Calorimetric Method for the Testing of Thermal Coefficients of the TIG Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Casting Procedure
2.2. Arc and Melting Efficiency
2.3. Fusion Geometry Examination
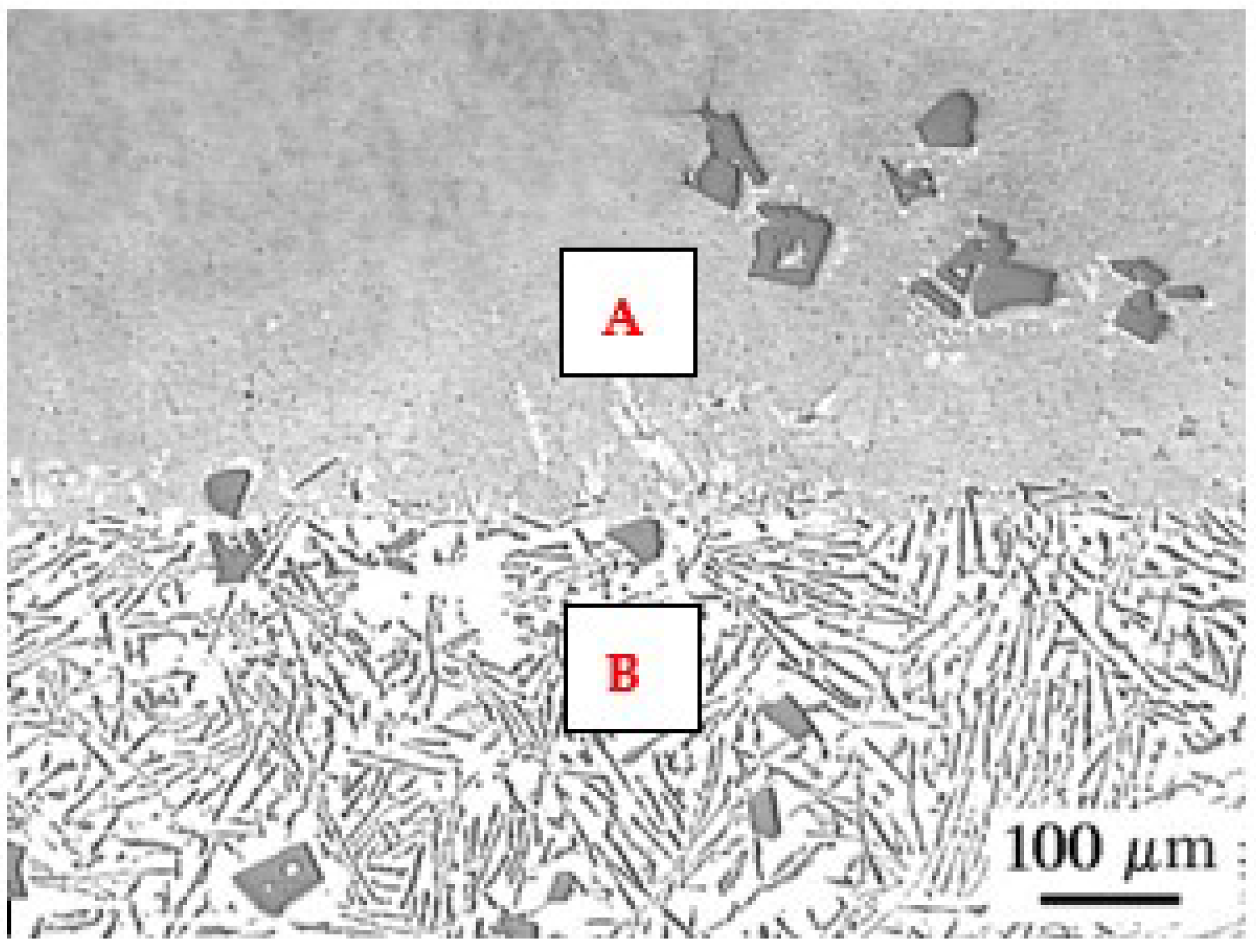
2.4. Microstructure Parameters
3. Results and discussion
3.1. Arc efficiency and melting efficiency
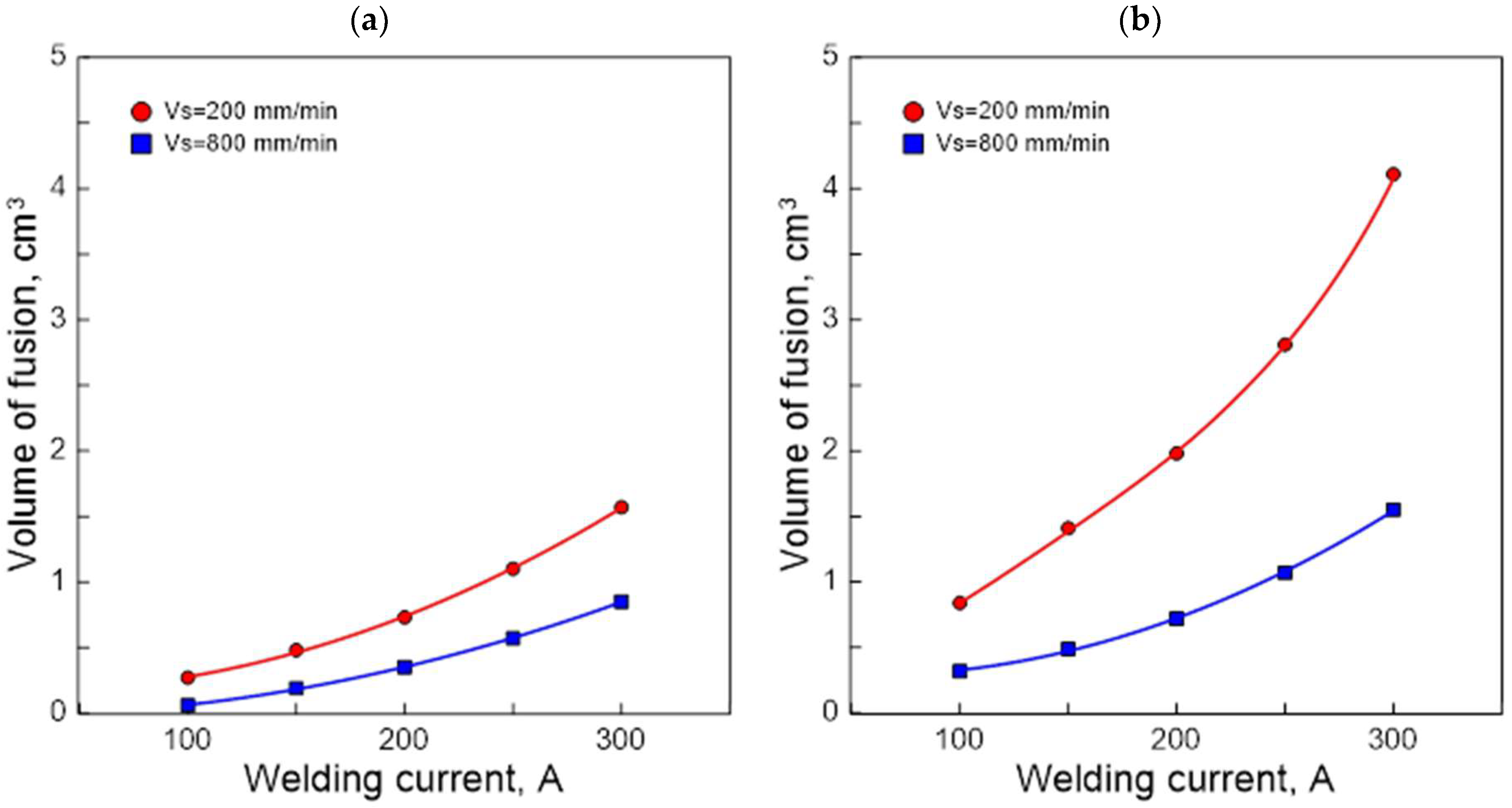
3.2. Fusion Geometry
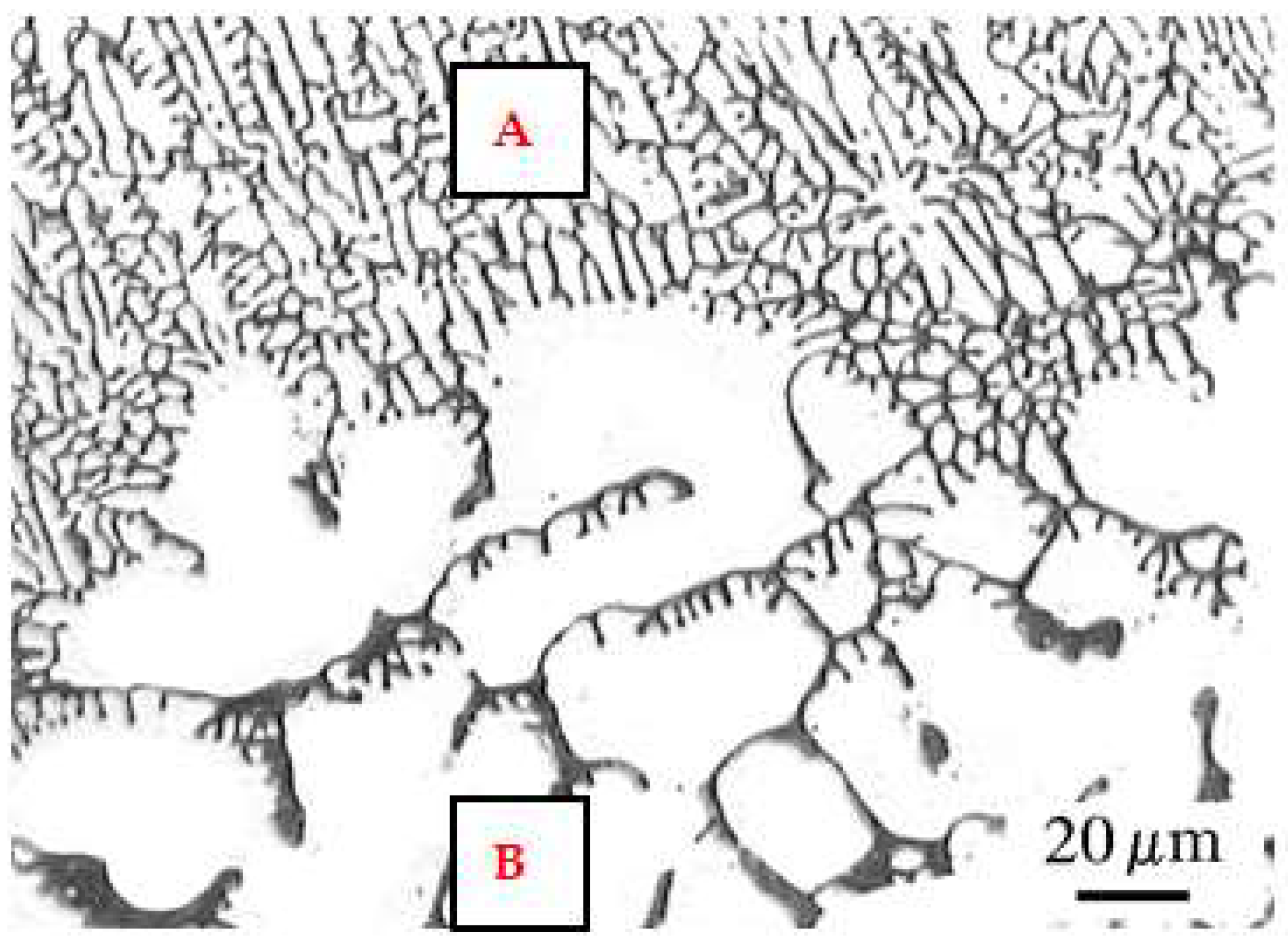
3.3. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferec, K.; Ferenc, J. Konstrukcje spawane. Połączenia; Wydanie WNT: Warsaw, Poland, 2003. [Google Scholar]
- Klimpel, A. Technologia spawania i cięcia metali; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 1998. [Google Scholar]
- Pilarczyk, J.; Adamiec, P.; Klimpel, A.; Włosiński, W.; Banasik, M.; Brózda, J.; Dębski, E.; Dobaj, E.; Dziuba, S.; Ferenc, K.; et al. Poradnik inżyniera; Wydawnictwo WNT: Warszawa, Poland, 2021. [Google Scholar]
- Tasak, E. Metalurgia spawania; Wydawnictwo JAK: Kraków, Poland, 2008. [Google Scholar]
- Ferenc, K. Spawalnictwo; Wydawnictwo WNT: Warszawa, Poland, 2007. [Google Scholar]
- Orłowicz, A.W.; Mróz, M. Sposób zmniejszania udziału ferrytu Widmanstättena w złączu spawanym elementów konstrukcyjnych wykonanych ze stali niskowęglowej. International Patent PL 237300, 11 September 2019. [Google Scholar]
- Orłowicz, A.W.; Mróz, M. Sposób zmniejszania udziału martenzytu oraz ferrytu Widmanstättena w dwuimiennym złączu spawanych elementów konstrukcyjnych wykonanych ze stali niskowęglowej. International Patent PL 236933, 11 September 2019. [Google Scholar]
- Giedt, W.H.; Tallerico, L.N.; Fuerschbach, P.W. GTA Welding efficiency: Calorimetric and temperature field measurements. Weld. J. Res. Suppl. 1989, 68, 28–32. [Google Scholar]
- Du Pont, J.N.; Marder, A.R. Thermal efficiency of arc welding processes. Weld. Res. Suppl. 1995, 74, 406–416. [Google Scholar]
- Glikstein, S.S.; Friedman, E. Temperature transients in gas tungsten arc weldments. Weld. Rev. 1983, 62, 72–75. [Google Scholar]
- Kou, S.; Sun, D.K. Three-dimensional heat flow and solidification during the autogenous GTA welding of aluminium plates. Metall. Trans. A 1983, 14, 2245–2253. [Google Scholar] [CrossRef]
- Kou, S.; Sun, D.K. Fluid flow and weld penetration in stationary arc welds. Metall. Trans. A 1985, 16, 203–213. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Ma, H.; Zhao, X.; Wu, C.; Gao, J. Numerical simulation of arc and droplet behaviors in TIG-MIG hybrid welding. Materials 2020, 13, 4520. [Google Scholar] [CrossRef]
- Karganroudi, S.S.; Maradi, M.; Attar, M.A.; Rasouli, S.A.; Ghoreishi, M.; Lawrence, J.; Ibrahim, H. Experimental and numerical analysis on TIG arc welding of stainless steel using RSM Approch. Materials 2021, 11, 1659. [Google Scholar] [CrossRef]
- Okada, A. Applications of melting efficiency and its problem. J. Jpn. Weld. Soc. 1977, 46, 53–61. [Google Scholar] [CrossRef]
- Glikstein, S.S.; Friedman, E. Weld modeling applications. Weld. J. 1984, 63, 38–42. [Google Scholar]
- Glikstein, S.S.; Friedman, E. An investigation of thermal response of stacionary gas tungsten arc welds. Weld. J. 1976, 55, 408–420. [Google Scholar]
- Havalda, A. Thermal Processes in Electric Welding; Wydawnictwo WNT: Warszawa, Poland, 1963. [Google Scholar]
- Krzyżanowski, M. Iron Alloy Surface Overwork with the Use of Plasma Heating; Wydawnictwo AGH: Cracow, Poland, 1995; pp. 287–297. [Google Scholar]
- Smartt, H.B.; Stewart, J.A.; Einerson, C.J. Heat transfer in gas tungsten arc welding. In Proceedings of the ASM metals congress/TMS-AIME fall meeting, Toronto, ON, Canada, 13 October 1985; Series nr 8511–011. pp. 1–14. [Google Scholar]
- Knorowski, G.A.; Fuerschbach, P.W. Calorimetry of Pulsed Versus Continous Gas Tungsten Arc Welding. In Proceedings of the ASM International Trends in Welding Research Meeting, Gatlinburg, TN, USA, 18 May 1986; pp. 393–400. [Google Scholar]
- Fuerschbach, P.W.; Knorovsky, G.A. A study of melting efficiency in plasma arc and gas tungsten arc welding. Weld. J. Res. Suppl. 1991, 70, 287–297. [Google Scholar]
- Orłowicz, A.W.; Mróz, M.F.; Trytek, A.; Tupaj, M.; Betlej, J. A Flow Calorimeter for Thermal Measurements on Welding Processes. Polish Patent No. 211283, 11 May 2012. [Google Scholar]
- Grączewski, A. Wytrzymałość i Trwałość Zmęczeniowa Złączy Spawanych Wykonanych Ze Stopu EN AW-5754. Ph.D Thesis, Bydgoszcz University of Technology, Bydgoszcz, Poland, 2022. [Google Scholar]
- Braszczyńska-Malik, K.; Mróz, M. Gas-tungsten arc welding of AZ 91 magnesium alloy. J. Alloy. Compd. 2011, 509, 9951–9958. [Google Scholar] [CrossRef]
- Orłowicz, A.W.; Mróz, M. Structure and operational characteristics of casting of LM25 alloy with surface layer refined by rapid solidification. Int. J. Cast Met. 2002, 15, 85–93. [Google Scholar] [CrossRef]
- Orłowicz, A.W.; Mróz, M. Study on susceptibility of AL-Si alloy castings to surface refinement with TIG arc. Z. Mtallkunde 2004, 12, 1391–1397. [Google Scholar] [CrossRef]
- Mróz, M. Orłowicz, A.W.; Tupaj, M. Współczynniki termiczne procesu GTAW. Arch. Foundry Eng. 2005, 5, 217–224. [Google Scholar]
- Orłowicz, A.W.; Trytek, A.; Malik, J. Formation of Microstructure and Service Properties of Cast Iron Castings by Surface Improvement with Electric arc Plasme; Rzeszow University of Technology: Rzeszów, Poland, 2013. [Google Scholar]
- Orłowicz, A.W.; Trytek, A. Susceptibility of iron castings to heat absorption from an electric arc and to hardened-layer shaping. Metall. Trans. A 2003, 34A, 2973–2984. [Google Scholar] [CrossRef]
- Orłowicz, A.W.; Trytek, A. Structural and operating aspects of rapid solidification of surface layer of spheroidal graphite cast iron. Int. J. Cast Met. 2003, 16, 496–505. [Google Scholar] [CrossRef]
- Opiekun, Z.; Orłowicz, A.W. Kształtowanie struktury warstwy wierzchniej odlewów ze stopów kobaltu plazmą łuku elektrycznego. Bull. Weld. Inst. 2004, 5, 43–49. [Google Scholar]
- Orłowicz, A.W.; Trytek, A. Nadtapianie powierzchniowe odlewów z superstopu na bazie kobaltu MAR-M509. Arch. Foundry Eng. 2004, 4, 348–355. [Google Scholar]
- Hogan, L.M.; Song, H. Interparticle spacings and undecooling in Al-Si eutectic microstructure. Metall. Trans. A 1987, 18, 707–713. [Google Scholar] [CrossRef]
- Cáceres, C.H.; Wang, Q.G. Solidifications conditions, heat treatment and tensile ductility of Al-Si-0.4Mg casting alloys. AFS Trans. 1996, 104, 1039–1043. [Google Scholar]
- Oroper, G.M.; Eagar, T.W.; Szekely, J. Convection in arc weld pools. Weld. J. 1983, 11, 307–312. [Google Scholar]




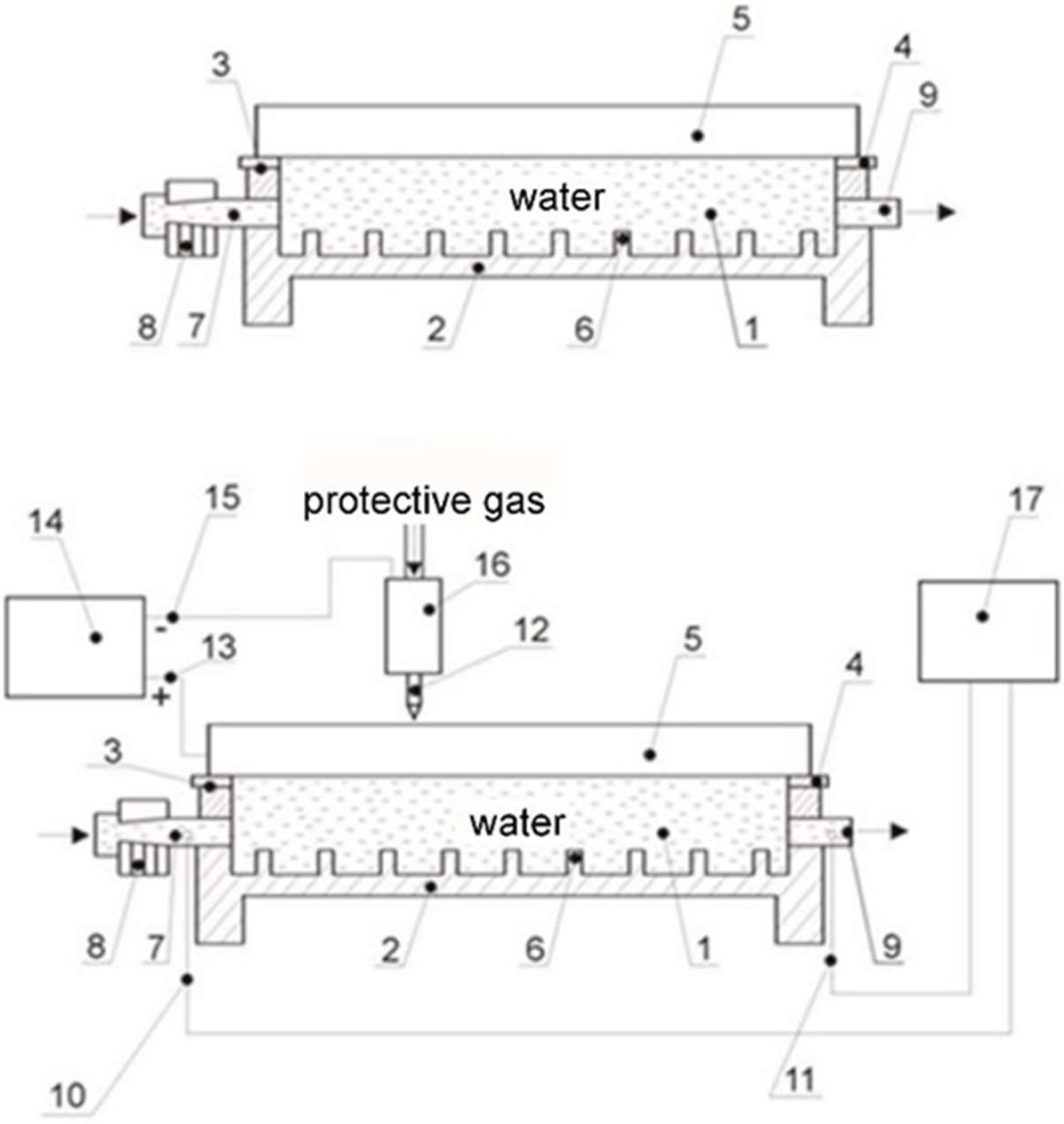

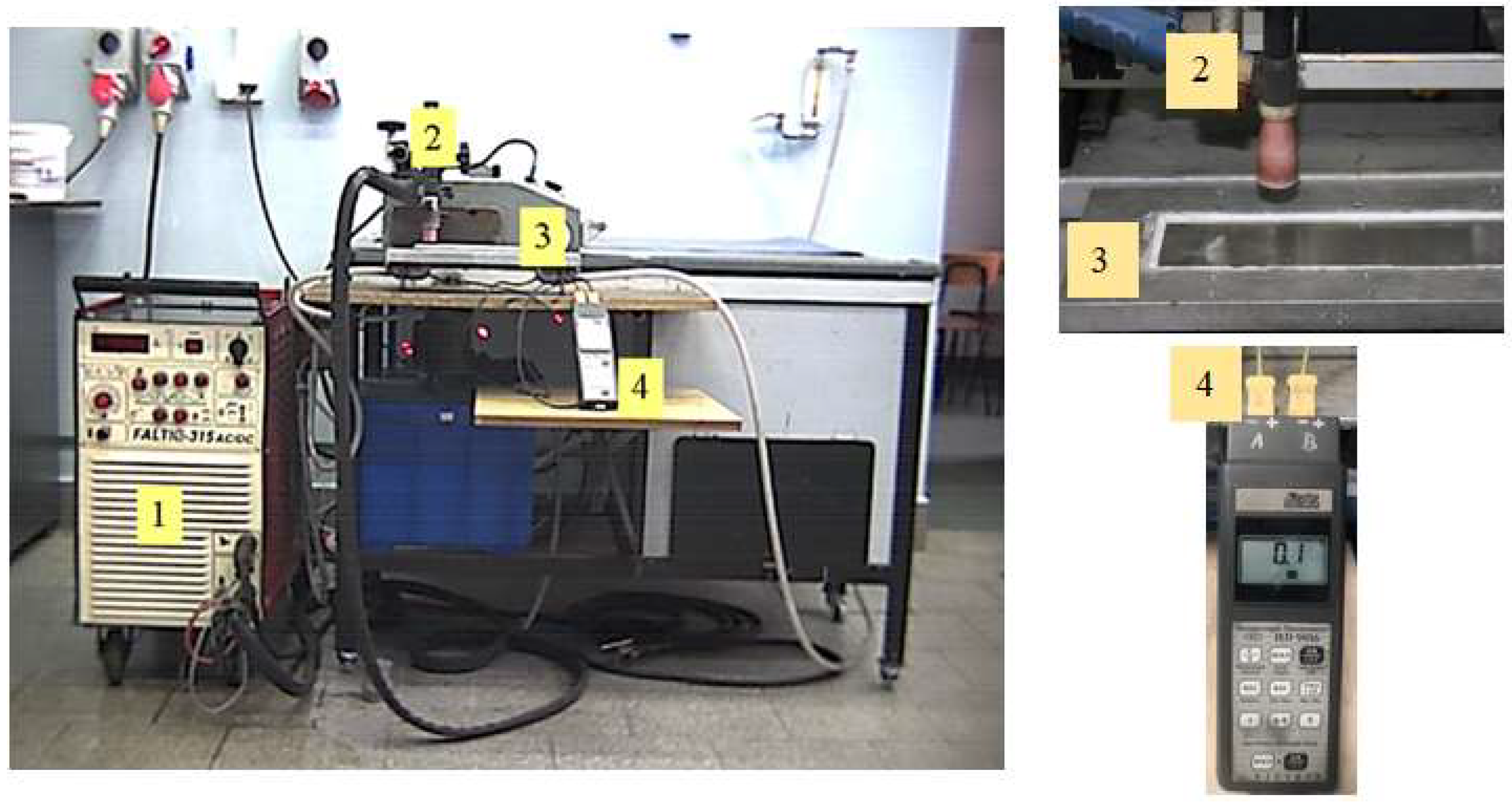





| Alloy | Elemental Content, % by Weight | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Mg | Mn | Cu | Fe | Ti | Cr | Al | |
| AK 51 | 5.56 | 0.38 | 0.34 | 0.24 | 0.26 | 0.110 | 0.009 | remainder |
| AK 20 | 21.65 | 0.04 | 0.02 | 0.10 | 0.28 | 0.020 | 0.010 | remainder |
| No. | GTAW Process Parameters | Efficiency | ||
|---|---|---|---|---|
| I, A | vs, mm/min | arc, η | Melting, ηm | |
| 1 | 100 | 200 | 0.74 | 0.017 |
| 2 | 150 | 200 | 0.77 | 0.018 |
| 3 | 200 | 200 | 0.80 | 0.020 |
| 4 | 250 | 200 | 0.82 | 0.022 |
| 5 | 300 | 200 | 0.84 | 0.024 |
| 6 | 100 | 800 | 0.56 | 0.059 |
| 7 | 150 | 800 | 0.58 | 0.087 |
| 8 | 200 | 800 | 0.60 | 0.111 |
| 9 | 250 | 800 | 0.61 | 0.138 |
| 10 | 300 | 800 | 0.62 | 0.169 |
| No. | GTAW Process Parameters | Efficiency | ||
|---|---|---|---|---|
| I, A | vs, mm/min | arc, η | Melting, ηm | |
| 1 | 100 | 200 | 0.81 | 0.046 |
| 2 | 150 | 200 | 0.83 | 0.048 |
| 3 | 200 | 200 | 0.84 | 0.051 |
| 4 | 250 | 200 | 0.86 | 0.054 |
| 5 | 300 | 200 | 0.88 | 0.059 |
| 6 | 100 | 800 | 0.57 | 0.207 |
| 7 | 150 | 800 | 0.61 | 0.234 |
| 8 | 200 | 800 | 0.62 | 0.258 |
| 9 | 250 | 800 | 0.64 | 0.286 |
| 10 | 300 | 800 | 0.65 | 0.328 |
| No. | GTAW Process Parameters | Surface Melting Area Indicators | ||||
|---|---|---|---|---|---|---|
| I, A | vs, mm/min | a, mm | h, mm | F, mm2 | Vn, cm2 | |
| 1 | 100 | 200 | 3.54 | 0.43 | 1.37 | 0.27 |
| 2 | 150 | 200 | 5.05 | 0.69 | 2.62 | 0.48 |
| 3 | 200 | 200 | 6.57 | 0.96 | 4.31 | 0.73 |
| 4 | 250 | 200 | 8.09 | 1.21 | 6.34 | 1.10 |
| 5 | 300 | 200 | 9.57 | 1.45 | 8.62 | 1.57 |
| 6 | 100 | 800 | 1.04 | 0.19 | 0.26 | 0.06 |
| 7 | 150 | 800 | 2.48 | 0.45 | 0.98 | 0.19 |
| 8 | 200 | 800 | 3.94 | 0.69 | 2.06 | 0.35 |
| 9 | 250 | 800 | 5.24 | 0.92 | 3.35 | 0.57 |
| 10 | 300 | 800 | 6.45 | 1.11 | 4.73 | 0.85 |
| No. | GTAW Process Parameters | Surface Melting Area Indicators | ||||
|---|---|---|---|---|---|---|
| I, A | vs, mm/min | a, mm | h, mm | F, mm2 | Vn, cm2 | |
| 1 | 100 | 200 | 5.63 | 2.10 | 7.26 | 0.84 |
| 2 | 150 | 200 | 6.47 | 2.65 | 10.24 | 1.41 |
| 3 | 200 | 200 | 7.28 | 3.25 | 13.86 | 1.98 |
| 4 | 250 | 200 | 8.17 | 3.82 | 17.99 | 2.81 |
| 5 | 300 | 200 | 8.97 | 4.38 | 22.38 | 4.11 |
| 6 | 100 | 800 | 3.17 | 1.37 | 2.97 | 0.32 |
| 7 | 150 | 800 | 3.88 | 1.90 | 4.79 | 0.49 |
| 8 | 200 | 800 | 4.55 | 2.49 | 7.13 | 0.72 |
| 9 | 250 | 800 | 5.36 | 2.99 | 9.80 | 1.07 |
| 10 | 300 | 800 | 6.25 | 3.57 | 13.32 | 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mróz, M.; Orłowicz, A.W.; Lenik, M.; Trytek, A.; Tupaj, M. Calorimetric Method for the Testing of Thermal Coefficients of the TIG Process. Materials 2022, 15, 7389. https://doi.org/10.3390/ma15207389
Mróz M, Orłowicz AW, Lenik M, Trytek A, Tupaj M. Calorimetric Method for the Testing of Thermal Coefficients of the TIG Process. Materials. 2022; 15(20):7389. https://doi.org/10.3390/ma15207389
Chicago/Turabian StyleMróz, Marek, Antoni Władysław Orłowicz, Magdalena Lenik, Andrzej Trytek, and Mirosław Tupaj. 2022. "Calorimetric Method for the Testing of Thermal Coefficients of the TIG Process" Materials 15, no. 20: 7389. https://doi.org/10.3390/ma15207389
APA StyleMróz, M., Orłowicz, A. W., Lenik, M., Trytek, A., & Tupaj, M. (2022). Calorimetric Method for the Testing of Thermal Coefficients of the TIG Process. Materials, 15(20), 7389. https://doi.org/10.3390/ma15207389











