D-π-A-Type Pyrazolo[1,5-a]pyrimidine-Based Hole-Transporting Materials for Perovskite Solar Cells: Effect of the Functionalization Position
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
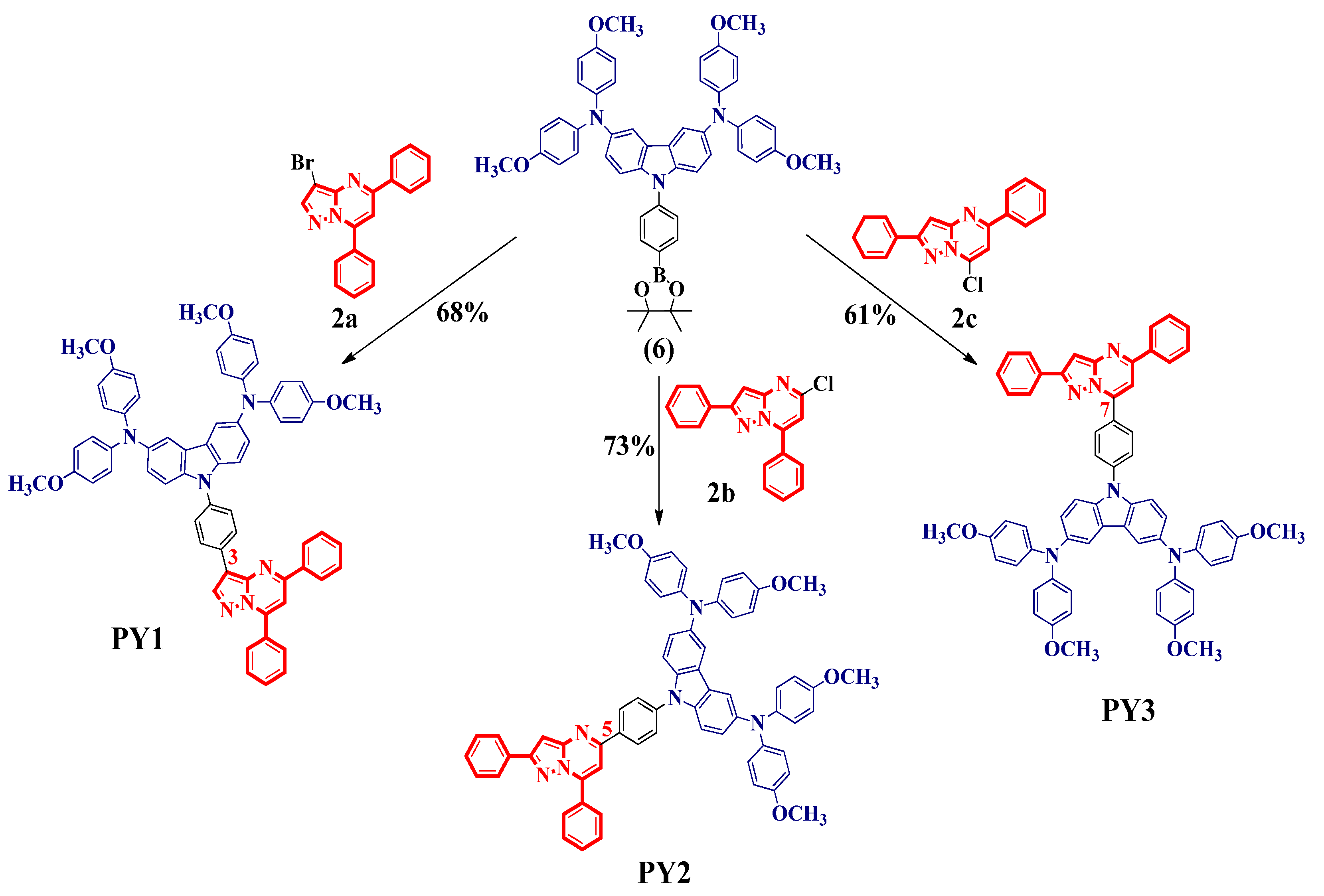
2.2. Synthesis of New HTMs PY1–PY3
2.3. Devices Fabrication Procedure
2.4. Instrumentation
3. Results and Discussion
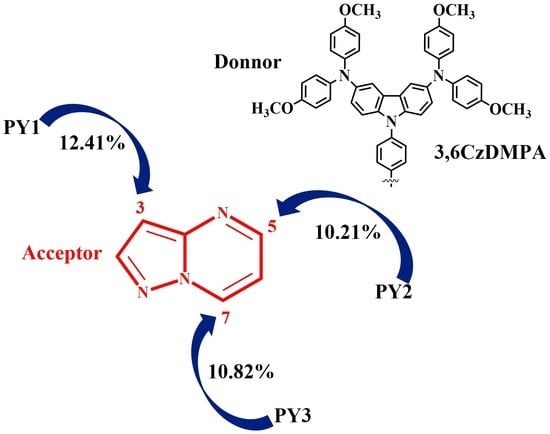
3.1. Design and Synthesis
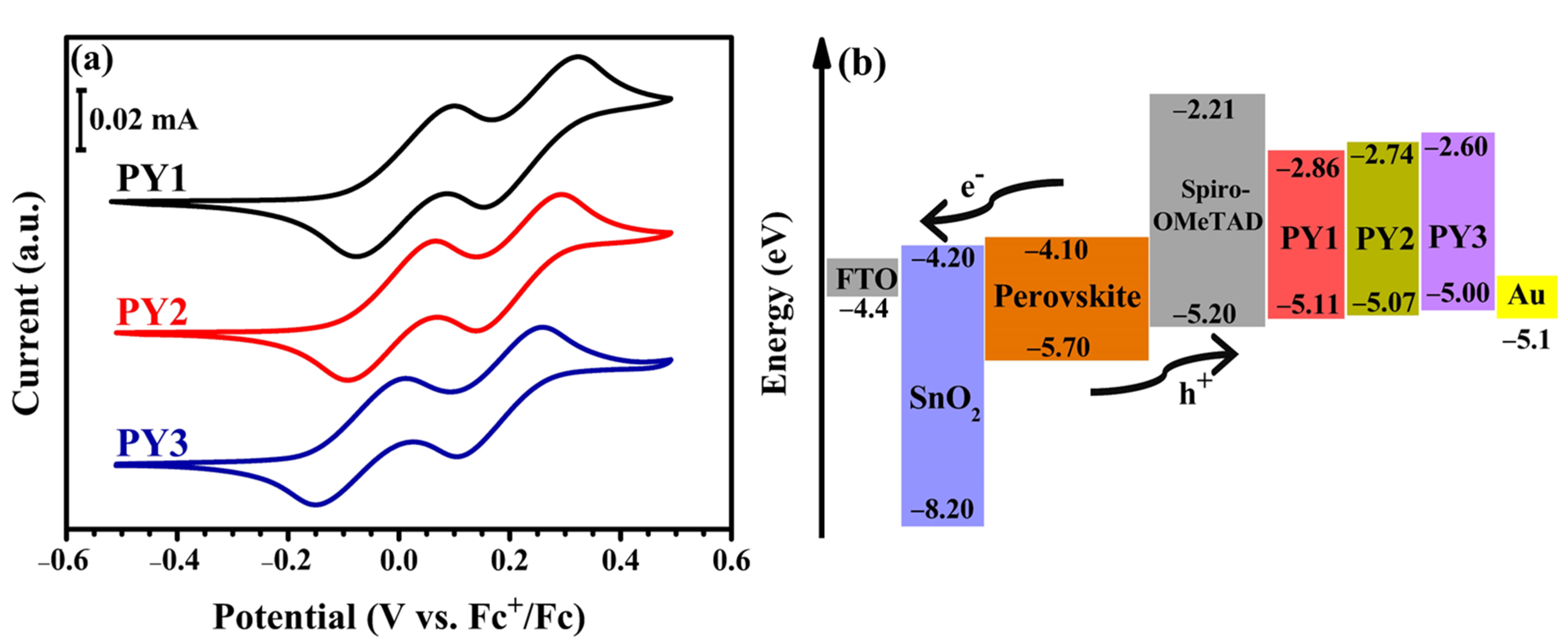
3.2. Optical, Electrochemical, Thermal and Photophysical Properties
3.3. DFT Calculations
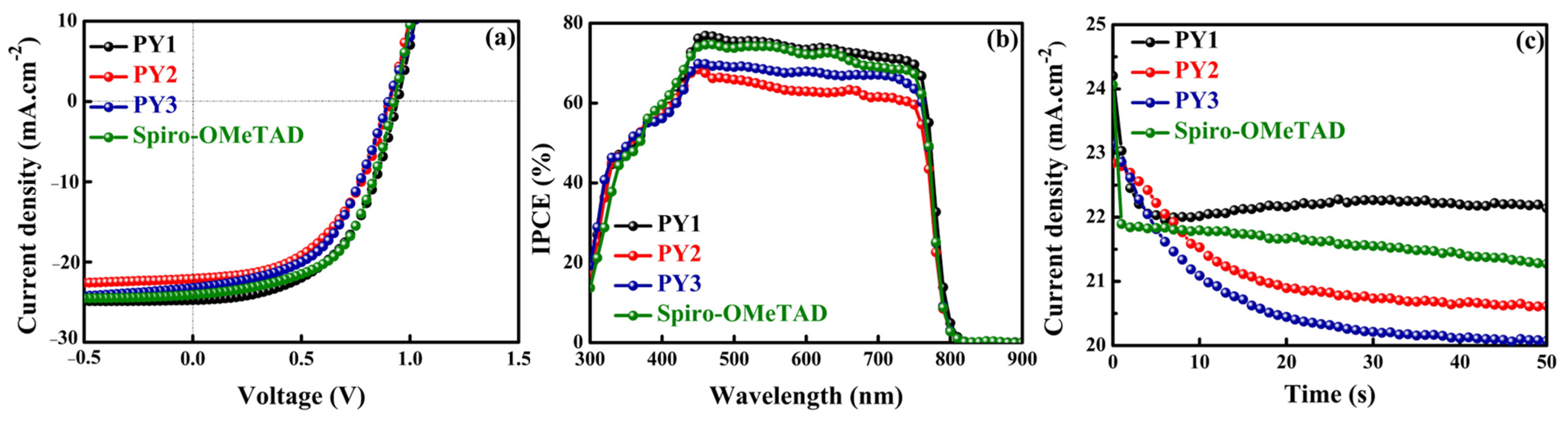
3.4. Photovoltaic Performances
4. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.B.; et al. Conformal quantum dot–SnO2 layers as electron transporters for efficient perovskite solar cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef]
- Iftikhar, F.J.; Wali, Q.; Yang, S.; Iqbal, Y.; Jose, R.; Munir, S.; Gondal, I.A.; Khan, M.E. Structural and optoelectronic properties of hybrid halide perovskites for solar cells. Org. Electron. 2021, 91, 106077. [Google Scholar] [CrossRef]
- Ašmontas, S.; Cerškus, A.; Gradauskas, J.; Griguceviciené, A.; Juškenas, R.; Leinartas, K.; Lucun, A.; Petrauskas, K.; Selskis, A.; Staišiunas, L.; et al. Photoelectric Properties of Planar and Mesoporous Structured Perovskite Solar Cells. Materials 2022, 15, 4300. [Google Scholar] [CrossRef]
- Zhang, D.; Li, D.; Hu, Y.; Mei, A.; Han, H. Degradation pathways in perovskite solar cells and how to meet international standards. Commun. Mater. 2022, 3, 58. [Google Scholar] [CrossRef]
- Wali, Q.; Aamir, M.; Ullah, A.; Iftikhar, F.J.; Khan, M.E.; Akhtar, J.; Yang, S. Fundamentals of Hysteresis in Perovskite Solar Cells: From Structure-Property Relationship to Neoteric Breakthroughs. Chem. Rec. 2022, 22, 202100150. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; He, H.; Berry, J.J.; Zhu, K.; Xu, T. On-device lead sequestration for perovskite solar cells. Nature 2020, 578, 555–558. [Google Scholar] [CrossRef]
- Sánchez, J.G.; Aktas, E.; Martínez-Ferreroun, E.; Capodilupo, A.L.; Corrente, G.A.; Beneduci, A.; Palomares, E. Increasing the stability of perovskite solar cells with dibenzofulvene-based hole transporting materials. Electrochim. Acta 2022, 432, 141190. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, Y.; Tang, R.; Zhang, J.; Chen, K.; Liu, H.; Wu, F.; Zhong, C.; Liu, X.; Zhu, L. Periphery group engineering in hole transport materials for efficient perovskite solar cells. Dyes Pigm. 2022, 206, 110671. [Google Scholar] [CrossRef]
- Han, M.; Liang, Y.; Chen, J.; Zhang, X.; Ghadari, R.; Liu, X.; Wu, N.; Wang, Y.; Zhou, Y.; Ding, Y.; et al. A N-Ethylcarbazole-Terminated Spiro-Type Hole-Transporting Material for Efficient and Stable Perovskite Solar Cells. ChemSusChem 2022, 15, 202201485. [Google Scholar] [CrossRef]
- Kadi, Z.; Wang, R.; Berton, N.; Kobeissi, M.; Jiang, Y.; Gao, J.; Schmaltz, B. Interface compatibility: How to outperform classical spiro-OMeTAD in perovskite solar cells with carbazole derivatives. J. Mater. Chem. C 2022, 10, 7680–7689. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Liang, Y.; Ghadari, R.; Lu, C.; Liu, X.; Zhang, Z.; Ma, S.; Ding, Y.; Cai, M.; et al. Hole transporting material with passivating group (C–N) for perovskite solar cells with improved stability. Dyes Pigm. 2021, 187, 109129. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Liu, Y.; Qin, T.; Zhang, K.; Li, N.; Zhao, J.; Ye, R.; Fan, Z.; Chi, Z.; et al. Dopant-Free Hole-Transporting Material with Enhanced Intermolecular Interaction for Efficient and Stable n-i-p Perovskite Solar Cells. Adv. Energy Mater. 2021, 11, 2100967. [Google Scholar] [CrossRef]
- Kim, G.W.; Choi, H.; Kim, M.; Lee, J.; Son, S.Y.; Park, T. Hole Transport Materials in Conventional Structural (n–i–p) Perovskite Solar Cells: From Past to the Future. Adv. Energy Mater. 2020, 10, 1903403. [Google Scholar] [CrossRef]
- Mattiello, S.; Lucarelli, G.; Calascibetta, A.; Polastri, L.; Ghiglietti, E.; Podapangi, S.K.; Brown, T.M.; Sassi, M.; Beverina, L. Sustainable, Efficient, and Scalable Preparation of Pure and Performing Spiro-OMeTAD for Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2022, 10, 4750–4757. [Google Scholar] [CrossRef]
- Abdellah, I.M.; Chowdhury, T.H.; Lee, J.J.; Islam, A.; Nazeeruddin, M.K.; Graetzel, M.; El-Shafe, A. Facile and low-cost synthesis of a novel dopant-free hole transporting material that rivals Spiro-OMeTAD for high efficiency perovskite solar cells. Sustain. Energy Fuels 2021, 5, 199–211. [Google Scholar] [CrossRef]
- Lamberti, F.; Schmitz, F.; Chen, W.; He, Z.; Gatti, T. The Non-Innocent Role of Hole-Transporting Materials in Perovskite Solar Cells. Sol. RRL 2021, 5, 2100514. [Google Scholar] [CrossRef]
- Mahajan, P.; Padha, B.; Verma, S.; Gupta, V.; Datt, R.; Tsoi, W.C.; Satapathi, S.; Arya, S. Review of current progress in hole-transporting materials for perovskite solar cells. J. Energy Chem. 2022, 68, 330–386. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, C.; Zhang, C.; Hu, H.; Wang, K.; De Wolf, S. Organic Hole-Transport Layers for Efficient, Stable, and Scalable Inverted Perovskite Solar Cells. Adv. Mater. 2022, 34, e2203794. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, C.; Meng, L.; Li, Y. Quinoxaline-Based D–A Copolymers for the Applications as Polymer Donor and Hole Transport Material in Polymer/ Perovskite Solar Cells. Adv. Mater. 2022, 34, 2104161. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, G.; Li, M.; Pan, Y.; Ma, H.; Sun, R.; Wang, J.; Liu, Y.; Chen, C.; Huang, W.; et al. Tailored Polymeric Hole-Transporting Materials Inducing High-Quality Crystallization of Perovskite for Efficient Inverted Photovoltaic Devices. Small 2022, 18, 2106632. [Google Scholar] [CrossRef]
- Kuznetsov, L.E.; Anokhin, D.V.; Piryazev, A.A.; Sideltsev, M.E.; Akhkiamova, A.F.; Novikov, A.V.; Kurbatov, V.G.; Ivanov, D.A.; Akkuratov, A.V. Tailoring the charge transport characteristics in ordered small-molecule organic semiconductors by side-chain engineering and fluorine substitution. Phys. Chem. Chem. Phys. 2022, 24, 16041–16049. [Google Scholar] [CrossRef]
- Igci, C.; Paek, S.; Rakstys, K.; Kanda, H.; Shibayama, N.; Jankauskas, V.; Carmona, C.R.; Kim, H.; Asiri, A.M.; Nazeeruddin, M.K. D–π–A-Type Triazatruxene-Based Dopant-Free Hole Transporting Materials for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2020, 4, 2000173. [Google Scholar] [CrossRef]
- Mai, C.L.; Zhou, Q.; Xiong, Q.; Chen, C.C.; Xu, J.; Zhang, Z.; Lee, H.W.; Yeh, C.Y.; Gao, P. Donor–π–Acceptor Type Porphyrin Derivatives Assisted Defect Passivation for Efficient Hybrid Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2007762. [Google Scholar] [CrossRef]
- Maddala, G.; Gade, R.; Ahemed, J.; Kalvapalli, S.; Simhachalam, N.B.; Chetti, P.; Pola, S.; Mitty, R. Efficient, dopant free phenazine based hole transporting materials for high performance perovskite solar cells. Sol. Energy 2021, 226, 501–512. [Google Scholar] [CrossRef]
- Latypova, A.F.; Emelianov, N.A.; Balakirev, D.O.; Sukhorukova, P.K.; Kalinichenko, N.K.; Kuznetsov, P.M.; Luponosov, Y.N.; Aldoshin, S.M.; Ponomarenko, S.A.; Troshin, P.A.; et al. Design Principles for Organic Small Molecule Hole-Transport Materials for Perovskite Solar Cells: Film Morphology Matters. ACS Appl. Energy Mater. 2022, 5, 5395–5403. [Google Scholar] [CrossRef]
- Ling, W.; Liu, F.; Li, Q.; Li, Z. The crucial roles of the configurations and electronic properties of organic hole-transporting molecules to the photovoltaic performance of perovskite solar cells. J. Mater. Chem. A 2021, 9, 18148–18163. [Google Scholar] [CrossRef]
- Tu, B.; Wang, Y.; Chen, W.; Liu, B.; Feng, X.; Zhu, Y.; Yang, K.; Zhang, Z.; Shi, Y.; Guo, X.; et al. Side-Chain Engineering of Donor−Acceptor Conjugated Small Molecules as Dopant-Free Hole-Transport Materials for Efficient Normal Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 48556–48563. [Google Scholar] [CrossRef]
- Steparuk, A.S.; Irgashev, R.A.; Zhilina, E.F.; Rusinov, G.L.; Petrova, S.A.; Saranin, D.S.; Aleksandrov, A.E.; Tameev, A.R. Thieno [3,2-b]indole–benzo[b]thieno [2,3-d]thiophen-3(2H)-one-based D–p–A molecules as electron transport materials for perovskite solar cells. New J. Chem. 2022, 46, 16612–16617. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Liu, R.; Yan, L.; Dong, P.; Zhao, H.; Wang, Z.; Wu, Y.; Yin, P.; Guo, K. D-π-D hole transport materials based on dioctylfluorene for highly efficient and stable perovskite solar cells without pre-oxidation. Dyes Pigm. 2022, 204, 110452. [Google Scholar] [CrossRef]
- Berton, N.; Nakar, R.; Schmaltz, B. DMPA-containing carbazole-based hole transporting materials for perovskite solar cells: Recent advances and perspectives. Synth Met 2019, 252, 91–106. [Google Scholar] [CrossRef]
- Luizys, P.; Xia, J.; Daskeviciene, M.; Kantminiene, K.; Kasparavicius, E.; Kanda, H.; Zhang, Y.; Jankauskas, V.; Rakstys, K.; Getautis, V.; et al. Branched Methoxydiphenylamine-Substituted Carbazole Derivatives for Efficient Perovskite Solar Cells: Bigger Is Not Always Better. Chem. Mater. 2021, 33, 7017–7027. [Google Scholar] [CrossRef]
- Puckyte, G.; Schmaltz, B.; Tomkeviciene, A.; Degbia, M.; Grazulevicius, J.V.; Melhem, H.; Bouclé, J.; Tran-Van, F. Carbazole-based molecular glasses for efficient solid-state dye-sensitized solar cells. J. Power Sources 2013, 233, 86–92. [Google Scholar] [CrossRef]
- Degbia, M.; Schmaltz, B.; Bouclé, J.; Grazulevicius, J.V.; Tran-Van, F. Carbazole based hole transporting materials for solid state dye sensitizer solar cells: Role of the methoxy groups. Polym. Int. 2014, 63, 1387–1393. [Google Scholar] [CrossRef]
- Benhatta, S.; Nakar, R.; Rodriguez Acosta, J.W.; Berton, N.; Faure-Vincent, J.; Bouclé, J.; Tran Van, F.; Schmaltz, B. Carbazole-based twin molecules as hole-transporting materials in dye-sensitized solar cells. Dyes Pigm. 2018, 151, 238–244. [Google Scholar] [CrossRef]
- Wang, R.; Nakar, R.; Jiang, Y.; Berton, N.; Wu, S.; Wang, Q.; Liu, J.M.; Zhou, G.; Kempa, K.; Schmaltz, B.; et al. Fluorinated Interfacial Layers in Perovskite Solar Cells: Efficient Enhancement of the Fill Factor. J. Mater. Chem. A 2020, 8, 16527–16533. [Google Scholar] [CrossRef]
- Al-Zohbi, F.; Jouane, Y.; Benhattab, S.; Faure-Vincent, J.; Tran-Van, F.; Vedraine, S.; Bouclé, J.; Berton, N.; Schmaltz, B. Simple carbazole-based hole transporting materials with fused benzene ring substituents for efficient perovskite solar cells. New J. Chem. 2019, 43, 12211–12214. [Google Scholar] [CrossRef]
- Benhattab, S.; Cho, A.N.; Nakar, R.; Berton, N.; Tran-Van, F.; Park, N.G.; Schmaltz, B. Simply designed carbazole-based hole transporting materials for efficient perovskite solar cells. Org. Electron. 2018, 56, 27–30. [Google Scholar] [CrossRef]
- Arias-Gómez, A.; Godoy, A.; Portilla, J. Functional Pyrazolo [1,5-a]pyrimidines: Current Approaches in Synthetic Transformations and Uses As an Antitumor Scaffold. Molecules 2021, 26, 2708. [Google Scholar] [CrossRef]
- Yi, K.J.; Yi, S.J. Doosan Corporation. WO2016126031, 2016. Available online: https://patents.google.com/patent/WO2016126031A2/en?oq=WO2016126031%2c+2016 (accessed on 5 November 2022).
- Tigreros, A.; Macías, M.; Portilla, J. Photophysical and crystallographic study of three integrated pyrazolo [1,5-a]pyrimidine–triphenylamine systems. Dyes Pigm. 2021, 184, 108730. [Google Scholar] [CrossRef]
- Karthick, S.; Velumani, S.; Bouclé, J. Experimental and SCAPS simulated formamidinium perovskite solar cells: A comparison of device performance. Sol. Energy 2020, 205, 349–357. [Google Scholar] [CrossRef]
- Kamal, A.; Tamboli, J.R.; Nayak, V.L.; Adil, S.F.; Vishnuvardhan, M.V.P.S.; Ramakrishna, S. Synthesis of pyrazolo [1,5-a]pyrimidine linked aminobenzothiazole conjugates as potential anticancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 3208–3215. [Google Scholar] [CrossRef]
- Choi, P.J.; Lu, G.L.; Sutherland, H.S.; Giddens, A.C.; Franzblau, S.G.; Cooper, C.B.; Denny, W.A.; Palmer, B.D. Synthetic studies towards isomeric pyrazolopyrimidines as potential ATP synthesis inhibitors of Mycobacterium tuberculosis. Structural correction of reported N-(6-(2-(dimethylamino)ethoxy)-5-fluoropyridin-3-yl)-2-(4-fluorophenyl)-5-(trifluoromethyl)pyrazolo [1,5-a]pyrimidin-7-amine. Tetrahedron Lett. 2022, 90, 153611. [Google Scholar]
- He, L.L.; Qi, Q.; Wang, X.; Li, Y.; Zhu, Y.; Wang, X.F.; Xu, L. Synthesis of two novel pyrazolo [1,5-a]pyrimidine compounds with antibacterial activity and biophysical insights into their interactions with plasma protein. Bioorg. Chem. 2020, 99, 103833. [Google Scholar] [CrossRef]
- Jismy, B.; Guillaumet, G.; Akssira, M.; Tikadd, A.; Abarbri, M. Efficient microwave-assisted Suzuki–Miyaura cross-coupling reaction of 3-bromo pyrazolo [1,5-a]pyrimidin-5(4H)-one: Towards a new access to 3,5-diarylated 7-(trifluoromethyl)pyrazolo [1,5-a] pyrimidine derivatives. RSC Adv. 2021, 11, 1287–1302. [Google Scholar] [CrossRef]
- Kodimuthali, A.; Nishad, T.C.N.; Prasunamba, P.L.; Pal, M. Reactivity of the –C(Cl)C–CN– moiety towards AlCl3-induced C–C bond forming reactions: A new synthesis of 7-(hetero)aryl-substituted pyrazolo [1,5-a]pyrimidines. Tetrahedron Lett. 2009, 50, 354–358. [Google Scholar] [CrossRef]
- Degbia, M.; Schmaltz, B.; Tran-Van, F. University of Tours. WO2016016221A1, 2016. Available online: https://patents.google.com/patent/WO2016016221A1/en?oq=WO2016016221A1%2c+2016 (accessed on 5 November 2022).
- Deksnys, T.; Simokaitiene, J.; Keruckas, J.; Volyniuk, D.; Bezvikonnyi, B.; Cherpak, V.; Stakhira, P.; Ivaniuk, K.; Helzhynskyy, I.; Baryshnikov, G.; et al. Synthesis and characterisation of carbazole-based bipolar exciplex-forming compound for efficient and color-tunable OLEDs. New J. Chem. 2017, 41, 559–568. [Google Scholar] [CrossRef]
- Lu, P.; Tang, X.; Liu, H.; Liu, F. CN108191847A, 2018. Available online: https://patents.google.com/patent/CN108191847A/en?oq=CN108191847A%2c+2018 (accessed on 5 November 2022).
- Castillo, J.C.; Tigreros, A.; Portilla, J. 3-Formylpyrazolo [1,5-a]pyrimidines as Key Intermediates for the Preparation of Functional Fluorophores. J. Org. Chem. 2018, 83, 10887–10897. [Google Scholar] [CrossRef]
- Yang, X.Z.; Sun, R.; Guo, X.; Wie, X.R.; Gao, J.; Xu, Y.J.; Ge, J.F. The application of bioactive pyrazolopyrimidine unit for the construction of fluorescent biomarkers. Dyes Pigm. 2020, 173, 107878. [Google Scholar] [CrossRef]
- Ren, J.; Ding, S.; Li, X.; Bi, R.; Zhao, Q. An Approach for the Synthesis of Pyrazolo [1,5-a]pyrimidines via Cu(II)-Catalyzed [3+3] Annulation of Saturated Ketones with Aminopyrazoles. J. Org. Chem. 2021, 86, 12762–12771. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, C.; Liang, J.; Zhong, X.; Li, Y.; He, J.; Zhao, A.; Li, L.; Shao, Y.; Zhang, X.; et al. Molecular Engineering of Efficient Singlet Oxygen Generators with Near-Infrared AIE Features for Mitochondrial Targeted Photodynamic Therapy. Adv. Funct. Mater. 2021, 31, 2104026. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Liu, B.; Gao, Y.; Wu, Z.; Shi, Y.; Tang, Y.; Yang, K.; Zhang, Y.; Weipeng, S.; et al. Engineering of dendritic dopant-free hole transport molecules: Enabling ultrahigh fill factor in perovskite solar cells with optimized dendron construction. Sci. Chin. Chem. 2021, 64, 41–51. [Google Scholar] [CrossRef]
- Vesce, L.; Stefanelli, M.; Di Carlo, A. Efficient and Stable Perovskite Large Area Cells by Low-Cost Fluorene-Xantene-Based Hole Transporting Layer. Energies 2021, 14, 6081. [Google Scholar] [CrossRef]
- Nakar, R.; Ramos, F.J.; Dalinot, C.; Marques, P.S.; Cabanetos, C.; Leriche, P.; Sanguinet, L.; Kobeissi, M.; Blanchard, P.; Faure-Vincent, J.; et al. Cyclopentadithiophene and Fluorene Spiro-Core-Based Hole-Transporting Materials for Perovskite Solar Cells. J. Phys. Chem. C 2019, 123, 22767–22774. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, R.; Jiang, Y.; Kong, X.; Lin, Y.; Xu, Z.; Zhou, G.; Liu, J.M.; Kempa, K.; Gao, J. Novel D-A-D type small-molecular hole transport materials for stable inverted perovskite solar cells. Org. Electron. 2021, 92, 106102. [Google Scholar] [CrossRef]
- Lu, H.; Xu, J.; Liu, X.; Wu, F.; Zhu, L. Effect of isomeric hole-transporting materials on perovskite solar cell performance. Mater. Today Energy 2021, 21, 100780. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Ling, S.; Ma, C.; Zhang, J.; Jiang, Y.; Zhao, R.; Li, H.; Lu, J.; Zhang, Q. Toward Highly Robust Nonvolatile Multilevel Memory by Fine Tuning of the Nanostructural Crystalline Solid-State Order. Small 2021, 17, 2100102. [Google Scholar] [CrossRef]
- Karon, K.; Lapkowski, M. Carbazole electrochemistry: A short review. J. Solid State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- An, M.W.; Wu, B.S.; Wang, S.; Chen, Z.C.; Su, Y.; Deng, L.L.; Li, S.H.; Nan, Z.A.; Tian, H.R.; Liu, W.L.; et al. Corannulene-based hole-transporting material for efficient and stable perovskite solar cells. Cell Rep. Phys. Sci. 2021, 2, 100662. [Google Scholar] [CrossRef]
- Daškevičiūtė, S.; Sakai, N.; Franckevičius, M.; Daškevičienė, M.; Magomedov, A.; Jankauskas, V.; Snaith, H.J.; Getautis, V. Nonspiro, Fluorene-Based, Amorphous Hole Transporting Materials for Efficient and Stable Perovskite Solar Cells. Adv. Sci. 2018, 5, 1700811. [Google Scholar] [CrossRef] [PubMed]
- Vaitukaityte, D.; Truong, M.A.; Rakstys, K.; R.Murdey, R.; Funasaki, T.; Yamada, T.; Kanemitsu, Y.; Jankauskas, V.; Getautis, V. Molecular Engineering of Enamine-Based HoleTransporting Materials for High-Performing Perovskite Solar Cells: Influence of the Central Heteroatom. Sol. RRL 2022, 2200590. [Google Scholar] [CrossRef]
- Hao, M.; Tan, D.; Chi, W.; Li, Z.S. A p-extended triphenylamine based dopant-free hole-transporting material for perovskite solar cells via heteroatom substitution. Phys. Chem. Chem. Phys. 2022, 24, 4635–4643. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liu, P.; Li, Y.; Xu, B.; Kloo, L.; Sun, L.; Hua, Y. D−A−D-Typed Hole Transport Materials for Efficient Perovskite Solar Cells: Tuning Photovoltaic Properties via the Acceptor Group. ACS Appl. Mater. Interfaces 2018, 10, 19697–19703. [Google Scholar] [CrossRef] [PubMed]
- Danyliv, Y.; Ivaniuk, K.; Danyliv, I.; Bezvikonnyi, O.; Volyniuk, D.; Galyna, S.; Lazauskas, A.; Skhirtladze, L.; Ågren, H.; Stakhira, P.; et al. Carbazole-σ-sulfobenzimide derivative exhibiting mechanochromic thermally activated delayed fluorescence as emitter for flexible OLEDs: Theoretical and experimental insights. Dyes Pigm. 2022, 208, 110841. [Google Scholar] [CrossRef]
- Kim, M.J.; Ahn, M.; Chae, M.; Kim, S.; Kim, D.; Wee, K.R. meta-Terphenyl linked donor–p–acceptor dyads: Intramolecular charge transfer controlled by electron acceptor group tuning. RSC Adv. 2021, 11, 34945–34954. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, J.; Li, Z.; Ji, M.; Zhao, M.; Shen, M.; Han, X.; Jia, T.; Li, C.; Wang, Y. Donor–Acceptor-Type Organic-Small-Molecule-Based Solar-Energy-Absorbing Material for Highly Efficient Water Evaporation and Thermoelectric Power Generation. Adv. Funct. Mater. 2021, 2106247. [Google Scholar] [CrossRef]
- Kumsampao, J.; Chaiwai, C.; Chasing, P.; Chawanpunyawat, T.; Namuangruk, S.; Sudyoadsuk, T.; Promarak, V. A Simple and Strong Electron-Deficient 5,6-Dicyano [2,1,3] benzothiadiazole-Cored Donor-Acceptor-Donor Compound for Efficient Near Infrared Thermally Activated Delayed Fluorescence. Chem. Asian J. 2020, 15, 3029–3036. [Google Scholar] [CrossRef]
- Nan, M.I.; Lakatos, E.; Giurgi, G.I.; Szolga, L.; Po, R.; Terec, A.; Jungsuttiwong, S.; Grosu, I.; Roncali, J. Mono- and di-substituted pyrene-based donor-π-acceptor systems with phenyl and thienyl π-conjugating bridges. Dyes Pigm. 2020, 181, 108527. [Google Scholar] [CrossRef]
- Altinkaya, C.; Aydin, E.; Ugur, E.; Isikgor, F.H.; Subbiah, A.S.; De Bastiani, M.; Liu, J.; Babayigit, A.; Allen, T.G.; Laquai, F.; et al. Tin Oxide Electron-Selective Layers for Efficient, Stable, and Scalable Perovskite Solar Cells. Adv. Mater. 2021, 33, 2005504. [Google Scholar] [CrossRef]
- Karthick, S.; Bouclé, J.; Velumani, S. Effect of bismuth iodide (BiI3) interfacial layer with different HTL’s in FAPI based perovskite solar cell–SCAPS–1D study. Sol. Energy 2021, 218, 157–168. [Google Scholar] [CrossRef]
- Zhou, S.; Daskeviciene, M.; Steponaitis, M.; Bubniene, G.; Jankauskas, V.; Schutt, K.; Holzhey, P.R.; Marshall, A.; Caprioglio, P.; Christoforo, G.; et al. Low-Cost Dopant-Free Carbazole Enamine Hole-Transporting Materials for Thermally Stable Perovskite Solar Cells. Sol. RRL 2022, 6, 2100984. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Deng, L.; Gao, Y.; Wang, C.; Xu, J.; Li, T.; Gao, P. Hot-Air Treatment-Regulated Diffusion of LiTFSI to Accelerate the Aging-Induced Efficiency Rising of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 4378–4388. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Pathak, R.; Qiao, Q. Origin and alleviation of J-V hysteresis in perovskite solar cells: A short review. Catal. Today 2021, 374, 86–101. [Google Scholar] [CrossRef]
- Minbashi, M.; Yazdani, E. Comprehensive study of anomalous hysteresis behavior in perovskite-based solar cells. Sci. Rep. 2022, 12, 14916. [Google Scholar] [CrossRef]
- Fan, Q.; Ma, R.; Su, W.; Zhu, Q.; Luo, Z.; Chen, K.; Tang, Y.; R. Lin, F.; Li, Y.; Yan, H.; et al. A new perspective to develop regiorandom polymer acceptors with high active layer ductility, excellent device stability, and high efficiency approaching 17%. Carbon Energy 2022, 1–9. [Google Scholar] [CrossRef]
- Belchi, R.; Habert, A.; Foy, E.; Gheno, A.; Vedraine, S.; Antony, R.; Ratier, B.; Bouclé, J.; Herlin-Boime, N. One-step synthesis of TiO2/graphene nanocomposites by laser pyrolysis with well-controlled properties and application in perovskite solar cells. ACS Omega 2019, 4, 11906–11913. [Google Scholar] [CrossRef] [PubMed]
- Karthick, S.; Hawashin, H.; Parou, N.; Vedraine, S.; Velumani, S.; Bouclé, J. Copper and Bismuth incorporated mixed cation perovskite solar cells by one-step solution process. Sol. Energy 2021, 218, 226–236. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Shen, C.; Zhang, D.; Liu, S.; Wu, Y.; Zhu, W. Coplanar π-Extended Quinoxaline Based Hole-Transporting Material Enabling over 21% Efficiency for Dopant-Free Perovskite Solar Cells. Angew. Chem. Int. Ed. 2021, 60, 2674–2679. [Google Scholar] [CrossRef]
- Niu, T.; Zhu, W.; Zhang, Y.; Xue, Q.; Jiao, X.; Wang, Z.; Xie, Y.M.; Li, P.; Chen, R.; Huang, F.; et al. D-A-p-A-D-type Dopant-free Hole Transport Material for Low-Cost, Efficient, and Stable Perovskite Solar Cells. Joule 2021, 5, 249–269. [Google Scholar] [CrossRef]
- Lee, K.M.; Chiu, W.H.; Tsai, Y.H.; Wang, C.S.; Tao, Y.T.; Lin, Y.D. High-performance perovskite solar cells based on dopant-free hole-transporting material fabricated by a thermal-assisted blade-coating method with efficiency exceeding 21%. J. Chem. Eng. 2022, 427, 131609. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Liu, S.; Yang, H.; Shao, Z. Fundamental Understanding of Photocurrent Hysteresis in Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803017. [Google Scholar] [CrossRef]
- Guerrero, A.; Bou, A.; Matt, G.; Almora, O.; Heumüller, T.; Garcia-Belmonte, G.; Bisquert, J.; Hou, Y.; Brabec, C. Switching Off Hysteresis in Perovskite Solar Cells by Fine-Tuning Energy Levels of Extraction Layers. Adv. Energy Mater. 2018, 1703376. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, L.; Tu, B.; Chen, T.C.; Liu, B.; Yang, K.; Koh, C.W.; Zhang, X.; Sun, H.; et al. Dopant-Free Small-Molecule Hole-Transporting Material for Inverted Perovskite Solar Cells with Efficiency Exceeding 21%. Adv. Mater. 2019, 1902781. [Google Scholar] [CrossRef] [PubMed]


| HTM | Egapopt (eV) 1 | E1/2ox (V/Fc+:Fc) 2 | △Ep1 (V/Fc+:Fc) 3 | △Ep2 (V/Fc+:Fc) 4 | EHOMO (eV) 5 | ELUMO (eV) 6 | Tg (°C) 7 | Td (°C) 8 | Hole Mobility (cm2 V−1 s−1) 9 |
|---|---|---|---|---|---|---|---|---|---|
| PY1 | 2.25 | 0.01 | 0.17 | 0.17 | −5.11 | −2.86 | 127 | 410 | 3 × 10−6 |
| PY2 | 2.33 | −0.03 | 0.18 | 0.18 | −5.07 | −2.74 | 136 | 430 | 1.3 × 10−6 |
| PY3 | 2.40 | −0.10 | 0.16 | 0.16 | −5.00 | −2.60 | 135 | 440 | 1.3 × 10−6 |
| Spiro- OMeTAD 10,11 | 2.99 | / | / | / | −5.20 | −2.21 | 126 | 449 | 2.5 × 10−5 |
| HTM | Scan Direction | JSC (mA cm−2) | VOC (V) | FF (%) | PCE (%) | HI |
|---|---|---|---|---|---|---|
| PY1 | FW | 22.44 1 22.34 2 ± 0.10 3 | 0.94 0.93 ± 0.01 | 49 48 ± 1 | 10.55 10.31 ± 0.23 | 0.15 |
| BW | 24.70 24.82 ± 0.12 | 0.93 0.92 ± 0.01 | 53 52 ± 1 | 12.41 12.11 ± 0.30 | ||
| PY2 | FW | 22.05 21.95 ± 0.10 | 0.90 0.88 ± 0.02 | 51 52 ± 1 | 10.21 10.11 ± 0.10 | −0.01 |
| BW | 22.90 22.80 ± 0.10 | 0.84 0.83 ± 0.01 | 52 53 ± 2 | 10.10 10.34 ± 0.23 | ||
| PY3 | FW | 22.46 22.34 ± 0.10 | 0.96 0.95 ± 0.01 | 49 50 ± 1 | 10.60 10.74 ± 0.14 | 0.02 |
| BW | 23.17 22.79 ± 0.37 | 0.90 0.89 ± 0.01 | 51 50 ± 2 | 10.82 10.39 ± 0.37 | ||
| Spiro- OMeTAD | FW | 23.11 23.02 ± 0.08 | 0.92 0.93 ± 0.01 | 48 50 ± 1 | 10.44 10.84 ± 0.40 | 0.17 |
| BW | 24.03 23.59 ± 0.43 | 0.92 0.93 ± 0.02 | 56 55 ± 1 | 12.58 12.46 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouihi, F.; Schmaltz, B.; Mathevet, F.; Kreher, D.; Faure-Vincent, J.; Yildirim, C.; Elhakmaoui, A.; Bouclé, J.; Akssira, M.; Tran-Van, F.; et al. D-π-A-Type Pyrazolo[1,5-a]pyrimidine-Based Hole-Transporting Materials for Perovskite Solar Cells: Effect of the Functionalization Position. Materials 2022, 15, 7992. https://doi.org/10.3390/ma15227992
Bouihi F, Schmaltz B, Mathevet F, Kreher D, Faure-Vincent J, Yildirim C, Elhakmaoui A, Bouclé J, Akssira M, Tran-Van F, et al. D-π-A-Type Pyrazolo[1,5-a]pyrimidine-Based Hole-Transporting Materials for Perovskite Solar Cells: Effect of the Functionalization Position. Materials. 2022; 15(22):7992. https://doi.org/10.3390/ma15227992
Chicago/Turabian StyleBouihi, Fatiha, Bruno Schmaltz, Fabrice Mathevet, David Kreher, Jérôme Faure-Vincent, Ceren Yildirim, Ahmed Elhakmaoui, Johann Bouclé, Mohamed Akssira, François Tran-Van, and et al. 2022. "D-π-A-Type Pyrazolo[1,5-a]pyrimidine-Based Hole-Transporting Materials for Perovskite Solar Cells: Effect of the Functionalization Position" Materials 15, no. 22: 7992. https://doi.org/10.3390/ma15227992
APA StyleBouihi, F., Schmaltz, B., Mathevet, F., Kreher, D., Faure-Vincent, J., Yildirim, C., Elhakmaoui, A., Bouclé, J., Akssira, M., Tran-Van, F., & Abarbri, M. (2022). D-π-A-Type Pyrazolo[1,5-a]pyrimidine-Based Hole-Transporting Materials for Perovskite Solar Cells: Effect of the Functionalization Position. Materials, 15(22), 7992. https://doi.org/10.3390/ma15227992