Effect of Secondary-Phase Precipitation on Mechanical Properties and Corrosion Resistance of 00Cr27Ni7Mo5N Hyper-Duplex Stainless Steel during Solution Treatment
Abstract
1. Introduction
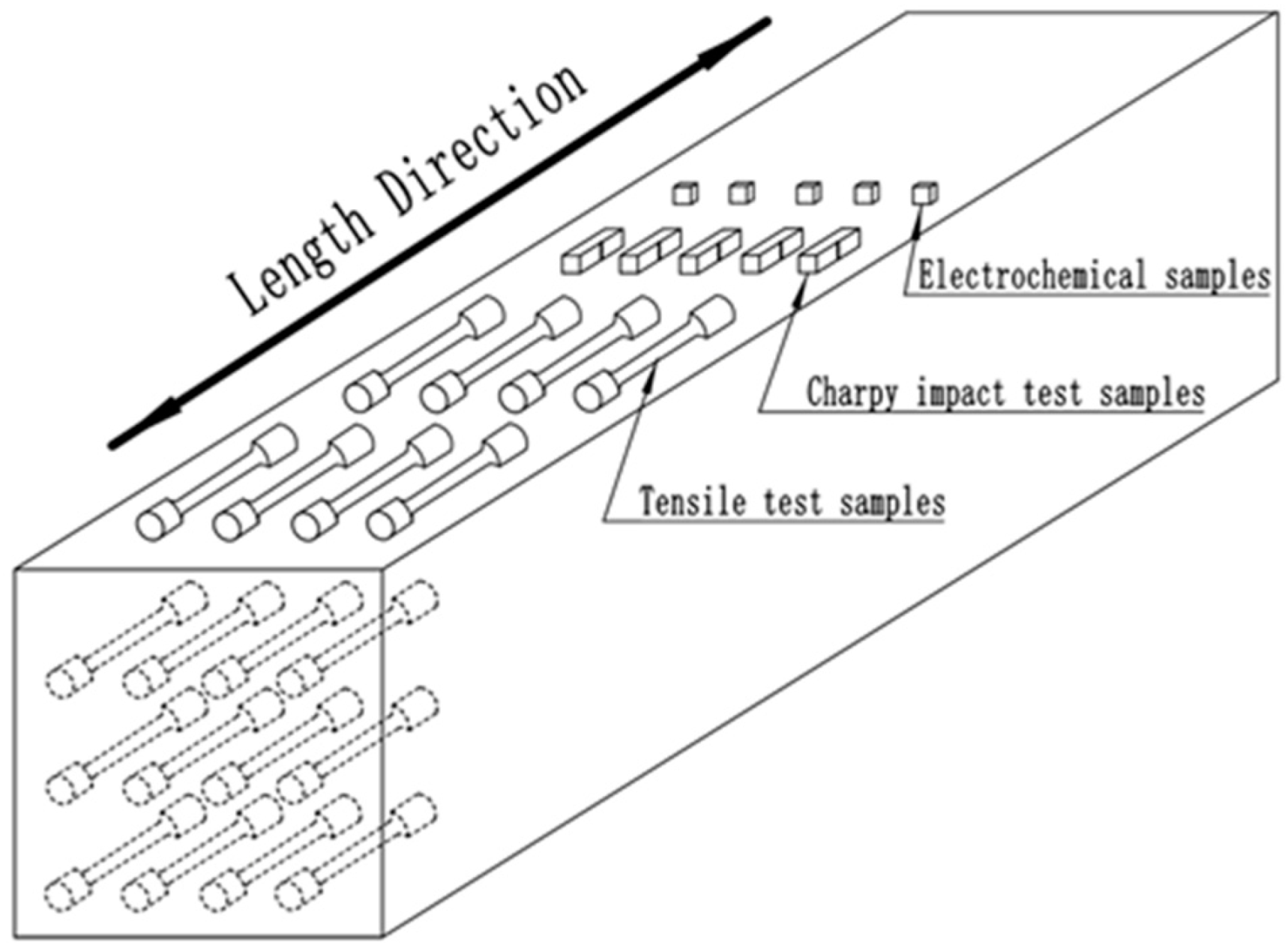
2. Materials and Methods
3. Results and Discussion
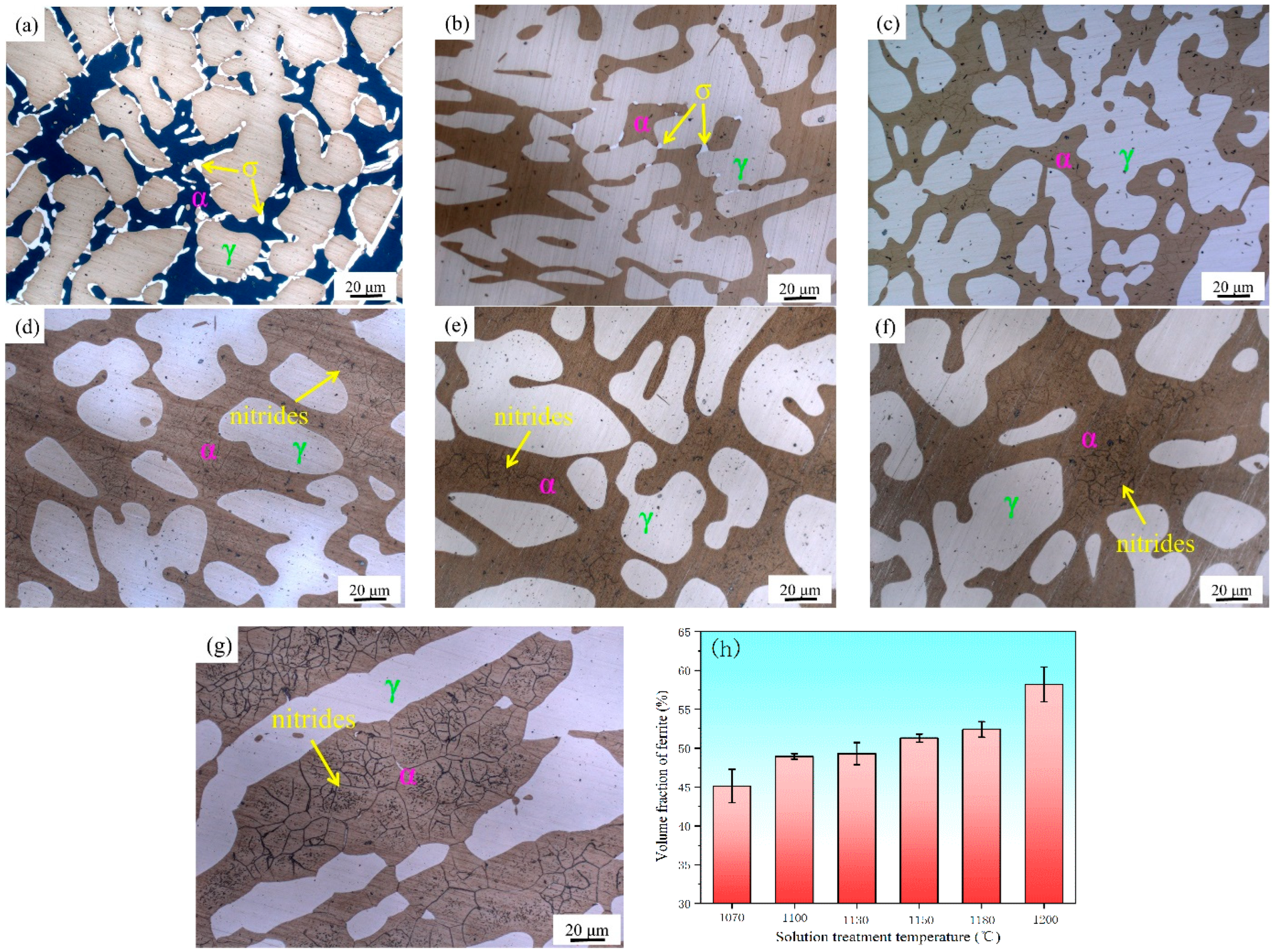
3.1. Effect of Solution Treatment Temperatures on Microstructure
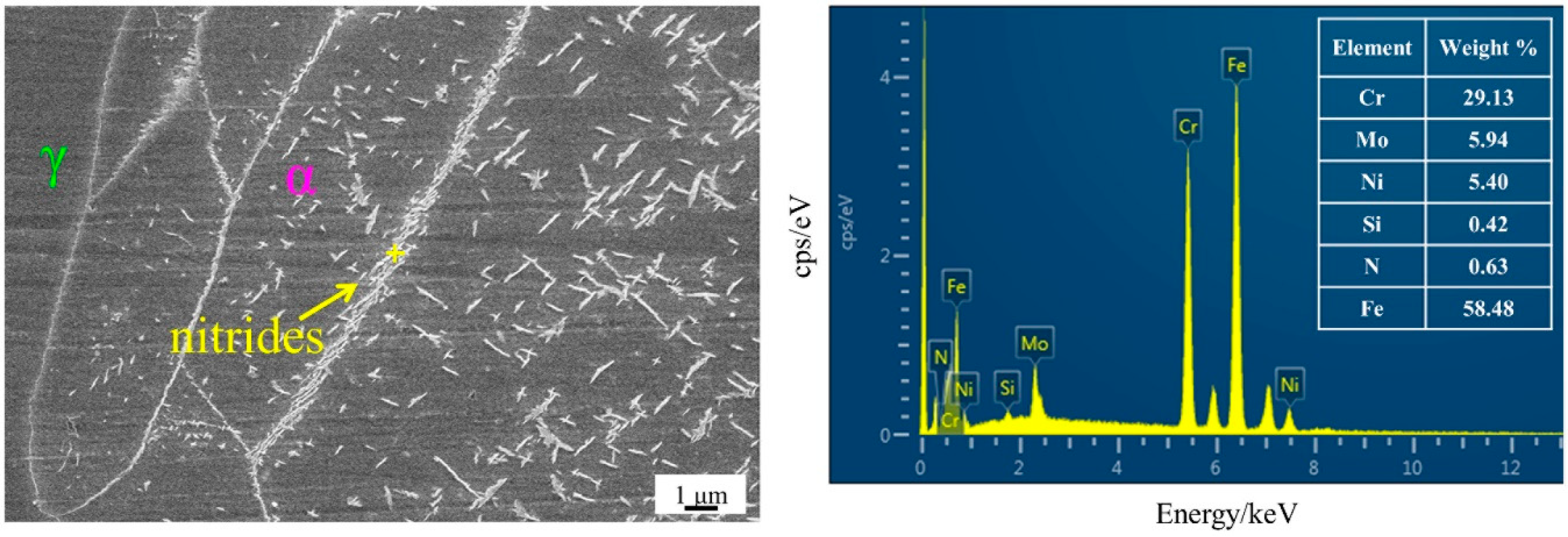
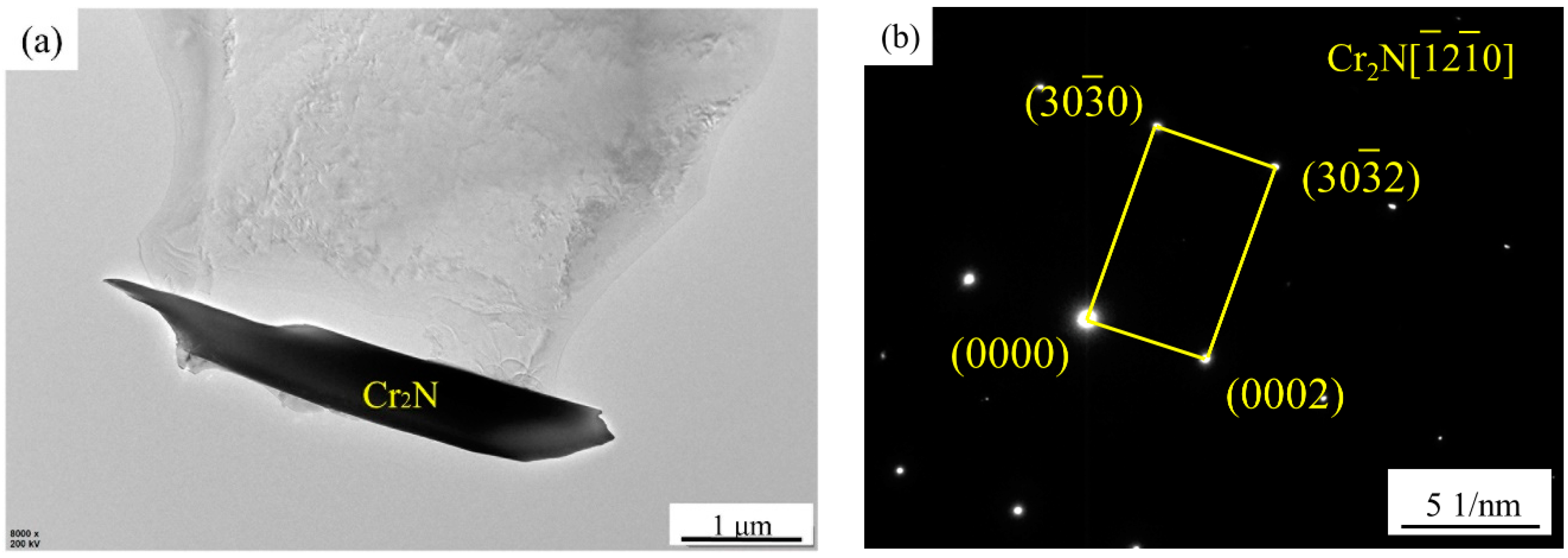
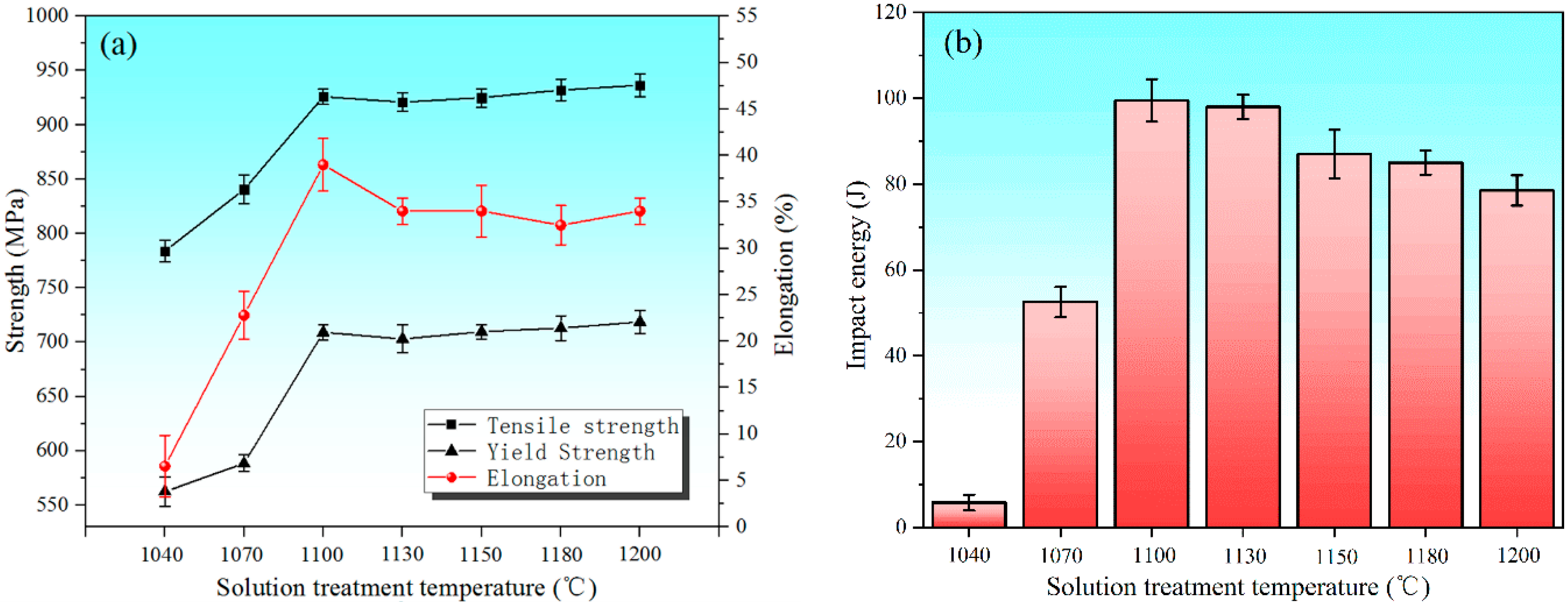
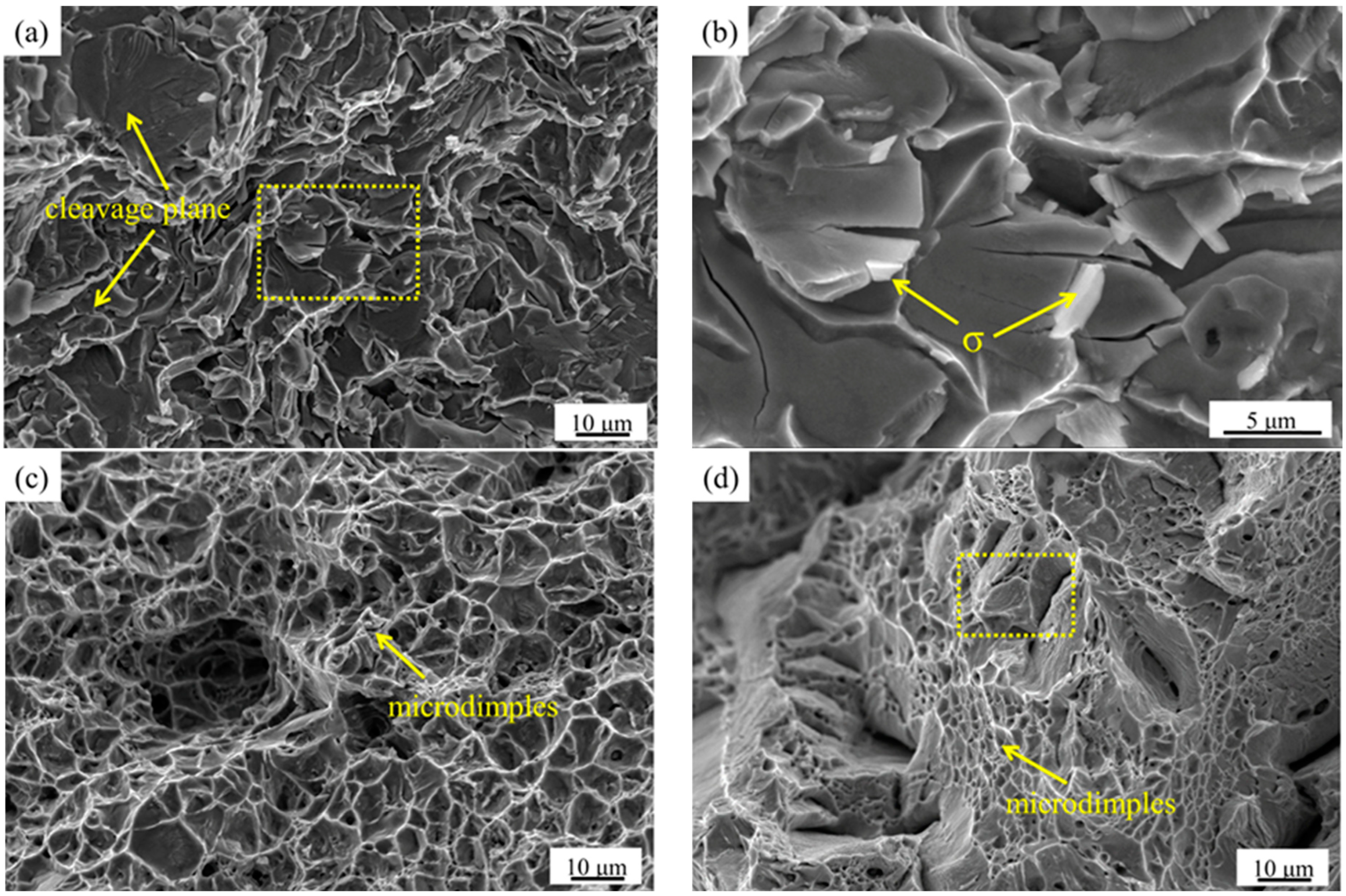
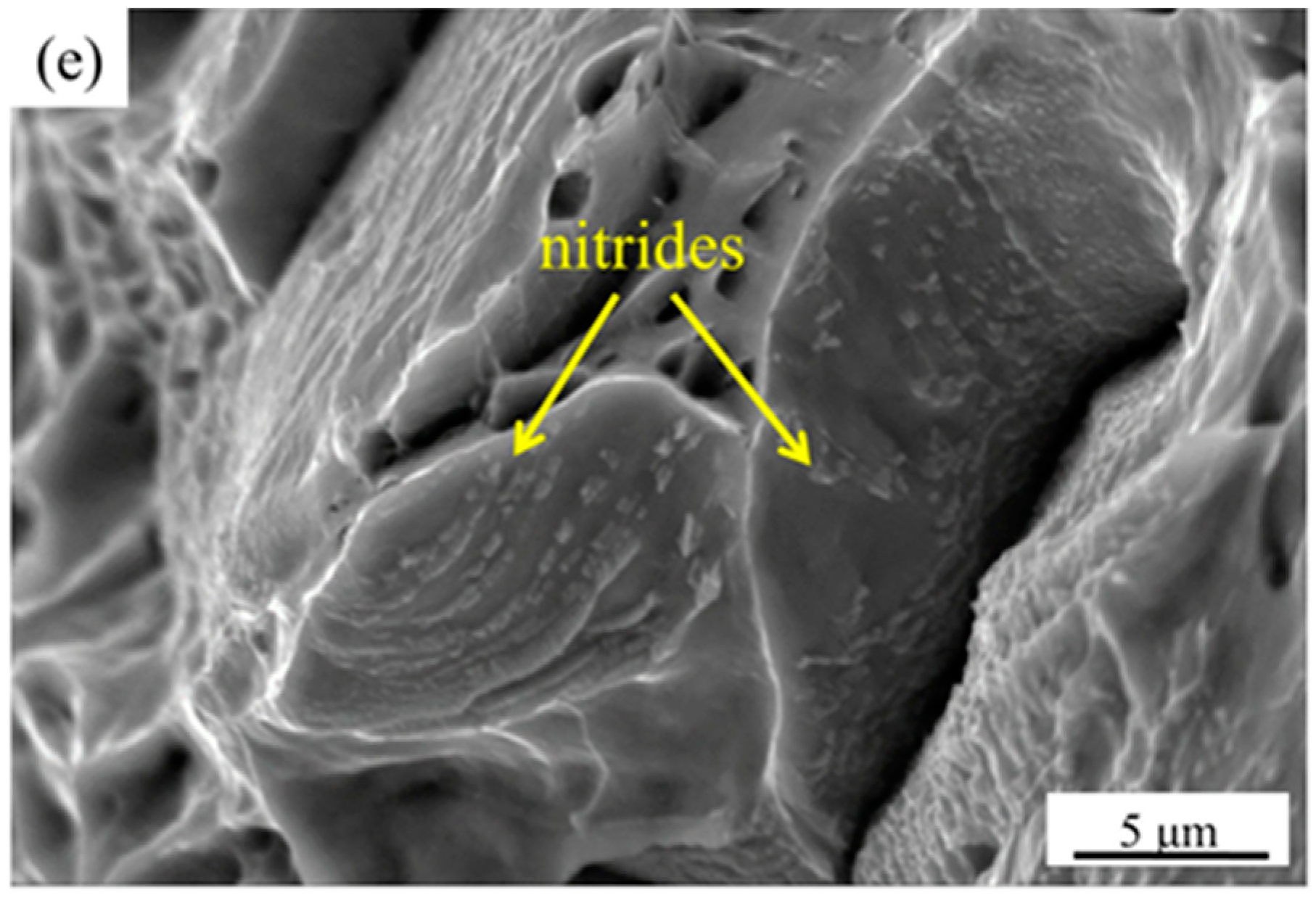
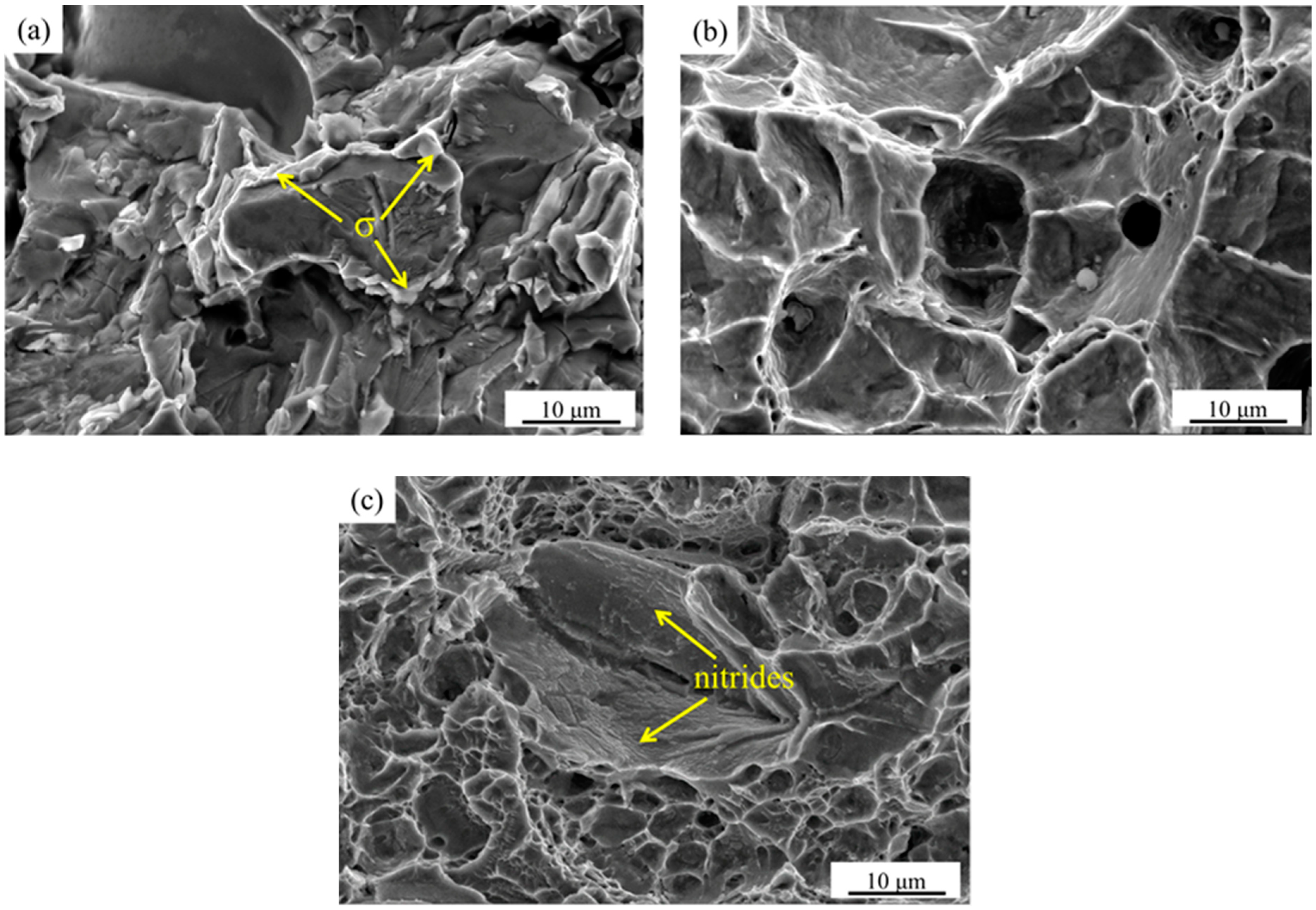
3.2. Effect of Secondary-Phase Precipitation on Mechanical Properties
3.3. Effect of Secondary-Phase Precipitation on the Resistance to Corrosion Resistance
4. Conclusions
- The best corrosion resistance of 00Cr27Ni7Mo5N HDSS can be obtained when it is solution-treated at 1100 °C. At the same time, it exhibits excellent mechanical properties, which can be better applied to severe corrosive working conditions.
- When the solution treatment temperature of the sample is lower than 1070 °C, σ phase exists in the interface between the α and γ phase, which is brittle and prone to create a Cr-depleted zone around it, significantly deteriorating the mechanical properties.
- There is no secondary-phase precipitation of the sample after solution treatment at 1100 °C. The yield strength and tensile strength increase slightly, but the plastic toughness decreases with the solution treatment temperature increasing from 1100 to 1200 °C, which can be explained by the volume fraction of α phase and the precipitation of Cr2N. The precipitation of Cr2N does not deteriorate the mechanical properties as significantly as the σ phase.
- With the increase in the solution treatment temperature, the corrosion current densities first decrease and then increase, reaching the lowest value at 1100 °C. The precipitation of σ phase and Cr2N can cause the generation of Cr-depleted zones around them, thus triggering selective corrosion and leading to a significant increase in pitting corrosion susceptibility.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nilsson, J.O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Zmitrowicz, P.; Kawiak, M.; Kochmański, P.; Baranowska, P. Microstructure and mechanical properties of welded joints of 1.4462 duplex steel made by the K-TIG method. Materials 2021, 14, 7868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Wang, C.Y.; Reddy, K.M.; Li, W.; Wang, X.D. Study on the deformation mechanism of a high-nitrogen duplex stainless steel with excellent mechanical properties originated from bimodal grain design. Acta Mater. 2022, 226, 117670. [Google Scholar] [CrossRef]
- NithinRaj, P.; Navaneethkrishnan, P.K.; Sekar, K.; Joseph, M.A. Comparative study of mechanical, corrosion and erosion-corrosion properties of cast hyper-duplex and super-duplex stainless steels. Int. J. Miner. Metall. Mater. 2020, 27, 954–961. [Google Scholar]
- Silva, R.; Vacchi, G.S.; Kugelmeier, C.L.; Santos, I.G.R.; Mendes Filho, A.A.; Magalhães, D.C.C.; Afonso, C.R.M.; Sordi, V.L.; Rovere, C.A.D. New insights into the hardening and corrosion resistance mechanisms of thermally aged duplex stainless steel at 475 °C: A comparative study between 2205 and 2101 steels. J. Mater. Sci. Technol. 2022, 98, 123–135. [Google Scholar] [CrossRef]
- Zhang, B.B.; Jiang, Z.H.; Li, H.B.; Zhang, S.C.; Feng, H.; Li, H. Precipitation behavior and phase transformation of hyper duplex stainless steel UNS S32707 at nose temperature. Mater. Charact. 2017, 129, 31–39. [Google Scholar] [CrossRef]
- Escriba, D.M.; Materna-Morris, E.; Plaut, R.L.; Padilha, A.F. Chi-phase precipitation in a duplex stainless steel. Mater. Charact. 2009, 60, 1214–1219. [Google Scholar] [CrossRef]
- Silva, R.; Kugelmeier, C.L.; Vacchi, G.S.; Martins Junior, C.B.; Dainezi, I.; Afonsoa, C.R.M.; Mendes Filho, A.A.; Rovere, C.A.D. A comprehensive study of the corrosion resistance mechanism of lean duplex stainless steel grade 2404 aged at 475 °C. Corros. Sci. 2021, 191, 109738. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandström, R. Sigma phase precipitation in duplex stainless steel 2205. Mater. Sci. Eng. A 2007, 444, 271–276. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Mondal, S. High temperature ageing behaviour of a duplex stainless steel. Mater. Charact. 2008, 59, 1776–1783. [Google Scholar] [CrossRef]
- Chen, T.H.; Yang, J.R. Effects of solution treated and continuous cooling on sigma-phase precipitation in a 2205 duplex stainless steel. Mater. Sci. Eng. A 2001, 311, 28–41. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson, R.F.A.; Wessman, S. Precipitation of chromium nitrides in the super duplex stainless steel 2507. Metall. Mater. Trans. A 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Zhang, S.C.; Li, H.B.; Jiang, Z.H.; Li, Z.X.; Wu, J.X.; Zhang, B.B.; Duan, F.; Feng, H.; Zhu, H.C. Influence of N on precipitation behavior, associated corrosion and mechanical properties of super austenitic stainless steel S32654. J. Mater. Sci. Technol. 2020, 42, 143–155. [Google Scholar] [CrossRef]
- Liang, X.Z.; Dodge, M.F.; Liang, W.; Dong, H.B. Precipitation of chromium nitride nano-rods on lamellar carbides along austenite-ferrite boundaries in super duplex stainless steel. Scr. Mater. 2017, 127, 45–48. [Google Scholar] [CrossRef]
- Lopez, N.; Cid, M.; Puiggali, M. Influence of σ-phase on mechanical properties and corrosion resistance of duplex stainless steels. Corros. Sci. 1999, 41, 1615–1631. [Google Scholar] [CrossRef]
- Xiang, H.L.; Liu, C.Y.; Deng, L.P.; Zheng, K.K. Effect of aging temperature on the microstructure and properties of economical duplex stainless steel. Materials 2019, 12, 2085. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.W.; Kim, H.B.; Park, C.N.; Choi, Y.I.; Park, C.J. Effects of the precipitation of secondary phases on the erosion-corrosion of 25% Cr duplex stainless steel. Corros. Sci. 2019, 152, 202–210. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.J.; Cui, W.F.; Liu, C.M. Precipitation kinetics of Cr2N in high nitrogen austenitic stainless steel. J. Iron Steel Res. Int. 2008, 15, 72–77. [Google Scholar] [CrossRef]
- Zhang, B.B.; Li, H.B.; Zhang, S.C.; Jiang, Z.H.; Lin, Y.; Feng, H.; Zhu, H.C. Effect of nitrogen on precipitation behavior of hyper duplex stainless steel S32707. Mater. Charact. 2021, 175, 111096. [Google Scholar] [CrossRef]
- Guo, Y.J.; Hu, J.C.; Li, J.; Jiang, L.Z.; Liu, T.W.; Wu, Y.P. Effect of annealing temperature on the mechanical and corrosion behavior of a newly developed novel lean duplex stainless steel. Materials 2014, 7, 6604–6619. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zhang, W.; Jiang, Y.M.; Deng, B.; Sun, D.M.; Li, J. Influence of annealing treatment on the corrosion resistance of lean duplex stainless steel 2101. Electrochim. Acta 2009, 54, 5387–5392. [Google Scholar] [CrossRef]
- Guo, L.Q.; Li, M.; Shi, X.L.; Yan, Y.; Li, X.Y.; Qiao, L.J. Effect of annealing temperature on the corrosion behavior of duplex stainless steel studied by in situ techniques. Corros. Sci. 2011, 53, 3733–3741. [Google Scholar] [CrossRef]
- Lee, T.H.; Ha, H.Y.; Hwang, B.C.; Kim, S.J. Isothermal decomposition of ferrite in a high-nitrogen nickel-free duplex stainless steel. Metall. Mater. Trans. A 2012, 43, 822–832. [Google Scholar] [CrossRef][Green Version]
- Chan, K.W.; Tjong, S.C. Effect of secondary phase precipitation on the corrosion behavior of duplex stainless steels. Materials 2014, 7, 5268–5304. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.C.; Magnabosco, R. Kinetic study to predict sigma phase formation in duplex stainless steels. Metall. Mater. Trans. A 2016, 47, 1554–1565. [Google Scholar] [CrossRef]
- Wu, J. Duplex Stainless Steel, 1st ed.; Metallurgical Industry Press: Beijing, China, 1999; p. 8. [Google Scholar]
- Deng, B.; Jiang, Y.M.; Gao, J.; Li, J. Effect of annealing treated on microstructure evolution and the associated corrosion behavior of a super-duplex stainless steel. J. Alloys. Compd. 2010, 493, 461–464. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fujimatsu, T.; Tsunekage, N.; Hiraoka, K.; Kida, K.; Santos, E.C. Study of rolling contact fatigue of bearing steels in relation to various oxide inclusions. Mater. Des. 2011, 32, 1605–1611. [Google Scholar] [CrossRef]
- Liu, H.H.; Fu, P.X.; Liu, H.W.; Cao, Y.F.; Sun, C.; Du, N.Y.; Li, D.Z. Effects of Rare Earth elements on microstructure evolution and mechanical properties of 718H pre-hardened mold steel. J. Mater. Sci. Technol. 2020, 50, 245–256. [Google Scholar] [CrossRef]
- Wang, F.P.; Kang, W.L.; Jing, H.P. Principles, Methods and Applications of Corrosion Electrochemistry, 1st ed.; Chemical Industry Press: Beijing, China, 2008; pp. 145–165. [Google Scholar]
- Garfias-Mesias, L.F.; Sykes, J.M.; Tuck, C.D.S. The effect of phase compositions on the pitting corrosion of 25 Cr duplex stainless steel in chloride solutions. Corros. Sci. 1996, 38, 1319–1330. [Google Scholar] [CrossRef]
- Migiakis, K.; Papadimitriou, G.D. Effect of nitrogen and nickel on the microstructure and mechanical properties of plasma welded UNS S32760 super-duplex stainless steels. J. Mater. Sci. 2009, 44, 6372–6383. [Google Scholar] [CrossRef]
- Suter, T.; Böhni, H. Microelectrodes for corrosion studies in microsystems. Electrochim. Acta 2001, 47, 191–199. [Google Scholar] [CrossRef]
- Szummer, A.; Janik-Czachor, M.; Hofmann, S. Discontinuity of the passivating film at nonmetallic inclusions in stainless steels. Mater. Chem. Phys. 1993, 34, 181–183. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Choi, M.S.; Kim, J.S.; Kim, K.T.; Park, Y.S. Effects of cerium on the compositional variations in and around inclusions and the initiation and propagation of corrosion resistance in hyper duplex stainless steels. Corros. Sci. 2013, 75, 367–375. [Google Scholar] [CrossRef]




| Solution Temperatures (°C) | Phase | Volume Fraction (%) | Chemical Compositions (Mass %) | PREN 1 | ΔPREN PREN(γ)-PREN(α) | ||
|---|---|---|---|---|---|---|---|
| Cr | Mo | N | |||||
| 1040 | Ferrite (α) | 27.2 | 28.91 | 6.26 | 0.05 | 51.1 | 7.6 |
| Austenite (γ) | 57.8 | 25.49 | 3.44 | 0.73 | 58.7 | ||
| 1100 | Ferrite (α) | 49.3 | 28.48 | 6.02 | 0.05 | 49.9 | 10.4 |
| Austenite (γ) | 50.7 | 25.52 | 3.46 | 0.78 | 60.3 | ||
| 1200 | Ferrite (α) | 58.2 | 27.63 | 5.76 | 0.05 | 48.1 | 11.4 |
| Austenite (γ) | 41.8 | 25.55 | 3.57 | 0.74 | 59.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, A.; Li, C.; Yu, X.; Xie, J.; Liu, C. Effect of Secondary-Phase Precipitation on Mechanical Properties and Corrosion Resistance of 00Cr27Ni7Mo5N Hyper-Duplex Stainless Steel during Solution Treatment. Materials 2022, 15, 7533. https://doi.org/10.3390/ma15217533
Wang H, Wang A, Li C, Yu X, Xie J, Liu C. Effect of Secondary-Phase Precipitation on Mechanical Properties and Corrosion Resistance of 00Cr27Ni7Mo5N Hyper-Duplex Stainless Steel during Solution Treatment. Materials. 2022; 15(21):7533. https://doi.org/10.3390/ma15217533
Chicago/Turabian StyleWang, Hang, Aiqin Wang, Changyi Li, Xingsheng Yu, Jingpei Xie, and Chenlu Liu. 2022. "Effect of Secondary-Phase Precipitation on Mechanical Properties and Corrosion Resistance of 00Cr27Ni7Mo5N Hyper-Duplex Stainless Steel during Solution Treatment" Materials 15, no. 21: 7533. https://doi.org/10.3390/ma15217533
APA StyleWang, H., Wang, A., Li, C., Yu, X., Xie, J., & Liu, C. (2022). Effect of Secondary-Phase Precipitation on Mechanical Properties and Corrosion Resistance of 00Cr27Ni7Mo5N Hyper-Duplex Stainless Steel during Solution Treatment. Materials, 15(21), 7533. https://doi.org/10.3390/ma15217533





