Adsorption and Surface Analysis of Sodium Phosphate Corrosion Inhibitor on Carbon Steel in Simulated Concrete Pore Solution
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Gjørv, O.E. Diffusion behavior of chloride ions in concrete. Cem. Concr. Res. 1996, 26, 907–917. [Google Scholar] [CrossRef]
- Garcés, P.; Saura, P.; Méndez, A.; Zornoza, E.; Andrade, C. Effect of nitrite in corrosion of reinforcing steel in neutral and acid solutions simulating the electrolytic environments of micropores of concrete in the propagation period. Corros. Sci. 2008, 50, 498–509. [Google Scholar] [CrossRef]
- Espinosa, R.M.; Franke, L.; Deckelmann, G. Model for the mechanical stress due to the salt crystallization in porous materials. Constr. Build. Mater. 2008, 22, 1350–1367. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Criado, M.; La Iglesia, V.M.; Fajardo, S.; La Iglesia, A.; Bastidas, J.M. Comparative study of three sodium phosphates as corrosion inhibitors for steel reinforcements. Cem. Concr. Compos. 2013, 43, 31–38. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Ormellese, M. Recent advances in the use of inhibitors to prevent chloride-induced corrosion in reinforced concrete. Cem. Concr. Res. 2022, 154, 106719. [Google Scholar] [CrossRef]
- Ormellese, M.; Berra, M.; Bolzoni, F.; Pastore, T. Corrosion inhibitors for chlorides induced corrosion in reinforced concrete structures. Cem. Concr. Res. 2006, 36, 536–547. [Google Scholar] [CrossRef]
- Mohamed, A.; Visco, D.P.; Bastidas, D.M. Effect of cations on the activity coefficient of NO2−/NO3− corrosion inhibitors in simulated concrete pore solution: An electrochemical thermodynamics study. Corros. Sci. 2022, 206, 110476. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Martin, U.; Bastidas, J.M.; Ress, J. Corrosion inhibition mechanism of steel reinforcements in mortar using soluble phosphates: A critical review. Materials 2021, 14, 6168. [Google Scholar] [CrossRef]
- Yohai, L.; Schreiner, W.; Valcarce, M.B.; Vázquez, M. Inhibiting steel corrosion in simulated concrete with low phosphate to chloride ratios. J. Electrochem. Soc. 2016, 163, C729–C737. [Google Scholar] [CrossRef]
- Söylev, T.A.; Richardson, M.G. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Mohamed, A.; Visco, D.P.; Bastidas, D.M. Significance of π–electrons in the design of corrosion inhibitors for carbon steel in simulated concrete pore solution. Corrosion 2021, 77, 976–990. [Google Scholar] [CrossRef]
- ASTM G106-89; Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. ASTM Internationa: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM International: West Conshohocken, PA, USA, 2009. [CrossRef]
- Lorenz, W.J.; Mansfeld, F. Interface and interphase corrosion inhibition. Electrochim. Acta 1986, 31, 467–476. [Google Scholar] [CrossRef]
- Liu, D.; Song, Y.; Shan, D.; Han, E.H. Self-healing coatings prepared by loading interphase inhibitors into MAO coating of AM60 Mg alloy. J. Electrochem. Soc. 2018, 165, C412–C421. [Google Scholar] [CrossRef]
- Macdonald, D.D. Some advantages and pitfalls of electrochemical impedance spectroscopy. Corrosion 1990, 46, 229–242. [Google Scholar] [CrossRef]
- Urquidi-Macdonald, M.; Real, S.; Macdonald, D.D. Applications of Kramers—Kronig transforms in the analysis of electrochemical impedance data—III. Stability and linearity. Electrochim. Acta 1990, 35, 1559–1566. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J. Corrosion resistance of carbon steel in alkaline concrete pore solutions containing phytate and chloride ions. Corros. Sci. 2022, 205, 110451. [Google Scholar] [CrossRef]
- Wang, D.; Ming, J.; Shi, J. Enhanced corrosion resistance of rebar in carbonated concrete pore solutions by Na2HPO4 and benzotriazole. Corros. Sci. 2020, 174, 108830. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pébère, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric properties of materials showing constant-phase-element (CPE) impedance response. J. Electrochem. Soc. 2013, 160, C215–C225. [Google Scholar] [CrossRef]
- Petrović, Ž.; Metikoš-Huković, M.; Babić, R. The electrochemical transfer reactions and the structure of the iron|oxide layer|electrolyte interface. Electrochim. Acta 2012, 75, 406–413. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, S.; Guo, L.; Feng, L.; Tan, B. Experimental and theoretical studies on the corrosion inhibition of carbon steel by two indazole derivatives in HCl medium. Materials 2019, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, D.M. Interpretation of impedance data for porous electrodes and diffusion processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Bosch, J.; Martin, U.; Aperador, W.; Bastidas, J.M.; Ress, J.; Bastidas, D.M. Corrosion behavior of high-Mn austenitic Fe–Mn–Al–Cr–C steels in NaCl and NaOH solutions. Materials 2021, 14, 425. [Google Scholar] [CrossRef]
- Mansfeld, F.; Kendig, M.W.; Lorenz, W.J. Corrosion inhibition in neutral, aerated media. J. Electrochem. Soc. 1985, 132, 290–296. [Google Scholar] [CrossRef]
- Byrne, C.; D’Alessandro, O.; Deyá, C. Tannins as interphase corrosion inhibitors for aluminum in near-neutral chloride solutions. Mater. Corros. 2022, 73, 798–810. [Google Scholar] [CrossRef]
- Du, X.S.; Su, Y.J.; Li, J.X.; Qiao, L.J.; Chu, W.Y. Inhibitive effects and mechanism of phosphates on the stress corrosion cracking of brass in ammonia solutions. Corros. Sci. 2012, 60, 69–75. [Google Scholar] [CrossRef]
- Hakiki, N.E.; Da Cunha Belo, M.; Simões, A.M.P.; Ferreira, M.G.S. Semiconducting properties of passive films formed on stainless steels: Influence of the alloying elements. J. Electrochem. Soc. 1998, 145, 3821–3829. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Cui, X.; Chen, D.; Li, G. Experimental and theoretical investigation on corrosion inhibitive properties of steel rebar by a newly designed environmentally friendly inhibitor formula. RSC Adv. 2018, 8, 6507–6518. [Google Scholar] [CrossRef]
- Macdonald, D.D. The Point Defect Model for the passive state. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Cui, J.; Yang, Y.; Li, X.; Yuan, W.; Pei, Y. Toward a slow-release borate inhibitor to control mild steel corrosion in simulated recirculating water. ACS Appl. Mater. Interfaces 2018, 10, 4183–4197. [Google Scholar] [CrossRef]
- Issaadi, S.; Douadi, T.; Chafaa, S. Adsorption and inhibitive properties of a new heterocyclic furan Schiff base on corrosion of copper in HCl 1M: Experimental and theoretical investigation. Appl. Surf. Sci. 2014, 316, 582–589. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Lai, J.; Qiang, S. Inhibition effect and mechanism explanation of perilla seed extract as a green corrosion inhibitor on Q235 carbon steel. Materials 2022, 15, 5394. [Google Scholar] [CrossRef]
- Al-Rashed, O.; Abdel Nazeer, A. Effectiveness of some novel ionic liquids on mild steel corrosion protection in acidic environment: Experimental and theoretical inspections. Materials 2022, 15, 2326. [Google Scholar] [CrossRef]
- Luo, W.; Lin, Q.; Ran, X.; Li, W.; Tan, B.; Fu, A.; Zhang, S. A new pyridazine derivative synthesized as an efficient corrosion inhibitor for copper in sulfuric acid medium: Experimental and theoretical calculation studies. J. Mol. Liq. 2021, 341, 117370. [Google Scholar] [CrossRef]
- Al-Senani, G.M. Synthesis of ZnO-NPs using a Convolvulus arvensis leaf extract and proving its efficiency as an inhibitor of carbon steel corrosion. Materials 2020, 13, 890. [Google Scholar] [CrossRef]
- Shi, J.J.; Sun, W. Effects of phosphate on the chloride-induced corrosion behavior of reinforcing steel in mortars. Cem. Concr. Compos. 2014, 45, 166–175. [Google Scholar] [CrossRef]
- Daou, T.J.; Begin-Colin, S.; Grenèche, J.M.; Thomas, F.; Derory, A.; Bernhardt, P.; Legaré, P.; Pourroy, G. Phosphate adsorption properties of magnetite-based nanoparticles. Chem. Mater. 2007, 19, 4494–4505. [Google Scholar] [CrossRef]
- Tejedor-Tejedor, M.I.; Anderson, M.A. The protonation of phosphate on the surface of goethite as studied by CIR-FTIR and electrophoretic mobility. Langmuir 1990, 6, 602–611. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gypser, S.; Leinweber, P.; Freese, D.; Kühn, O. Infrared spectroscopic characterization of phosphate binding at the goethite–water interface. Phys. Chem. Che. Phys. 2019, 21, 4421–4434. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, S.; Wiens, M.; Neufurth, M.; Ackermann, M.; Relkovic, D.; Kokkinopoulou, M.; Feng, Q.; Schröder, H.C.; Wang, X. Uptake of polyphosphate microparticles in vitro (SaOS-2 and HUVEC cells) followed by an increase of the intracellular ATP pool size. PLoS ONE 2017, 12, e0188977. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, Z.; Pan, H.; Cheng, X. The initiation and formation of a double-layer phosphate conversion coating on steel. Electrochem. Commun. 2020, 114, 106676. [Google Scholar] [CrossRef]
- Wang, J.-C.; Ren, J.; Yao, H.-C.; Zhang, L.; Wang, J.-S.; Zang, S.-Q.; Han, L.-F.; Li, Z.-J. Synergistic photocatalysis of Cr(VI) reduction and 4-chlorophenol degradation over hydroxylated α-Fe2O3 under visible light irradiation. J. Hazard. Mater. 2016, 311, 11–19. [Google Scholar] [CrossRef]
- Kalina, L.; Bílek, V.; Novotný, R.; Mončeková, M.; Másilko, J.; Koplík, J. Effect of Na3PO4 on the hydration process of alkali-activated blast furnace slag. Materials 2016, 9, 395. [Google Scholar] [CrossRef]
- Hao, S.; Wang, H.; Yang, R.; Liu, D.; Liu, X.; Zhang, Q.; Chen, X. Corn-like mesoporous SnO2/α–Fe2O3 heterostructure for superior TEA sensing performance. Appl. Phys. A 2021, 127, 252. [Google Scholar] [CrossRef]
- Madkour, L.H.; Kaya, S.; Guo, L.; Kaya, C. Quantum chemical calculations, molecular dynamic (MD) simulations and experimental studies of using some azo dyes as corrosion inhibitors for iron. Part 2: Bis–azo dye derivatives. J. Mol. Struct. 2018, 1163, 397–417. [Google Scholar] [CrossRef]
- Arrousse, N.; Salim, R.; Obot, I.B.; Abdellaoui, A.; El Hajjaji, F.; Mabrouk, E.; Taleb, M. Effect of molecular structure of two fluorescein molecules on the corrosion inhibition of mild steel in 1 M HCl solution. J. Mol. Liq. 2022, 359, 119311. [Google Scholar] [CrossRef]
- Lin, B.; Zuo, Y. Corrosion inhibition of carboxylate inhibitors with different alkylene chain lengths on carbon steel in an alkaline solution. RSC Adv. 2019, 9, 7065–7077. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Hammouti, B.; Dafali, A.; Bouachrine, M.; Zarrok, H.; Boukhris, S.; Al-Deyab, S.S. A theoretical study on the inhibition efficiencies of some quinoxalines as corrosion inhibitors of copper in nitric acid. J. Saudi Chem. Soc. 2014, 18, 450–455. [Google Scholar] [CrossRef]
- Gao, G.; Liang, C. Electrochemical and DFT studies of β-amino-alcohols as corrosion inhibitors for brass. Electrochim. Acta 2007, 52, 4554–4559. [Google Scholar] [CrossRef]
- Fazayel, A.S.; Khorasani, M.; Sarabi, A.A. The effect of functionalized polycarboxylate structures as corrosion inhibitors in a simulated concrete pore solution. Appl. Surf. Sci. 2018, 441, 895–913. [Google Scholar] [CrossRef]
- Teymouri, F.; Allahkaram, S.R.; Shekarchi, M.; Azamian, I.; Johari, M. A comprehensive study on the inhibition behaviour of four carboxylate-based corrosion inhibitors focusing on efficiency drop after the optimum concentration for carbon steel in the simulated concrete pore solution. Constr. Build. Mater. 2021, 296, 123702. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Adekunle, A.S.; Olasunkanmi, L.O.; Bahadur, I.; Baskar, R.; Kabanda, M.M.; Obot, I.B.; Ebenso, E.E. Experimental, quantum chemical and Monte Carlo simulation studies on the corrosion inhibition of some alkyl imidazolium ionic liquids containing tetrafluoroborate anion on mild steel in acidic medium. J. Mol. Liq. 2015, 211, 105–118. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Verma, C.; Lgaz, H.; Salghi, R.; Quraishi, M.A. N-Methyl-N,N,N-trioctylammonium chloride as a novel and green corrosion inhibitor for mild steel in an acid chloride medium: Electrochemical, DFT and MD studies. New J. Chem. 2017, 41, 13647–13662. [Google Scholar] [CrossRef]
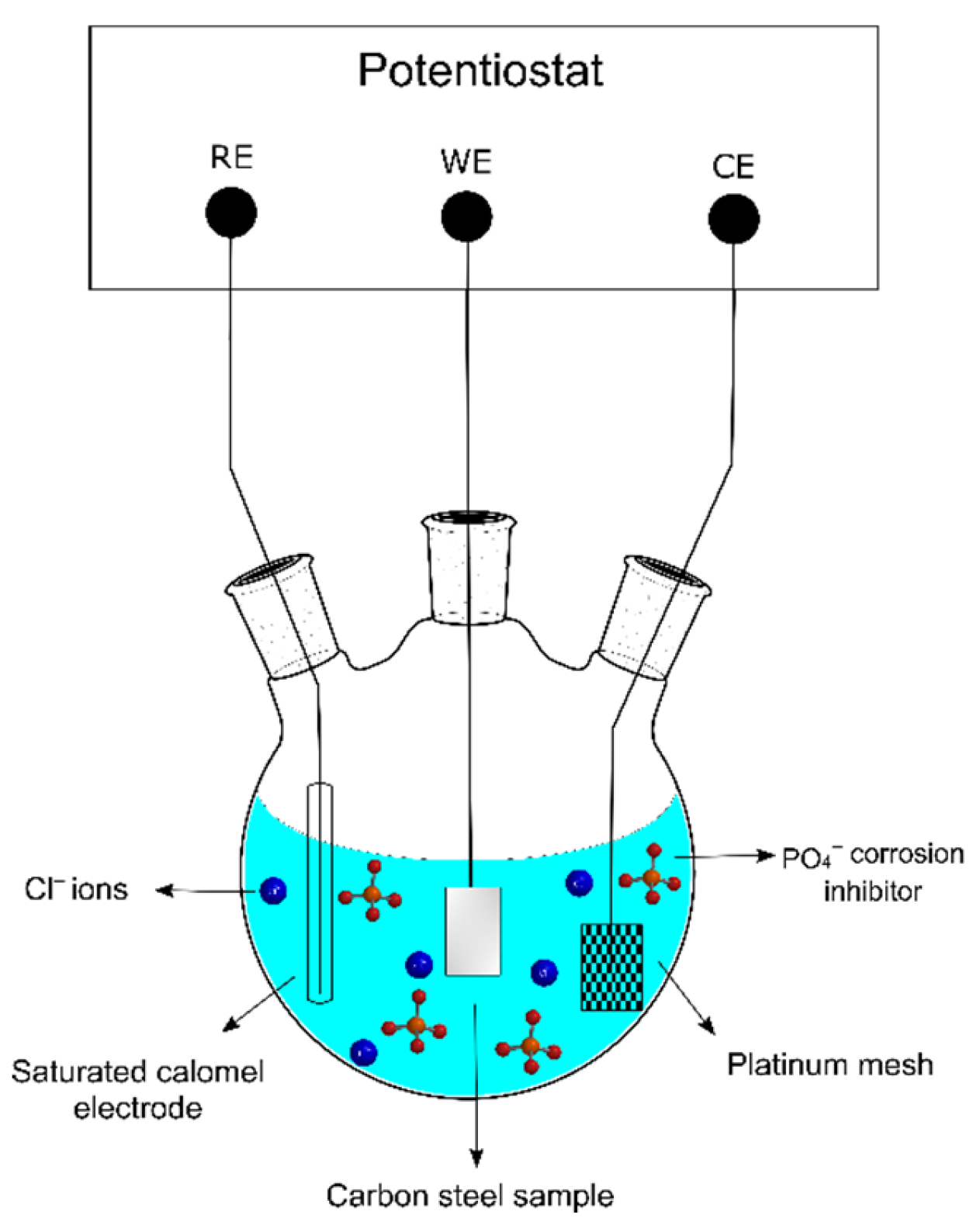

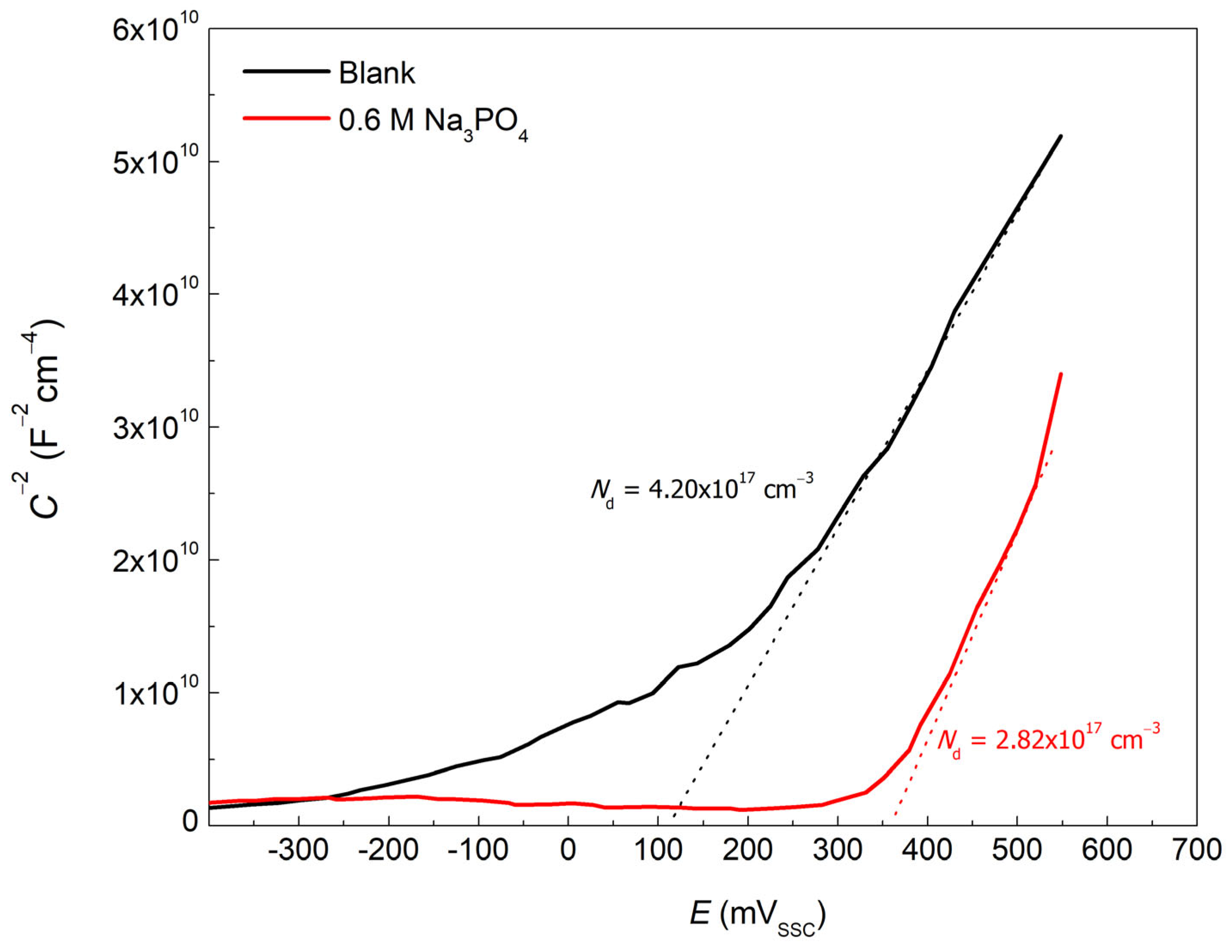

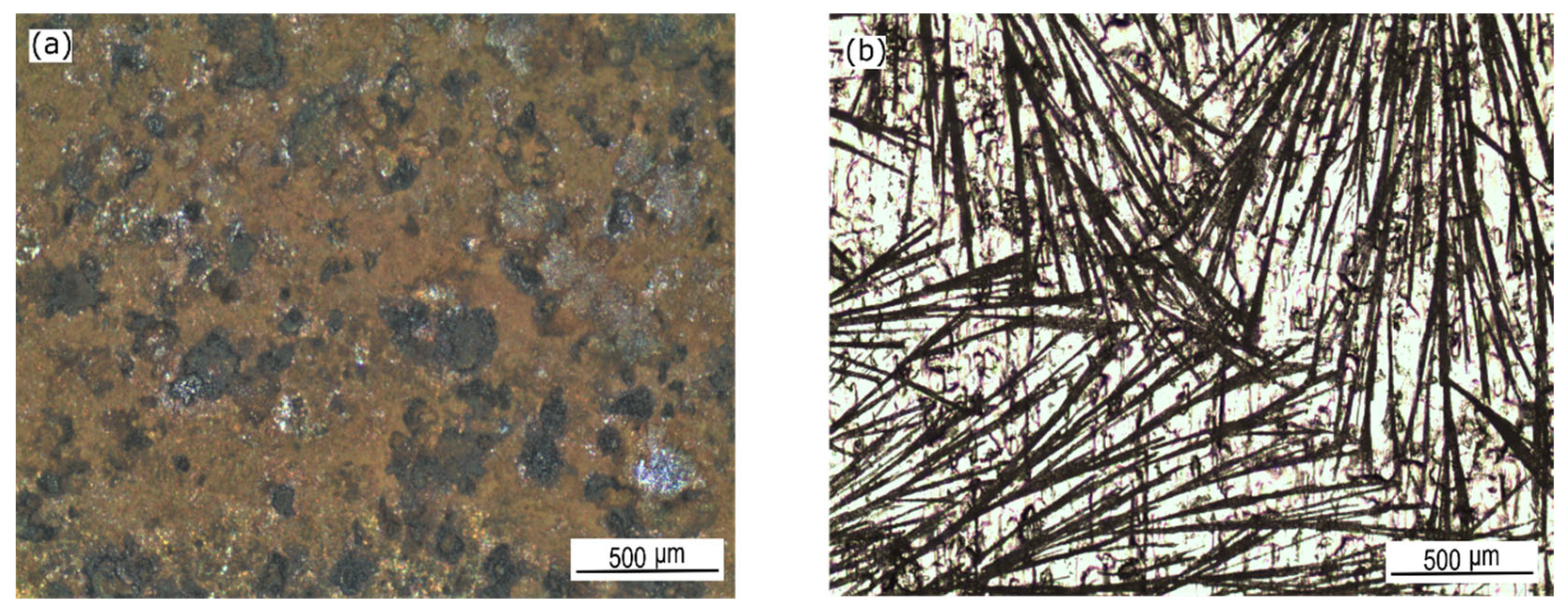
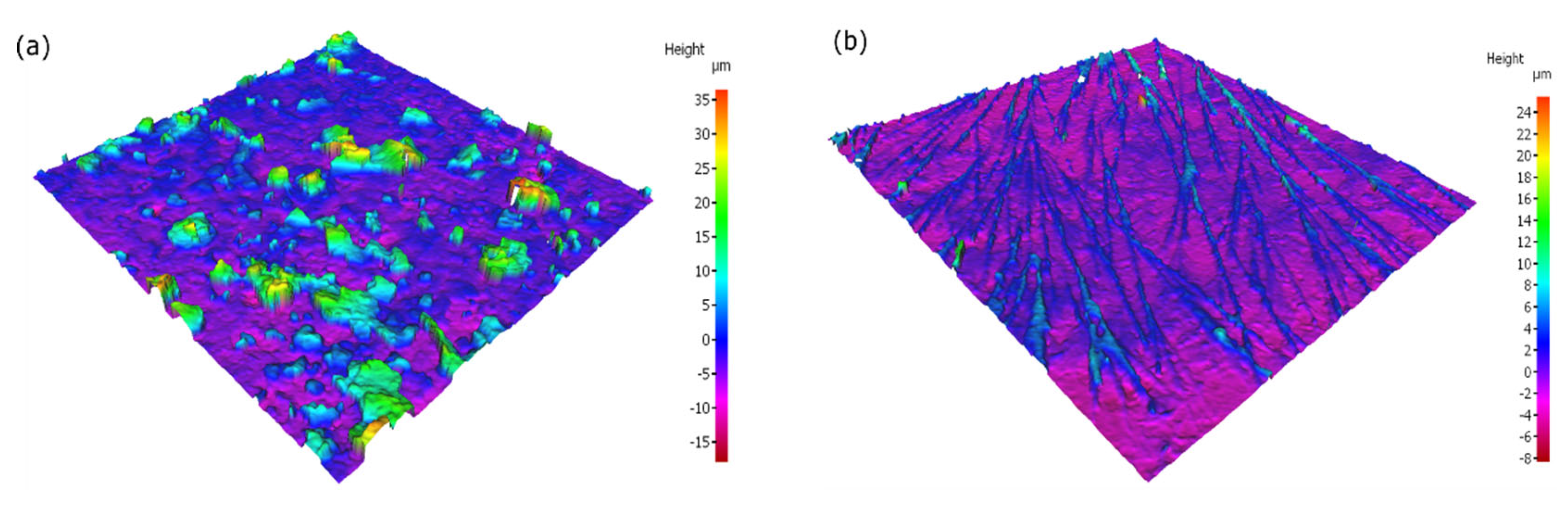
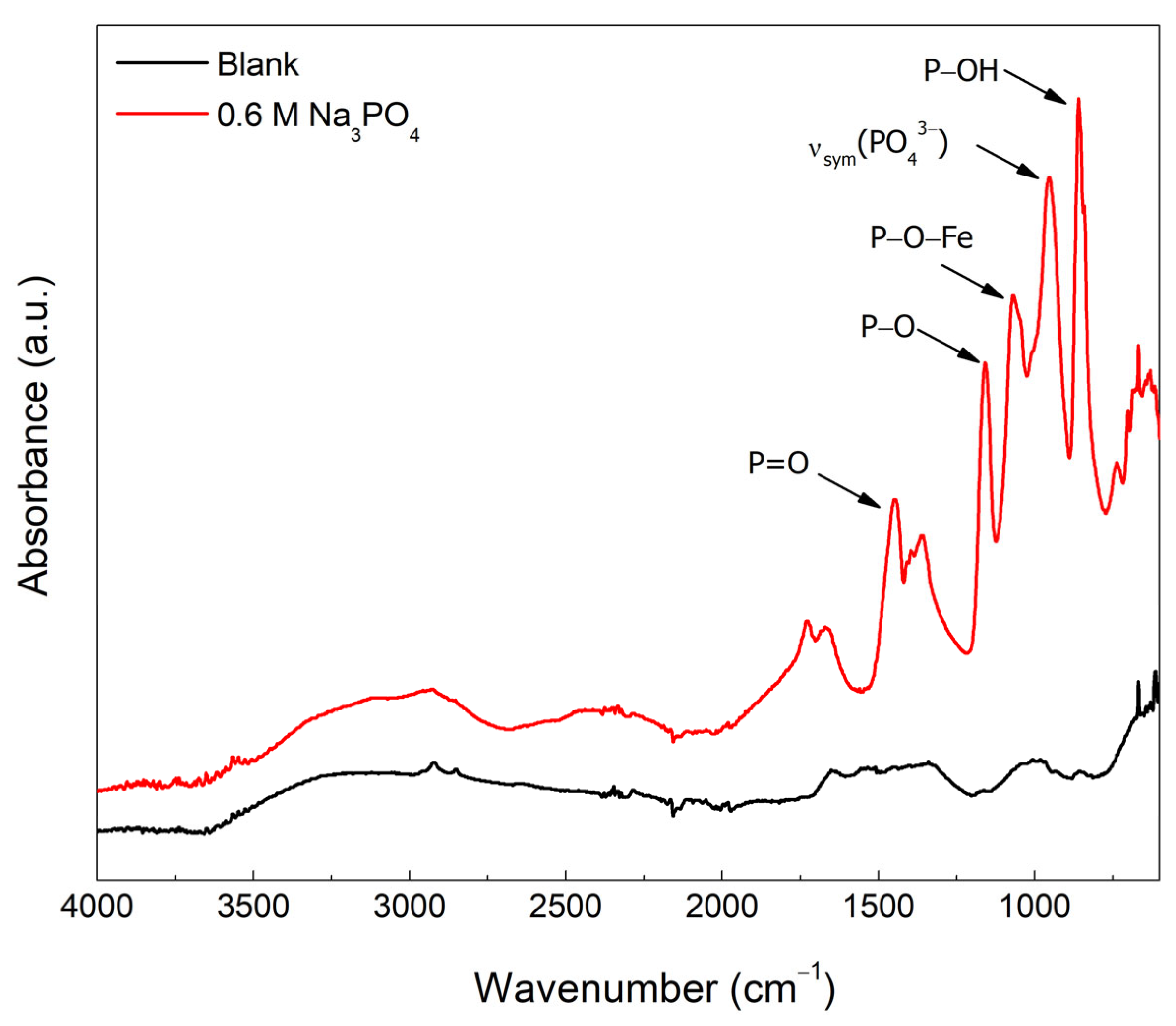

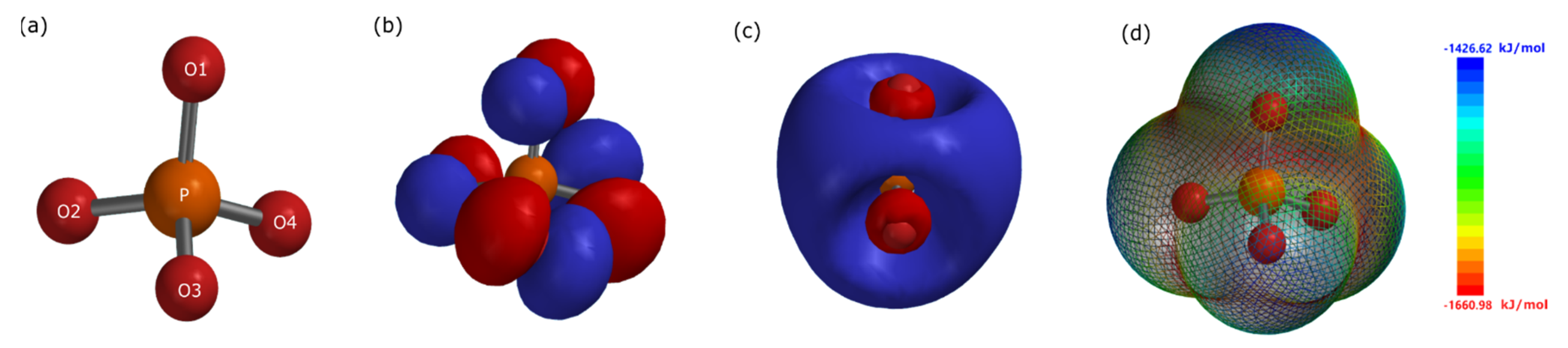
| C | Mn | P | S | Si | Cu | Ni | Cr | Mo | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.28 | 1.08 | 0.019 | 0.043 | 0.20 | 0.37 | 0.16 | 0.16 | 0.050 | 0.0379 | Bal. |
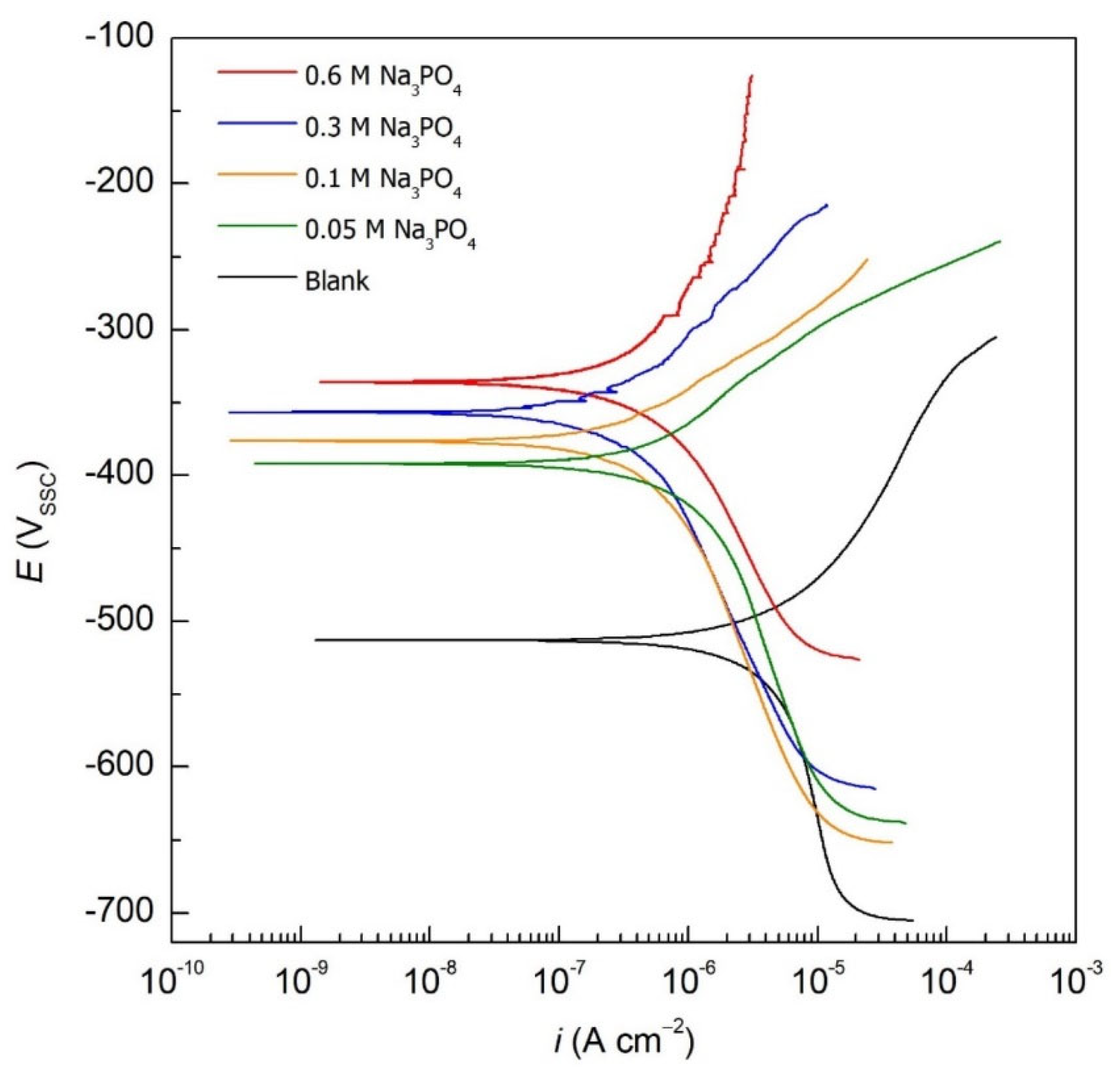
| [Na3PO4] (M) | Ecorr (mVSSC) | icorr (µA cm−2) | IE (%) | θ | βc (mV/dec) | βa (mV/dec) | |
|---|---|---|---|---|---|---|---|
| Blank | - | −514 | 5.20 | - | - | 190 | 189 |
| Na3PO4 | 0.05 | −390 | 0.43 | 80.8 | 0.808 | 194 | 86 |
| 0.10 | −341 | 0.50 | 87.5 | 0.875 | 194 | 75 | |
| 0.30 | −378 | 0.65 | 90.4 | 0.904 | 211 | 122 | |
| 0.60 | −371 | 1.00 | 91.7 | 0.917 | 242 | 396 |
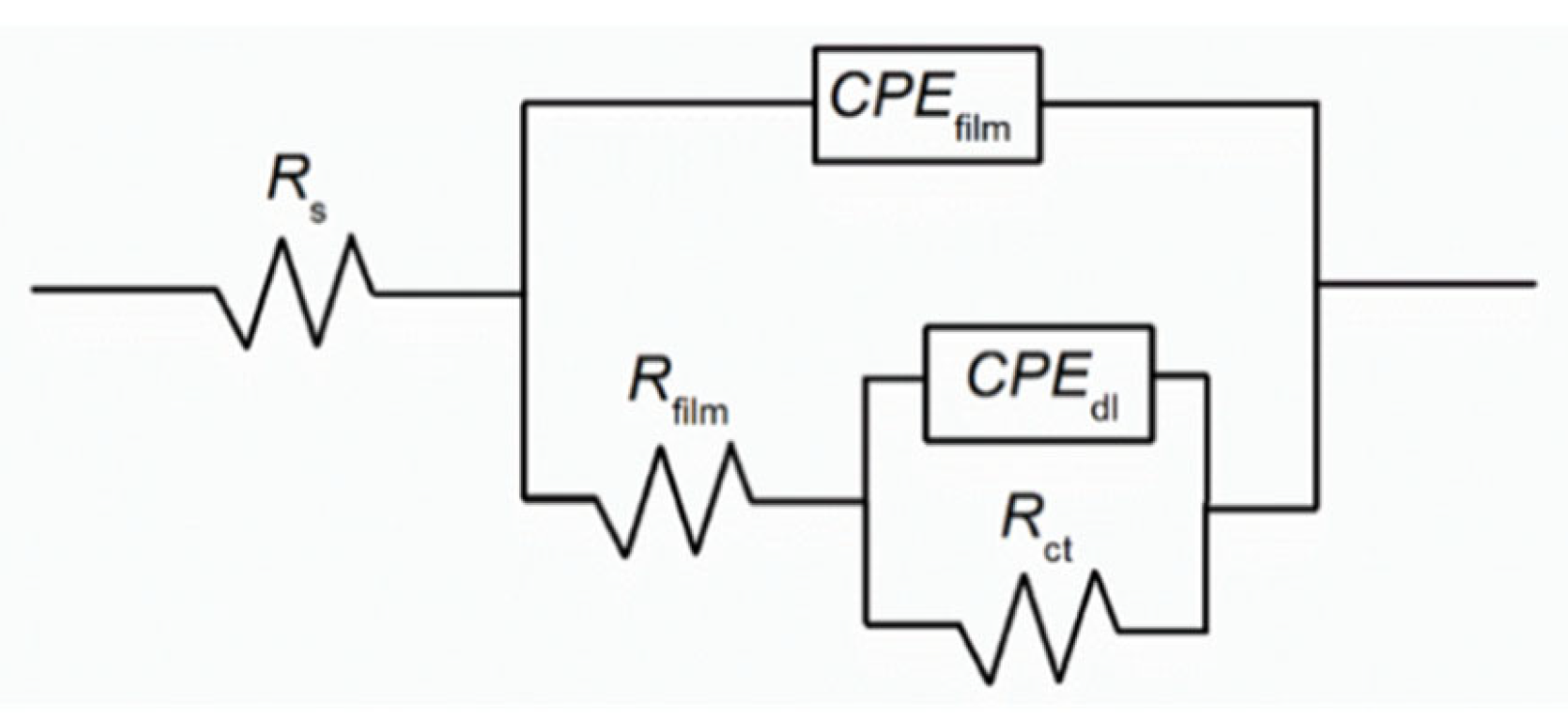
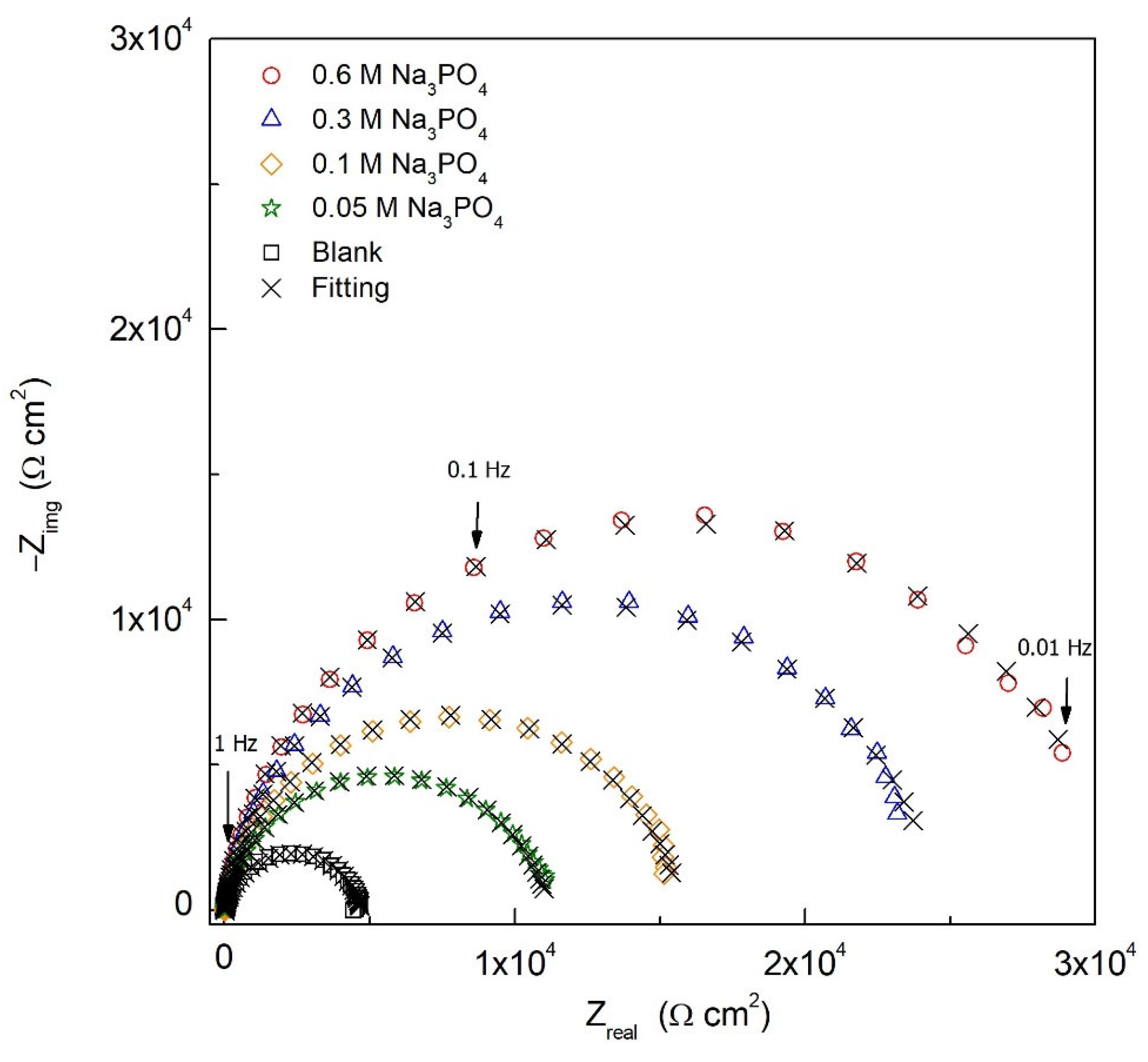
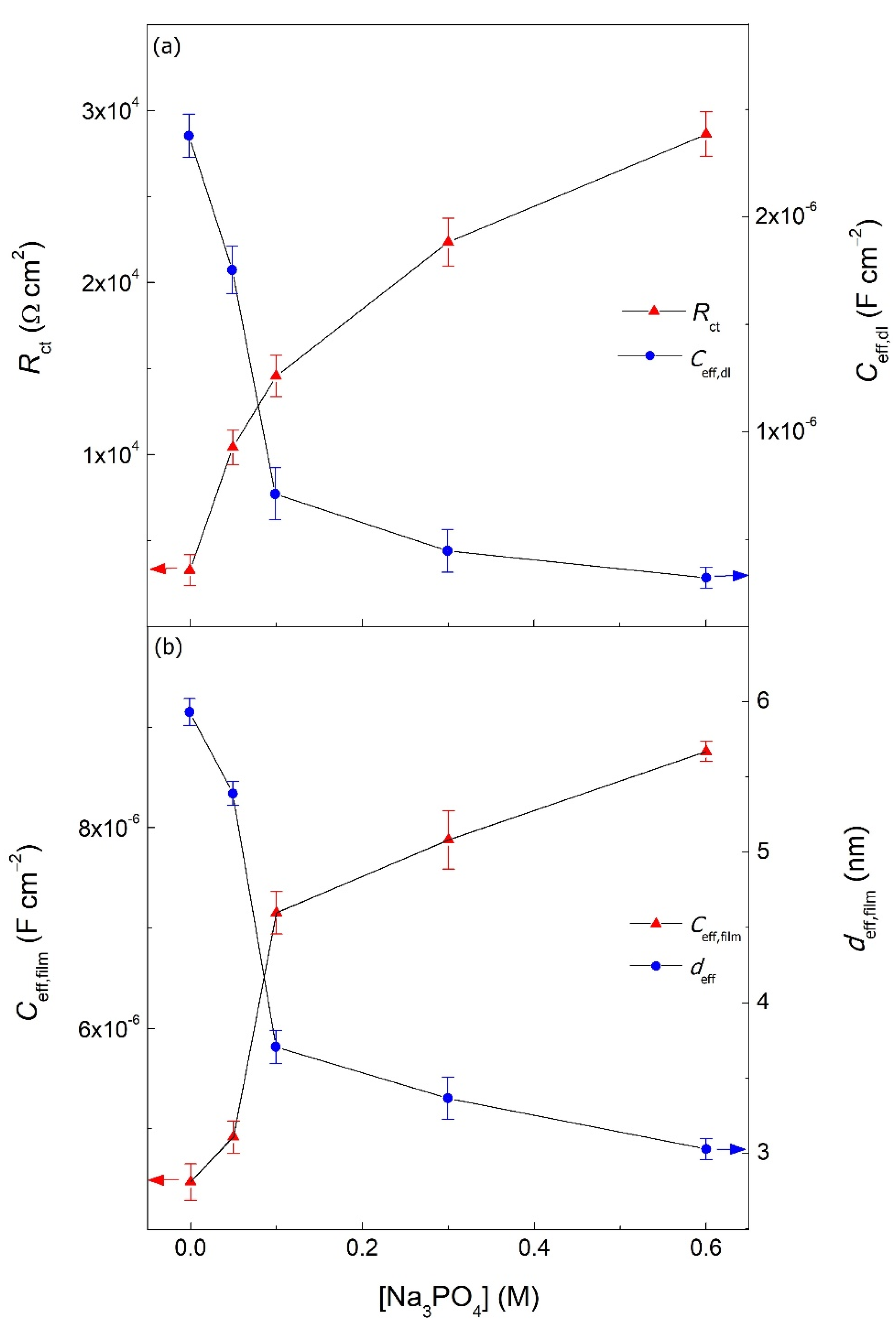
| [Na3PO4] (M) | Rs (Ω cm2) | Rfilm (Ω cm2) | Yfilm (S cm−2 snfilm) | nfilm | Rct (Ω cm2) | Ydl (S cm−2 sndl) | ndl | IE (%) | χ2 (*) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Blank | - | 6.22 | 4.80 × 102 | 4.35 × 10−6 | 0.95 | 3.27 × 103 | 2.71 × 10−5 | 0.78 | - | 6.64 × 10−4 |
| Na3PO4 | 0.05 | 5.98 | 7.68 × 102 | 4.58 × 10−6 | 0.96 | 1.04 × 104 | 2.77 × 10−5 | 0.76 | 68.6 | 5.50 × 10−4 |
| 0.10 | 8.64 | 1.10 × 103 | 5.96 × 10−6 | 0.90 | 1.45 × 104 | 5.47 × 10−6 | 0.83 | 77.4 | 1.34 × 10−4 | |
| 0.30 | 8.89 | 1.92 × 103 | 6.42 × 10−6 | 0.91 | 2.23 × 104 | 1.55 × 10−6 | 0.90 | 85.4 | 5.54 × 10−4 | |
| 0.60 | 8.46 | 2.69 × 103 | 6.84 × 10−6 | 0.91 | 2.86 × 104 | 4.17 × 10−6 | 0.80 | 88.6 | 5.47 × 10−4 |
| [Na3PO4] (M) | Ceff,dl (F cm−2) | Ceff,film (F cm−2) | deff,film (nm) | |
|---|---|---|---|---|
| Blank | - | 2.38 × 10−6 | 4.47 × 10−6 | 5.92 |
| Na3PO4 | 0.05 | 1.75 × 10−6 | 4.92 × 10−6 | 5.39 |
| 0.10 | 7.12 × 10−7 | 7.15 × 10−6 | 3.71 | |
| 0.30 | 4.47 × 10−7 | 7.88 × 10−6 | 3.36 | |
| 0.60 | 3.21 × 10−7 | 8.76 × 10−6 | 3.03 |
| Adsorption Isotherm | R2 | Equation |
|---|---|---|
| Langmuir | 0.999 | |
| Temkin | 0.866 | |
| Freundlich | 0.912 | |
| Frumkin | 0.920 | |
| El-Awady | 0.932 |
| EHOMO (eV) | ELUMO (eV) | ΔEgap (eV) | µD (Debye) |
|---|---|---|---|
| −2.88 | 5.76 | 8.64 | 1.79 |
| Atom | O1 | O2 | O3 | O4 | P |
| Mulliken charge | −1.212 | −1.323 | −1.323 | −1.323 | 2.182 |
| Corrosion Inhibitor | Environment | Substrate | Concentration | IE (%) | EHOMO (eV) | ELUMO (eV) | ΔEgap (eV) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Polymethacrylic acid | 0.3 M Cl− SCPS | Carbon steel | 1 wt.% | 71.51 | −7.56 | −1.39 | 6.17 | [56] |
| Polymethacrylic acid-co-2-Acrylamido-2methylpropane sulfonic acid | 0.3 M Cl− SCPS | Carbon steel | 1 wt.% | 87.96 | −7.36 | −1.46 | 5.90 | [56] |
| Potassium Sodium Tartrate | 0.5 M Cl− SCPS | Carbon steel | 0.1 M | 87.20 | −8.11 | −1.48 | 6.63 | [57] |
| Sodium Acetate | 0.5 M Cl− SCPS | Carbon steel | 0.125 M | 81.00 | −7.87 | −0.48 | 7.38 | [57] |
| 1-ethyl-3-methylimidazolium tetrafluoroborate | 1 M HCl | Mild Steel | 500 ppm | 82.41 | −8.29 | −1.40 | 6.89 | [58] |
| N-Methyl-N,N,N-trioctylammonium chloride | 1 M HCl | Mild steel | 4.95 µM | 93.20 | −6.06 | 0.04 | 6.10 | [59] |
| PO43− (Na3PO4) | 0.6 M Cl− SCPS | Carbon steel | 0.6 M | 91.70 | −2.88 | 5.76 | 8.64 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.; Martin, U.; Bastidas, D.M. Adsorption and Surface Analysis of Sodium Phosphate Corrosion Inhibitor on Carbon Steel in Simulated Concrete Pore Solution. Materials 2022, 15, 7429. https://doi.org/10.3390/ma15217429
Mohamed A, Martin U, Bastidas DM. Adsorption and Surface Analysis of Sodium Phosphate Corrosion Inhibitor on Carbon Steel in Simulated Concrete Pore Solution. Materials. 2022; 15(21):7429. https://doi.org/10.3390/ma15217429
Chicago/Turabian StyleMohamed, Ahmed, Ulises Martin, and David M. Bastidas. 2022. "Adsorption and Surface Analysis of Sodium Phosphate Corrosion Inhibitor on Carbon Steel in Simulated Concrete Pore Solution" Materials 15, no. 21: 7429. https://doi.org/10.3390/ma15217429
APA StyleMohamed, A., Martin, U., & Bastidas, D. M. (2022). Adsorption and Surface Analysis of Sodium Phosphate Corrosion Inhibitor on Carbon Steel in Simulated Concrete Pore Solution. Materials, 15(21), 7429. https://doi.org/10.3390/ma15217429







