Mixing Enthalpies of Liquid Ag–Mg–Pb Alloys: Experiment vs. Thermodynamic Modeling
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37. [Google Scholar] [CrossRef]
- Plevachuk, Y. Electrophysical Properties of Mg-Pb Based Liquid Alloys and Their Application; Kainer, K.U., Ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Singh, P.; Rana, P.J.S.; Mukherejee, R.; Srivastava, P. A step towards environmental benign Mg/Pb based binary metal mixed halide perovskite material. Sol. Energy 2018, 170, 769. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloy. Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Baran, A.; Polański, M. Magnesium-based materials for hydrogen storage—A scope review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef] [PubMed]
- Mendis, C.L.; Oh-ishi, K.; Hono, K. Enhanced age hardening in a Mg–2.4 at.% Zn alloy by trace additions of Ag and Ca. Scr. Mater. 2007, 57, 485. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282. [Google Scholar] [CrossRef]
- Pęska, M.; Smektalska, K.; Dworecka-Wójcik, J.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Dębski, A.; Polański, M. Hydrogen sorption behavior of mechanically synthesized Mg–Ag alloys. Int. J. Hydrogen Energy 2021, 46, 33152. [Google Scholar] [CrossRef]
- Dębski, A.; Terlicka, S.; Sypien, A.; Gąsior, W.; Pęska, M.; Polański, M. Hydrogen Sorption Behavior of Cast Ag-Mg Alloys. Materials 2022, 15, 270. [Google Scholar] [CrossRef]
- Dębski, A.; Gierlotka, W.; Gąsior, W. Calorimetric studies and thermodynamic calculations of the Ag-Mg system. J. Alloys Compd. 2022, 891, 161937. [Google Scholar] [CrossRef]
- Terlicka, S.; Dębski, A.; Sypien, A.; Gąsior, W.; Budziak, A. Determination of thermophysical and thermodynamic properties, of Ag-Mg alloys. Mater. Today Commun. 2021, 29, 102946. [Google Scholar] [CrossRef]
- Dębski, A.; Gierlotka, W.; Terlicka, S.; Gąsior, W. On the Mg-Pb system. Calorimetric studies and thermodynamic calculations. J. Alloys Compd. 2021, 861, 158396. [Google Scholar] [CrossRef]
- Zimmermann, B. Calculative and Experimental Optimization of Binary and Ternary Ag, Bi, Pb and T1 Systems. Ph.D. Thesis, Universitat Stuttgart, Stuttgart, German, 1976. [Google Scholar]
- Elliott, R.P.; Shunk, F.A. The Ag–Pb (Silver-Lead) System. Bull. Alloy Phase Diagr. 1980, 1, 56. [Google Scholar] [CrossRef]
- Ashtakala, S.; Pelton, A.D.; Bale, C.W. The Ag-Pb (Silver-Lead) System. Bull. Alloy Phase Diagr. 1981, 2, 81. [Google Scholar] [CrossRef]
- Karakaya, I.; Thompson, W.T. The Ag-Pb (Silver-Lead) system. Bull. Alloy Phase Diagr. 1987, 8, 326. [Google Scholar] [CrossRef]
- Gierlotka, W.; Łapsa, J.; Fitzner, K. Thermodynamic Description of the Ag-Pb-Te Ternary System. J. Phase Equlib. Diff. 2010, 31, 509. [Google Scholar] [CrossRef]
- Hassam, S.; Boa, D.; Rogez, B.P.J. Critical assessment and optimization of the Ag–Au–Pb system. Thermochim. Acta 2010, 510, 37. [Google Scholar] [CrossRef]
- Du, J.-Y.; Zemanova, A.; Hutabalian, Y.; Kroupa, A.; Chen, S.-W. Phase diagram of Ag–Pb–Sn system. Calphad 2020, 71, 101997. [Google Scholar] [CrossRef]
- Heycock, C.T.; Neville, F.H. Complete Freezing-point Curves of Binary Alloys, containing Silver or Copper together with another metal. Philos. Trans. R. Soc. Lond. Ser. A 1897, 189, 25. [Google Scholar]
- Petrenko, G.J. On the Alloying of Silver with Lead and Tin. Z. Anorg. Chem. 1907, 53, 200. [Google Scholar]
- Friedrich, K. Lead and Silver. Metallurgie 1906, 3, 396. [Google Scholar]
- Yoldi, F. The Lead-Silver System. An. Soc. Espan. Fis. Quim. 1930, 28, 1055. [Google Scholar]
- Kleppa, O.J. Thermodynamic Properties of Moderately Dilute Liquid Solutions of Copper, Silver and Gold in Thallium, Lead and Bismuth. J. Phys. Chem. 1956, 60, 446. [Google Scholar] [CrossRef]
- Preckshot, G.W.; Hudrlik, R.E. Diffusion in the solid silver-molten lead system. Trans. AIME 1960, 218, 516. [Google Scholar]
- Glazov, V.M.; Akopyan, R.A.; Timoshina, G.G. Determination of the Position of the Retrograde Solidus in the Ag-Pb and Ag-Bi Systems. Izvest. Akad. Nauk SSSR Met. 1975, 1, 162. [Google Scholar]
- Akopyan, R.A.; Mamedova, S.K.; Kerimov, E.R. A Study of the Retrograde Solidus Curves in the Systems Ag−Pb and Ag−Bi. Izv V. U. Z. Tsvetn. Metall. 1983, 6, 83. [Google Scholar]
- Pollock, D.D. Solubility Limits of Some Silver-Rich Binary Solid Solutions near Room Temperature. Trans. AIME 1967, 239, 1768. [Google Scholar]
- Kusunoki, K.; Tsumuraya, K.; Nishikawa, S. Diffusion of Ag in dilute Pb (Ag) alloys. Trans. Jpn. Inst. Met. 1981, 22, 501. [Google Scholar] [CrossRef][Green Version]
- Kawakami, M. A Further Investigation of the Heat of Mixture in Molten Metals. Sci. Rep. Tohoku Imp. Univ. 1930, 19, 521. [Google Scholar]
- von Samson-Himmelstjerna, H.O. Heat capacity and heat of formation of molten alloys. Z. Metallkd. 1936, 28, 197. [Google Scholar]
- Ehrlich, K. The Enthalpy of Mixing of Silver and Magnesium with Some B Metals in Liquid Binary Systems. Inaugural Thesis, Ludwig-Maximilians-Universität, München, Germany, 1965. [Google Scholar]
- Kozuka, Z.; Oishi, T.; Moriyama, J. Measurements of the Thermodynamic Properties of Molten Ag-Pb Alloys by Calorimetry. Nippon Kihz. Gakk. 1968, 32, 136. [Google Scholar]
- Castanet, R.; Claire, Y.; Lafitte, M. Enthalpie de formation à 1 280 k des alliages liquides d’argent avec le germanium, l’étain et le plomb. J. Chim. Phys. 1969, 66, 1276. [Google Scholar] [CrossRef]
- Itagaki, K.; Yazawa, A. Measurements of Heats of Mixing in Liquid Silver Binary Alloys. J. Jpn. Inst. Met. 1968, 32, 1294. [Google Scholar] [CrossRef][Green Version]
- Hultgren, R.; Sommelet, P. The magnitude of some of the errors in determining heats of formation of liquid alloys by drop calorimetry; the silver-lead system. In Proceedings of the First International Conference on Calorimetry and Thermodynamics, Warsaw, Poland, 31 August–4 September 1969; p. 1027. [Google Scholar]
- Hager, J.P.; Wilkomirsky, I.A. Galvanic cell studies using a molten oxide electrolyte. Pt. 1. Thermodynamic properties of the lead-silver system. Trans. AIME 1968, 242, 183. [Google Scholar]
- Terpilowski, J. Thermodynamic Properties of Liquid Metallic Solutions. (I) Ag−Pb System. Arch. Hutnictwa 1957, 2, 289. [Google Scholar]
- Eremenko, V.N. Thermodynamic Activity of Pb in Melted Pb−Ag Alloys. Ukr. Khim. Zh. 1957, 23, 6. [Google Scholar]
- Iwase, M.; Fujimura, K.; Mori, T. Thermodynamic study of liquid lead-silver alloys by means of solid-oxide galvanic cell. Trans. Jpn. Inst. Met. 1978, 19, 377. [Google Scholar] [CrossRef]
- Jacob, K.T.; Jeffes, J.H.E. Activities of oxygen and lead in liquid Pb + Ag + O solutions. J. Chem. Thermodyn. 1971, 3, 433. [Google Scholar] [CrossRef]
- Aldred, A.T.; Pratt, J.N. Thermodynamic properties of liquid silver-lead alloys. With an appendix on the vapour pressure of lead. Trans. Faraday Soc. 1961, 57, 611. [Google Scholar] [CrossRef]
- Khobdabergenov, R.Z.; Nesterov, V.I.; Ivragimov, E.T.; Shendyapin, A.S.; Vasharatyan, E.I.; Kalinin, E.I. The Vapour Pressure of Lead in the Silver-Lead System. Trans. Inst. Met. i Obogashch. Akad. Nauk Kaz. SSR 1967, 26, 37. [Google Scholar]
- Granovskaya, A.P.; Lyubimov, A.P. Measurement of Small Vapor Pressures at High Temperatures (IV) Partial Vapor Pressures of Components of the Ag− Pb System. Z. Fiz. Khim. 1953, 27, 1437. [Google Scholar]
- Scientific Group Thermodata Europe. Unary SGTE Database 5.0. Available online: https://www.sgte.net/en/free-pure-substance-database (accessed on 19 September 2022).
- Muggianu, Y.M.; Gambino, M.; Bros, J.-P. Enthalpies de formation des alliages liquides bismuth-etain-gallium a 723 k. choix d’une representation analytique des grandeurs d’exces integrales e t partielles de melange. J. Chim. Phys. 1975, 72, 83. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 1948, 40, 345. [Google Scholar] [CrossRef]
- Gąsior, W.; Dębski, A.; Zabrocki, M. Thermodynamic description of the Ge-In-Li liquid alloys. J. Mol. Liq. 2018, 260, 415. [Google Scholar] [CrossRef]
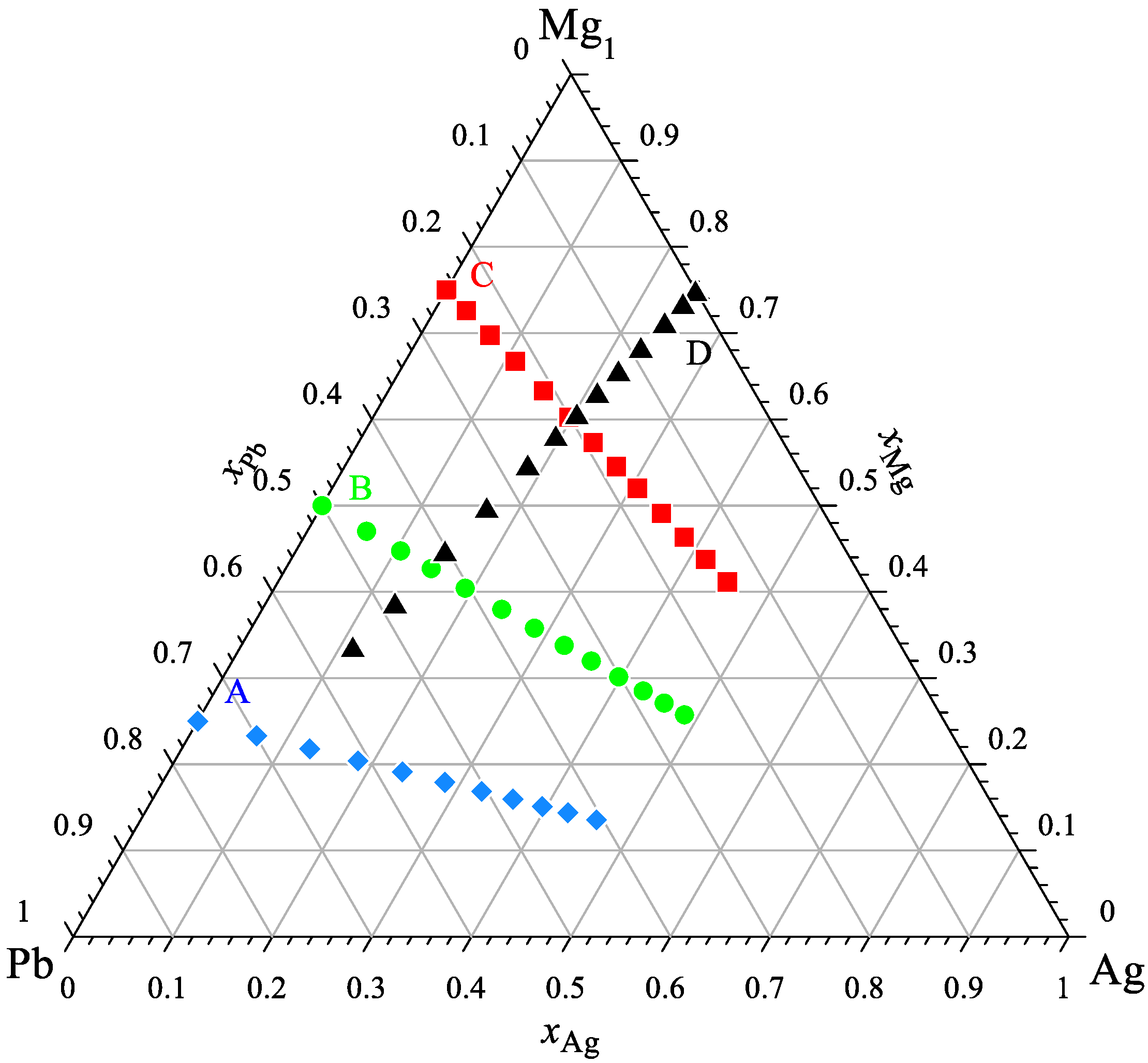
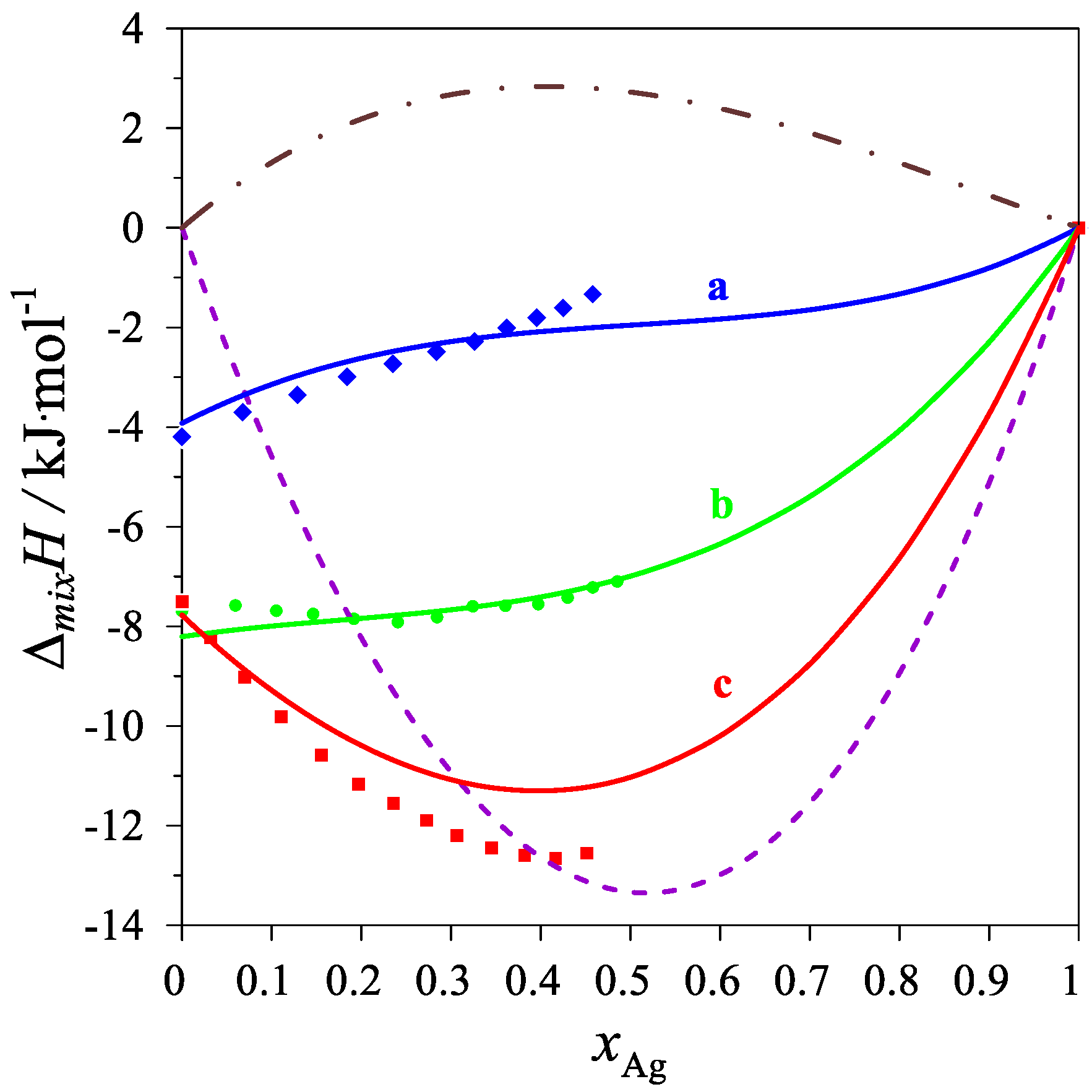
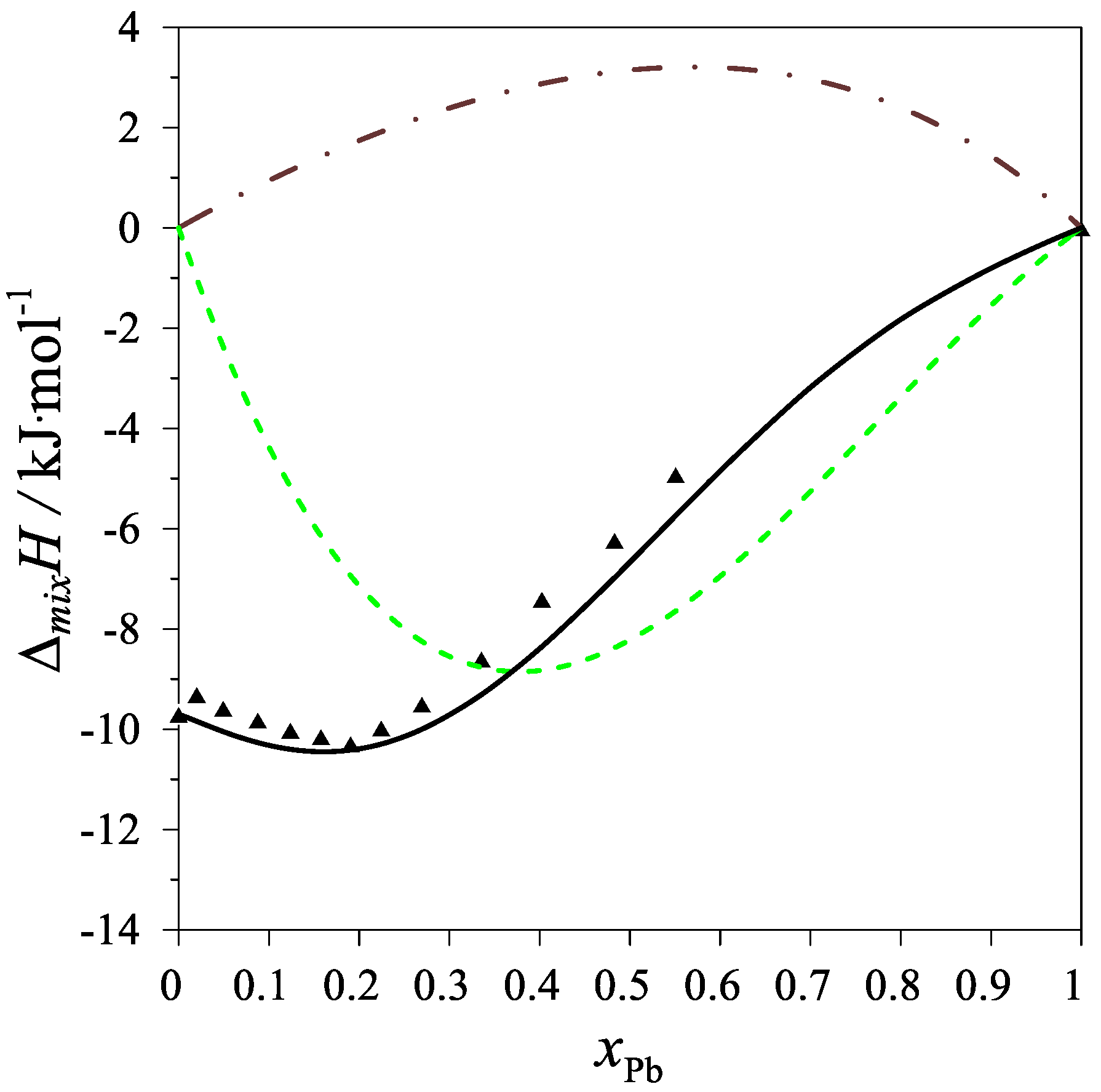


| Chemical Name | Source | Purity [Mass%] | Purification Method | Analysis Method |
|---|---|---|---|---|
| Magnesium | Alfa Aesar | 99.9 | None | Certified purity |
| Lead | POCH | 99.999 | None | Certified purity |
| Silver | Innovator Sp. z o.o. | 99.9 | None | Certified purity |
| Argon | Air Products | 99.9999 | None | Certified purity |
| Number of Dropped Moles of Ag [mol] | Heat Effect ΔHSignal·K [kJ] | Mole Fraction xAg | Integral Molar Enthalpy ΔmixH [kJ/mol] | Standard Uncertainties u(ΔmixH) [kJ/mol] | ||
|---|---|---|---|---|---|---|
| = 0.04 kJ/mol. | ||||||
| 0.000929 | 0.034250 | 0.003 | 0.0677 | −3.7 | 3.1 | 0.06 |
| 0.000959 | 0.033922 | 0.002 | 0.1287 | −3.4 | 1.6 | 0.08 |
| 0.000996 | 0.035925 | 0.002 | 0.1840 | −3.0 | 2.3 | 0.11 |
| 0.001044 | 0.036470 | 0.001 | 0.2350 | −2.7 | 1.2 | 0.13 |
| 0.001137 | 0.039690 | 0.001 | 0.2837 | −2.5 | 1.1 | 0.15 |
| 0.001119 | 0.038816 | 0.001 | 0.3260 | −2.3 | 0.9 | 0.16 |
| 0.001072 | 0.039129 | 0.003 | 0.3620 | −2.0 | 2.8 | 0.18 |
| 0.001115 | 0.039747 | 0.002 | 0.3956 | −1.8 | 1.9 | 0.20 |
| 0.001077 | 0.038824 | 0.002 | 0.4249 | −1.6 | 2.3 | 0.22 |
| 0.001353 | 0.049990 | 0.004 | 0.4579 | −1.3 | 3.2 | 0.24 |
| = 0.16 kJ/mol. | ||||||
| 0.001148 | 0.032040 | −0.007 | 0.0596 | −7.6 | −5.8 | 0.19 |
| 0.000980 | 0.023487 | −0.010 | 0.1051 | −7.7 | −9.8 | 0.21 |
| 0.000974 | 0.024040 | −0.009 | 0.1462 | −7.8 | −9.1 | 0.23 |
| 0.001192 | 0.028836 | −0.011 | 0.1916 | −7.8 | −9.6 | 0.25 |
| 0.001446 | 0.035855 | −0.013 | 0.2407 | −7.9 | −9.0 | 0.28 |
| 0.001457 | 0.040151 | −0.009 | 0.2844 | −7.8 | −6.2 | 0.30 |
| 0.001494 | 0.044598 | −0.006 | 0.3243 | −7.6 | −3.9 | 0.33 |
| 0.001521 | 0.040084 | −0.011 | 0.3606 | −7.6 | −7.4 | 0.36 |
| 0.001724 | 0.046074 | −0.012 | 0.3973 | −7.6 | −7.0 | 0.39 |
| 0.001727 | 0.049376 | −0.009 | 0.4300 | −7.4 | −5.2 | 0.41 |
| 0.001647 | 0.050232 | −0.005 | 0.4581 | −7.2 | −3.3 | 0.44 |
| 0.001765 | 0.051050 | −0.009 | 0.4853 | −7.1 | −4.8 | 0.47 |
| = 0.44 kJ/mol. | ||||||
| 0.0011199 | 0.003841 | −0.034 | 0.0317 | −8.2 | −30.3 | 0.44 |
| 0.0014406 | 0.007875 | −0.041 | 0.0697 | −9.0 | −28.3 | 0.44 |
| 0.0016789 | 0.010824 | −0.046 | 0.1104 | −9.8 | −27.3 | 0.45 |
| 0.0020599 | 0.018174 | −0.051 | 0.1557 | −10.6 | −24.9 | 0.46 |
| 0.0020673 | 0.023159 | −0.047 | 0.1967 | −11.2 | −22.5 | 0.47 |
| 0.0021758 | 0.032061 | −0.041 | 0.2358 | −11.5 | −19.0 | 0.48 |
| 0.0022833 | 0.034419 | −0.043 | 0.2729 | −11.9 | −18.7 | 0.49 |
| 0.0022861 | 0.034784 | −0.042 | 0.3067 | −12.2 | −18.5 | 0.51 |
| 0.0029063 | 0.049882 | −0.048 | 0.3453 | −12.4 | −16.6 | 0.52 |
| 0.0031056 | 0.057853 | −0.047 | 0.3820 | −12.6 | −15.1 | 0.54 |
| 0.0032734 | 0.065761 | −0.045 | 0.4166 | −12.7 | −13.7 | 0.56 |
| 0.0037230 | 0.085006 | −0.041 | 0.4515 | −12.6 | −10.9 | 0.59 |
| Number of Dropped Moles of Pb [mol] | Heat Effect ΔHSignal·K [kJ] | Mole Fraction xPb | Integral Molar Enthalpy ΔmixH [kJ/mol] | Standard Uncertainties u(ΔmixH) [kJ/mol] | ||
|---|---|---|---|---|---|---|
| = 0.54 kJ/mol. | ||||||
| 0.0006636 | −0.012041 | −0.031 | 0.0200 | −9.3 | −46.8 | 0.55 |
| 0.0010212 | 0.010405 | −0.019 | 0.0494 | −9.6 | −18.5 | 0.56 |
| 0.0014334 | 0.018966 | −0.022 | 0.0877 | −9.8 | −15.4 | 0.57 |
| 0.0014633 | 0.020177 | −0.022 | 0.1237 | −10.0 | −14.9 | 0.58 |
| 0.0014899 | 0.022766 | −0.020 | 0.1576 | −10.1 | −13.4 | 0.59 |
| 0.0015642 | 0.023316 | −0.022 | 0.1905 | −10.3 | −13.8 | 0.60 |
| 0.0017505 | 0.045275 | −0.005 | 0.2244 | −10.0 | −2.8 | 0.62 |
| 0.0025801 | 0.069316 | −0.005 | 0.2695 | −9.5 | −1.8 | 0.65 |
| 0.0044069 | 0.128546 | 0.002 | 0.3354 | −8.6 | 0.5 | 0.70 |
| 0.0054725 | 0.174444 | 0.018 | 0.4024 | −7.4 | 3.2 | 0.76 |
| 0.0084517 | 0.253278 | 0.011 | 0.4829 | −6.2 | 1.3 | 0.84 |
| 0.0094570 | 0.306745 | 0.036 | 0.5506 | −4.9 | 3.8 | 0.92 |
| System | References | |
|---|---|---|
| Ag–Mg | 53,346.5 −3694 905.8 | [10] |
| Ag–Pb | 12,902.274 | [17] |
| −4008.088 −2576.139 | ||
| Mg–Pb | −39,272.641-5.773712·T | [12] |
| −43,546.211-16.706741·T | ||
| −4329.298-8.616737·T | ||
| 17,406.133 | ||
| 4967.802 | ||
| Ag–Mg–Pb | 4700.8075 | This study |
| 518.60761 | ||
| 39,007.392 |
| xAg | xMg | xPb | ΔmixH | |||
|---|---|---|---|---|---|---|
| kJ/mol | ||||||
| (Mg0.25Pb0.75)1−xAgx alloys at T = 1116 K | ||||||
| 0 | 0.25 | 0.75 | 5.318 | −17.286 | 0.532 | −3.923 |
| 0.05 | 0.2375 | 0.7125 | 3.880 | −18.909 | 1.121 | −3.498 |
| 0.1 | 0.225 | 0.675 | 2.646 | −20.519 | 1.790 | −3.144 |
| 0.2 | 0.2 | 0.6 | 0.744 | −23.735 | 3.302 | −2.617 |
| 0.3 | 0.175 | 0.525 | −0.490 | −27.000 | 4.930 | −2.284 |
| 0.4 | 0.15 | 0.45 | −1.161 | −30.386 | 6.529 | −2.084 |
| 0.5 | 0.125 | 0.375 | −1.379 | −33.969 | 7.949 | −1.955 |
| 0.6 | 0.1 | 0.3 | −1.261 | −37.832 | 9.027 | −1.832 |
| 0.7 | 0.075 | 0.225 | −0.929 | −42.063 | 9.594 | −1.647 |
| 0.8 | 0.05 | 0.15 | −0.514 | −46.756 | 9.465 | −1.330 |
| 0.9 | 0.025 | 0.075 | −0.155 | −52.013 | 8.444 | −0.806 |
| 1 | 0 | 0 | 0 | −57.946 | 6.318 | 0 |
| (Mg0.50Pb0.50)1−xAgx alloys at T = 1116 K | ||||||
| 0 | 0.5 | 0.5 | −5.584 | −14.433 | −1.982 | −8.207 |
| 0.05 | 0.475 | 0.475 | −6.112 | −15.669 | −0.720 | −8.090 |
| 0.1 | 0.45 | 0.45 | −6.450 | −16.889 | 0.553 | −7.996 |
| 0.2 | 0.4 | 0.4 | −6.624 | −19.354 | 3.066 | −7.840 |
| 0.3 | 0.35 | 0.35 | −6.246 | −21.982 | 5.427 | −7.669 |
| 0.4 | 0.3 | 0.3 | −5.455 | −24.926 | 7.500 | −7.410 |
| 0.5 | 0.25 | 0.25 | −4.392 | −28.340 | 9.152 | −6.993 |
| 0.6 | 0.2 | 0.2 | −3.199 | −32.379 | 10.248 | −6.346 |
| 0.7 | 0.15 | 0.15 | −2.018 | −37.201 | 10.651 | −5.396 |
| 0.8 | 0.1 | 0.1 | −0.995 | −42.964 | 10.223 | −4.070 |
| 0.9 | 0.05 | 0.05 | −0.273 | −49.826 | 8.825 | −2.296 |
| 1 | 0 | 0 | 0 | −57.946 | 6.318 | 0 |
| (Mg0.75Pb0.25)1−xAgx alloys at T = 1116 K | ||||||
| 0 | 0.75 | 0.25 | −24.819 | −2.974 | −22.182 | −7.776 |
| 0.05 | 0.7125 | 0.2375 | −22.916 | −4.358 | −18.227 | −8.579 |
| 0.1 | 0.675 | 0.225 | −21.085 | −5.781 | −14.551 | −9.285 |
| 0.2 | 0.6 | 0.2 | −17.600 | −8.792 | −7.987 | −10.393 |
| 0.3 | 0.525 | 0.175 | −14.311 | −12.114 | −2.420 | −11.077 |
| 0.4 | 0.45 | 0.15 | −11.210 | −15.881 | 2.177 | −11.304 |
| 0.5 | 0.375 | 0.125 | −8.320 | −20.248 | 5.791 | −11.030 |
| 0.6 | 0.3 | 0.1 | −5.699 | −25.395 | 8.372 | −10.201 |
| 0.7 | 0.225 | 0.075 | −3.433 | −31.518 | 9.838 | −8.757 |
| 0.8 | 0.15 | 0.05 | −1.634 | −38.831 | 10.080 | −6.627 |
| 0.9 | 0.075 | 0.025 | −0.437 | −47.560 | 8.960 | −3.736 |
| 1 | 0 | 0 | 0 | −57.946 | 6.318 | 0 |
| (Ag0.25Mg0.75)1−xPbx alloys at T = 1116 K | ||||||
| 0.25 | 0.75 | 0 | −29.880 | −2.972 | −17.047 | −9.699 |
| 0.2375 | 0.7125 | 0.05 | −27.155 | −3.919 | −16.231 | −10.053 |
| 0.225 | 0.675 | 0.1 | −23.995 | −5.219 | −14.018 | −10.323 |
| 0.2 | 0.6 | 0.2 | −17.600 | −8.792 | −7.987 | −10.393 |
| 0.175 | 0.525 | 0.3 | −12.104 | −13.056 | −2.478 | −9.716 |
| 0.15 | 0.45 | 0.4 | −7.707 | −17.004 | 1.042 | −8.391 |
| 0.125 | 0.375 | 0.5 | −3.991 | −19.697 | 2.432 | −6.669 |
| 0.1 | 0.3 | 0.6 | −0.384 | −20.577 | 2.273 | −4.847 |
| 0.075 | 0.225 | 0.7 | 3.502 | −19.653 | 1.398 | −3.181 |
| 0.05 | 0.15 | 0.8 | 7.656 | −17.564 | 0.532 | −1.826 |
| 0.025 | 0.075 | 0.9 | 11.581 | −15.514 | 0.075 | −0.806 |
| 0 | 0 | 1 | 14.334 | −15.079 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębski, A.; Gąsior, W.; Gierlotka, W.; Polański, M. Mixing Enthalpies of Liquid Ag–Mg–Pb Alloys: Experiment vs. Thermodynamic Modeling. Materials 2022, 15, 7360. https://doi.org/10.3390/ma15207360
Dębski A, Gąsior W, Gierlotka W, Polański M. Mixing Enthalpies of Liquid Ag–Mg–Pb Alloys: Experiment vs. Thermodynamic Modeling. Materials. 2022; 15(20):7360. https://doi.org/10.3390/ma15207360
Chicago/Turabian StyleDębski, Adam, Władysław Gąsior, Wojciech Gierlotka, and Marek Polański. 2022. "Mixing Enthalpies of Liquid Ag–Mg–Pb Alloys: Experiment vs. Thermodynamic Modeling" Materials 15, no. 20: 7360. https://doi.org/10.3390/ma15207360
APA StyleDębski, A., Gąsior, W., Gierlotka, W., & Polański, M. (2022). Mixing Enthalpies of Liquid Ag–Mg–Pb Alloys: Experiment vs. Thermodynamic Modeling. Materials, 15(20), 7360. https://doi.org/10.3390/ma15207360







