Formation of Nanoscale Al2O3 Protective Layer by Preheating Treatment for Improving Corrosion Resistance of Dilute Fe-Al Alloys
Abstract
1. Introduction
2. Materials and Experimental Procedures
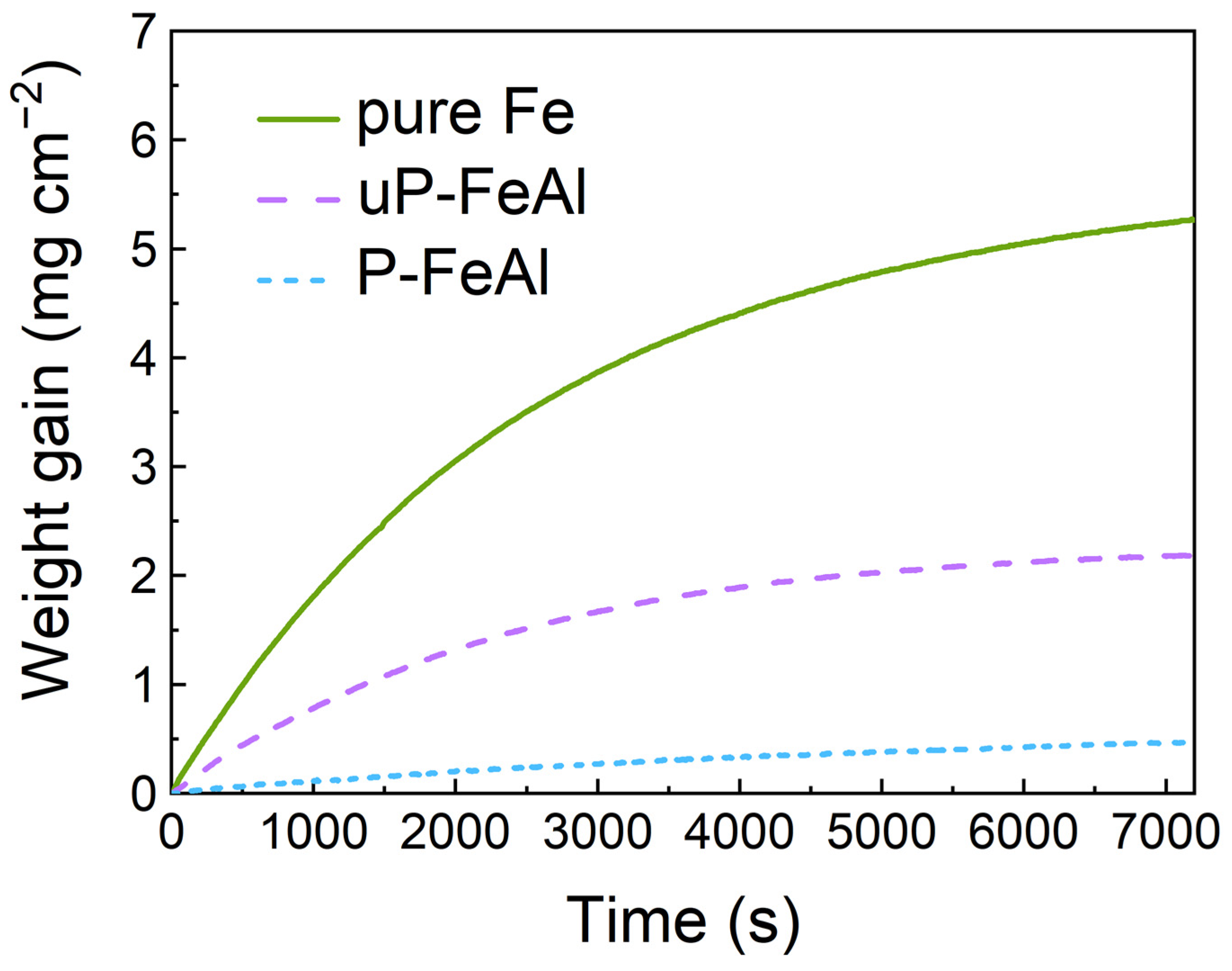
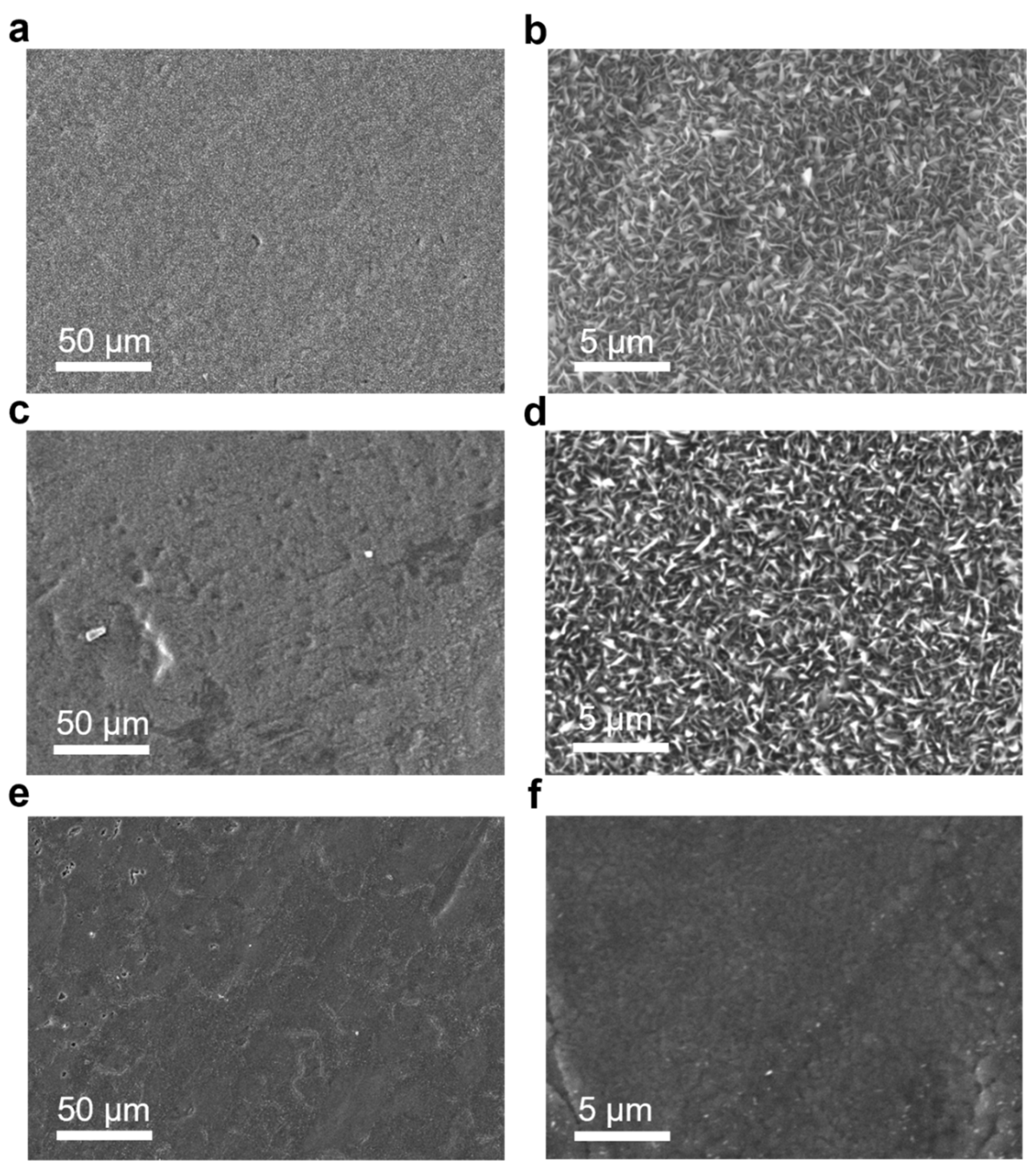
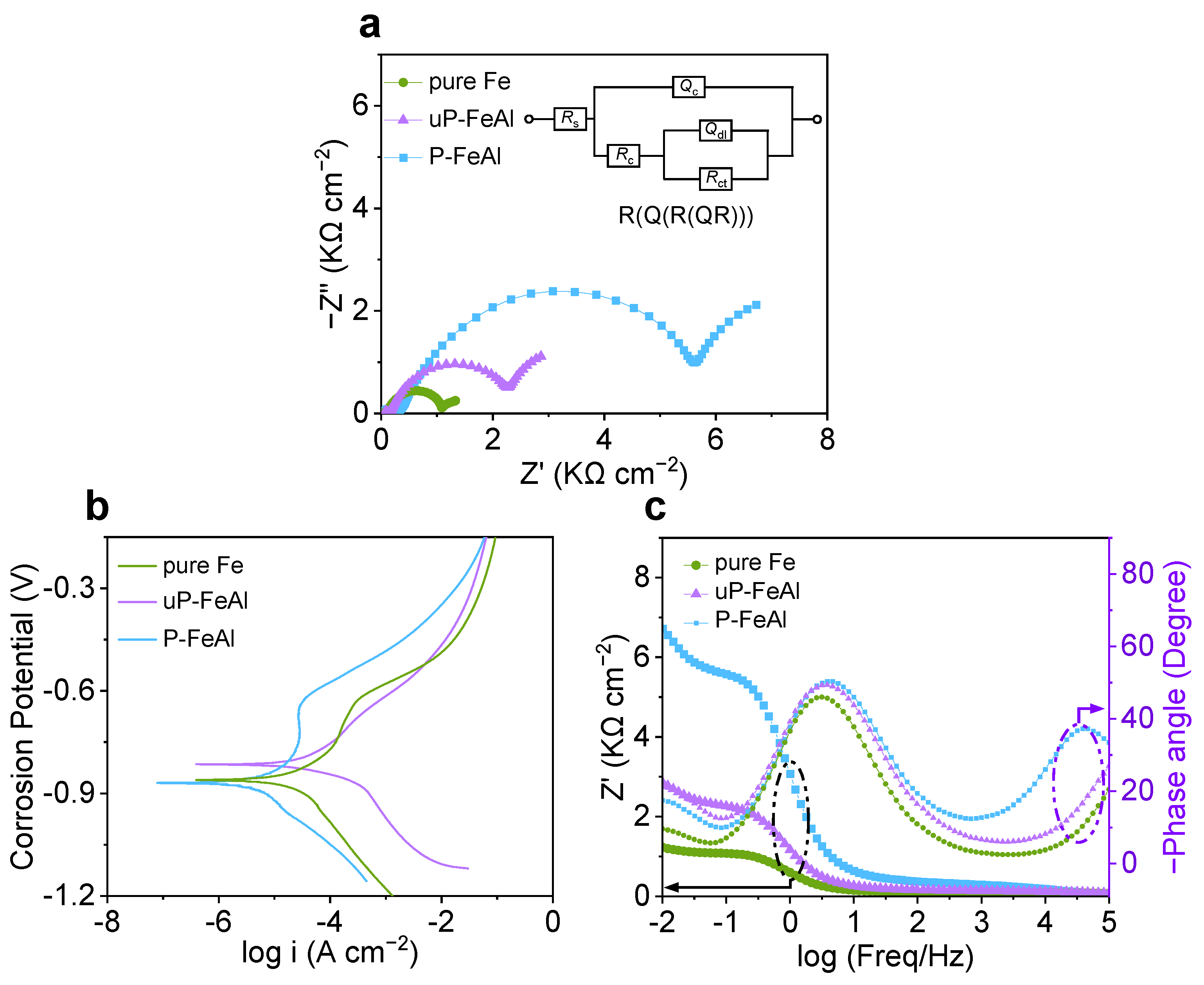
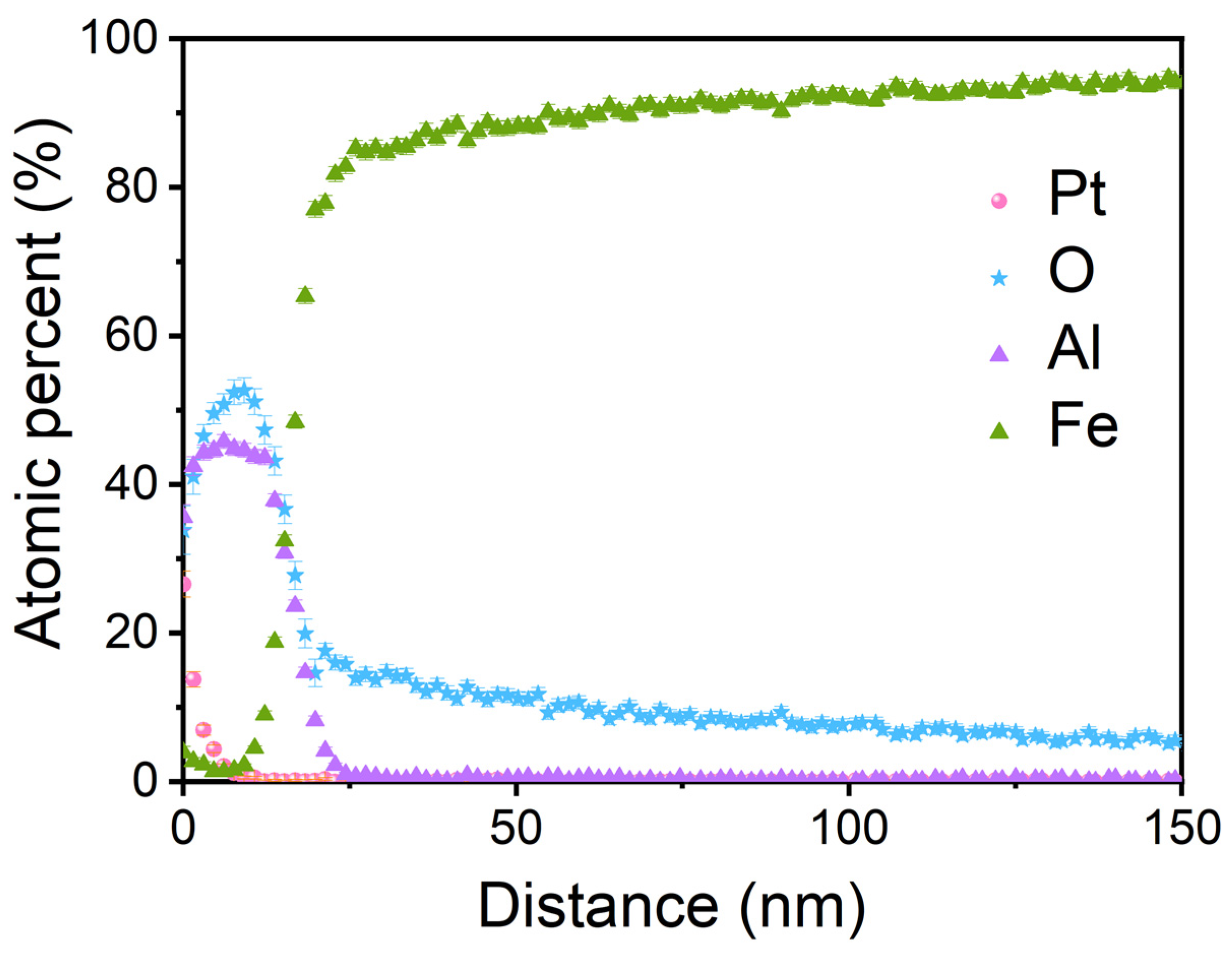
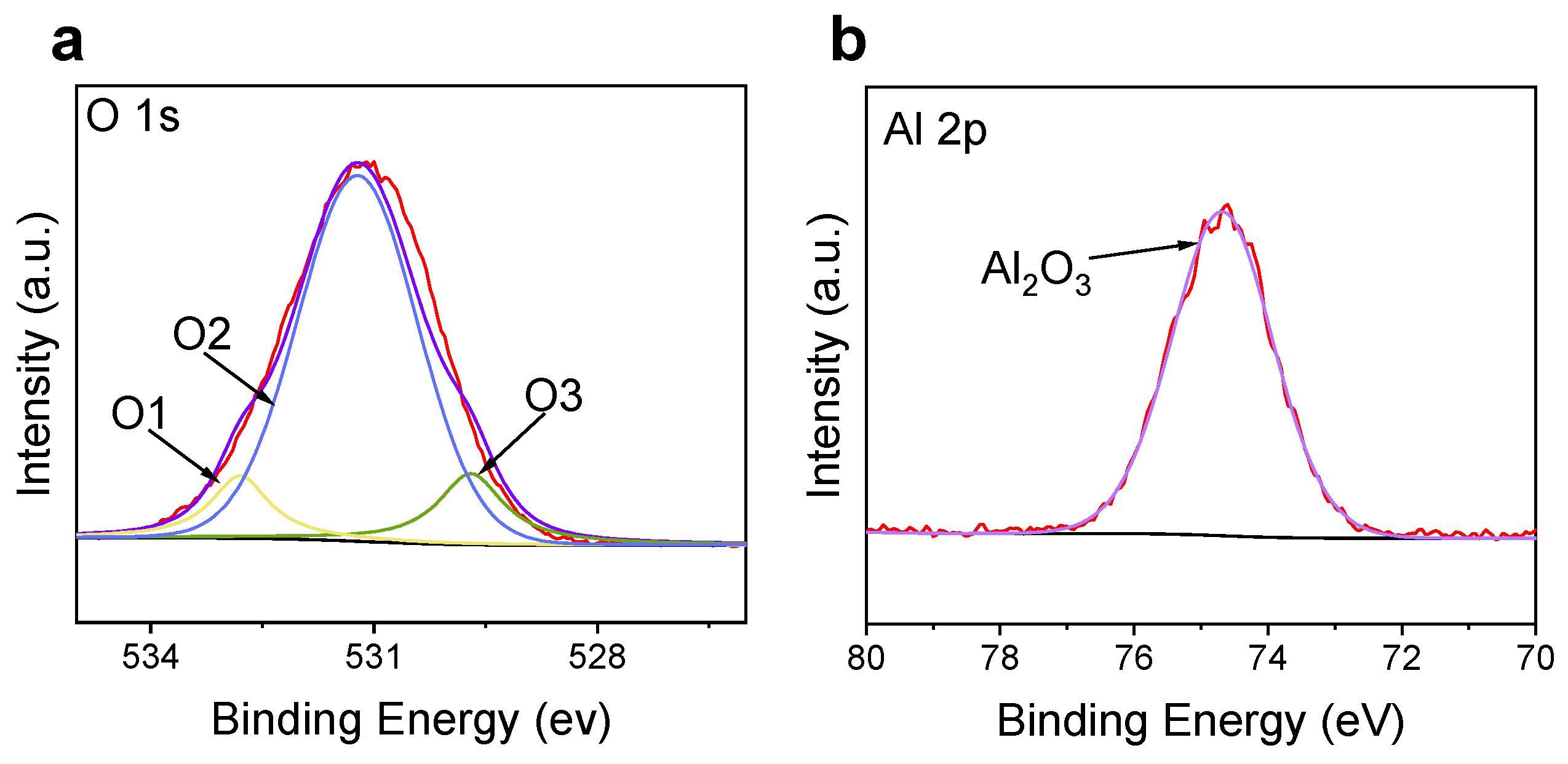
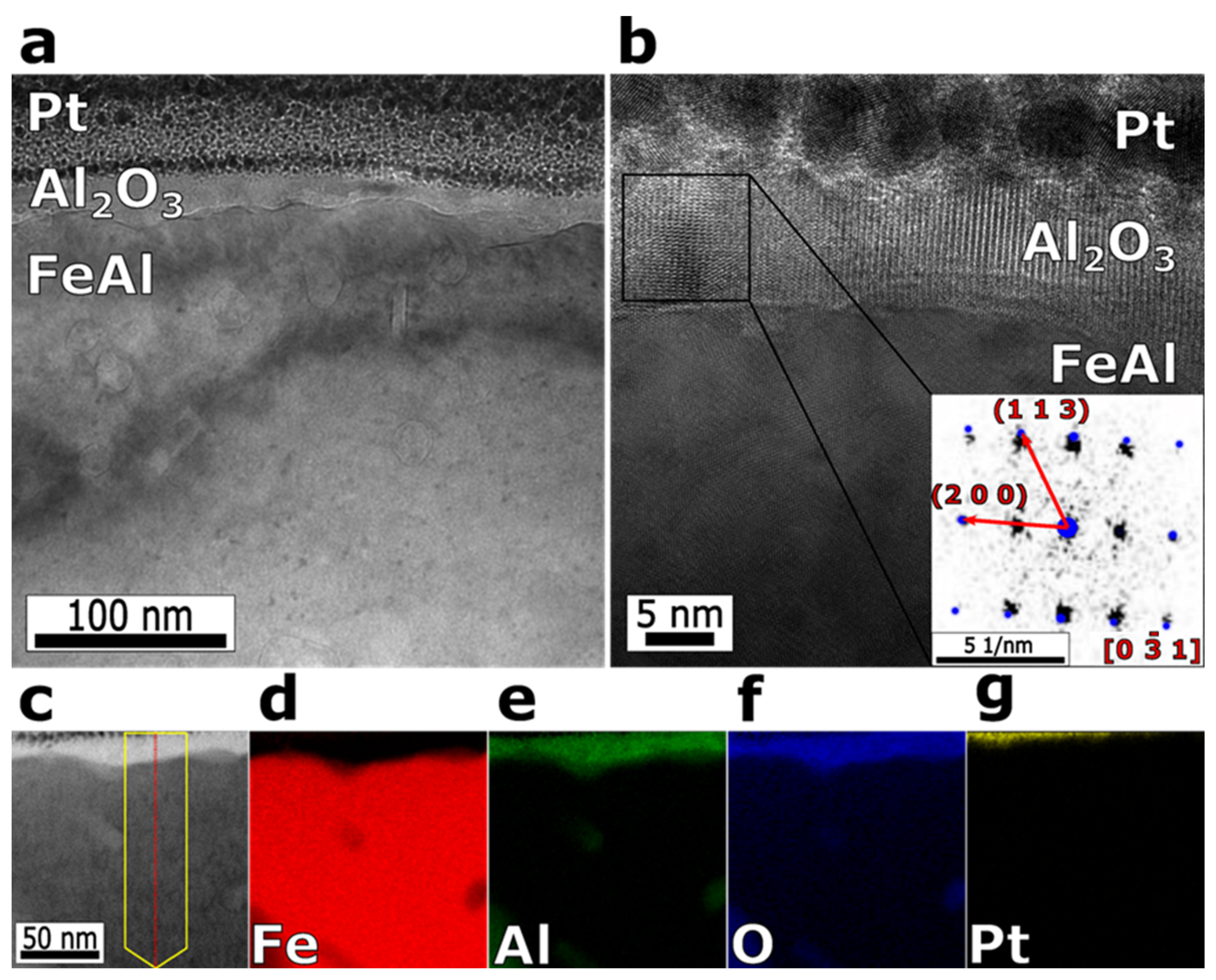
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, Y.X.; Wang, X.Y.; Yang, L.L.; Wang, X.J.; Chen, J.; Wang, Z.B.; Zhou, H.L.; Zou, J.S.; Wang, F.H. Effect of aging treatment on microstructure and corrosion behavior of a Fe-18Cr-15Mn-0.66N stainless steel. J. Mater. Sci. Technol. 2022, 107, 197–206. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Zhang, Y.X.; Xue, Y.N. Recent Advances in Corrosion Research of Biomedical NiTi Shape Memory Alloy. Rare Metal Mat. Eng. 2021, 50, 4165–4173. [Google Scholar]
- Raman, R.K.S. Mechanical Alloying of Elemental Powders into Nanocrystalline (NC) Fe-Cr Alloys: Remarkable Oxidation Resistance of NC Alloys. Metals 2021, 11, 695. [Google Scholar] [CrossRef]
- Backman, D.G.; Williams, J.C. Advanced materials for aircraft engine applications. Science 1992, 255, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Aye, K.K.; Nguyen, T.D.; Zhang, J.; Young, D.J. Effect of silicon on corrosion of Fe-20Cr and Fe-20Cr-20Ni alloys in wet CO2 with and without HCl at 650 °C. Corros. Sci. 2021, 179, 109096. [Google Scholar] [CrossRef]
- Liu, M. Finite element analysis of pitting corrosion on mechanical behavior of E690 steel panel. Anti-Corros. Method M. 2022, 69, 351–361. [Google Scholar] [CrossRef]
- Gong, Y.; Young, D.J.; Kontis, P.; Chiu, Y.L.; Larsson, H.; Shin, A.; Pearson, J.M.; Moody, M.P.; Reed, R.C. On the breakaway oxidation of Fe9Cr1Mo steel in high pressure CO2. Acta Mater. 2017, 130, 361–374. [Google Scholar] [CrossRef]
- Liu, M. Effect of uniform corrosion on mechanical behavior of E690 high-strength steel lattice corrugated panel in marine environment: A finite element analysis. Mater. Res. Express 2021, 8, 066510. [Google Scholar] [CrossRef]
- Wang, F.; Shu, Y. Influence of Cr content on the corrosion of Fe-Cr alloys: The synergistic effect of NaCl and water vapor. Oxid. Met. 2003, 59, 201–214. [Google Scholar] [CrossRef]
- Jung, K.; Ahn, S.; Kim, Y.; Oh, S.; Ryu, W.H.; Kwon, H. Alloy design employing high Cr concentrations for Mo-free stainless steels with enhanced corrosion resistance. Corros Sci. 2018, 140, 61–72. [Google Scholar] [CrossRef]
- Huang, C.A.; Chang, J.H.; Chen, C.Y.; Liao, K.Y.; Mayer, J. Microstructure and electrochemical corrosion behavior of Cr-Ni-Fe alloy deposits electroplated in the presence of trivalent Cr ions. Thin Solid Films 2013, 544, 69–73. [Google Scholar] [CrossRef]
- Choi, Y.I.; Shin, E.S.; Kuroda, K.; Okido, M.; Park, C.J. Improved surface morphology and corrosion resistance for galvannealed coatings by pre-electroplating iron. Corros. Sci. 2012, 58, 152–158. [Google Scholar] [CrossRef]
- Hu, W.B.; Cai, H.L.; Yang, M.H.; Tong, X.L.; Zhou, C.M.; Chen, W. Fe-C-coated fibre Bragg grating sensor for steel corrosion monitoring. Corros Sci. 2011, 53, 1933–1938. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.H. A Study on Cavitation Erosion and Corrosion Behavior of Al-, Zn-, Cu-, and Fe-Based Coatings Prepared by Arc Spraying. J. Therm. Spray Technol. 2010, 19, 1224–1230. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kainuma, S.; Ahn, J.H. Structural response of orthotropic bridge deck depending on the corroded deck surface. Constr. Build Mater. 2013, 43, 87–97. [Google Scholar] [CrossRef]
- Yu, C.; Nguyen, T.D.; Zhang, J.; Young, D.J. Sulfur Effect on Corrosion Behavior of Fe-20Cr-(Mn, Si) and Fe-20Ni-20Cr-(Mn, Si) in CO2-H2O at 650 °C. J. Electrochem. Soc. 2015, 163, C106–C115. [Google Scholar] [CrossRef]
- Rao, V.S. The influence of temperature on the oxidation behaviour of Fe3AI-Fe3AlC0.69 and FeAl-Fe3AlC0.69 intermetallics. Intermetallics 2003, 11, 713–719. [Google Scholar] [CrossRef]
- Wei, W.; Geng, S.J.; Chen, G.; Wang, F.H. Growth mechanism of surface scales on Ni-Fe-Cr alloys at 960 degrees C in air. Corros. Sci. 2020, 173, 108737. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Huang, S.; Shen, J.; Chen, N.-X. Structural and mechanical properties of Fe–Al compounds: An atomistic study by EAM simulation. Intermetallics 2014, 52, 86–91. [Google Scholar] [CrossRef]
- Hong, S.H.; Zhu, Y.F.; Mimura, K.; Isshiki, M. Role of Al2O3 layer in oxidation resistance of Cu-Al dilute alloys pre-annealed in H2 atmospheres. Corros. Sci. 2006, 48, 3692–3702. [Google Scholar] [CrossRef]
- Novak, P.; Nova, K. Oxidation Behavior of Fe-Al, Fe-Si and Fe-Al-Si Intermetallics. Materials 2019, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Jang, P.; Shin, S.; Jung, C.S.; Kim, K.H.; Seomoon, K. Fabrication of Fe-Al nanoparticles by selective oxidation of Fe-Al thin films. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.Q.; Young, D.J. Oxidation of Fe-Si, Fe-Al and Fe-Si-Al alloys in CO2-H2O gas at 800 degrees C. Corros. Sci. 2012, 54, 127–138. [Google Scholar] [CrossRef]
- Xu, Y.F.; Jeurgens, L.P.H.; Bo, H.; Lin, L.C.; Zhu, S.L.; Huang, Y.; Liu, Y.C.; Qiao, J.W.; Wang, Z.M. On the competition between synchronous oxidation and preferential oxidation in Cu-Zr-Al metallic glasses. Corros. Sci. 2020, 177, 108996. [Google Scholar] [CrossRef]
- Pang, X.J.; Li, S.S.; Qin, L.; Pei, Y.L.; Gong, S.K. Effect of trace Ce on high-temperature oxidation behavior of an Al-Si-coated Ni-based single crystal superalloz. J. IronSteel Res. Int. 2019, 26, 78–83. [Google Scholar]
- Wang, X.H.; Li, F.Z.; Chen, J.X.; Zhou, Y.C. Insights into high temperature oxidation of Al2O3-forming Ti3AlC2. Corros Sci. 2012, 58, 95–103. [Google Scholar] [CrossRef]
- Lu, S.D.; Li, X.X.; Liang, X.Y.; Shao, W.T.; Yang, W.; Chen, J. Effect of Al content on the oxidation behavior of refractory high-entropy alloy AlMo0.5NbTa0.5TiZr at elevated temperatures. Int. J. Refract Met. H. 2022, 105, 105812. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Mimura, K.; Isshiki, M. Oxidation mechanism of copper at 623–1073 K. Mater. Trans. 2002, 43, 2173–2176. [Google Scholar] [CrossRef]
- Ogbuji, L.U. The oxidation behavior of an ODS copper alloy Cu-Al2O3. Oxid. Met. 2004, 62, 141–151. [Google Scholar] [CrossRef]
- Liu, Y.X.; Yin, F.C.; Hu, J.X.; Li, Z.; Cheng, S.H. Phase equilibria of Al-Fe-Sn ternary system. T Nonferr. Metal. Soc. 2018, 28, 282–289. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Zhou, D.; Pu, J.; Yu, M.; Guo, W. A new insight into the NaCl-induced hot corrosion mechanism of TiN coatings at 500 °C. Corros. Sci. 2020, 174, 108794. [Google Scholar] [CrossRef]
- Dafali, A.; Hammouti, B.; Mokhlisse, R.; Kertit, S. Substituted uracils as corrosion inhibitors for copper in 3% NaCl solution. Corros. Sci. 2003, 45, 1619–1630. [Google Scholar] [CrossRef]
- Ozcan, M.; Dehri, I.; Erbil, M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: Correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004, 236, 155–164. [Google Scholar] [CrossRef]
- Saha, S.K.; Banerjee, P. Introduction of newly synthesized Schiff base molecules as efficient corrosion inhibitors for mild steel in 1 M HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Mater. Chem. Front. 2018, 2, 1674–1691. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardas, G.; Culha, M.; Yazici, B.; Erbil, M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: Experimental and theoretical approach, Phys. Chem. Chem. Phys. 2016, 18, 17898–17911. [Google Scholar] [CrossRef]
- Visser, P.; Terryn, H.; Mol, J.M.C. On the importance of irreversibility of corrosion inhibitors for active coating protection of AA2024-T3. Corros. Sci. 2018, 140, 272–285. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, C.; Chen, Z.; Wang, Y.; Jiang, S. Corrosion behaviors of (Cr,Fe)3Si/Cr13Fe5Si2 composite coating under condition of synergistic effects of electrochemical corrosion and mechanical erosion. J. Alloys Compd. 2010, 496, 429–432. [Google Scholar] [CrossRef]
- Zhu, X.X.; Sun, M.Y.; Zhao, R.; Li, Y.Q.; Zhang, B.; Zhang, Y.L.; Lang, X.Y.; Zhu, Y.F.; Jiang, Q. 3D hierarchical self-supported NiO/Co3O4@C/CoS2 nanocomposites as electrode materials for high-performance supercapacitors. Nanoscale Adv. 2020, 2, 2785–2791. [Google Scholar] [CrossRef]
- van den Brand, J.; Sloof, W.G.; Terryn, H.; de Wit, J.H.W. Correlation between hydroxyl fraction and O/Al atomic ratio as determined from XPS spectra of aluminium oxide layers. Surf. Interface Anal. 2004, 36, 81–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Freiberg, K.; Tang, Y.; Lippmann, S.; Zhu, Y. Formation of Nanoscale Al2O3 Protective Layer by Preheating Treatment for Improving Corrosion Resistance of Dilute Fe-Al Alloys. Materials 2022, 15, 7978. https://doi.org/10.3390/ma15227978
Li C, Freiberg K, Tang Y, Lippmann S, Zhu Y. Formation of Nanoscale Al2O3 Protective Layer by Preheating Treatment for Improving Corrosion Resistance of Dilute Fe-Al Alloys. Materials. 2022; 15(22):7978. https://doi.org/10.3390/ma15227978
Chicago/Turabian StyleLi, Chenglong, Katharina Freiberg, Yuntong Tang, Stephanie Lippmann, and Yongfu Zhu. 2022. "Formation of Nanoscale Al2O3 Protective Layer by Preheating Treatment for Improving Corrosion Resistance of Dilute Fe-Al Alloys" Materials 15, no. 22: 7978. https://doi.org/10.3390/ma15227978
APA StyleLi, C., Freiberg, K., Tang, Y., Lippmann, S., & Zhu, Y. (2022). Formation of Nanoscale Al2O3 Protective Layer by Preheating Treatment for Improving Corrosion Resistance of Dilute Fe-Al Alloys. Materials, 15(22), 7978. https://doi.org/10.3390/ma15227978






