Syntheses of APTMS-Coated ZnO: An Investigation towards Penconazole Detection
Abstract
1. Introduction
2. Materials, Equipment, and Synthetic Procedures
2.1. Materials and Equipment
2.2. Synthetic Procedures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Available online: https://www.sfa.gov.sg/food-information/risk-at-a-glance/use-of-pesticide-in-food (accessed on 18 October 2022).
- Available online: https://www.sustainability-times.com/green-consumerism/report-finds-pesticides-may-make-many-u-s-foods-unsafe/ (accessed on 8 October 2022).
- Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2017.4853 (accessed on 18 October 2022).
- van Asselt, E.D.; Banach, J.L.; van der Fels-Klerx, H.J. Prioritization of chemical hazards in spices and herbs for European monitoring programs. Food Control 2018, 83, 7–17. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.M.; Ferrer, C.; Malato, O.; Agüera, A.; Fernández-Alba, A.R. Liquid chromatography-high-resolution mass spectrometry for pesticide residue analysis in fruit and vegetables: Screening and quantitative studies. J. Chromatogr. A 2013, 1287, 24–37. [Google Scholar] [CrossRef]
- Polledri, E.; Mercadante, R.; Fustinoni, S. Determination of tebuconazole and penconazole fungicides in rat and human hair by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 1243–1249. [Google Scholar] [CrossRef]
- Abdallah, O.I.; Alrasheed, A.M.; Al-Mundarij, A.A.; Omar, A.F.; Alhewairini, S.S.; Al-Jamhan, K.A. Levels of residues and dietary risk assessment of the fungicides myclobutanil, penconazole, tebuconazole, and triadimenol in squash. Biomed. Chromatogr. 2021, 35, e5126. [Google Scholar] [CrossRef]
- Babazadeh, S.; Moghaddam, P.A.; Keshipour, S.; Mollazade, K. Analysis of imidacloprid and penconazole residues during their pre-harvest intervals in the greenhouse cucumbers by HPLC–DAD. J. Iran Chem. Soc. 2020, 17, 1439–1446. [Google Scholar] [CrossRef]
- Zeid, M.I.A.; Awad, M.K.; Melki, K.C.; Jawdah, Y.A.; Jammoul, A.M. Pesticides residues on Loquat: A minor crop in Lebanon. Food Control 2021, 130, 108297. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Luo, F.; Sheng, H.; Zhou, L.; Zhong, Q.; Lou, Z.; Sun, H.; Yang, M.; Cui, X.; et al. Application and enantioselective residue determination of chiral pesticide penconazole in grape, tea, aquatic vegetables and soil by ultra performance liquid chromatography-tandem mass spectrometry. Ecotox. Environ. Saf. 2019, 172, 530–537. [Google Scholar] [CrossRef]
- Abd-Alrahman, S.H.; Ahmed, N.S. Dissipation of Penconazole in Peach, Plum, Apricot, and Mango by HPLC–DAD. Bull. Environ. Contam. Toxicol. 2013, 90, 260–263. [Google Scholar] [CrossRef]
- Chen, M.; Chen, L.; Pan, L.; Liu, R.; Guo, J.; Fan, M.; Wang, X.; Liu, H.; Liu, S. Simultaneous analysis of multiple pesticide residues in tobacco by magnetic carbon composite-based QuEChERS method and liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2022, 1668, 462913. [Google Scholar] [CrossRef] [PubMed]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Seebunrueng, K.; Tamuang, S.; Ruangchai, S.; Sansuk, S.; Srijaranai, S. In situ self-assembled coating of surfactant-mixed metal hydroxide on Fe3O4@SiO2 magnetic composite for dispersive solid phase microextraction prior to HPLC analysis of triazole fungicides. Microchem. J. 2021, 168, 106396. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Djozan, D.; Nouri, N.; Bamorowat, M.; Shalamzari, M.S. Coupling stir bar sorptive extraction-dispersive liquid–liquid microextraction for preconcentration of triazole pesticides from aqueous samples followed by GC-FID and GC-MS determinations. J. Sep. Sci. 2010, 33, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, R.; Iacob, B.-C.; Farcău, C.; Bodoki, E.; Oprean, R. Strategies for SERS Detection of Organochlorine Pesticides. Nanomaterials 2021, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Bernat, A.; Samiwala, M.; Albo, J.; Jiang, X.; Rao, Q. Challenges in SERS-based pesticide detection and plausible solutions. J. Agric. Food Chem. 2019, 67, 12341–12347. [Google Scholar] [CrossRef]
- Pang, S.; Yang, T.; He, L. Review of surface enhanced Raman spectroscopic (SERS) detection of synthetic chemical pesticides. TrAC Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef]
- Carbone, M.; Sabbatella, G.; Antonaroli, S.; Remita, H.; Orlando, V.; Biagioni, S.; Nucara, A. Exogenous control over intracellular acidification: Enhancement via proton caged compounds coupled to gold nanoparticles. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2304–2307. [Google Scholar] [CrossRef]
- Limosani, F.; Remita, H.; Tagliatesta, P.; Bauer, E.M.; Leoni, A.; Carbone, M. Functionalization of Gold Nanoparticles with Ru-Porphyrin and Their Selectivity in the Oligomerization of Alkynes. Materials 2022, 15, 1207. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Khanna, S.; Utsav Paneliya, S.; Takhar, V.; Banerjee, R.; Mukhopadhyay, I. Fabrication of silver nanodome embedded zinc oxide nanorods for enhanced Raman spectroscopy. Colloids Surface A 2022, 639, 128336. [Google Scholar] [CrossRef]
- Guerrini, L.; Izquierdo-Lorenzo, I.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Self-assembly of α, ω-aliphatic diamines on Ag nanoparticles as an effective localized surface plasmon nanosensor based in interparticle hot spots. Phys. Chem. Chem. Phys. 2009, 11, 7363–7371. [Google Scholar] [CrossRef]
- Guerrini, L.; Izquierdo Lorenzo, I.; Rodriguez-Oliveros, R.; Sánchez-Gil, J.A.; Sanchez-Cortes, S.; Garcia-Ramos, J.V.; Domingo, C. α, ω-Aliphatic Diamines as Molecular Linkers for Engineering Ag Nanoparticle Clusters: Tuning of the Interparticle Distance and Sensing Application. Plasmonics 2010, 5, 273–286. [Google Scholar] [CrossRef]
- Kubackova, J.; Fabriciova, G.; Miskovsky, P.; Jancura, D.; Sanchez-Cortes, S. Sensitive Surface-Enhanced Raman Spectroscopy (SERS) Detection of Organochlorine Pesticides by Alkyl Dithiol-Functionalized Metal Nanoparticles-Induced Plasmonic Hot Spots. Anal. Chem. 2015, 87, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Aliaga, A.E.; Cárcamo, J.; Gómez-Jeria, J.S.; Sanchez-Cortes, S.; Campos-Vallette, M.M.; García-Ramos, J.V. Functionalization of Ag nanoparticles with the bis-acridinium lucigenin as a chemical assembler in the detection of persistent organic pollutants by surface-enhanced Raman scattering. Anal. Chim. Acta 2008, 624, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Porous zeolite imidazole framework-wrapped urchin-like Au-Ag nanocrystals for SERS detection of trace hexachlorocyclohexane pesticides via efficient enrichment. J. Hazard. Mater. 2019, 368, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Donia, D.T.; Carbone, M. Fate of nanoparticles in environmental cycles. Int. J. Environ. Sci. Technol. 2019, 16, 583–600. [Google Scholar] [CrossRef]
- Ramos-Ruiz, A.; Wilkening, J.V.; Field, J.A.; Sierra-Alvarez, R. Leaching of cadmium and tellurium from cadmium telluride (CdTe) thin-film solar panels under simulated landfill conditions. J. Hazard. Mater. 2017, 336, 57–64. [Google Scholar] [CrossRef]
- Babazadeh, S.; Bisauriya, R.; Carbone, M.; Roselli, L.; Cecchetti, D.; Bauer, E.M.; Sennato, S.; Prosposito, P.; Pizzoferrato, R. Colorimetric Detection of Chromium(VI) Ions in Water Using Unfolded-Fullerene Carbon Nanoparticles. Sensors 2021, 21, 6353. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Palleschi, G.; Flammini, R.; Bauer, E.M.; Nasillo, G.; Caponetti, E. Oxidized Graphene in Ionic Liquids for Assembling Chemically Modified Electrodes: A Structural and Electrochemical Characterization Study. Anal. Chem. 2012, 84, 5823–5831. [Google Scholar] [CrossRef]
- Gontrani, L.; Pulci, O.; Carbone, M.; Pizzoferrato, R.; Prosposito, P. Detection of Heavy Metals in Water Using Graphene Oxide Quantum Dots: An Experimental and Theoretical Study. Molecules 2021, 26, 5519. [Google Scholar] [CrossRef]
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Prosposito, P.; Carbone, M. Top-Down N-Doped Carbon Quantum Dots for Multiple Purposes: Heavy Metal Detection and Intracellular Fluorescence. Nanomaterials 2021, 11, 2249. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.S.; Wang, Z.L. Dissolving Behavior and Stability of ZnO Wires in Biofluids: A Study on Biodegradability and Biocompatibility of ZnO Nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical Properties of ZnO Nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Li, T.; Tao, T. ZnO quantum dots for fluorescent detection of environmental contaminants. J. Environ. Chem. Eng. 2021, 9, 106800. [Google Scholar] [CrossRef]
- Sinha, R.; Ganesana, M.; Andreescu, S.; Stanciu, L. AChE biosensor based on zinc oxide sol-gel for the detection of pesticides. Anal. Chim. Acta 2010, 661, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Mandal, A.; Mitra, T.; Chakraborty, K.; Bardhan, M.; Dasgupta, A.K. Nanosensing of Pesticides by Zinc Oxide Quantum Dot: An Optical and Electrochemical Approach for the Detection of Pesticides in Water. J. Agric. Food Chem. 2018, 66, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.R.; Mitra, S.; Sahoo, D. Metal oxide QD based ultrasensitive microsphere fluorescent sensor for copper, chromium and iron ions in water. RSC Adv. 2020, 10, 9512–9524. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Koch, U.; Fojtik, A.; Weller, H.; Henglein, A. Photochemistry of semiconductor colloids. Preparation of extremely small ZnO particles, fluorescence phenomena and size quantization effects. Chem. Phys. Lett. 1985, 122, 507–510. [Google Scholar] [CrossRef]
- Monticone, S.; Tufeu, R.; Kanaev, A.V. Complex Nature of the UV and Visible Fluorescence of Colloidal ZnO Nanoparticles. J. Phys. Chem. B 1998, 102, 2854–2862. [Google Scholar] [CrossRef]
- Irimpan, L.; Nampoori, V.P.N.; Radhakrishnan, P.; Deepthy, A.; Krishnan, B. Size dependent fluorescence spectroscopy of nanocolloids of ZnO. J. Appl. Phys. 2007, 102, 063524. [Google Scholar] [CrossRef]
- Carbone, M. CQDs@NiO: An Efficient Tool for CH4 Sensing. Appl. Sci. 2020, 10, 6251. [Google Scholar] [CrossRef]
- John, R.A.B.; Kumar, A.R. A review on resistive-based gas sensors for the detection of volatile organic compounds using metal-oxide nanostructures. Inorg. Chem. Commun. 2021, 133, 108893. [Google Scholar] [CrossRef]
- Carbone, M.; Aneggi, E.; Figueredo, F.; Susmel, S. NiO-nanoflowers decorating a plastic electrode for the non-enzymatic amperometric detection of H2O2 in milk: Old issue, new challenge. Food Control 2022, 132, 108549. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-Temperature Gas Sensors under Photoactivation: From Metal Oxides to 2D Materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Bauer, E.M.; Ditaranto, N.; Sabbatini, L.; Caponetti, E.; Chillura-Martino, D. Graphene and ionic liquids new gel paste electrodes for caffeic acid quantification. Sens. Actuators B Chem. 2015, 212, 248–255. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Y.; Debliquy, M.; Zhang, C. Graphene-enhanced metal oxide gas sensors at room temperature: A review. Beilstein J. Nanotechnol. 2018, 9, 2832–2844. [Google Scholar] [CrossRef]
- Valentini, F.; Ciambella, E.; Boaretto, A.; Rizzitelli, G.; Carbone, M.; Conte, V.; Cataldo, F.; Russo, V.; Casari, C.S.; Chillura-Martino, D.F.; et al. Sensor Properties of Pristine and Functionalized Carbon Nanohorns. Electroanalysis 2016, 28, 2489–2499. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Hu, Y.; Gu, H. Ultraviolet Detectors Based on Wide Bandgap Semiconductor Nanowire: A Review. Sensors 2018, 18, 2072. [Google Scholar] [CrossRef]
- Carbone, M.; Tagliatesta, P. NiO Grained-Flowers and Nanoparticles for Ethanol Sensing. Materials 2020, 13, 1880. [Google Scholar] [CrossRef]
- Carbone, M. NiO-Based Electronic Flexible Devices. Appl. Sci. 2022, 12, 2839. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Gonçalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef]
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does the Shape Win? Materials 2020, 13, 1417. [Google Scholar] [CrossRef]
- Carbone, M. Zn defective ZnCo2O4 nanorods as high capacity anode for lithium ion batteries. J. Electroanal. Chem. 2018, 815, 151–157. [Google Scholar] [CrossRef]
- Carbone, M.; Bauer, E.M.; Micheli, L.; Missori, M. NiO morphology dependent optical and electrochemical properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 178–182. [Google Scholar] [CrossRef]
- Carbone, M. Cu-Zn-Co nanosized mixed oxides prepared from hydroxycarbonate precursors. J. Alloys Compd. 2016, 688, 202–209. [Google Scholar] [CrossRef]
- Carbone, M.; Nesticò, A.; Bellucci, N.; Micheli, L.; Palleschi, G. Enhanced performances of sensors based on screen printed electrodes modified with nanosized NiO particles. Electrochim. Acta 2017, 246, 580–587. [Google Scholar] [CrossRef]
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial power of Cu/Zn mixed oxide nanoparticles to Escherichia coli. Environ. Nanotech. Monitor. Manag. 2017, 7, 97–102. [Google Scholar] [CrossRef]
- Kadam, V.V.; Balakrishnan, R.M.; Ettiyappan, J.P.; Thomas, N.S.; Souza, S.A.D.; Parappan, S. Sensing of p-nitrophenol in aqueous solution using zinc oxide quantum dots coated with APTES. Environ. Nanotech. Monitor. Manag. 2021, 16, 100474. [Google Scholar] [CrossRef]
- Egghe, T.; Narimisa, M.; Ghobeira, R.; Nisol, B.; Onyshchenko, Y.; Hoogenboom, R.; Morent, R.; De Geyter, N. Comparison of the physicochemical properties and aging behavior of two different APTES-derived plasma polymer-based coatings. Surf. Coat. Technol. 2022, 449, 128945. [Google Scholar] [CrossRef]
- Knorr, D.B., Jr.; Williams, K.S.; Baril, N.F.; Weiland, C.; Andzelm, J.W.; Lenhart, J.L.; Woicik, J.C.; Fischer, D.A.; Tidrow, M.Z.; Bandara, S.V.; et al. Use of 3-aminopropyltriethoxysilane deposited from aqueous solution for surface modification of III-V materials. Appl. Surf. Sci. 2014, 320, 414–428. [Google Scholar] [CrossRef]
- Rahman, I.A.; Jafarzadeh, M.; Sipaut, C.S. Synthesis of organo-functionalized nanosilica via a co-condensation modification using γ-aminopropyltriethoxysilane (APTES). Ceram. Int. 2009, 35, 1883–1888. [Google Scholar] [CrossRef]
- Bertani, F.R.; Businaro, L.; Gambacorta, L.; Mencattini, A.; Brenda, D.; Di Giuseppe, D.; De Ninno, A.; Solfrizzo, M.; Martinelli, E.; Gerardino, A. Optical detection of aflatoxins B in grained almonds using fluorescence spectroscopy and machine learning algorithms. Food Control 2020, 112, 107073. [Google Scholar] [CrossRef]
- Weesepoel, Y.; Alewijn, M.; Wijtten, M.; Müller-Maatsch, J. Detecting Food Fraud in Extra Virgin Olive Oil Using a Prototype Portable Hyphenated Photonics Sensor. J. AOAC Int. 2021, 104, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Koppel, D.E. Analysis of Macromolecular Polydispersity in Intensity Correlation Spectroscopy: The Method of Cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Tscharnuter, W.W. Mobility measurements by phase analysis. Appl. Opt. 2001, 40, 3995–4003. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor clusters in the sol-gel process: Quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J. Am Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Donia, D.T.; Bauer, E.M.; Missori, M.; Roselli, L.; Cecchetti, D.; Tagliatesta, P.; Gontrani, L.; Carbone, M. Room Temperature Syntheses of ZnO and Their Structures. Symmetry 2021, 13, 733. [Google Scholar] [CrossRef]
- Widjonarko, N.E. Introduction to Advanced X-ray Diffraction Techniques for Polymeric Thin Films. Coating 2016, 6, 54. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel Green Route of Synthesis of ZnO Nanoparticles by Using Natural Biodegradable Polymer and Its Application as a Catalyst for Oxidation of Aldehydes. J. Macromol. Sci. A 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, I.; Szczygieł, B. XPS and FT-IR Characterization of Selected Synthetic Corrosion Products of Zinc Expected in Neutral Environment Containing Chloride Ions. J. Spectrosc. 2018, 2018, 2079278. [Google Scholar] [CrossRef]
- Musić, S.; Popović, S.; Maljković, M.; Dragčević, D. Influence of synthesis procedure on the formation and properties of zinc oxide. J. Alloys Compd. 2002, 347, 324–332. [Google Scholar] [CrossRef]
- Bistričit, L.; Volovšek, V.; Dananić, V. Conformational and vibrational analysis of gamma-aminopropyltriethoxysilane. J. Mol. Struct. 2007, 834–836, 355–363. [Google Scholar] [CrossRef]
- Nicolay, A.; Lanzutti, A.; Poelman, M.; Ruelle, B.; Fedrizzi, L.; Dubois, P.h.; Olivier, M.-G. Elaboration and characterization of a multifunctional silane/ZnO hybrid nanocomposite coating. Appl. Surf. Sci. 2015, 327, 379–388. [Google Scholar] [CrossRef]
- Sakohara, S.; Ishida, M.; Anderson, M.A. Visible Luminescence and Surface Properties of Nanosized ZnO Colloids Prepared by Hydrolyzing Zinc Acetate. J. Phys. Chem. B 1998, 102, 10169–10175. [Google Scholar] [CrossRef]
- Toscani, S.; Hernandez, O.; Aparicio, C.; Spanhel, L. Glass formation and confined melting in sol-gel derived nano-ZnO aggregates. J. Sol-Gel Sci. Technol. 2014, 69, 457–463. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Baez-Cruza, R.; Montoya, L.F.; Medinam, C.; Pérez-Tijerina, E.; Salazar, F.; Rojas, D.; Melendrez, M.F. Estimation of the surface interaction mechanism of ZnO nanoparticles modified with organosilane groups by Raman Spectroscopy. Ceram. Int. 2017, 43, 11838–11847. [Google Scholar] [CrossRef]
- Frost, R.L.; Kloprogge, J.T. Raman spectroscopy of the acetates of sodium, potassium and magnesium at liquid nitrogen temperature. J. Mol. Struct. 2000, 526, 131–141. [Google Scholar] [CrossRef]
- Gültekin, D.; Akbulut, H. Raman Studies of ZnO Products Synthesized by Solution Based Methods. Acta Phys. Pol. A 2016, 129, 803–805. [Google Scholar] [CrossRef]
- Noma, H.; Miwa, Y.; Yokoyama, I.; Machida, K. Infrared and Raman intensity parameters of sodium acetate and their intensity distributions. J. Mol. Struct. 1991, 242, 207–219. [Google Scholar] [CrossRef]
- Yang, R.D.; Tripathy, S.; Li, Y.; Sue, H.-J. Photoluminescence and micro-Raman scattering in ZnO nanoparticles: The influence of acetate adsorption. Chem. Phys. Lett. 2005, 411, 150–154. [Google Scholar] [CrossRef]
- Pochapski, D.J.; dos Santos, C.C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.S.A.; Bakry, A.; Al-Harbi, L.M.; Khowdiary, M.M.; El-Henawy, A.A.; Yoon, J. Core/shell PA6@Fe3O4 nanofibers: Magnetic and shielding behavior. J. Disper. Sci. Technol. 2019, 41, 1711–1719. [Google Scholar] [CrossRef]
- Wooten, A.J.; Werder, D.J.; Williams, D.J.; Casson, J.L.; Hollingsworth, J.A. Solution-Liquid-Solid Growth of Ternary Cu-In-Se Semiconductor Nanowires from Multiple- and Single-Source Precursors. J. Am. Chem. Soc. 2009, 131, 16177–16188. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile Synthesis of Quasi Spherical ZnO Nanoparticles with Excellent Photocatalytic Activity. J. Clust. Sci. 2015, 26, 1187–1201. [Google Scholar] [CrossRef]
- Hanif, M.A.; Kim, Y.S.; Ameen, S.; Kim, H.G.; Kwac, L.K. Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment. Coatings 2022, 12, 579. [Google Scholar] [CrossRef]
- Zhao, N.; Qi, L. Low-Temperature Synthesis of Star-Shaped PbS Nanocrystals in Aqueous Solutions of Mixed Cationic/Anionic Surfactants. Adv. Mater. 2006, 18, 359–362. [Google Scholar] [CrossRef]
- Navaneethan, M.; Archana, J.; Arivanandhan, M.; Hayakawa, Y. Functional properties of amine-passivated ZnO nanostructures and dye-sensitized solar cell characteristics. Chem. Eng. J. 2012, 213, 70–77. [Google Scholar] [CrossRef]
- Chi, H.; Wang, C.; Wang, Z.; Zhu, H.; Mesias, V.S.D.; Dai, X.; Chen, Q.; Liu, W.; Huang, J. Highly reusable nanoporous silver sheet for sensitive SERS detection of pesticides. Analyst 2020, 145, 5158–5165. [Google Scholar] [CrossRef]

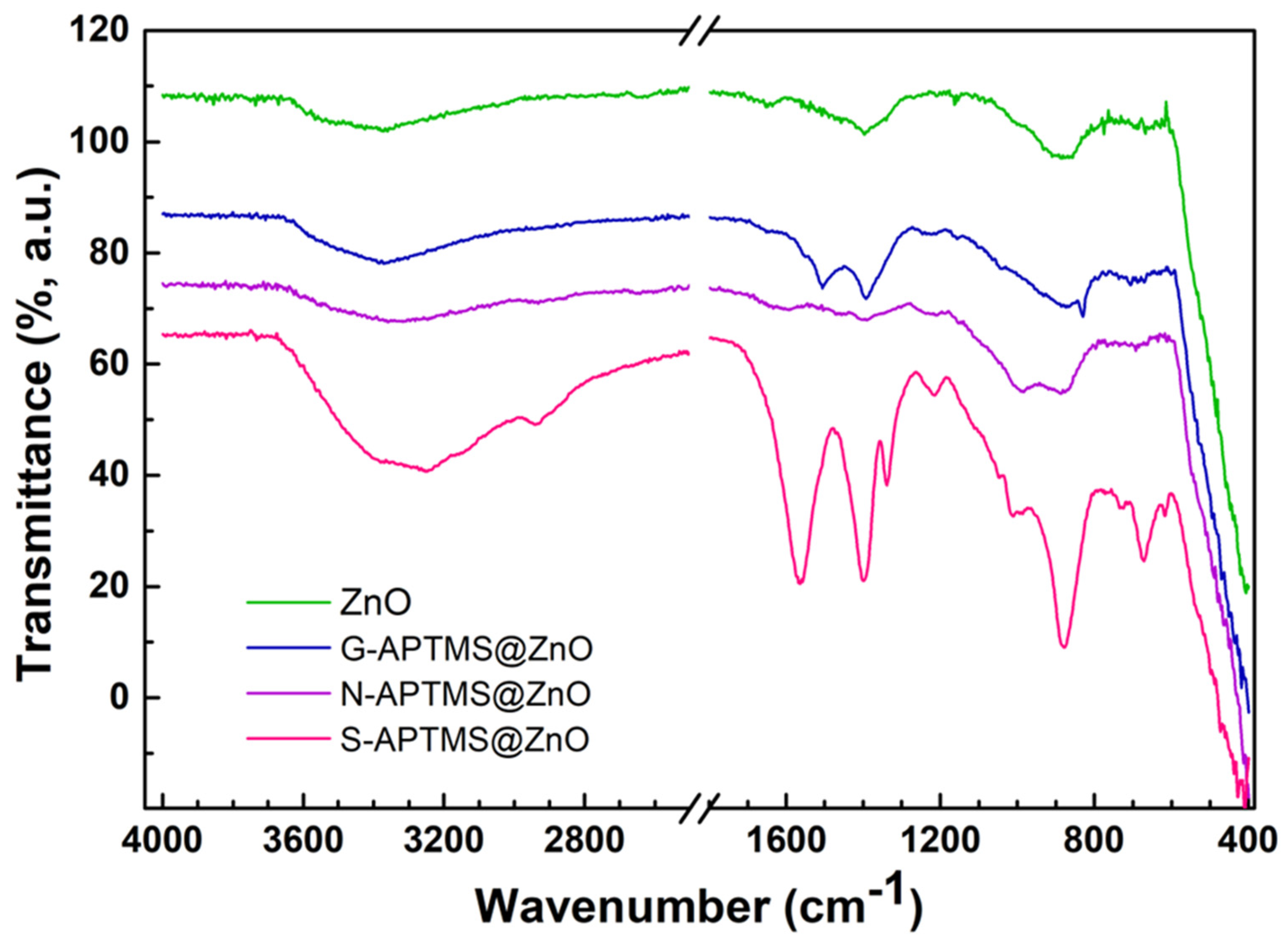
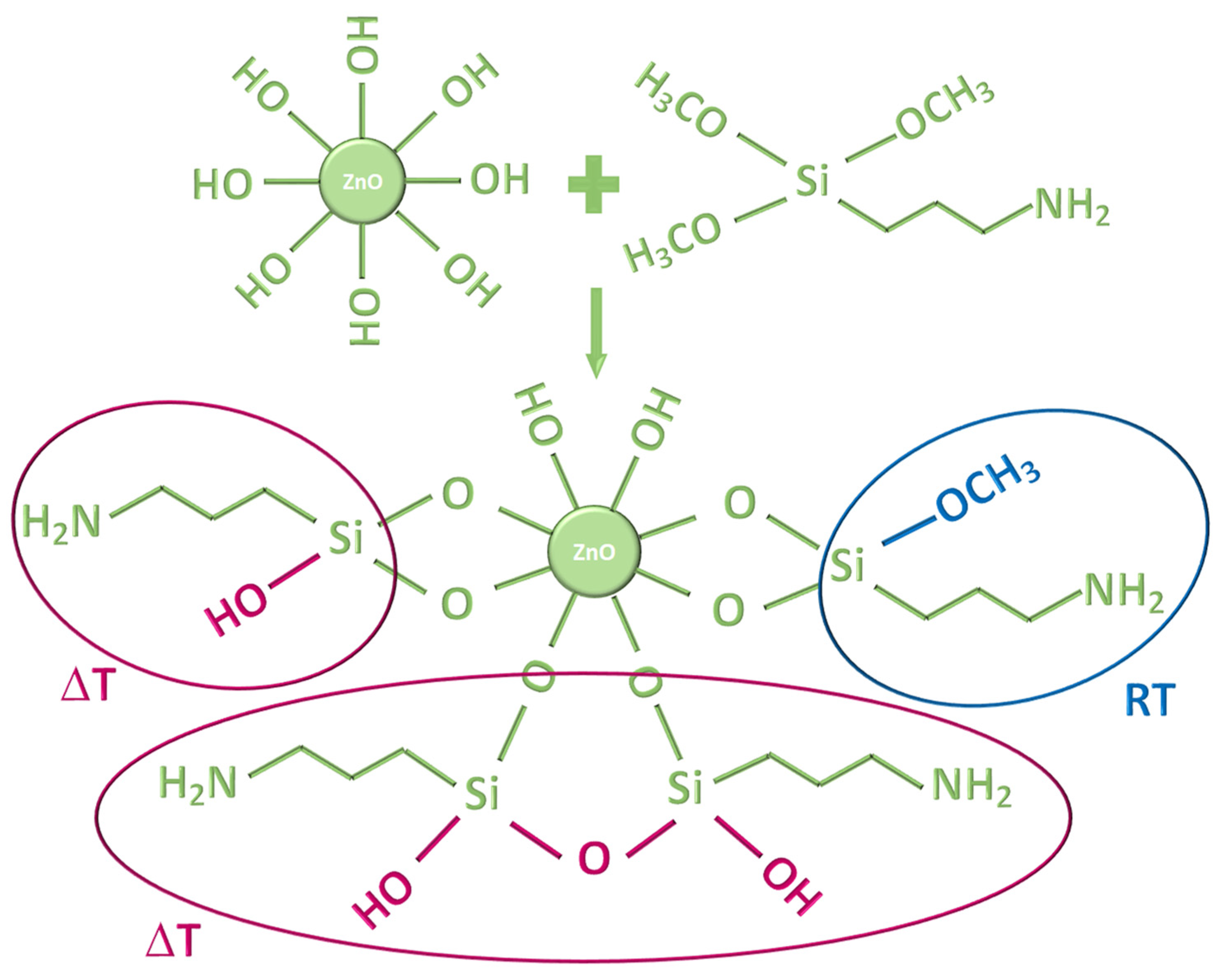


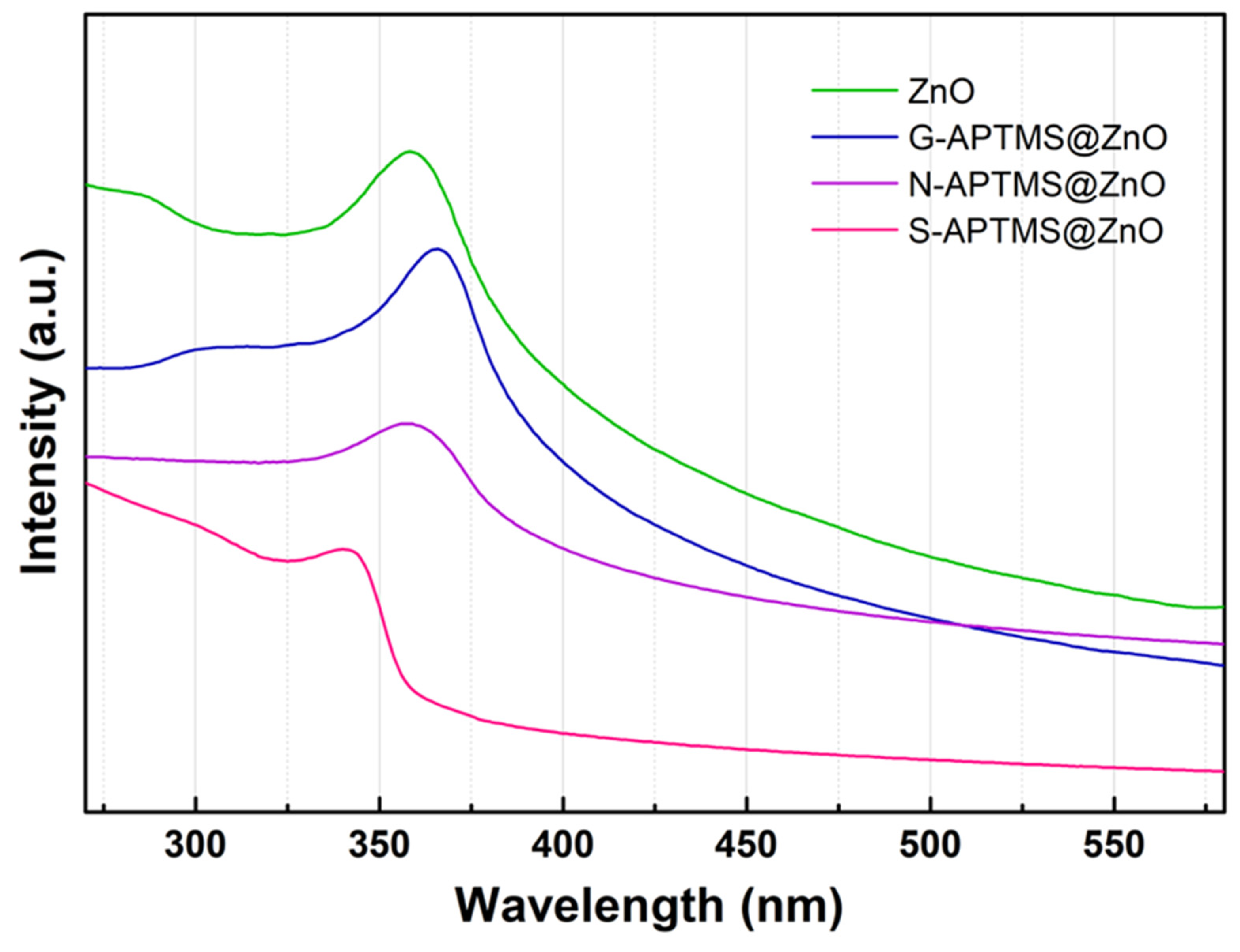
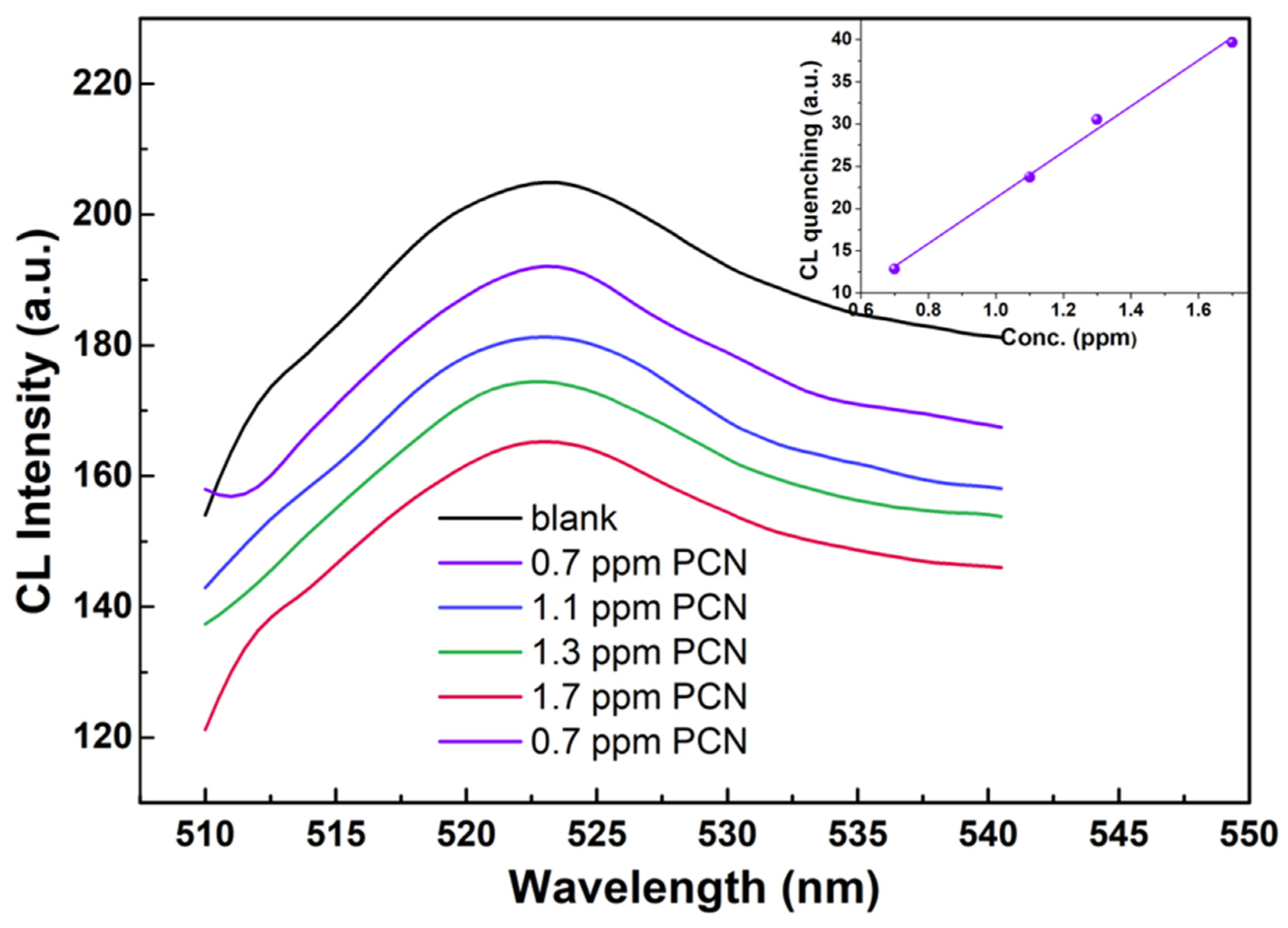
| Sample | Step | Salt | [Zn2+] | Base | [OH−] | APTMS | ZnO | Solv. | Temp. | Time | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZnO | 1st | Nitrate | 0.1 M | NaOH | 0.1 M | H2O | 40 °C | ovn | |||
| G-APTMS@ZnO | 2nd | 40 μL | 0.125 M | H2O | 40 °C | 24 h | 6.5/8.22 | ||||
| N-APTMS@ZnO | 2nd | 40 μL | 0.125 M | H2O | 100 °C | ovn | |||||
| S-APTMS@ZnO | Single | Acetate | 0.19 M | KOH | 0.8 M | 60 μL/5 mL EtOH | EtOH | 68 °C | 2 h |
| ZnO-cm−1 | S-APTMS@ZnO-cm−1 | N-APTMS@ZnO-cm−1 | G-APTMS@ZnO-cm−1 | Assignment |
|---|---|---|---|---|
| 3360 br | 3387 sd | -OH ν | ||
| 3330 br | 3330 br | -OH ν + -NH ν + -CH2 ν | ||
| 3248 br | -NH ν | |||
| 2935 m-w | 2934 w | -CH2 ν | ||
| 1598 w | -NH2 δ | |||
| 1564 v st | -C=O (C-O) ν | |||
| 1550 sd | -NH2 δ | |||
| 1510 | 1506 st | -CO32− νas split | ||
| 1454 vw | -CH δ | |||
| 1394 br | -CO32− νas | |||
| 1400 v st | C-O (C=O) ν | |||
| 1398 w | 1392 st | -CH2 χ | ||
| 1339 st | -C-O (C=O) ν | |||
| 1215 m | 1217 w | 1215 w | -C-O- ν NH2 ω | |
| 1015 w | 993 m | 1039 sd | Si-O-Si ν | |
| 878 st | 881 m | 881 m | Zn-O-Si ν | |
| 860 br | Zn-OH libr | |||
| 829 sh | Si-O-CH3 ν | |||
| 672 st | 668 vw | 670 vw | NH2 wag SiC str | |
| 617 w | -CH δ | |||
| 543 sd | 543 sd | 543 sd | 543 sd | Zn-O ν |
| Sample | C | N | O | Zn | Si | K | Zn/O | Zn/C | Zn/N | N/Si |
|---|---|---|---|---|---|---|---|---|---|---|
| G-APTMS@ZnO | 15.0 | 1.4 | 45.0 | 38.0 | 0.6 | / | 0.8 | 2.5 | 27.1 | 2.3 |
| N-APTMS@ZnO | 20.1 | 1.6 | 40.1 | 37.1 | 1.1 | / | 0.9 | 1.8 | 23.2 | 1.5 |
| S-APTMS@ZnO | 53.2 | 2.1 | 29.2 | 13.2 | 2.0 | 0.3 | 0.5 | 0.2 | 6.3 | 1.05 |
| Sample | 2R (nm) | PDI | Z Pot (mV) |
|---|---|---|---|
| ZnO | 214.4 ± 6.9 | 0.182 ± 0.032 | 25.8 ± 0.70 |
| N-APTMS@ZnO | 201.0 ± 2.5 | 0.161 ± 0.018 | 26.8 ± 0.30 |
| G-APTMS@ZnO | 175.7 ± 3.5 | 0.148 ± 0.011 | 27.4 ± 0.75 |
| S-APTMS@ZnO | 226.0 ± 47.0 | 0.449 ± 0.054 | 38.4 ± 0.50 |
| Sample | λm-nm | Band Gap-eV | Ref. |
|---|---|---|---|
| ZnO Water | 378 | 3.28 | [88] |
| ZnO EtOH | 368 | 3.37 | [89] |
| ZnO | 358 | 3.46 | this work |
| G-APTMS@ZnO | 366 | 3.39 | this work |
| N-APTMS@ZnO | 357 | 3.48 | this work |
| S-APTMS@ZnO | 340 | 3.65 | this work |
| Sample | Pesticide | Matrix | Method | LOD (ppm) | Ref. |
|---|---|---|---|---|---|
| G-APTMS@ZnO | Penconazole | Water | Fluorescence | 0.1 | This work |
| Au/Ag | Dieldrin | Water | SERS | 0.3 | [24] |
| Ag NPs sheets | Lindane | Water | SERS | 0.0872 | [24] |
| Ag | Endosulfan | Water | SERS | 0.167 | [24] |
| Ag/Au | HCH | Water | SERS | 1 | [92] |
| Penconazole | Water | HPLC | 2.5 × 10−3 | [14] | |
| Penconazole | Grape, Tea | ES-HPLC | 0.3–1.5 × 10−3 | [10] | |
| Penconazole | Peach, Plum | HPLC/DAD | 0.1 × 10−3 | [11] | |
| Penconazole | tobacco | LE-GC | 0.011 | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, E.M.; Bogliardi, G.; Ricci, C.; Cecchetti, D.; De Caro, T.; Sennato, S.; Nucara, A.; Carbone, M. Syntheses of APTMS-Coated ZnO: An Investigation towards Penconazole Detection. Materials 2022, 15, 8050. https://doi.org/10.3390/ma15228050
Bauer EM, Bogliardi G, Ricci C, Cecchetti D, De Caro T, Sennato S, Nucara A, Carbone M. Syntheses of APTMS-Coated ZnO: An Investigation towards Penconazole Detection. Materials. 2022; 15(22):8050. https://doi.org/10.3390/ma15228050
Chicago/Turabian StyleBauer, Elvira Maria, Gabriele Bogliardi, Cosimo Ricci, Daniele Cecchetti, Tilde De Caro, Simona Sennato, Alessandro Nucara, and Marilena Carbone. 2022. "Syntheses of APTMS-Coated ZnO: An Investigation towards Penconazole Detection" Materials 15, no. 22: 8050. https://doi.org/10.3390/ma15228050
APA StyleBauer, E. M., Bogliardi, G., Ricci, C., Cecchetti, D., De Caro, T., Sennato, S., Nucara, A., & Carbone, M. (2022). Syntheses of APTMS-Coated ZnO: An Investigation towards Penconazole Detection. Materials, 15(22), 8050. https://doi.org/10.3390/ma15228050








