, D. Pulsed electromagnetic fields modify the adverse effects of glucocorticoids on bone architecture, bone strength and porous implant osseointegration by rescuing bone-anabolic actions. Bone 2020, 133, 115266. [Google Scholar] [CrossRef]
- Cheaney, B.; El Hashemi, M.; Obayashi, J.; Than, K.D. Combined magnetic field results in higher fusion rates than pulsed electromagnetic field bone stimulation after thoracolumbar fusion surgery. J. Clin. Neurosci. 2020, 74, 115–119. [Google Scholar] [CrossRef]
- Ehnert, S.; Schröter, S.; Aspera-Werz, R.H.; Eisler, W.; Falldorf, K.; Ronniger, M.; Nussler, A.K. Translational Insights into Extremely Low Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery. J. Clin. Med. 2019, 8, 2028. [Google Scholar] [CrossRef]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular mechanisms of skeletal response. Jaaos Glob. Res. Rev. 2020, 4, e19.00155. [Google Scholar] [CrossRef]
- Oltean-Dan, D.; Dogaru, G.B.; Apostu, D.; Mester, A.; Benea, H.R.C.; Paiusan, M.G.; Popa, C.O.; Jianu, E.M.; Bodizs, G.I.; Berce, C.; et al. Enhancement of bone consolidation using high-frequency pulsed electromagnetic fields (HF-PEMFs): An experimental study on rats. Bosn. J. Basic Med. Sci. 2019, 19, 201–209. [Google Scholar] [CrossRef]
- Mi, J.; Xu, J.; Yao, Z.; Yao, H.; Li, Y.; He, X.; Dai, B.; Zou, L.; Tong, W.; Zhang, X.; et al. Implantable Electrical Stimulation at Dorsal Root Ganglions Accelerates Osteoporotic Fracture Healing via Calcitonin Gene-Related Peptide. Adv. Sci. 2021, 9, 2103005–2103015. [Google Scholar] [CrossRef]
- Daish, C.; Blanchard, R.; Fox, K.; Pivonka, P.; Pirogova, E. The Application of Pulsed Electromagnetic Fields (PEMFs) for Bone Fracture Repair: Past and perspective findings. Ann. Biomed. Eng. 2018, 46, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Sawaji, Y.; Iwaki, T.; Masaoka, T.; Fukada, E.; Date, M.; Yamamoto, K. Intermittent pulsed electromagnetic field stimulation activates the mTOR pathway and stimulates the proliferation of osteoblast-like cells. Bioelectromagnetics 2019, 40, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, B.; Kaliannagounder, V.K.; Jang, S.R.; Awasthi, G.P.; Bhattarai, D.P.; Choukrani, G.; Park, C.H.; Kim, C.S. In-situ polymerized polypyrrole nanoparticles immobilized poly(ε-caprolactone) electrospun conductive scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 114, 111056–111065. [Google Scholar] [CrossRef]
- Erikson, U.; Grimsmo, L.; Digre, H. Establishing a Method for Electrical Immobilization of Whitefish on Board Fishing Vessels. J. Aquat. Food Prod. Technol. 2021, 30, 694–705. [Google Scholar] [CrossRef]
- Yao, G.; Kang, L.; Li, C.; Chen, S.; Wang, Q.; Yang, J.; Long, Y.; Li, J.; Zhao, K.; Xu, W.; et al. A self-powered implantable and bioresorbable electrostimulation device for biofeedback bone fracture healing. Proc. Natl. Acad. Sci. USA 2021, 118, e2100772118. [Google Scholar] [CrossRef]
- Mahmood, I.; Chandler, H.; Kottam, L.; Eardley, W.; Rangan, A.; Baker, P. Neuromuscular Electrostimulation Device Reduces Preoperative Edema and Accelerates Readiness for Theater in Patients Requiring Open Reduction Internal Fixation for Acute Ankle Fracture. Tech. Foot Ankle Surg. 2019, 19, 215–219. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, W.; Tremblay, P.L.; Zhang, T. Electrostimulation of fibroblast proliferation by an electrospun poly (lactide-co-glycolide)/polydopamine/chitosan membrane in a humid environment. Colloids Surf. B Biointerfaces 2022, 220, 112902–112912. [Google Scholar] [CrossRef] [PubMed]
- Skrzetuska, E.; Michalak, D.; Krucińska, I. Design and Analysis of Electrodes for Electrostimulation (TENS) Using the Technique of Film Printing and Embroidery in Textiles. Sensors 2021, 21, 4789. [Google Scholar] [CrossRef]
- Bhavsar, M.B.; Han, Z.; DeCoster, T.; Leppik, L.; Costa Oliveira, K.M.; Barker, J.H. Electrical stimulation-based bone fracture treatment, if it works so well why do not more surgeons use it? Eur. J. Trauma Emerg. Surg. 2020, 46, 245–264. [Google Scholar] [CrossRef]
- Wähnert, D.; Greiner, J.; Brianza, S.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. Strategies to Improve Bone Healing: Innovative Surgical Implants Meet Nano-/Micro-Topography of Bone Scaffolds. Biomedicines 2021, 9, 746. [Google Scholar] [CrossRef]
- Van Lieshout, E.M.M.; Verhofstad, M.H.J.; Beens, L.M.; Van Bekkum, J.J.J.; Willemsen, F.; Janzing, H.M.J.; Van Vledder, M.G. Personalized 3D-printed forearm braces as an alternative for a traditional plaster cast or splint; A systematic review. Injury 2022, 53, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Górski, F.; Zawadzki, P.; Wichniarek, R.; Kuczko, W.; Żukowska, M.; Wesołowska, I.; Wierzbicka, N. Automated Design of Customized 3D-Printed Wrist Orthoses on the Basis of 3D Scanning. Comput. Exp. Simul. Eng. 2019, 75, 1133–1143. [Google Scholar]
- Mrozek, A.; Tomaszewska, E.; Rychlik, M. Concept of Individual Orthosis with 3D Pattern Structure for Upper Limb Using 3D Printing Technology. Lect. Notes Mech. Eng. 2022, 5, 16–30. [Google Scholar]
- Castro, T.C. Avaliação da Resistência ao Rasgamento do Silicone Submetido à Ação de Suor Artificial. Master’s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2008. [Google Scholar]
- Chohan, J.S.; Singh, R.; Boparai, K.S. Post-processing of ABS Replicas with Vapour Smoothing for Investment Casting Applications. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2020, 92, 97–102. [Google Scholar] [CrossRef]
- Valerga, A.P.; Batista, M.; Fernandez-Vidal, S.R.; Gamez, A.J. Impact of Chemical Post-Processing in Fused Deposition Modelling (FDM) on Polylactic Acid (PLA) Surface Quality and Structure. Polymers 2019, 11, 566. [Google Scholar] [CrossRef]
- Fletcher, J.W.A.; Williams, S.; Whitehouse, M.R.; Gill, H.S.; Preatoni, E. Juvenile bovine bone is an appropriate surrogate for normal and reduced density human bone in biomechanical testing: A validation study. Sci. Rep. 2018, 8, 10181. [Google Scholar] [CrossRef]
- Ning, T.; Zhang, K.; Heng, B.C.; Ge, Z. Diverse effects of pulsed electrical stimulation on cells-with a focus on chondrocytes and cartilage regeneration. Eur. Cells Mater. 2019, 38, 79–93. [Google Scholar] [CrossRef]
- Tirono, M.; Hananto, F.S.; Abtokhi, A. Pulse Voltage Electrical Stimulation for Bacterial Inactivation and Wound Healing in Mice with Diabetes. Avicenna J. Med. Biotechnol. 2022, 14, 95–101. [Google Scholar] [CrossRef]
- Devante, D.A.; Jones, P.D.; Adams, M.S.; Lotz, J.C.; Diederich, C.J. LIPUS far-field exposimetry system for uniform stimulation of tissues in-vitro: Development and validation with bovine intervertebral disc cells. Biomed. Phys. Eng. Express 2020, 6, 035033–035044. [Google Scholar]
- Choi, W.J.; Hwang, K.S.; Kwon, H.J.; Lee, C.; Kim, C.H.; Kim, T.H.; Heo, S.W.; Kim, J.-H.; Lee, J.-Y. Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater. Sci. Eng. C 2020, 110, 110693. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of inorganic nanoparticles reinforcement on its performance and food packaging applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Vanaei, H.R.; Raissi, K.; Deligant, M.; Shirinbayan, M.; Fitoussi, J.; Khelladi, S.; Tcharkhtchi, A. Toward the understanding of temperature effect on bonding strength, dimensions and geometry of 3D-printed parts. J. Mater. Sci. 2020, 55, 14677–14689. [Google Scholar] [CrossRef]
- Bhandari, S.; Lopez-Anido, R.A.; Gardner, D.J. Enhancing the interlayer tensile strength of 3D printed short carbon fiber reinforced PETG and PLA composites via annealing. Addit. Manuf. 2019, 30, 100922–100934. [Google Scholar] [CrossRef]
- Wu, F.; Zheng, J.; Li, Z.; Liu, M. Halloysite nanotubes coated 3D printed PLA pattern for guiding human mesenchymal stem cells (hMSCs) orientation. Chem. Eng. J. 2019, 359, 672–683. [Google Scholar] [CrossRef]
- Bressan, L.P.; Lima, T.M.; Da Silveira, G.D.; Da Silva, J.A.F. Low-cost and simple FDM-based 3D-printed microfluidic device for the synthesis of metallic core–shell nanoparticles. Sn Appl. Sci. 2020, 2, 984. [Google Scholar] [CrossRef]
- Niknejad, A.S.; Bazgir, S.; Sadeghzadeh, A.; Shirazi, M.M.A. Evaluation of a novel and highly hydrophobic acrylonitrile-butadiene-styrene membrane for direct contact membrane distillation: Electroblowing/air-assisted electrospraying techniques. Desalination 2020, 500, 114893–114902. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Osika, P.; Wasilewski, T.; Bujak, T. Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics. Molecules 2017, 22, 320. [Google Scholar] [CrossRef]
- Toth, L.; Schiffer, A.; Nyitrai, M.; Pentek, A.; Told, R.; Maroti, P. Developing an anti-spastic orthosis for daily home-use of stroke patients using smart memory alloys and 3D printing technologies. Mater. Des. 2020, 195, 109029–109039. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; Di Bartolomeo, A.; Iemmo, L.; Zampino, D.C.; Vittoria, V.; Gorrasi, G. Ionic Liquid as Dispersing Agent of LDH-Carbon Nanotubes into a Biodegradable Vinyl Alcohol Polymer. Polymers 2020, 12, 495. [Google Scholar] [CrossRef]
- Hussain, S.; Park, S.-Y. Sweat-Based Noninvasive Skin-Patchable Urea Biosensors with Photonic Interpenetrating Polymer Network Films Integrated into PDMS Chips. Acs Sens. 2020, 5, 3988–3998. [Google Scholar] [CrossRef]
- Min, H.S.; Kim, H.J.; Naito, M.; Ogura, S.; Toh, K.; Hayashi, K.; Kim, B.S.; Fukushima, S.; Anraku, Y.; Miyata, K.; et al. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood–Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew. Chem. 2020, 132, 8250–8257. [Google Scholar] [CrossRef]
- Parfenov, V.A.; Khesuani, Y.D.; Petrov, S.V.; Karalkin, P.A.; Koudan, E.V.; Nezhurina, E.K.; DAS Pereira, F.; Krokhmal, A.A.; Gryadunova, A.A.; Bulanova, E.A.; et al. Magnetic Levitational Bioassembly of 3D Tissue Construct in Space. Sci. Adv. 2020, 6, eaba4174. [Google Scholar] [CrossRef] [PubMed]
- Yokouchi, T.; Kagawa, F.; Hirschberger, M.; Otani, Y.; Nagaosa, N.; Tokura, Y. Emergent electromagnetic induction in a helical-spin magnet. Nature 2020, 586, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Mogk, J.P.; Keir, P. Wrist and carpal tunnel size and shape measurements: Effects of posture. Clin. Biomech. 2008, 23, 1112–1120. [Google Scholar] [CrossRef]
- Portnova, A.A.; Mukherjee, G.; Peters, K.M.; Yamane, A.; Steele, K. Design of a 3D-printed, open-source wrist-driven orthosis for individuals with spinal cord injury. PLoS ONE 2018, 13, e0193106–e0193118. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, S. Case study: Hybrid model for the customized wrist orthosis using 3d printing. J. Mech. Sci. Technol. 2015, 29, 5151–5156. [Google Scholar] [CrossRef]
- Chae, D.-S.; Kim, D.-H.; Kang, K.-Y.; Kim, D.-Y.; Park, S.-W.; Park, S.-J.; Kim, J.-H. The functional effect of 3D-printing individualized orthosis for patients with peripheral nerve injuries. Medicine 2020, 99, e19791–e19804. [Google Scholar] [CrossRef]
- York, G.; Chakrabarty, S. A survey on foot drop and functional electrical stimulation. Int. J. Intell. Robot. Appl. 2019, 3, 4–10. [Google Scholar] [CrossRef]
- Mohammadi, M.; Zolfagharian, A.; Bodaghi, M.; Xiang, Y.; Kouzani, A.Z. 4D printing of soft orthoses for tremor suppression. Bio-Des. Manuf. 2022, 5, 786–807. [Google Scholar] [CrossRef]
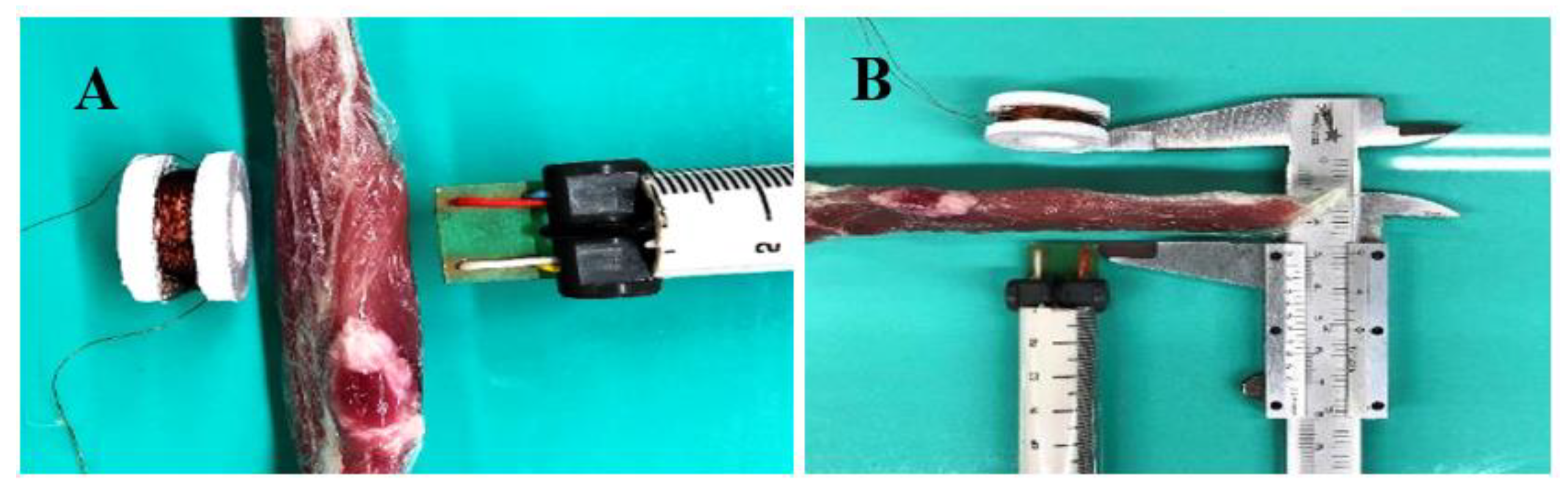

| Scenario | Intensity of Magnetic Field (µT) | Distance (mm) |
|---|---|---|
| Alternating magnetic field (76.6 Hz), combined with continuous magnetic one, peak voltage (Vp) of 12V | 40 | 35.3 ± 1.09 |
| 20 | 39.2 ± 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, F.B.; Silva, E.S.; de Lourdes Noronha Motta Melo, M.; Oliveira, R.M.P.; Capellato, P.; Sachs, D. Polymeric Orthosis with Electromagnetic Stimulator Controlled by Mobile Application for Bone Fracture Healing: Evaluation of Design Concepts for Medical Use. Materials 2022, 15, 8141. https://doi.org/10.3390/ma15228141
Vilela FB, Silva ES, de Lourdes Noronha Motta Melo M, Oliveira RMP, Capellato P, Sachs D. Polymeric Orthosis with Electromagnetic Stimulator Controlled by Mobile Application for Bone Fracture Healing: Evaluation of Design Concepts for Medical Use. Materials. 2022; 15(22):8141. https://doi.org/10.3390/ma15228141
Chicago/Turabian StyleVilela, Filipe Bueno, Eduardo Serafim Silva, Mirian de Lourdes Noronha Motta Melo, Rochelly Mariana Pedroso Oliveira, Patricia Capellato, and Daniela Sachs. 2022. "Polymeric Orthosis with Electromagnetic Stimulator Controlled by Mobile Application for Bone Fracture Healing: Evaluation of Design Concepts for Medical Use" Materials 15, no. 22: 8141. https://doi.org/10.3390/ma15228141
APA StyleVilela, F. B., Silva, E. S., de Lourdes Noronha Motta Melo, M., Oliveira, R. M. P., Capellato, P., & Sachs, D. (2022). Polymeric Orthosis with Electromagnetic Stimulator Controlled by Mobile Application for Bone Fracture Healing: Evaluation of Design Concepts for Medical Use. Materials, 15(22), 8141. https://doi.org/10.3390/ma15228141








