In-Situ Growth of Graphene Films to Improve Sensing Performances
Abstract
1. Introduction
2. Surface Contamination and Defects Come from Transfer Procedures
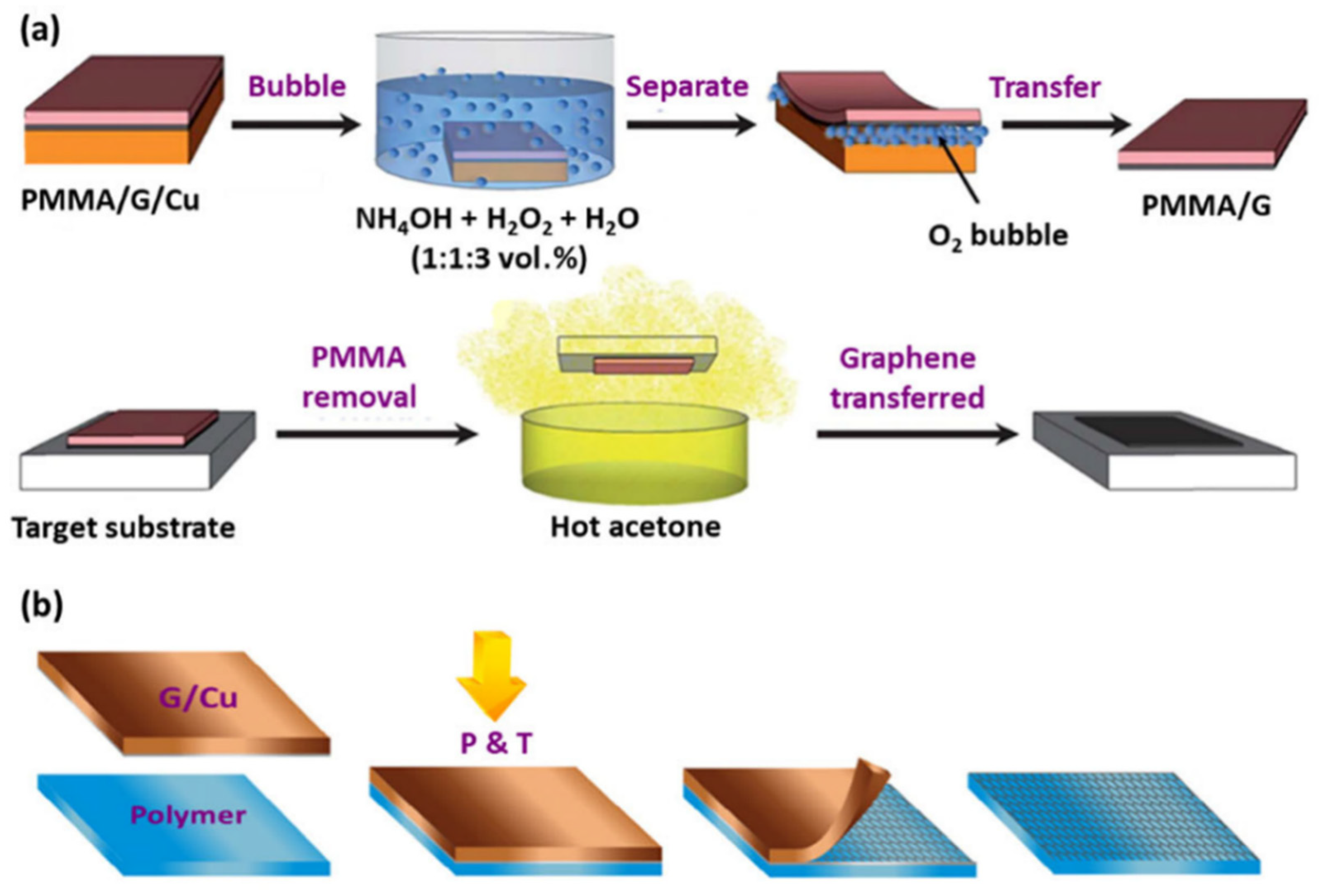
2.1. Wet Transfer
2.2. Dry Transfer
3. In-Situ Growth Makes Graphene Film of Fewer Defects Available
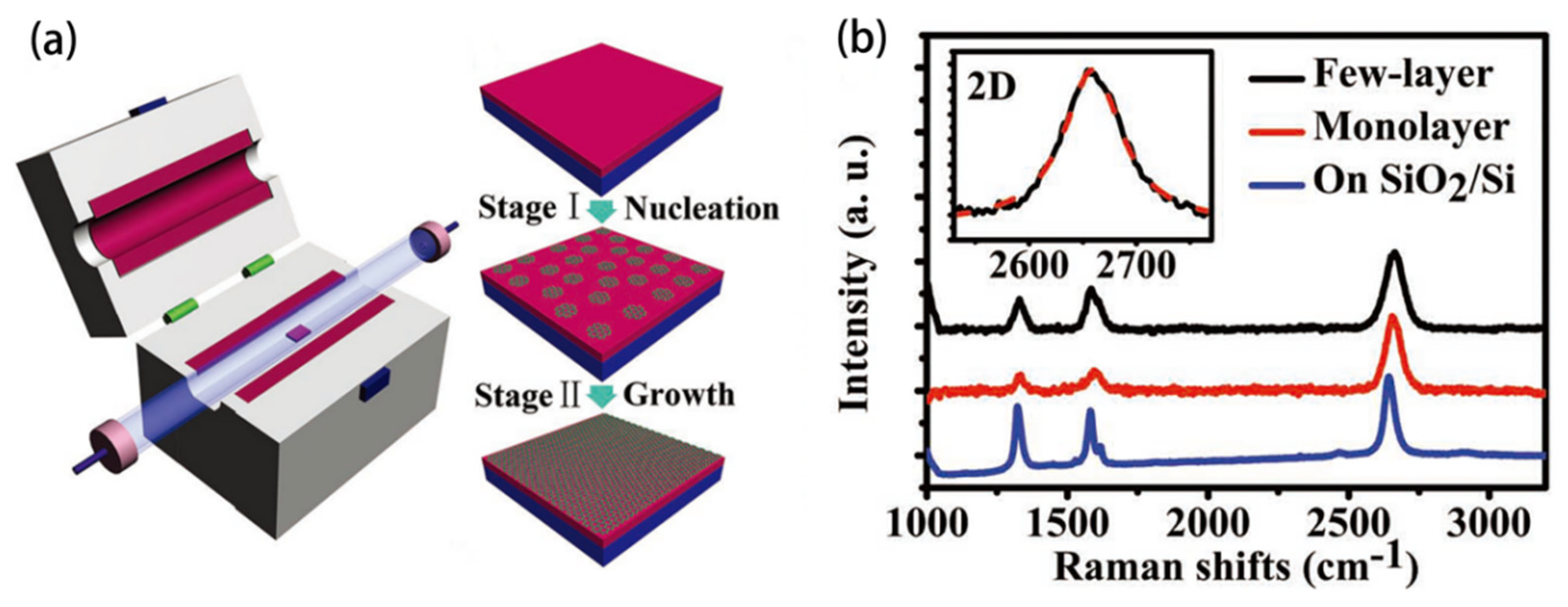
3.1. Metal-Free Catalytic Growth
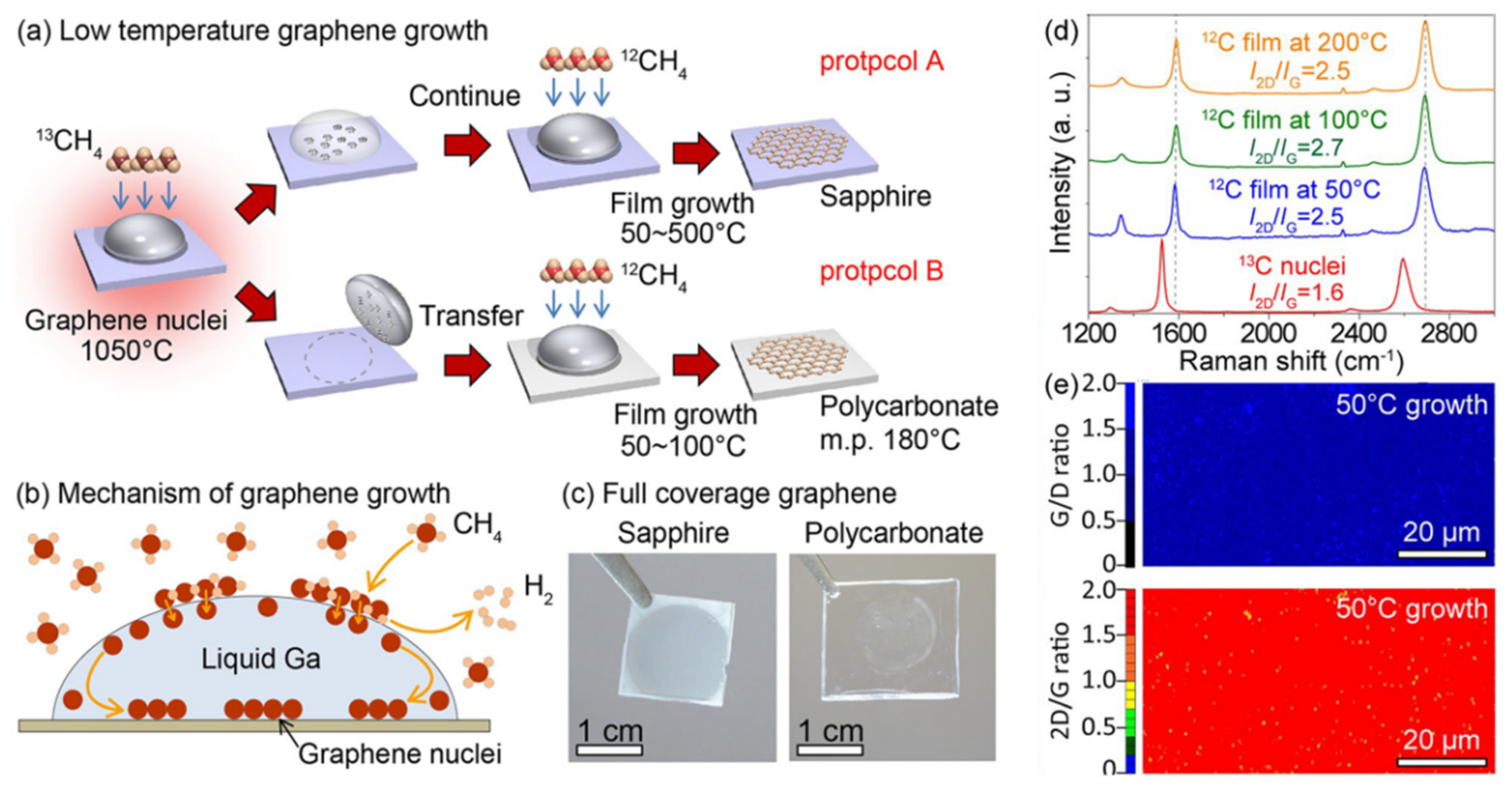
3.2. Metal-Assisted Growth
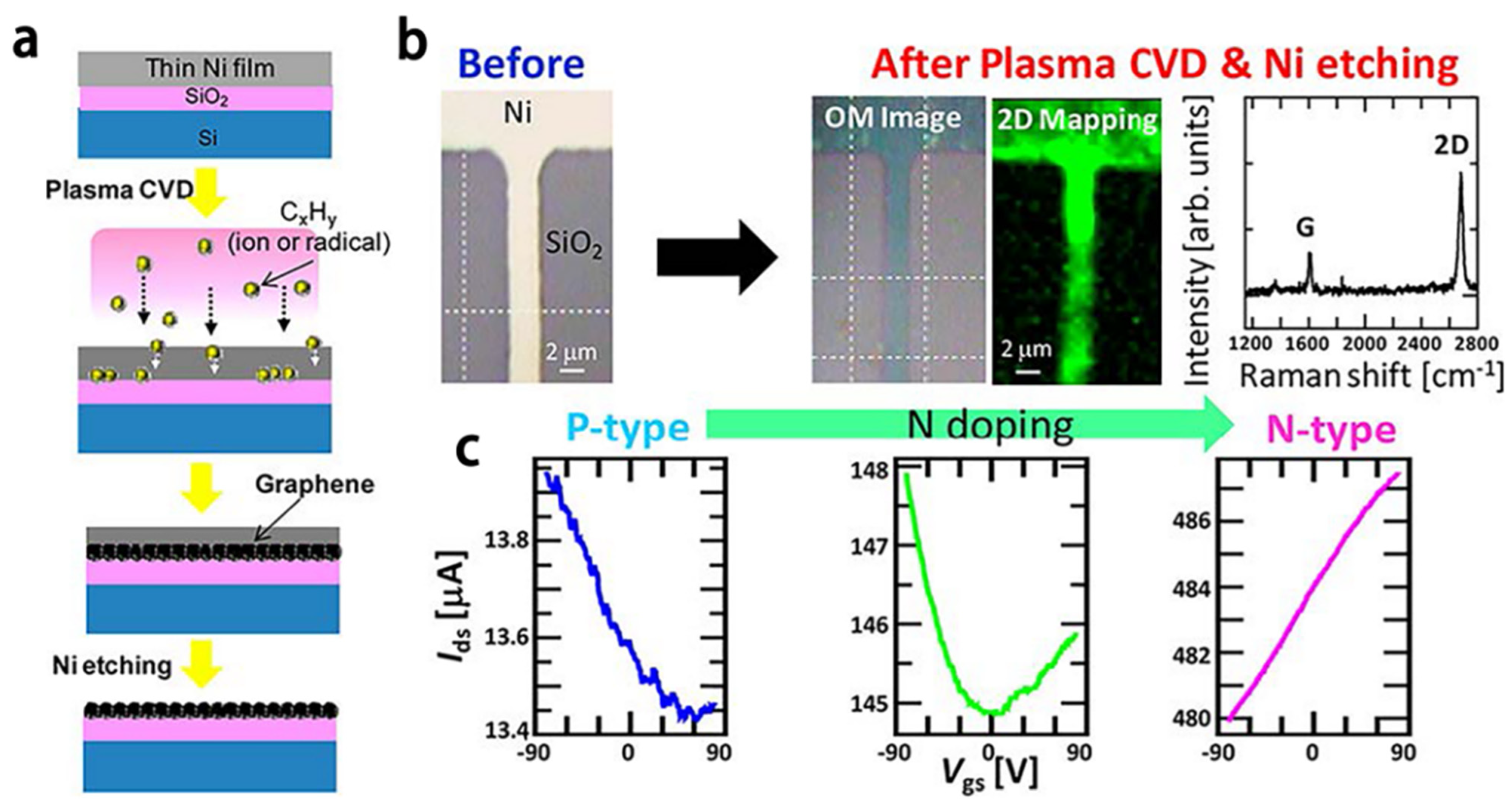
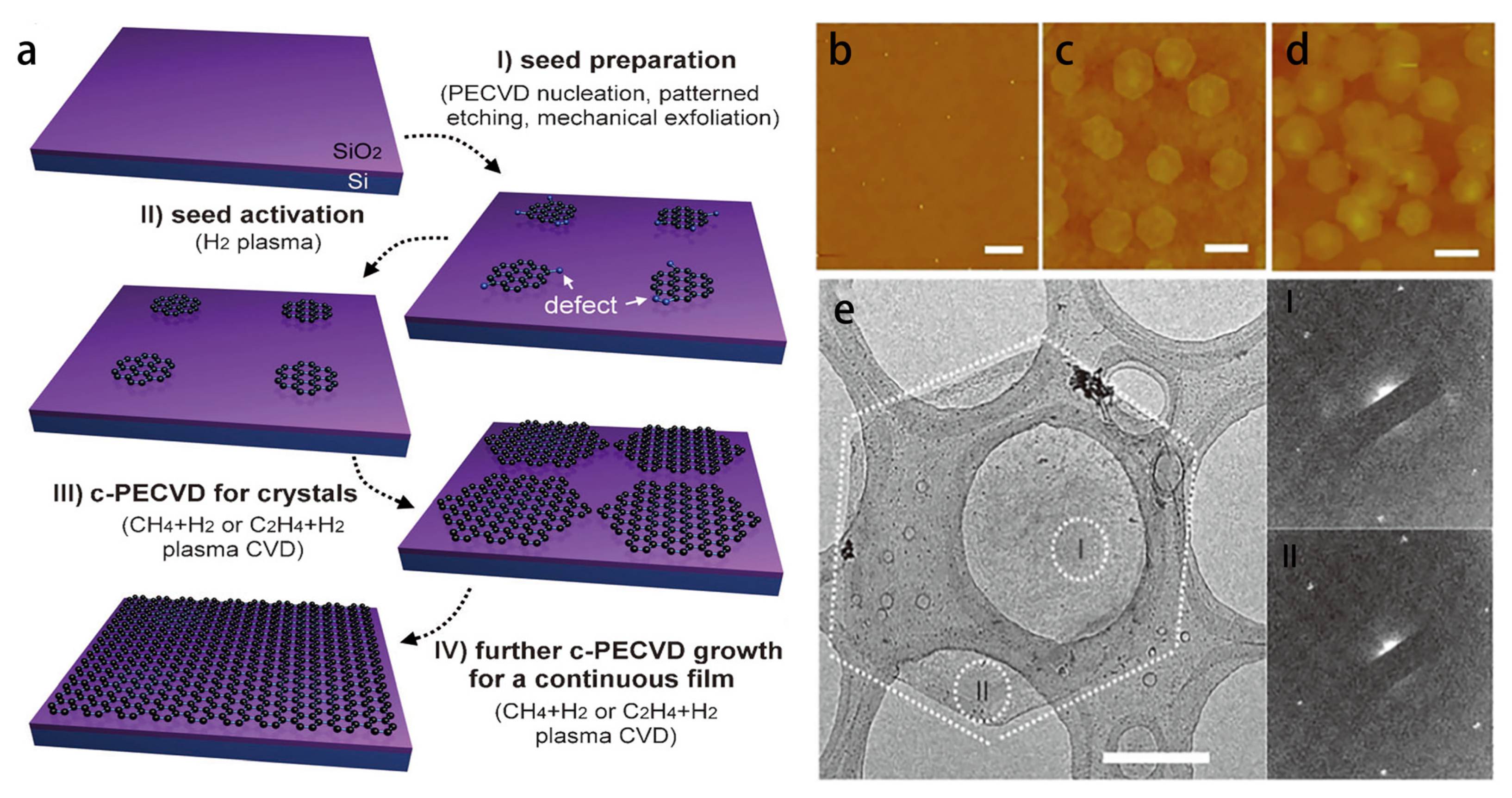
3.3. Plasma-Enhanced Growth
3.4. Comparison of In-Situ Growth Methods
4. Use In-Situ Grown Graphene Film to Improve Sensing Performances
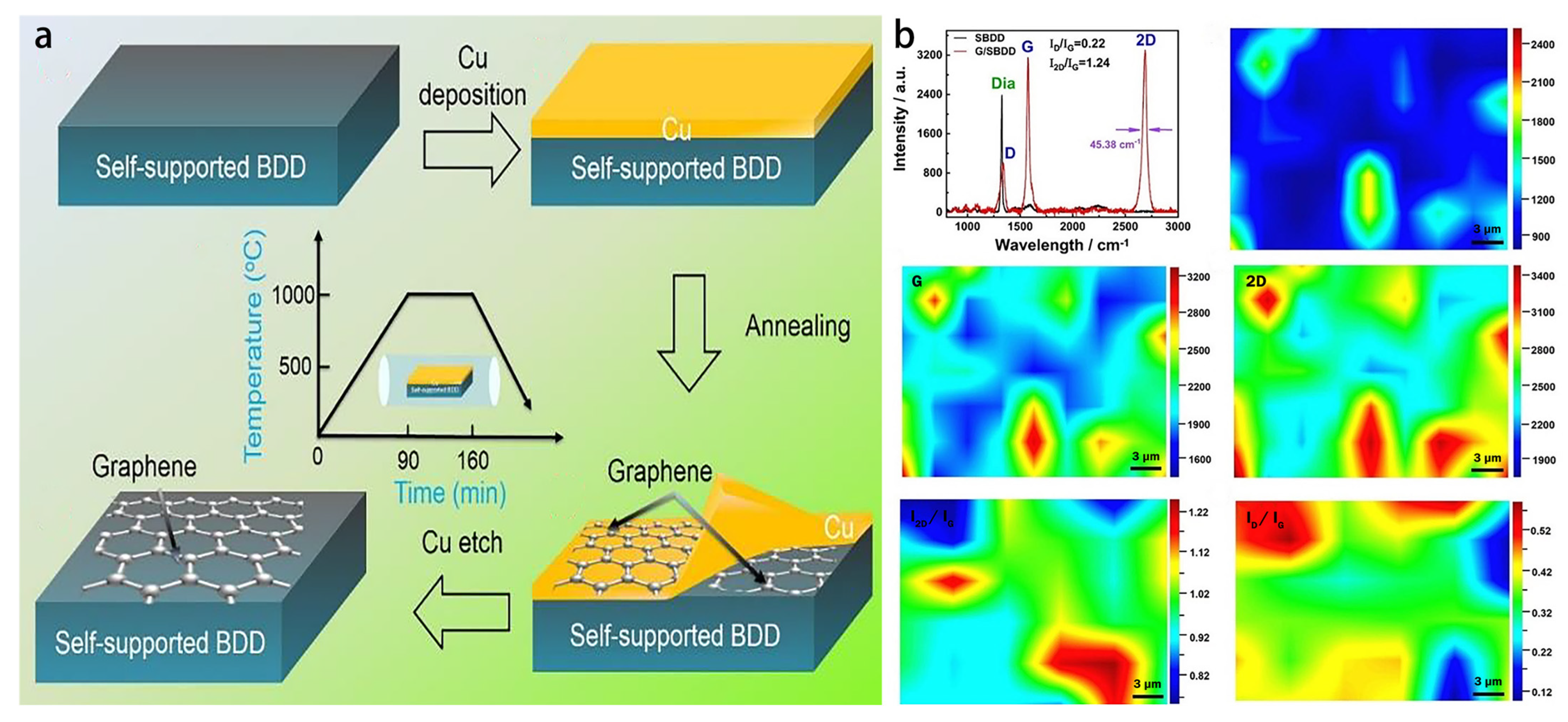
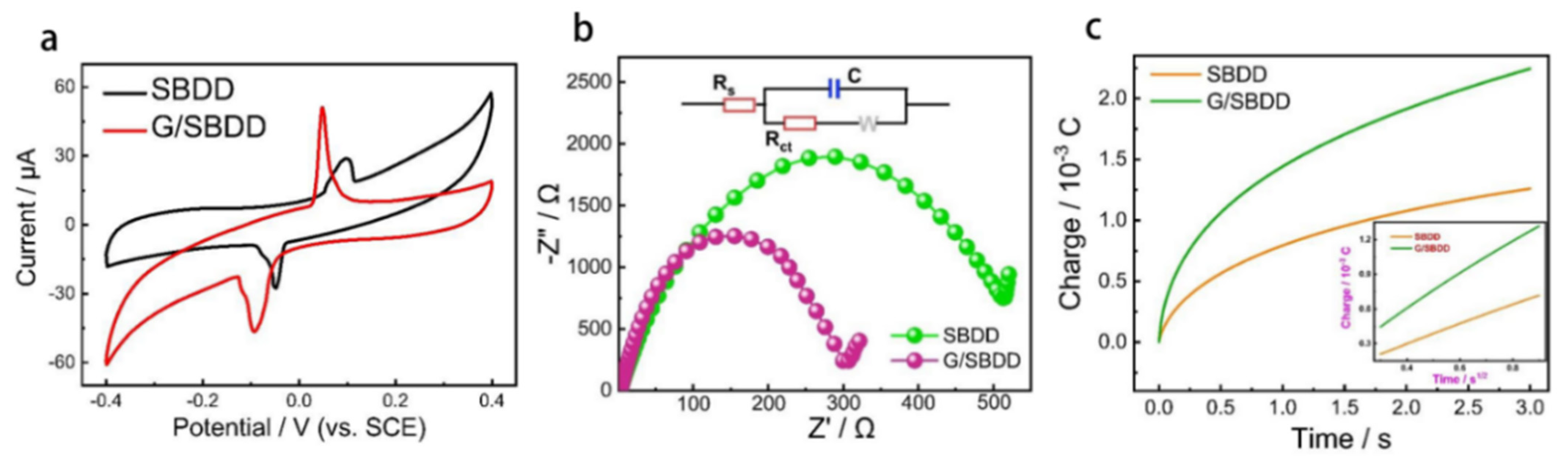
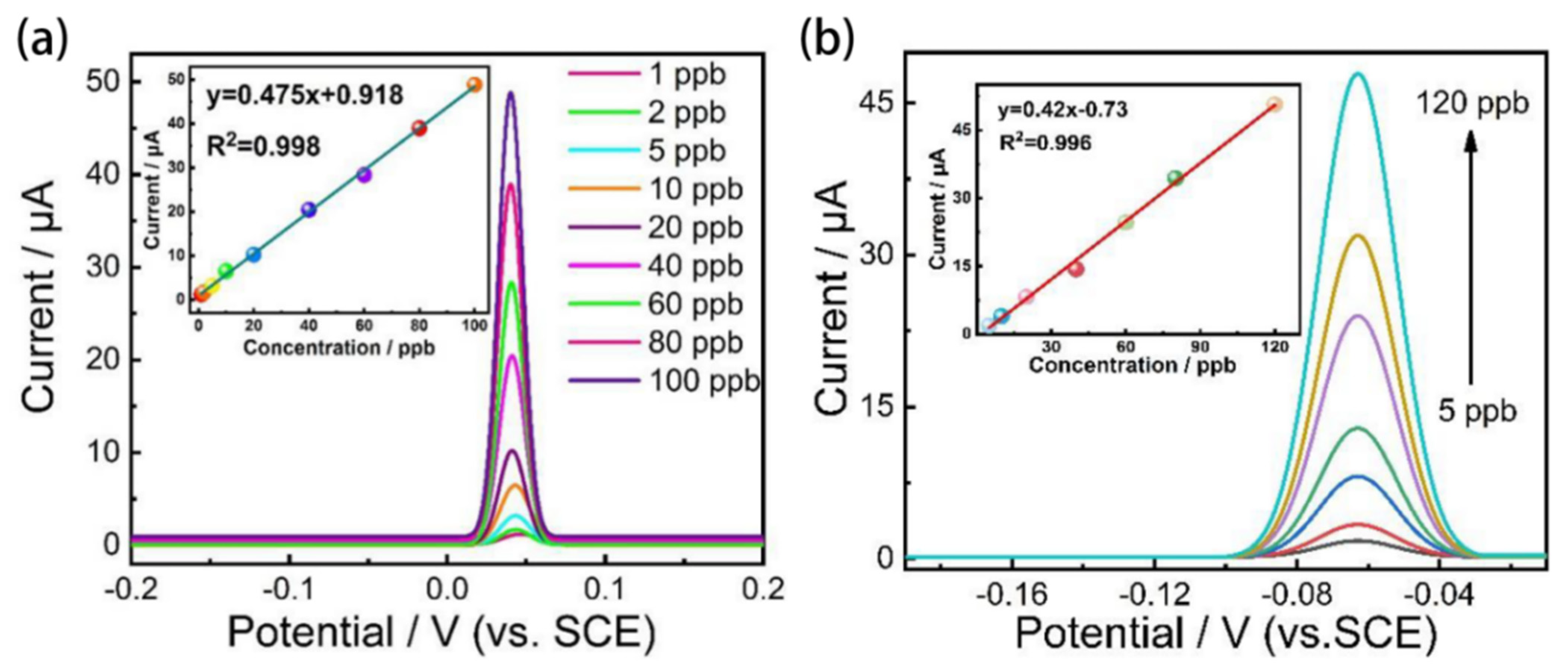
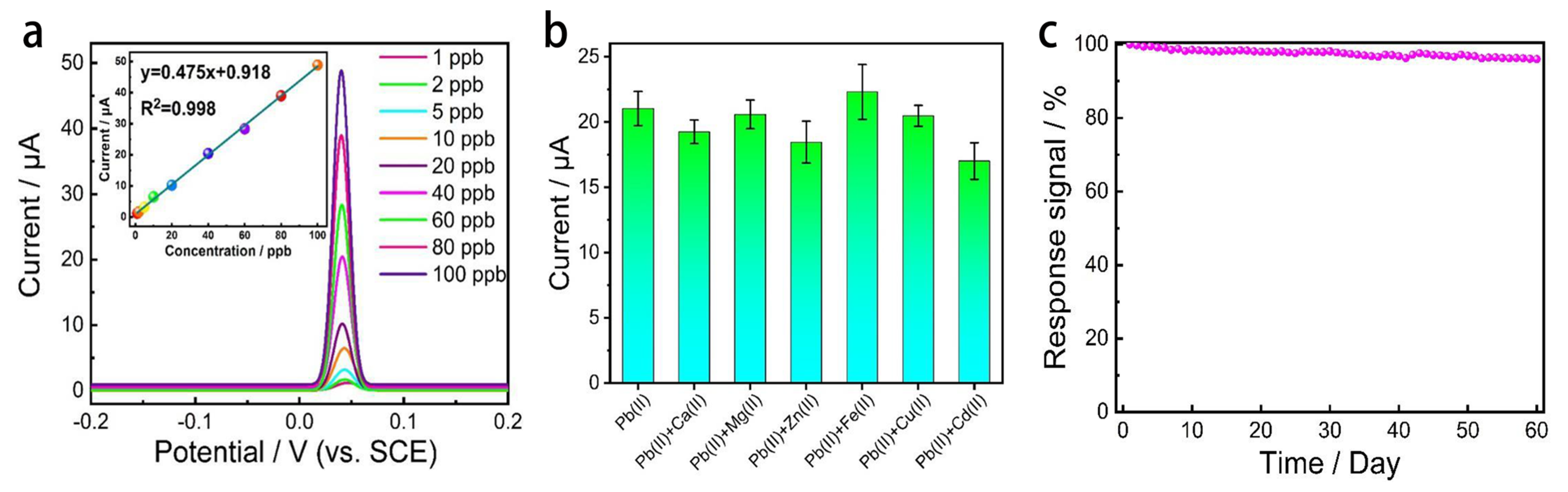
4.1. In-Situ Graphene Modified Self-Supported Boron-Doped Diamond Electrode for Pb (II) Electrochemical Detection in Seawater
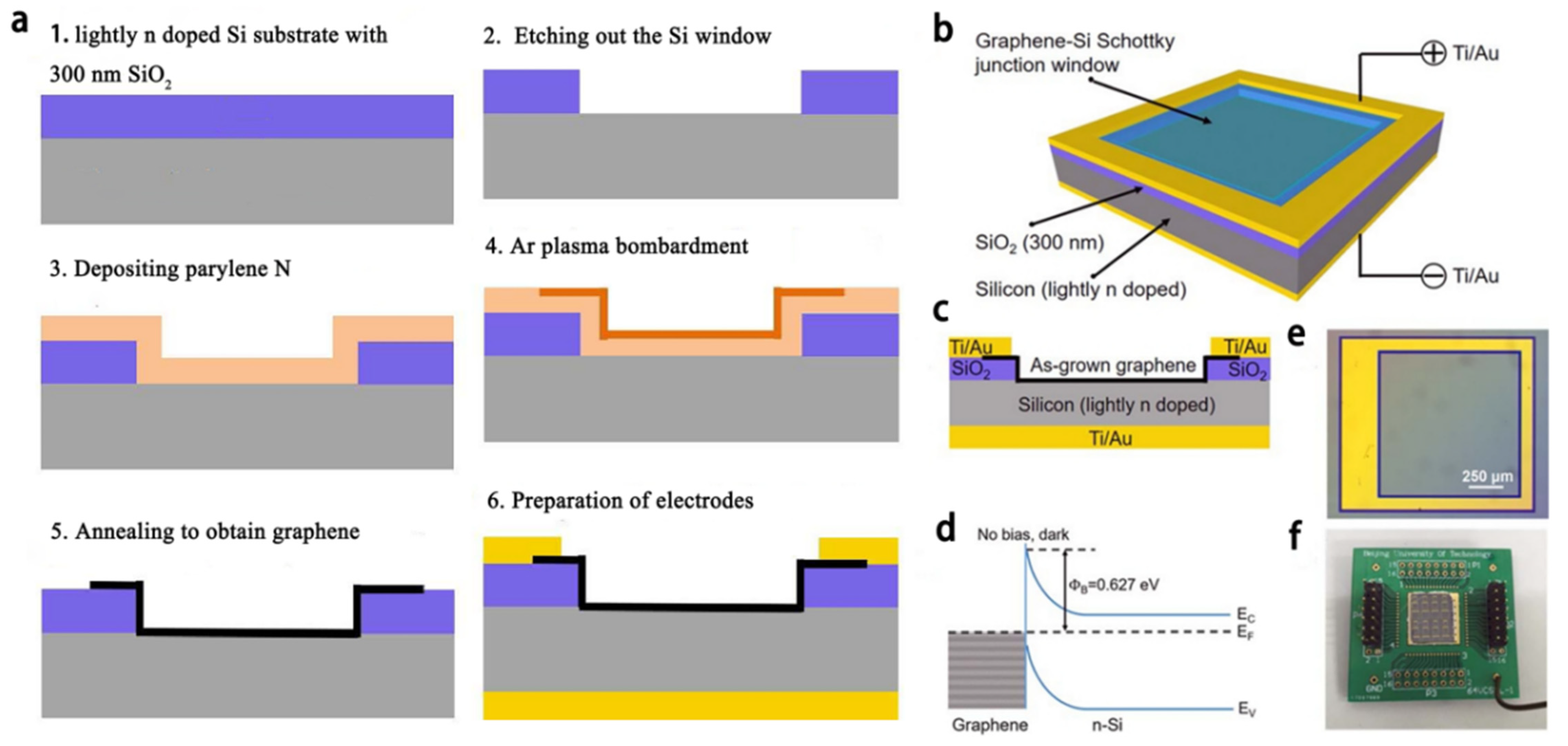
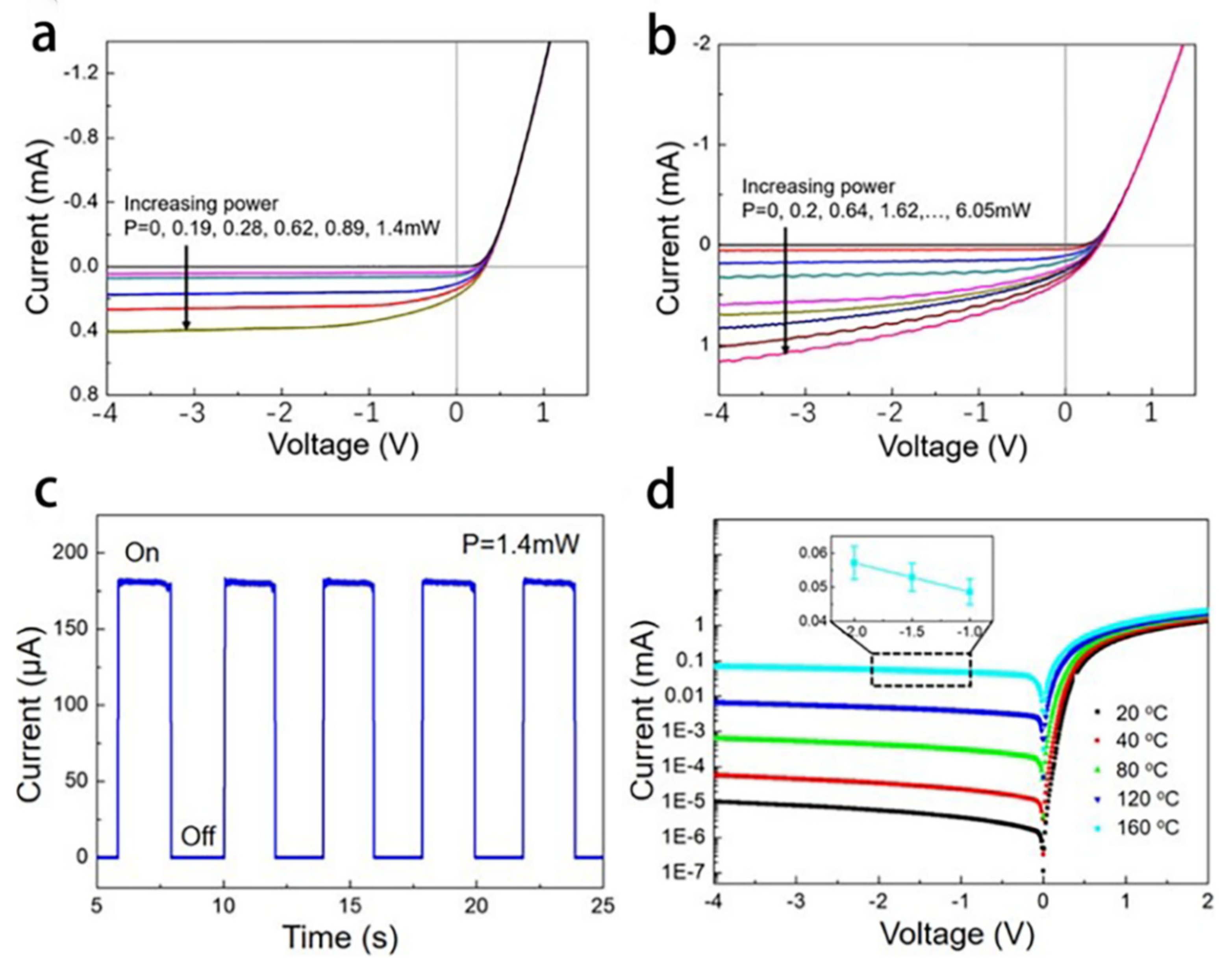
4.2. Infrared Sensor Based on Graphene-Silicone Schottky Contact for Earth Observation Mission
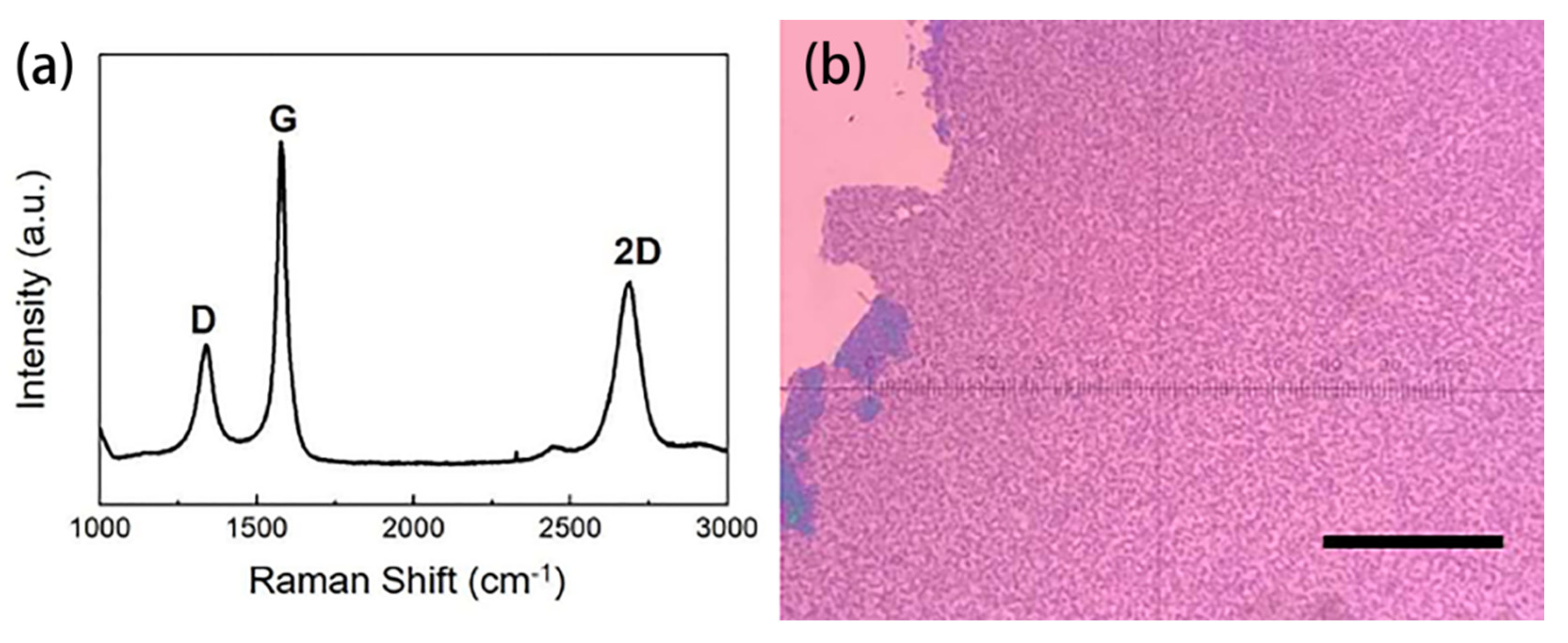
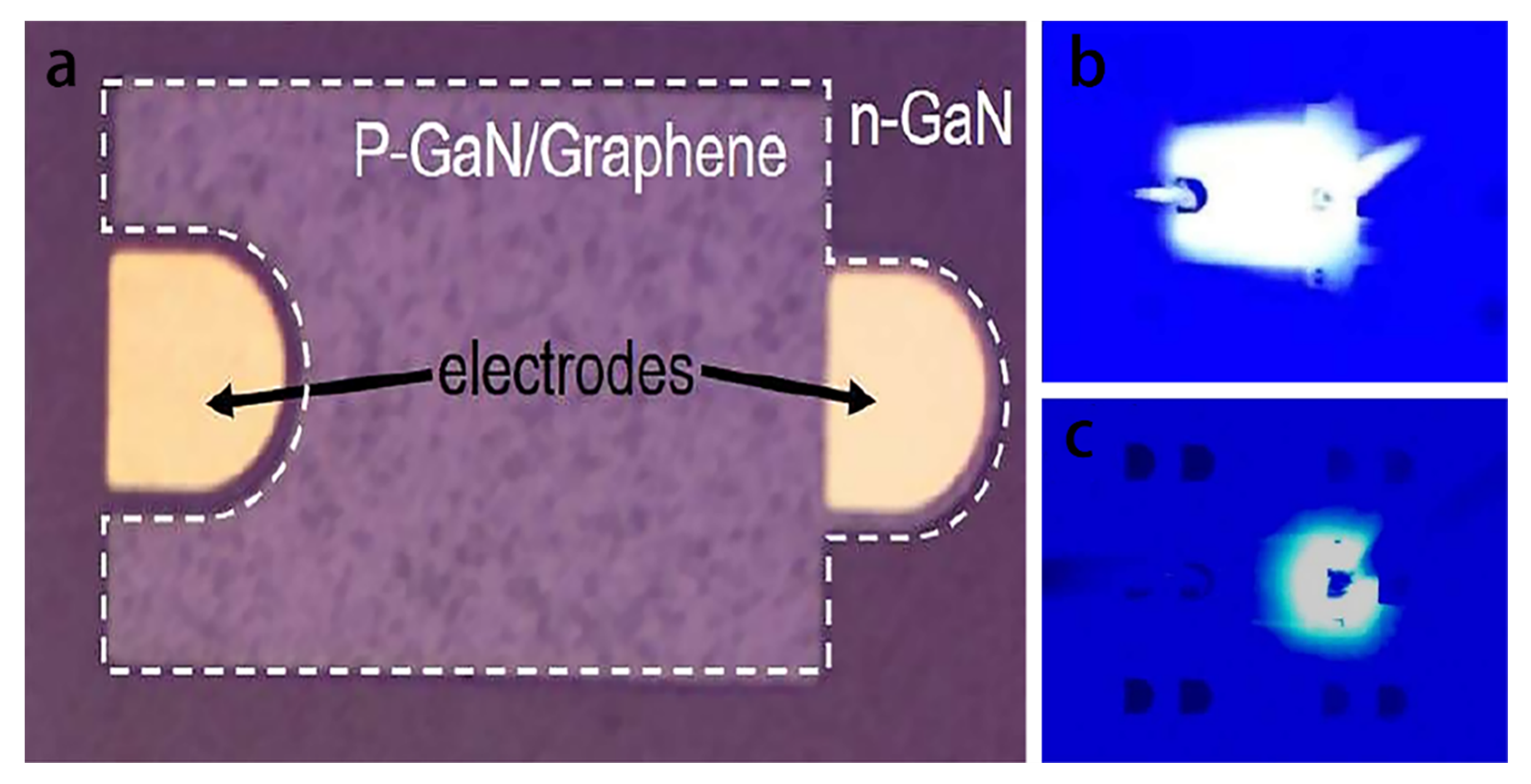
4.3. The Graphene Modified Blue LED Sensor
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Chen, Q.; Shen, H.J.; Li, G.R. Review-wearable graphene devices for sensing. J. Electrochem. Soc. 2020, 167, 037541. [Google Scholar] [CrossRef]
- Pyun, K.R.; Ko, S.H. Graphene as a material for energy generation and control: Recent progress in the control of graphene thermal conductivity by graphene defect engineering. Mater. Today Energy 2019, 12, 431–442. [Google Scholar] [CrossRef]
- Liu, J.R.; Qin, H.S.; Liu, Y.L. Multi-scale structure-mechanical property relations of graphene-based layer materials. Materials 2021, 14, 4757. [Google Scholar] [CrossRef] [PubMed]
- Anindya, N.; Arkadeep, M.; Subhas, C.M. Graphene and its sensor-based applications: A review. Sens. Actuat. A-Phys. 2018, 270, 177–194. [Google Scholar]
- Sang-Hoon, B.; Youngbin, L.; Bhupendra, K.S.; Hak-Joo, L.; Jae-Hyun, K.; Jong-Hyun, A. Graphene-based transparent strain sensor. Carbon 2013, 51, 236–242. [Google Scholar]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Lu, Y.M.; Tseng, C.F.; Lan, B.Y.; Chia-Fen, H. Fabrication of graphene/zinc oxide nano-heterostructure for hydrogen sensing. Materials 2021, 14, 6943. [Google Scholar] [CrossRef]
- Su, X.J.; Luo, C.L.; Yan, W.G.; Jiao, J.Y.; Zhong, D.Z. Microdome-tunable graphene/carbon nanotubes pressure sensors based on polystyrene array for wearable electronics. Materials 2021, 14, 7385. [Google Scholar] [CrossRef]
- López, V.; Sundaram, R.S.; Gómez-Navarro, C.; Olea, D.; Burghard, M.; Gómez-Herrero, J.; Zamora, F.; Kern, K. Chemical Vapor Deposition repair of graphene oxide: A route to highly-conductive graphene monolayers. Adv. Mater. 2009, 21, 4683–4686. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.Y.; Lei, R.B.; Lai, J.F.; Ma, T.M.; Li, Y. Highly thermally conductive graphene film produced using glucose under low-temperature thermal annealing. J. Mater. Sci. 2019, 54, 7553–7562. [Google Scholar] [CrossRef]
- Schuenemann, C.; Schaeffel, F.; Bachmatiuk, A.; Queitsch, U.; Sparing, M.; Rellinghaus, B.; Lafdi, K.; Schultz, L.; Buechner, B.; Ruemmeli, M.H. Catalyst poisoning by amorphous carbon during carbon nanotube growth: Fact or fiction? ACS Nano 2011, 5, 8928. [Google Scholar]
- Cheng, M.; Yang, R.; Zhang, L.C.; Shi, Z.W.; Yang, W.; Wang, D.M.; Xie, G.B. Restoration of graphene from graphene oxide by defect repair. Carbon 2012, 50, 2581–2587. [Google Scholar] [CrossRef]
- Qing, F.Z.; Zhang, Y.F.; Niu, Y.T.; Stehle, R.; Chen, Y.F.; Li, X.S. Towards large-scale graphene transfer. Nanoscale 2020, 12, 10890–10911. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Yu, G. Direct CVD graphene growth on semiconductors and dielectrics for transfer-free device fabrication. Adv. Mater. 2016, 28, 4956–4975. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Kang, J.W.; Hong, S.H.; Kim, N.Y.; Parka, S.J. Selective growth and in situ transfer of graphene on GaN using patterned SiO2 supporting layers. ECS J. Solid State Sci. Technol. 2015, 12, 73–76. [Google Scholar] [CrossRef][Green Version]
- Zhu, M.M.; Du, Z.H.; Yin, Z.Y.; Zhou, W.W.; Liu, Z.D.; Tsang, S.H. Low-temperature in situ growth of graphene on metallic substrates and its application in anticorrosion. ACS Appl. Mater. Interfaces 2015, 8, 502–510. [Google Scholar] [CrossRef]
- Genki, O.; Shigeki, O.; Chuhei, O.; Masahiko, S.; Tsuneo, Y.; Takanori, K. In-situ observation of graphene growth on Ni(111). Surf. Sci. 2011, 605, 1095–1098. [Google Scholar]
- Vocciante, M.; Finocchi, A.; Auris, A.D.F.D.; Conte, A.; Tonziello, J.; Pola, A.; Reverberi, A.P. Enhanced oil spill remediation by adsorption with interlinked multilayered graphene. Materials 2019, 12, 2231. [Google Scholar] [CrossRef]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The influence of graphene in improvement of physico-mechanical properties in PMMA denture base resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef]
- Kostogrud, I.A.; Boyko, E.V.; Smovzh, D.V. The main sources of graphene damage at transfer from copper to PET/EVA polymer. Mater. Chem. Phys. 2018, 219, 67–73. [Google Scholar] [CrossRef]
- Amiri, M.H.; Heidler, J.; Hasnain, A.; Anwar, S.; Lu, H.; Müllen, K.; Asadi, K. Doping free transfer of graphene using aqueous ammonia flow. RSC. Adv. 2020, 10, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Liu, W.W.; Chai, S.P.; Mohamed, A.R.; Aziz, A.; Khe, C.S.; Hidayah, N.M.; Hashim, U. Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv. 2017, 7, 15644–15693. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. The CVD graphene transfer procedure introduces metallic impurities which alter the graphene electrochemical properties. Nanoscale 2014, 6, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Hou, Y.; Liu, Y.; Khodkov, T.; Goossens, S.; Tang, J.; Wang, Y.; Yan, R.; Du, Y.; Koppens, F.H. Growth of Ultraflat Graphene with Greatly Enhanced Mechanical Properties. Nano Lett. 2020, 20, 6798. [Google Scholar] [CrossRef]
- Kang, S.; Yoon, T.; Kim, S.; Kim, T.S. Role of crack deflection on rate dependent mechanical transfer of multilayer graphene and its application to transparent electrodes. ACS Appl. Nano Mater. 2019, 2, 1980–1985. [Google Scholar] [CrossRef]
- Chandrashekar, B.N.; Smitha, A.S.; Wu, Y.C.; Cai, N.D.; Li, Y.L.; Huang, Z.Y.; Wang, W.J.; Shi, R.; Wang, J.W.; Liu, S.Y. A universal stamping method of graphene transfer for conducting flexible and transparent polymers. Sci. Rep. 2019, 9, 3999. [Google Scholar] [CrossRef]
- Ullah, S.; Yang, X.Q.; Ta, H.Q.; Hasan, M.; Bachmatiuk, A.; Tokarska, K.; Trzebicka, B.; Fu, L.; Rummeli, M.H. Graphene transfer methods: A review. Nano Res. 2021, 11, 5974. [Google Scholar] [CrossRef]
- Leong, W.S.; Wang, H.Z.; Yeo, J.J.; Martin-Martinez, F.J.; Zubair, A.; Shen, P.C.; Mao, Y.W.; Palacios, T.; Buehler, M.J.; Hong, J.Y. Paraffin-enabled graphene transfer. Nat. Commun. 2019, 10, 867. [Google Scholar] [CrossRef]
- Deng, S.K.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Ma, L.P.; Ren, W.; Cheng, H.M. Transfer methods of graphene from metal substrates: A review. Small Methods 2019, 3, 1900049. [Google Scholar] [CrossRef]
- Liu, L.L.; Qing, M.Q.; Wang, Y.B.; Chen, S.M. Defects in graphene: Generation, healing, and their effects on the properties of graphene: A review. J. Mater. Sci. Technol. 2015, 31, 599–606. [Google Scholar] [CrossRef]
- Lin, Q.J.; Zhang, F.Z.; Zhao, N.; Zhao, L.B.; Wang, Z.W.; Yang, P.; Lu, D.J.; Dong, T.; Jiang, Z.D. A flexible and wearable nylon fiber sensor modified by reduced graphene oxide and ZnO quantum dots for wide-range NO2 gas detection at room temperature. Materials 2022, 15, 3772. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Guo, Y.L.; Wen, Y.G.; Huang, L.P.; Xue, Y.Z.; Geng, D.C.; Wu, B.; Luo, B.R.; Yu, G.; Liu, Y.Q. Two-stage metal-catalyst-free growth of high-quality polycrystalline graphene films on silicon nitride substrates. Adv. Mater. 2013, 25, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.C.; Man, B.Y.; Jiang, S.Z.; Chen, C.S.; Yang, C.; Liu, M.; Gao, X.G.; Sun, Z.C.; Zhang, C. Direct synthesis of graphene on SiO2 substrates by chemical vapor deposition. CrystEngComm 2013, 15, 1840. [Google Scholar] [CrossRef]
- Shan, J.Y.; Fang, S.M.; Wang, W.D.; Zhao, W.; Zhang, R.; Liu, B.Z.; Lin, L.; Jiang, B.; Ci, H.N.; Liu, R.J. Copper acetate-facilitated transfer-free growth of high-quality graphene for hydrovoltaic generators. Natl. Sci. Rev. 2022, 9, nwab169. [Google Scholar] [CrossRef]
- Pham, V.P.; Jang, H.S.; Whang, D.; Choi, J.Y. Direct growth of graphene on rigid and flexible substrates: Progress, applications, and challenges. Chem. Soc. Rev. 2017, 46, 6276–6300. [Google Scholar] [CrossRef]
- Peng, Z.W.; Yan, Z.; Sun, Z.Z.; Tour, J.M. Direct growth of bilayer graphene on SiO2 substrates by carbon diffusion through nickel. ACS. Nano 2011, 5, 8241–8247. [Google Scholar] [CrossRef]
- Baraton, L.; He, Z.; Lee, C.S.; Maurice, J.L.; Cojocaru, C.S.; Gourgues-Lorenzon, A.F.; Lee, Y.H.; Pribat, D. Synthesis of few-layered graphene by ion implantation of carbon in nickel thin films. Nat. Nanotechnol. 2011, 22, 085601. [Google Scholar] [CrossRef]
- Fujita, J.I.; Hiyama, T.; Hirukawa, A.; Kondo, T.; Nakamura, J.; Ito, S.I.; Araki, R.; Ito, Y.; Takeguchi, M.; Pai, W.W. Near room temperature chemical vapor deposition of graphene with diluted methane and molten gallium catalyst. Sci. Rep. 2017, 7, 12371. [Google Scholar] [CrossRef]
- Kato, T.; Hatakeyama, R. Direct growth of doping-density controlled hexagonal graphene on SiO2 substrate by rapid-heating plasma CVD. ACS Nano 2012, 6, 8508–8515. [Google Scholar] [CrossRef]
- Wei, D.C.; Lu, Y.H.; Han, C.; Niu, T.C.; Chen, W.; Wee, A.T.S. Critical crystal growth of graphene on dielectric substrates at low temperature for electronic devices. Nanomaterials 2013, 52, 14121–14126. [Google Scholar]
- Riaz, S.; Park, S.J. Thermal and mechanical interfacial behaviors of graphene oxide-reinforced epoxy composites cured by thermal latent catalyst. Materials 2019, 12, 1354. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, M.A.; Yussuf, A.A.; Al-Enezi, S.; Kazemi, R.; Wahit, M.U.; Al-Shammari, T.; Al-Banna, A. Polypropylene/graphene nanocomposites: Effects of GNP loading and compatibilizers on the mechanical and thermal properties. Materials 2019, 12, 3924. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.S. A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano-Micro Lett. 2015, 8, 95–119. [Google Scholar] [CrossRef]
- Demon, S.Z.N.; Kamisan, A.I.; Abdullah, N.; Noor, S.A.M.; Khim, O.K.; Kasim, N.A.M.; Yahya, M.Z.; Manaf, N.A.A.; Azmi, A.F.M.; Halim, N.A. Graphene-based Materials in Gas Sensor Applications: A Review. Sens. Mater. 2020, 32, 759–777. [Google Scholar] [CrossRef]
- Chen, X.R.; Gao, J.; Zhao, G.Q.; Wu, C. In situ growth of FeOOH nanoparticles on physically-exfoliated graphene nanosheets as high performance H2O2 electrochemical sensor. Sens. Actuat. B-Chem. 2020, 313, 128038. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.Y.; Liu, S.; Zhang, T. In situ growth of Ag-reduced graphene oxide-carbon nanotube on indium tin oxide and its application for electrochemical sensing. Mater. Res. Bull. 2016, 84, 355–362. [Google Scholar] [CrossRef]
- Yorifuji, T. Lessons from an early-stage epidemiological study on minamata disease. J. Epidemiol. 2019, 30, 12–14. [Google Scholar] [CrossRef]
- Selvan, K.S.; Narayanan, S.S. Synthesis and characterization of carbon nanotubes/asymmetric novel tetradentate ligand forming complexes on PIGE modified electrode for simultaneous determination of Pb(II) and Hg(II) in sea water, lake water and well water using anodic stripping voltammetry. J. Electroanal. Chem. 2018, 810, 176–184. [Google Scholar]
- Memon, A.G.; Zhou, X.H.; Xing, Y.P.; Wang, R.Y.; Liu, L.H.; Khan, M.; He, M. Label-free colorimetric nanosensor with improved sensitivity for Pb2 + in water by using a truncated 8–17 DNAzyme. Front. Environ. Sci. Eng. 2019, 13, 125–130. [Google Scholar] [CrossRef]
- Hanna, A.M.; La, C.J.; Sadler, R.C.; Champney, S.A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am. J. Public Health 2016, 106, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.Y.; Li, T.J.; Hu, J.W.; Cao, R.S.; Wu, Q.; Wei, X.H.; Li, L.Y.; Shi, X.D.; Ruan, W.Q. Synthesis and characterization of reduced graphene oxide-supported nanoscale zero-valent Iron (nZVI/rGO) composites used for Pb(II) removal. Materials 2016, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.X.; Yu, X.; Zhang, Z.Q.; Zhang, J.; Wei, S.B.; Boukherroub, R. In-situ graphene modified self-supported boron-doped diamond electrode for Pb (II) electrochemical detection in seawater. Appl. Surf. Sci. 2020, 527, 146761. [Google Scholar] [CrossRef]
- Bernal, M.M.; Tortello, M.; Colonna, S.; Saracco, G.; Fina, A. Thermally and electrically conductive nanopapers from reduced graphene oxide: Effect of nanoflakes thermal annealing on the film structure and properties. Nanomaterials 2017, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Sultan, S.; Zahid, A.; Aftab, S.; Nisar, J.; Nayab, S.; Qureshi, R.; Khan, G.S.; Hussain, H.; Ozkan, S.A. Highly sensitive and selective electrochemical sensor for the trace level detection of mercury and cadmium. Electrochim. Acta 2017, 258, 1397–1403. [Google Scholar] [CrossRef]
- He, Y.; Xiao, S.; Nie, P.; Dong, T.; Qu, F.; Lin, L. Research on the optimum water content of detecting soil nitrogen wsing near infrared sensor. Sensors 2017, 17, 2045. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Hou, K.X.; Zhu, W.T.; Wang, X.; Yang, X.; Dong, R.H.; Cheng, H.J.; Guo, L.J.; Liu, X.L. Human respiratory monitoring based on Schottky resistance humidity sensors. Materials 2020, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.P.; Li, Z. Schottky-contacted nanowire sensors. Adv. Mater. 2020, 32, 2000130. [Google Scholar] [CrossRef]
- Singh, A.; Uddin, M.A.; Sudarshan, T.; Koley, G. Tunable reverse-biased graphene/silicon heterojunction Schottky diode sensor. Small 2013, 10, 1555–1565. [Google Scholar] [CrossRef]
- Biswas, M.R.U.D.; Oh, W.C. Comparative study on gas sensing by a Schottky diode electrode prepared with graphene–semiconductor–polymer nanocomposites. RSC Adv. 2019, 9, 11484–11492. [Google Scholar] [CrossRef]
- Heber, J. Nobel Prize 2014: Akasaki, Amano & Nakamura. Nat. Phys. 2014, 10, 791. [Google Scholar]
- Cho, E.C.; Huang, J.H.; Li, C.P.; Chang-Jian, C.W.; Lee, K.C.; Hsiao, Y.S.; Huang, J.H. Graphene-based thermoplastic composites and their application for LED thermal management. Carbon 2016, 102, 66–73. [Google Scholar] [CrossRef]
- Tang, P.H.; Xiong, F.Z.; Du, Z.F.; Li, K.; Mei, Y.; Guo, W.L.; Sun, J. Nickel-assisted transfer-free technology of graphene chemical vapor deposition on GaN for improving the electrical performance of light-emitting diodes. Crystals 2022, 12, 1497. [Google Scholar] [CrossRef]
- Filintoglou, K.; Pinakidou, F.; Arvanitidis, J.; Christofilos, D.; Paloura, E.C. Size control of GaN nanocrystals formed by ion implantation in thermally grown silicon dioxide. J. Appl. Phys. 2020, 127, 034302. [Google Scholar] [CrossRef]
- Reddy, V.R.; Reddy, P.R.S.; Reddy, I.N.; Choi, C.J. Microstructural, electrical and carrier transport properties of Au/NiO/n-GaN heterojunction with a nickel oxide interlayer. RSC Adv. 2016, 6, 105761–105770. [Google Scholar] [CrossRef]


| Growth Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| CVD method | Mature technology Large-area film Good controllability | Complex transfer process | [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] |
| Metal-free growth | No substrate selectivity | Long period High working temperature Poor quality | [33,34] |
| Metal-assisted growth | Fast growth Good quality Graphical growth | Complex and high-cost Metal pollution | [35,36,37,38,39] |
| Plasma-enhanced growth | Low working temperature | Poor controllability | [40,41] |
| Device structure | Material | Responsivity | Specific Detection Rate | Light Source |
|---|---|---|---|---|
| Graphene/planar silicon | Grown in situ | 267.3 mA/W (−2Vbias voltage) | 4.93 × 109 | 792 nm laser |
| Graphene/planar silicon | Transferred graphene | 225 mA/W (−2Vbias voltage) | 7.69 × 109 | 488 nm laser |
| Graphene/planar silicon | Transferred graphene | 3.259 μA/KGy cm2 (1Vbias voltage) | _ | γray |
| Graphene/planar silicon | Transferred graphene | 83 A/W (10Vbias voltage) | 108 | 1.55 μm laser |
| Graphene oxide/silicon nanowires | Transferred graphene | 9.0 mA/W (1Vbias voltage) | _ | 10.6 μm laser |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wu, L.; Yu, X.; Peng, H.; Xu, S.; Zhou, Z. In-Situ Growth of Graphene Films to Improve Sensing Performances. Materials 2022, 15, 7814. https://doi.org/10.3390/ma15217814
Liu X, Wu L, Yu X, Peng H, Xu S, Zhou Z. In-Situ Growth of Graphene Films to Improve Sensing Performances. Materials. 2022; 15(21):7814. https://doi.org/10.3390/ma15217814
Chicago/Turabian StyleLiu, Xinghong, Liang Wu, Xiang Yu, Haoran Peng, Shijue Xu, and Zilong Zhou. 2022. "In-Situ Growth of Graphene Films to Improve Sensing Performances" Materials 15, no. 21: 7814. https://doi.org/10.3390/ma15217814
APA StyleLiu, X., Wu, L., Yu, X., Peng, H., Xu, S., & Zhou, Z. (2022). In-Situ Growth of Graphene Films to Improve Sensing Performances. Materials, 15(21), 7814. https://doi.org/10.3390/ma15217814







