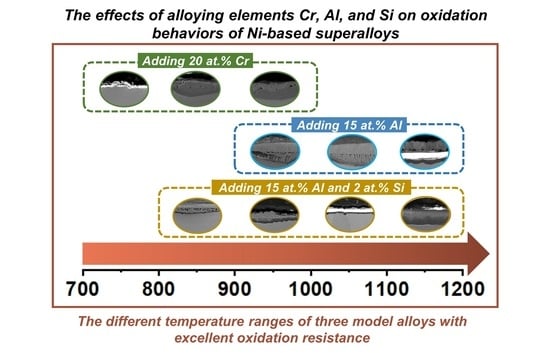
The Effects of Alloying Elements Cr, Al, and Si on Oxidation Behaviors of Ni-Based Superalloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isothermal Oxidation
2.3. Microstructure Characterization
2.4. Thermodynamic Calculation
3. Results
3.1. Initial Microstructures
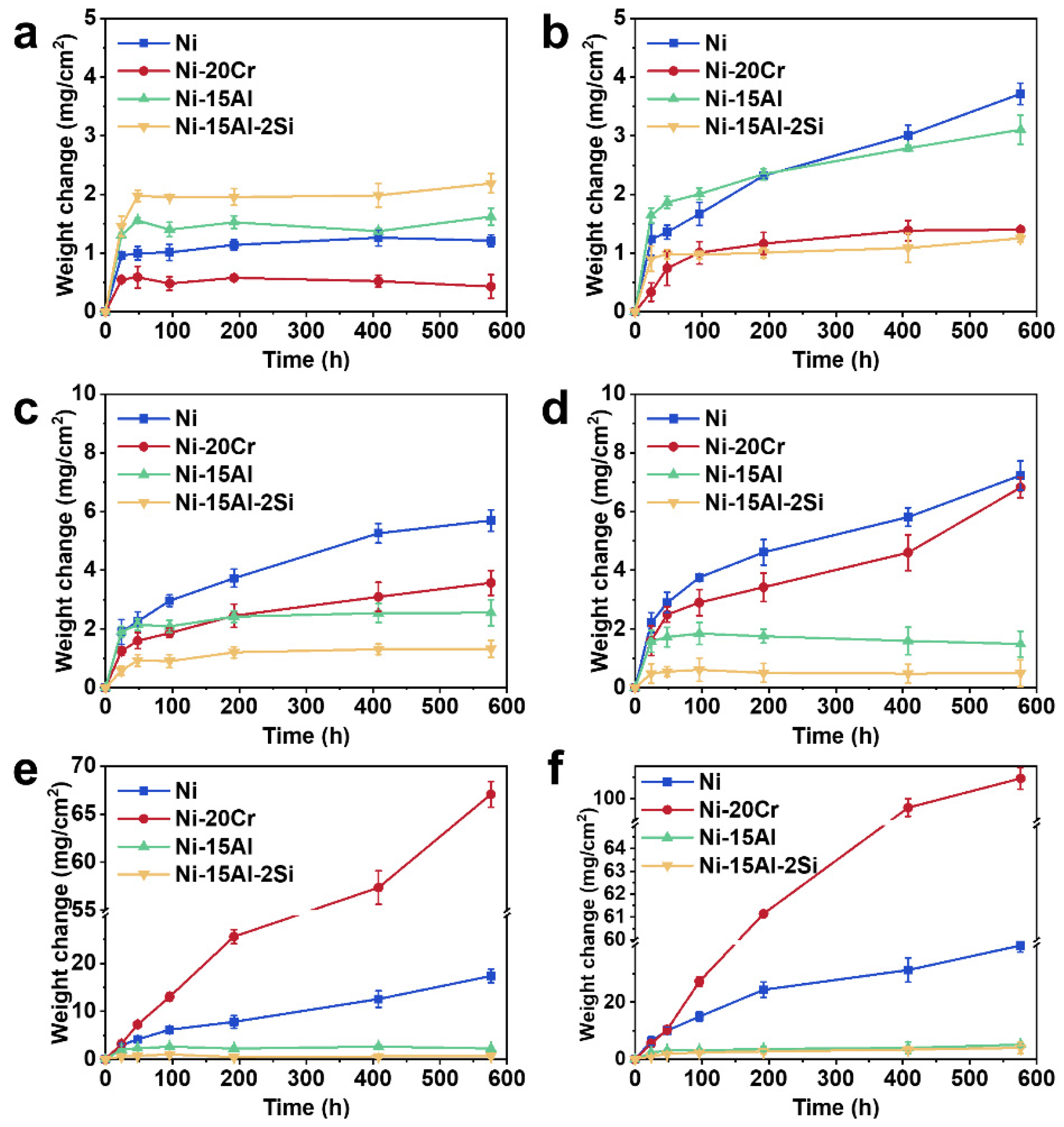
3.2. Oxidation Kinetics Curves
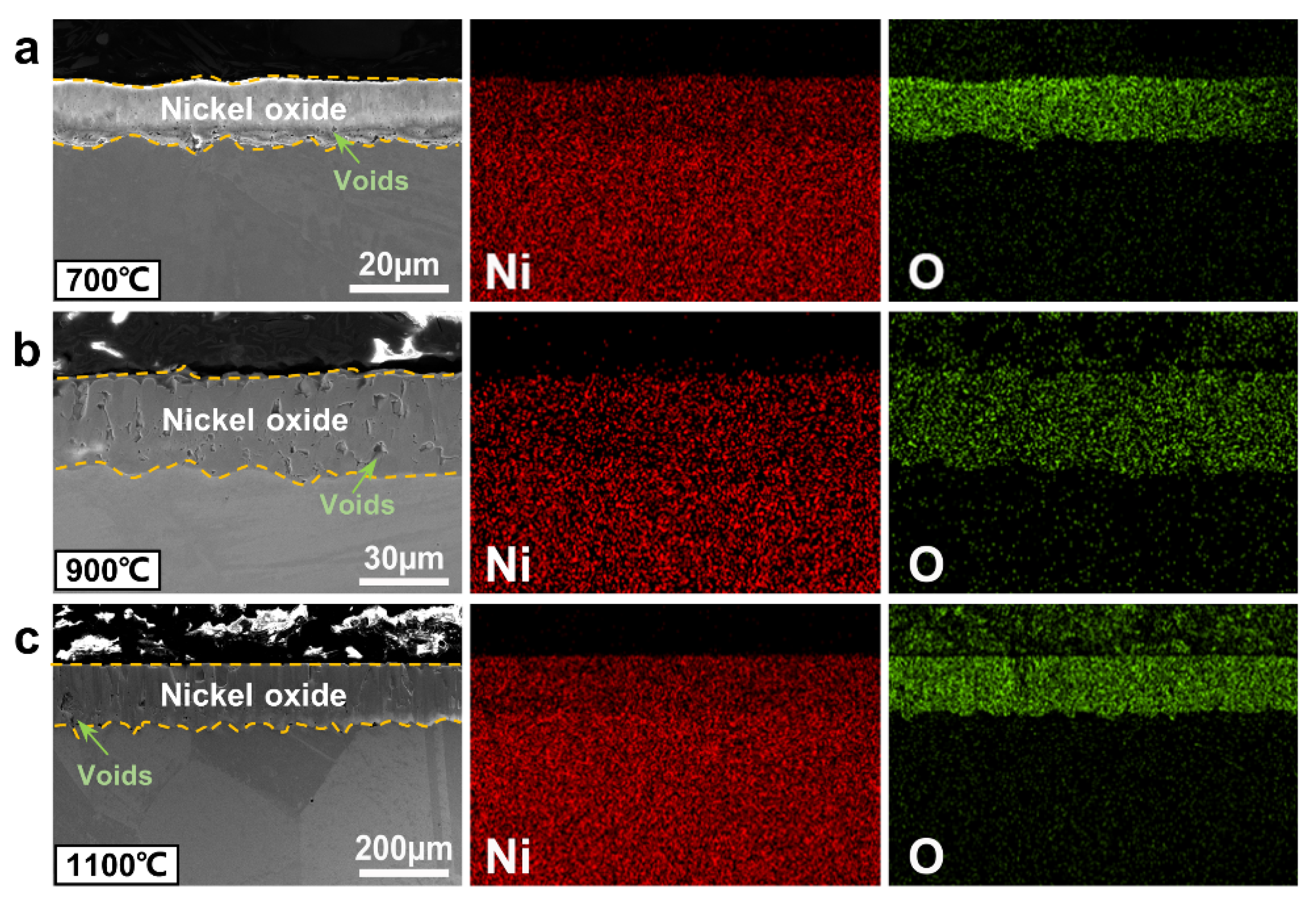
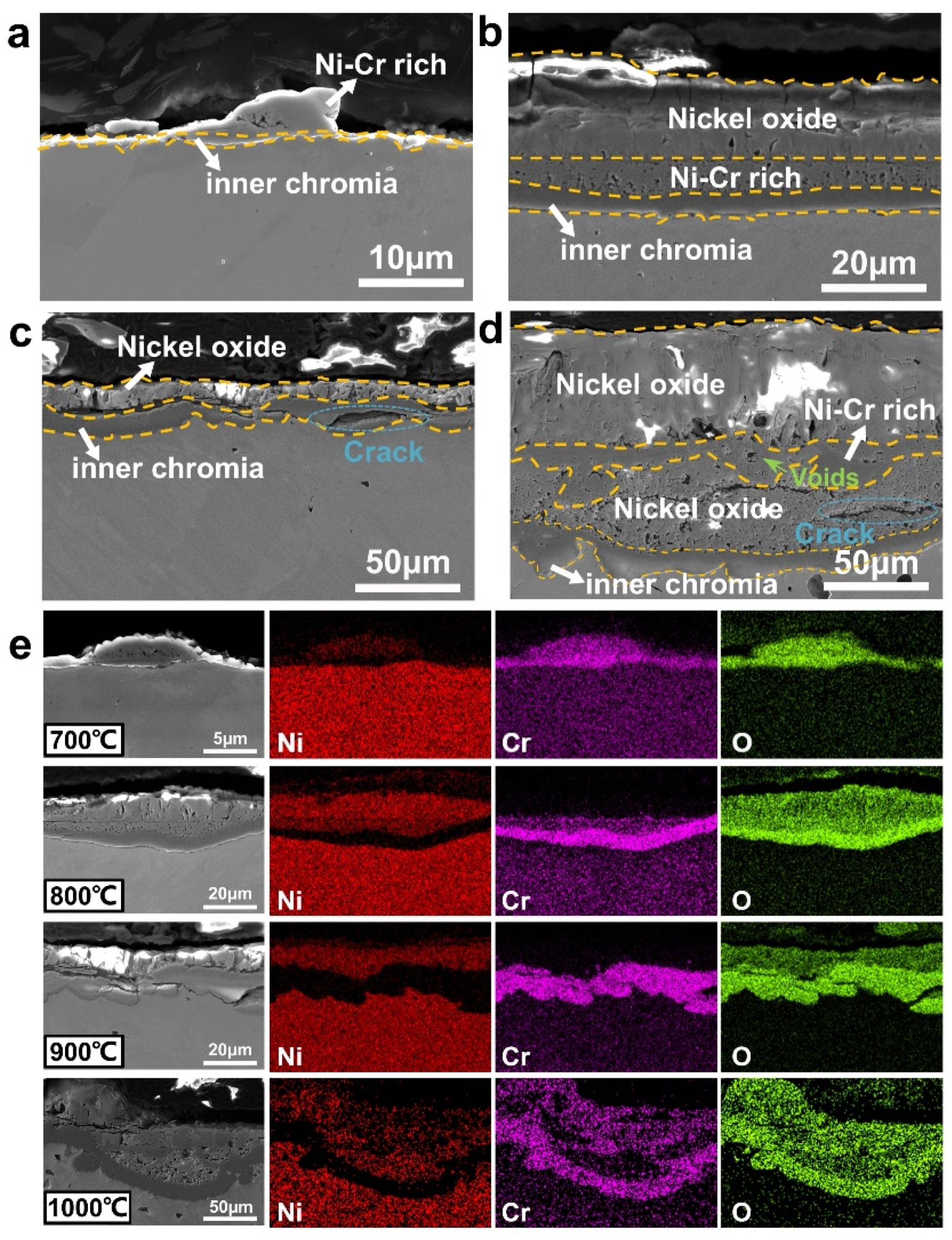
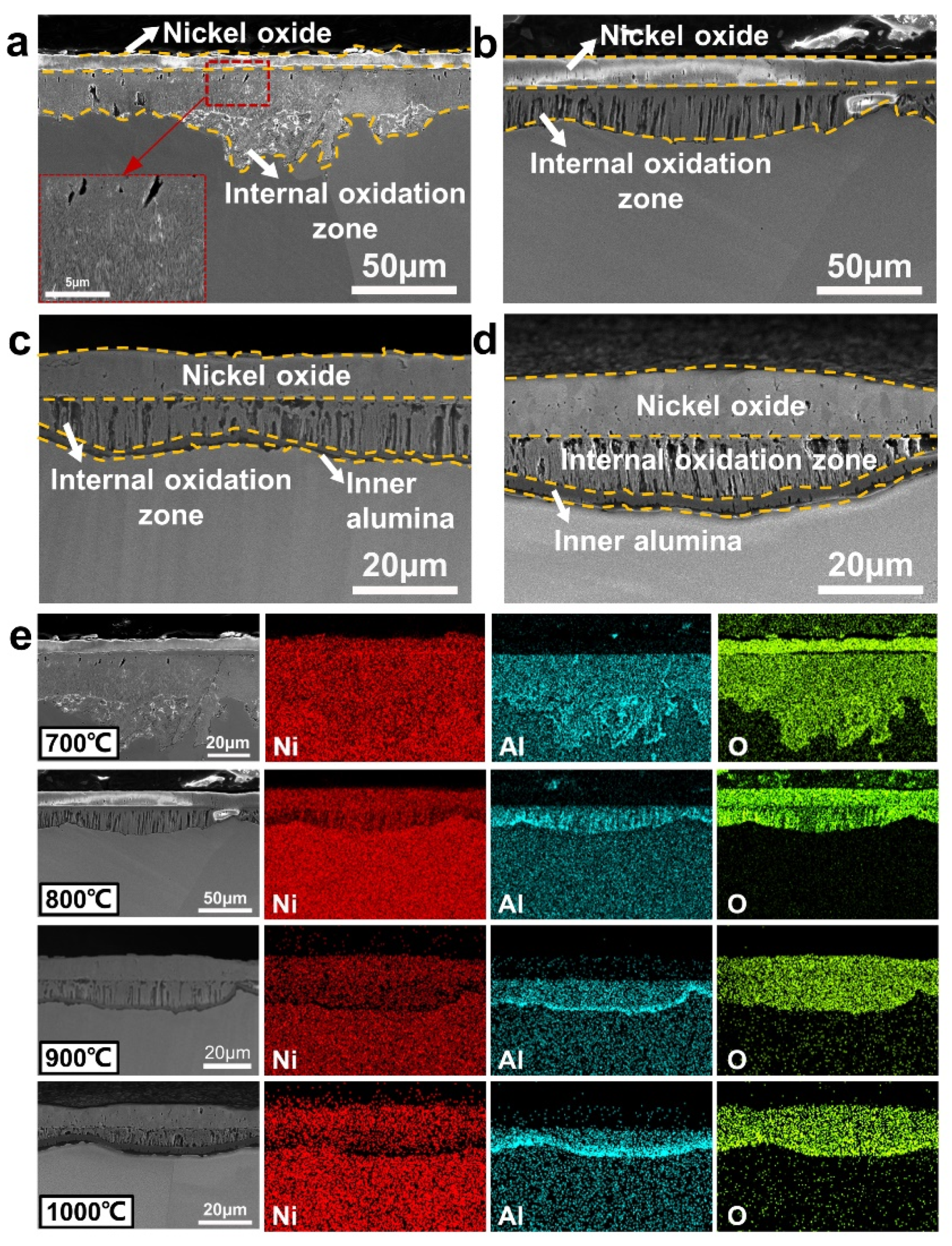
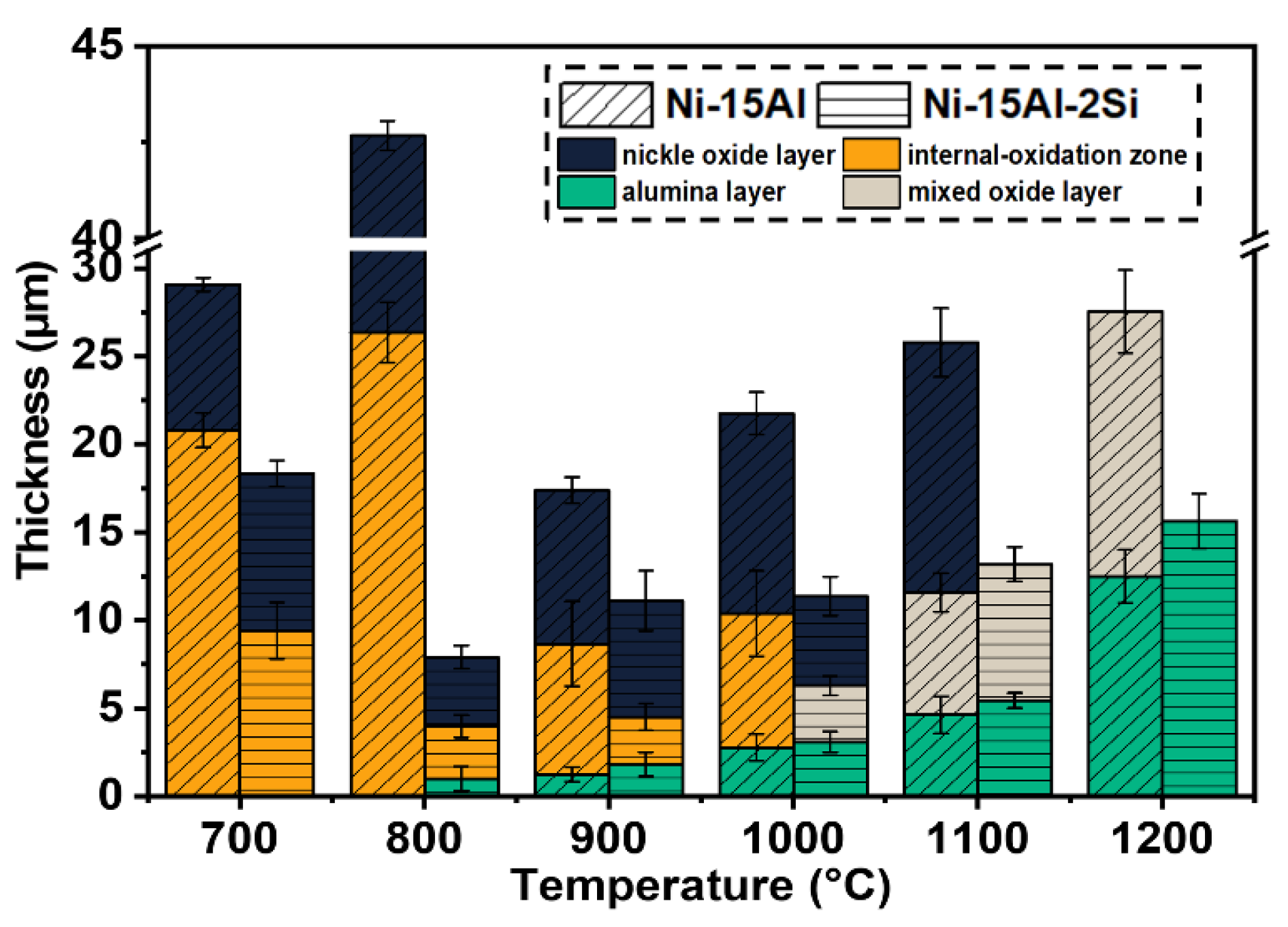
3.3. Cross-Sectional Microstructure and Composition of Oxide Scales
3.3.1. Analysis of Ni, Ni-20Cr, and Ni-15Al Oxide Scales
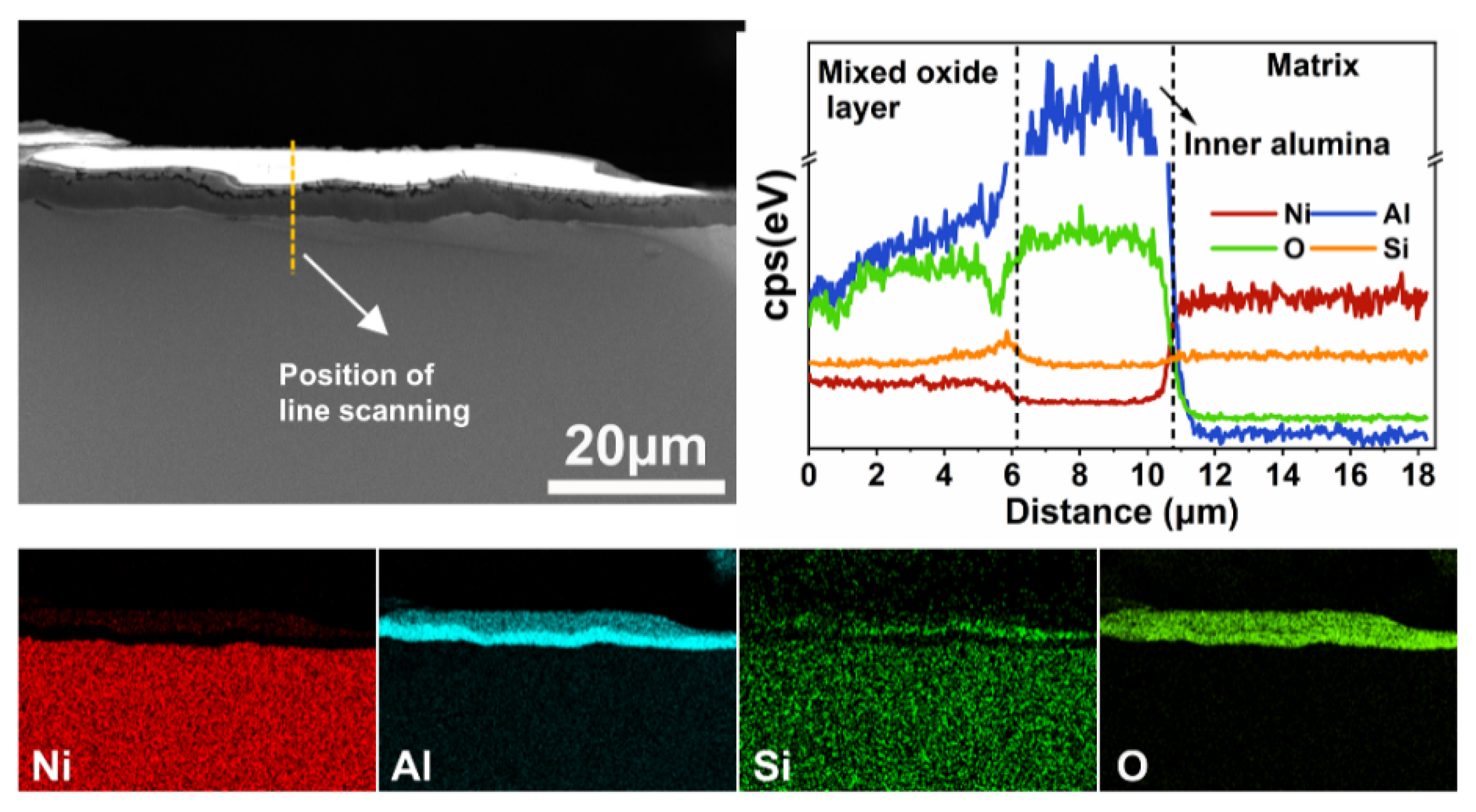
3.3.2. Analysis of Ni-15Al-2Si Oxide Scales
4. Discussion
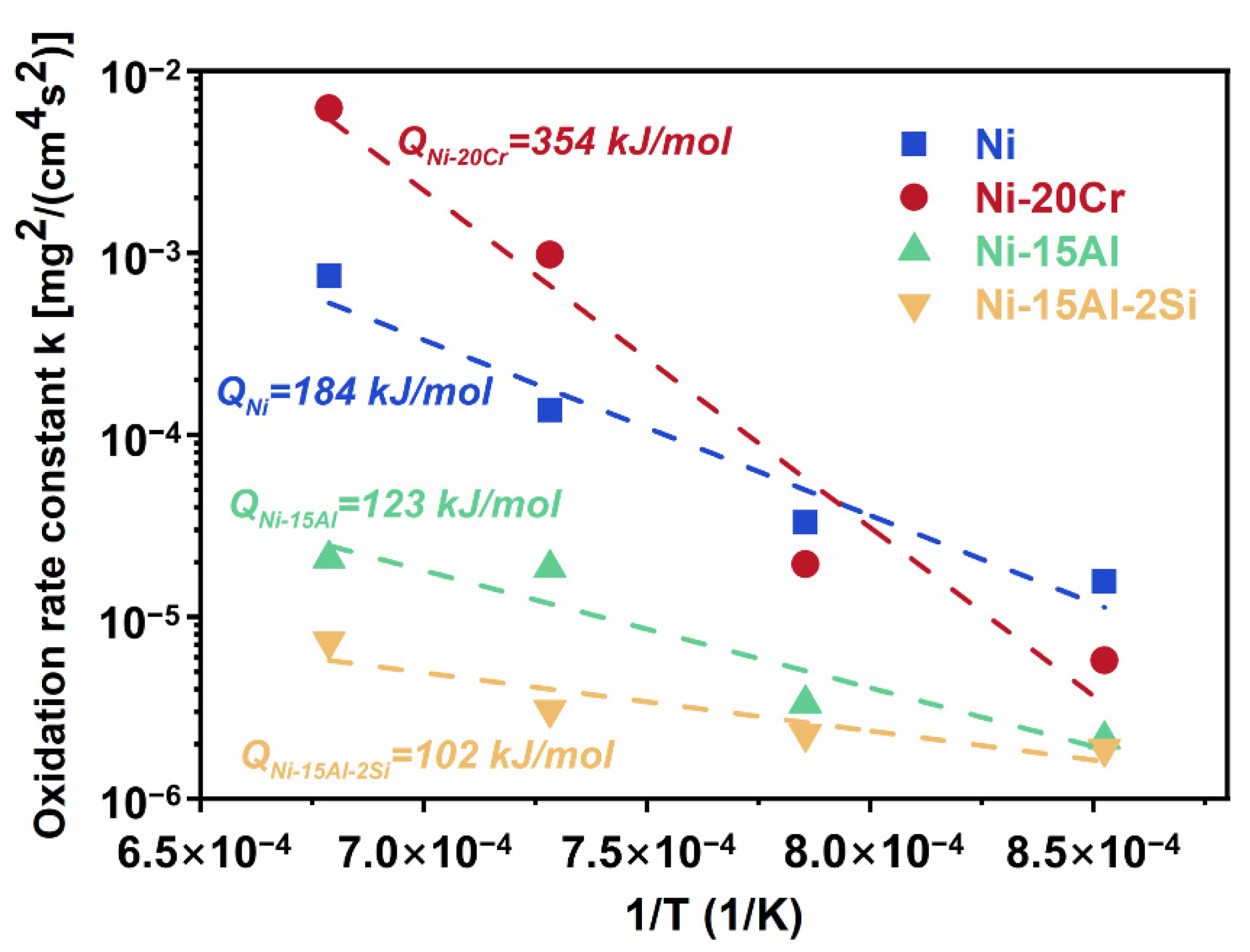
4.1. Oxidation Kinetics
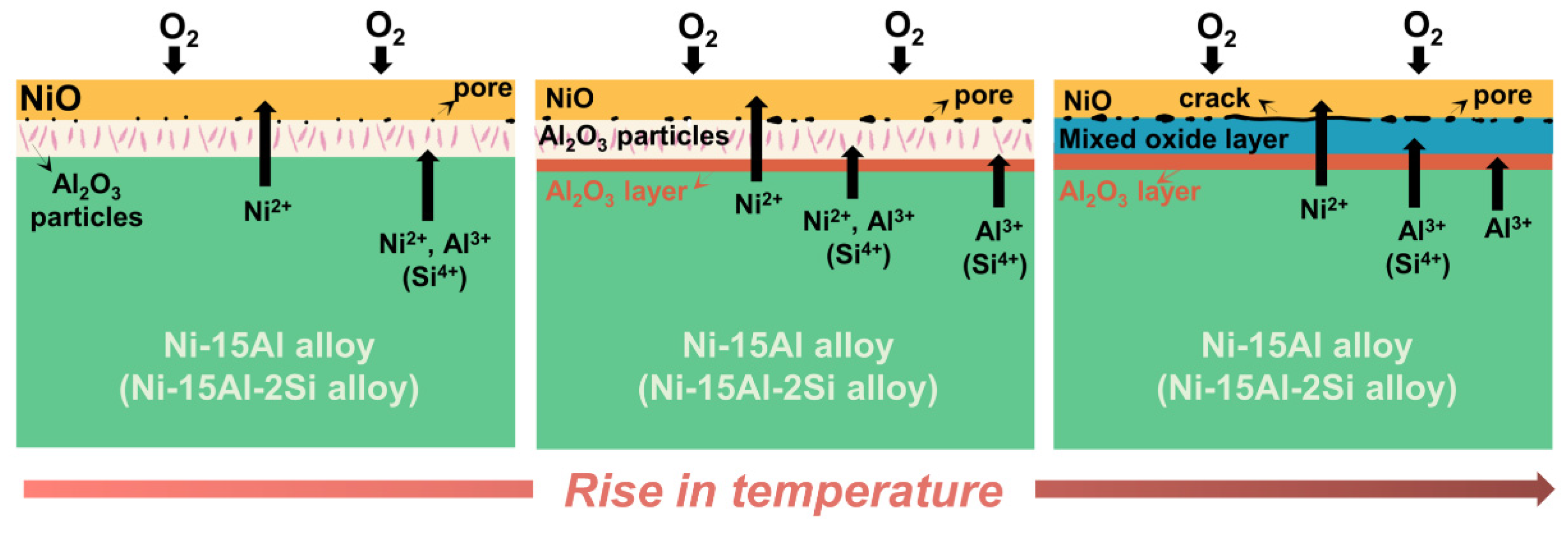
4.2. Oxidation Mechanism and Oxide Layer Formation
5. Summary and Conclusions
- The addition of 20 at.% Cr can effectively improve the oxidation resistance of Ni-based alloy by forming a thin chromia film below 900 °C. However, the protection of chromia is reduced due to formation of volatile CrO3 above 1000 °C, resulting in catastrophic oxidation.
- The addition of 15 at.% Al can form an inner alumina layer above 900 °C to provide effective protection, thus showing excellent oxidation resistance at higher temperatures. However, at temperatures below 800 °C, Al addition intensifies oxidation due to the high oxygen affinity of Al, and continuous protective scales cannot form.
- At 900 °C, the additions of Al and Cr can both improve the oxidation resistance, while the effect of Al on oxidation resistance is more pronounced than that of Cr.
- Si can form a synergistic effect with Al to affect the oxidation resistance, rather than forming a silicon oxide layer at temperatures between 700 °C and 1200 °C. By promoting alumina formation, the addition of 2 at.% Si can improve the oxidation resistance of Ni-Al alloys above 800 °C.
- At the relatively low temperature of 700 °C, the addition of Si cannot promote the formation of the protective alumina layer in Ni-Al alloy, where coarse alumina particles in the internal oxidation zone are formed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reed, R.C. The Superalloys: Fundamentals and Applications, 1st ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 1–33. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 27–141. [Google Scholar]
- Akande, I.G.; Oluwole, O.O.; Fayomi, O.S.I.; Odunlami, O.A. Overview of mechanical, microstructural, oxidation properties and high-temperature applications of superalloys. Mater. Today Proc. 2021, 43, 2222–2231. [Google Scholar] [CrossRef]
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure and properties. J. Propuls. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 1–127. [Google Scholar]
- Bose, S. High Temperature Coatings, 2nd ed.; Garcia, A.C., Ed.; Butterworth-Heinemann: Langford, Australia, 2017; pp. 53–96. [Google Scholar]
- Khanna, A.S. Handbook of Environmental Degradation of Materials, 3rd ed.; Elsevier, William Andrew: Noewich, NY, USA, 2018; pp. 117–132. [Google Scholar]
- Wang, E.; Sun, D.; Liu, H.; Lu, M.; Guo, W.; Zheng, B.; Zhang, X. The oxidation behavior of Ni–15Cr–5Al–xSi (x = 0, 1, 3, 5 wt.%) alloys in air at 1100 °C. Oxid. Met. 2019, 92, 151–166. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Ni, C.S.; Niu, Y. The effect of Si additions on the high temperature oxidation of a ternary Ni–10Cr–4Al alloy in 1atm O2 at 1100 °C. Corros. Sci. 2009, 51, 511–517. [Google Scholar] [CrossRef]
- Li, D.; Chen, G.; Li, D.; Zheng, Q.; Gao, P.; Zhang, L. Oxidation resistance of nickel-based superalloy Inconel 600 in air at different temperatures. Rare Met. 2021, 40, 3235–3240. [Google Scholar] [CrossRef]
- Bataillou, L.; Martinelli, L.; Desgranges, C.; Bosonnet, S.; Ginestar, K. Growth kinetics and characterization of chromia scales formed on Ni–30Cr alloy in impure argon at 700 °C. Oxid. Met. 2020, 93, 329–353. [Google Scholar] [CrossRef]
- Hou, P.Y. Oxidation of metals and alloys. In Shreir’s Corrosion, 1st ed.; Cottis, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 195–239. [Google Scholar]
- Tang, Z.; Hu, R.; Li, J. Isothermal oxidation behavior of Ni-20Cr-18W superalloy at 1100 °C. Rare Met. Mater. Eng. 2012, 41, 2081–2085. [Google Scholar]
- Vargel, C. Corrosion of aluminium. In Corrosion of Aluminium, 2nd ed.; Legrand, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 91–111. [Google Scholar]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al alloys between 1000 °C and 1200 °C. J. Electrochem. Soc. 1971, 118, 1782–1790. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The formation of aluminum oxide scales on high-temperature alloys. Oxid. Met. 1992, 38, 233–254. [Google Scholar] [CrossRef]
- Hayashi, S.; Narita, S.; Narita, T. Oxidation behavior of Ni-3, 6, 10 wt.% Al alloys at 800 °C. Oxid. Met. 2006, 66, 191–207. [Google Scholar] [CrossRef]
- Pei, H.; Wen, Z.; Zhang, Y.; Yue, Z. Oxidation behavior and mechanism of a Ni-based single crystal superalloy with single α-Al2O3 film at 1000 °C. Appl. Surf. Sci. 2017, 411, 124–135. [Google Scholar] [CrossRef]
- Gao, W. Developments in High Temperature Corrosion and Protection of Materials; CRC Press: Boca Raton, FL, USA, 2008; pp. 399–432. [Google Scholar]
- Wright, I.G. Oxidation of Iron-, Nickel-, and Cobalt-Base Alloys. Corrosion 1965, 21, 316–326. [Google Scholar]
- Chattopadhyay, B.; Wood, G.C. The transient oxidation of alloys. Oxid. Met. 1970, 2, 373–399. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, L.; Zhang, H.; Bai, L.; Wan, C.; Yang, G. Comparison of Isothermal Oxidation Behavior of Co-10Cr-xSi(x=0,5,10)Alloys at 1 073K. J. Beijing Inst. Technol. 2018, 27, 65–70. [Google Scholar]
- Stott, F.H. Influence of alloy additions on oxidation. Mater. Sci. Technol. 1989, 5, 734–740. [Google Scholar] [CrossRef]
- Šulhánek, P.; Drienovský, M.; Černičková, I.; Ďuriška, L.; Skaudžius, R.; Gerhátová, Ž.; Palcut, M. Oxidation of Al-Co Alloys at High Temperatures. Materials 2020, 13, 3152. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The oxidation of iron-aluminum alloys. Oxid. Met. 1992, 38, 73–87. [Google Scholar] [CrossRef]
- Wehner, B.I.; Köster, U. Microstructural Evolution of Alumina Layers on an Al–Cu–Fe Quasicrystal during High-Temperature Oxidation. Oxid. Met. 2000, 54, 445–456. [Google Scholar] [CrossRef]
- Yeh, A.C.; Kawagishi, K.; Harada, H.; Yokokawa, T.; Koizumi, Y. Development of Si-bearing 4th generation Ni-base single crystal superalloys. Carbon N. Y. 2008, 20, 619–628. [Google Scholar]
- Sato, A.; Harada, H.; Koizumi, Y.; Kobayashi, T.; Kawagishi, K.; Imai, H. Oxidation resistances of silicon-containing 5th generation Ni-base single crystal superalloys. J. Jpn. Inst. Met. 2006, 70, 180–183. [Google Scholar] [CrossRef][Green Version]
- Hindam, H.; Whittle, D.P. High temperature internal oxidation behaviour of dilute Ni-Al alloys. J. Mater. Sci. 1983, 18, 1389–1404. [Google Scholar] [CrossRef]
- Wu, Y.; Gesmundo, F.; Niu, Y. The effect of silicon on the oxidation of a Ni-6 at.% Al alloy in 1 atm of pure O2 at 900 °C. Oxid. Met. 2006, 65, 53–74. [Google Scholar] [CrossRef]
- Niu, Y.; Wu, Y.; Gesmundo, F. The oxidation of three Ni–6Si–xAl alloys in 1atm O2 at 1000 °C. Corros. Sci. 2006, 48, 1–22. [Google Scholar] [CrossRef]
- Wu, Y.; Niu, Y. High temperature scaling of Ni–xSi–10 at.% Al alloys in 1atm of pure O2. Corros. Sci. 2007, 49, 1656–1672. [Google Scholar] [CrossRef]
- Rehman, K.; Sheng, N.; Sang, Z.; Xun, S.; Wang, Z.; Xie, J.; Hou, G.; Zhou, Y.; Sun, X. Comparative study of the reactive elements effects on oxidation behavior of a Ni-based superalloy. Vacuum 2021, 191, 110382. [Google Scholar] [CrossRef]
- Taylor, M.; Ding, R.; Mignanelli, P.; Hardy, M. Oxidation behaviour of a developmental nickel-based alloy and the role of minor elements. Corros. Sci. 2022, 196, 110002. [Google Scholar] [CrossRef]
- Cheng, C.; Hu, Y.; Cao, T.; Zhang, L.; Zhu, Y.; Zhao, J. Two typical oxidation models on nickel-based superalloys under different initial surface roughness. Corros. Sci. 2020, 176, 108942. [Google Scholar] [CrossRef]
- Tan, Z.H.; Wang, X.G.; Song, W.; Yang, Y.H.; Liu, J.L. Oxidation behavior of a novel nickel-based single crystal superalloy at elevated temperature. Vacuum 2020, 175, 109284. [Google Scholar] [CrossRef]
- Douglass, D.L. The oxidation mechanism of dilute Ni-Cr alloys. Corros. Sci. 1968, 8, 665–678. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Wysong, W.S. Thin Oxide Films on Aluminum. J. Phys. Colloid Chem. 1947, 51, 1087–1103. [Google Scholar] [CrossRef]


| Samples | Ni | Cr | Al | Si |
|---|---|---|---|---|
| Ni | 100.0 | - | - | - |
| Ni-20Cr | 80.0 | 20.0 | - | - |
| Ni-15Al | 85.0 | - | 15.0 | - |
| Ni-15Al-2Si | 83.0 | - | 15.0 | 2.0 |
| Temperature | Ni | Ni-20Cr | Ni-15Al |
|---|---|---|---|
| 700 °C | 12 (±1) | 2.2 (±0.8) | 29.1 (±1.4) |
| 800 °C | 40 (±3) | 26.1 (±2.1) | 42.7 (±2.1) |
| 900 °C | 48.7 (±4.9) | 20.7 (±2.3) | 18.9 (±4.2) |
| 1000 °C | 51.6 (±3.2) | 107.9 (±11.0) | 22.7 (±4.4) |
| 1100 °C | 141.7 (±11.0) | >300 | 26.9 (±3.2) |
| 1200 °C | 336.9 (±57.2) | >400 | 27.6 (±3.7) |
| Temperatures | Ni | Ni-20Cr | Ni-15Al | Ni-15Al-2Si |
|---|---|---|---|---|
| 900 °C | 2.1 × 10−5 | 5.8 × 10−6 | 2.1 × 10−6 | 1.9 × 10−6 |
| 1000 °C | 4.4 × 10−5 | 2.0 × 10−5 | 3.3 × 10−6 | 2.3 × 10−6 |
| 1100 °C | 9.0 × 10−5 | 9.8 × 10−4 | 1.9 × 10−5 | 3.1 × 10−6 |
| 1200 °C | 6.6 × 10−4 | 6.2 × 10−3 | 2.1 × 10−5 | 7.3 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Ding, Q.; Wei, X.; Zhang, Z.; Bei, H. The Effects of Alloying Elements Cr, Al, and Si on Oxidation Behaviors of Ni-Based Superalloys. Materials 2022, 15, 7352. https://doi.org/10.3390/ma15207352
Ma S, Ding Q, Wei X, Zhang Z, Bei H. The Effects of Alloying Elements Cr, Al, and Si on Oxidation Behaviors of Ni-Based Superalloys. Materials. 2022; 15(20):7352. https://doi.org/10.3390/ma15207352
Chicago/Turabian StyleMa, Suyu, Qingqing Ding, Xiao Wei, Ze Zhang, and Hongbin Bei. 2022. "The Effects of Alloying Elements Cr, Al, and Si on Oxidation Behaviors of Ni-Based Superalloys" Materials 15, no. 20: 7352. https://doi.org/10.3390/ma15207352
APA StyleMa, S., Ding, Q., Wei, X., Zhang, Z., & Bei, H. (2022). The Effects of Alloying Elements Cr, Al, and Si on Oxidation Behaviors of Ni-Based Superalloys. Materials, 15(20), 7352. https://doi.org/10.3390/ma15207352