Lithium Accumulation in Salvinia natans Free-Floating Aquatic Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Element Analysis
2.3. Photosynthetic Pigments
2.4. Antioxidant Content
2.5. FT–IR Analysis
2.6. Bioaccumulation Factor
2.7. Data Analysis
3. Results and Discussion
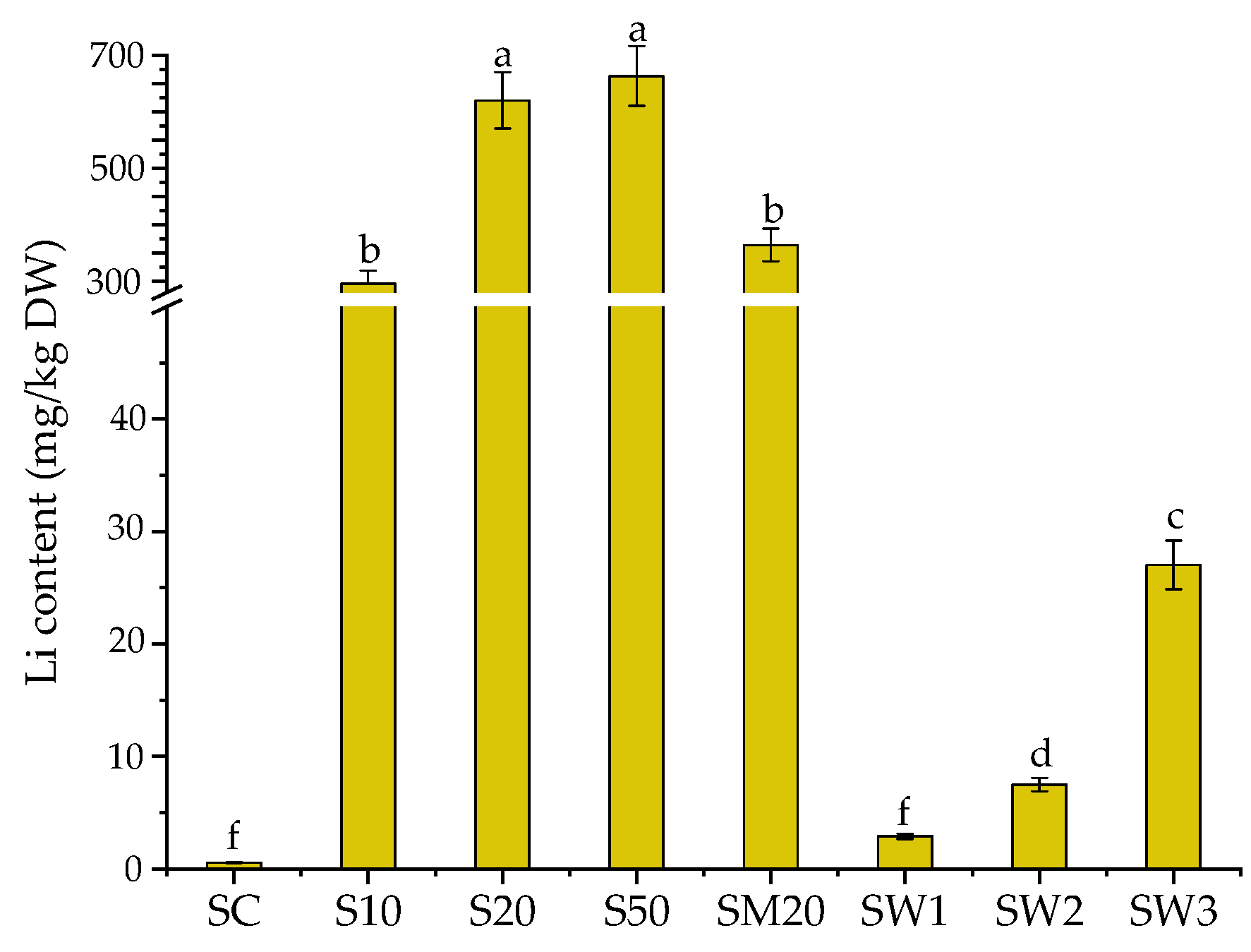
3.1. Li Uptake by S. natans
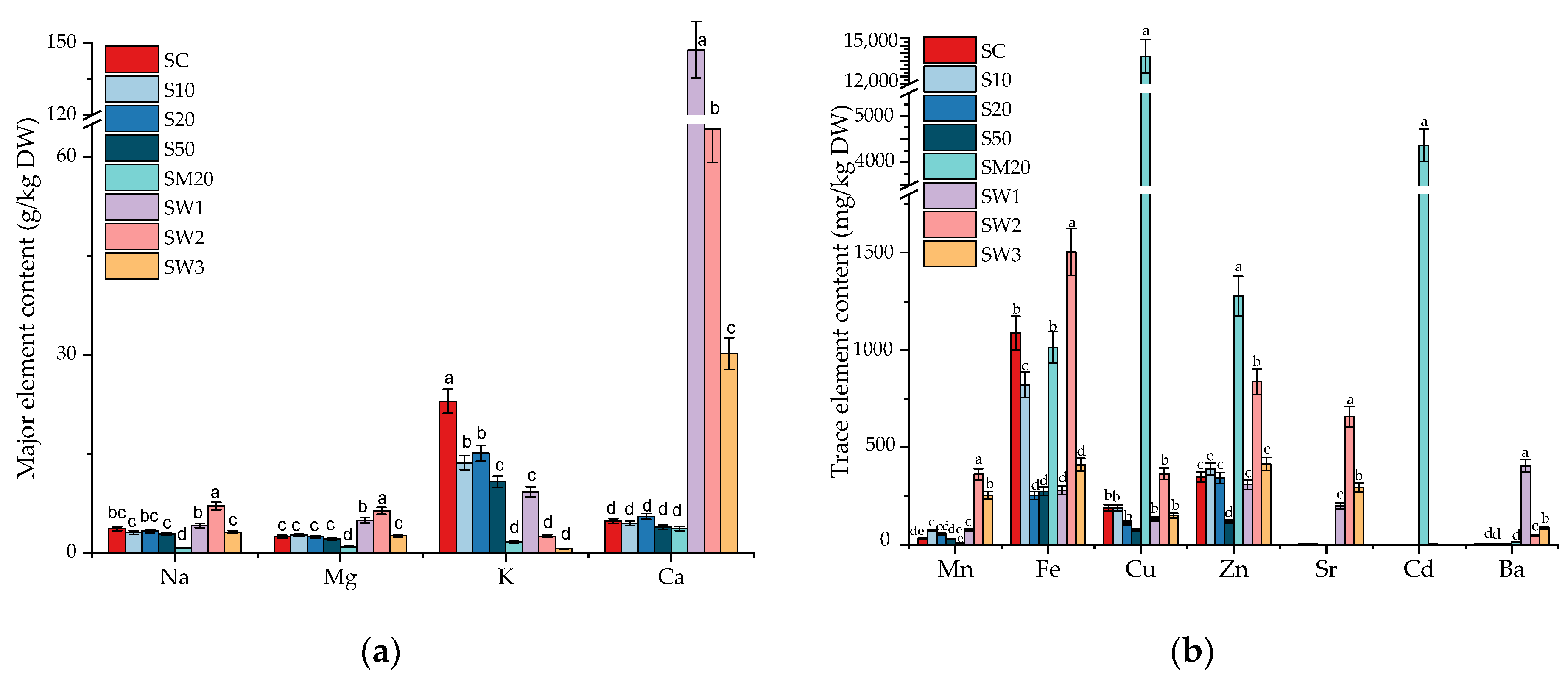
3.2. Major and Trace Element Contents in S. natans Exposed to Li
3.3. Bioaccumulation Factor
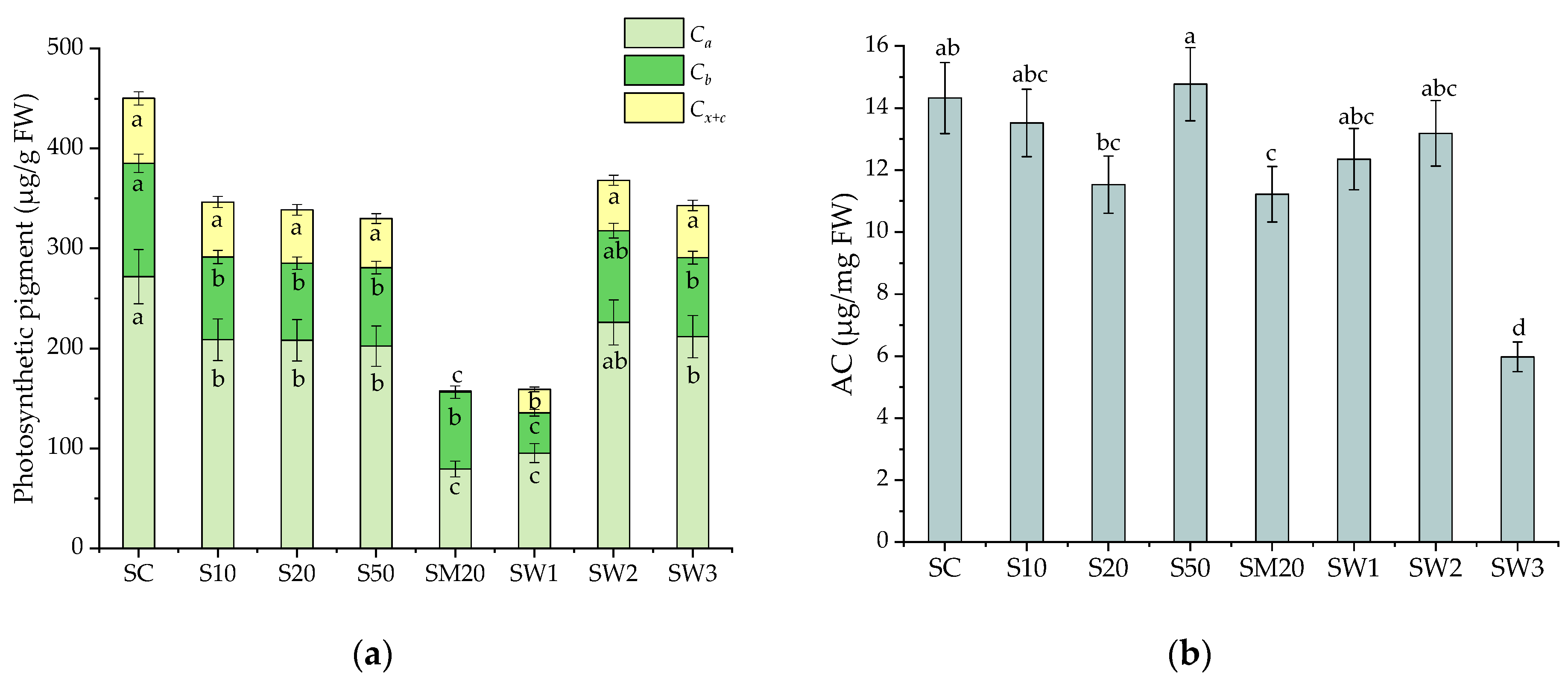
3.4. Photosynthetic Pigments and Antioxidant Capacity of S. natans after Li Stress Conditions
3.5. Elemental Composition
3.6. FT–IR Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Md Din, M.F.; Taib, S.M.; Sabbagh, F.; Sairan, F.M. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J. Environ. Manag. 2015, 163, 125–133. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lee, J.; Haiping, G.; Kim, K.-H.; Wanxi, P.; Bhardwaj, N.; Oh, J.-M.; Brown, R.J.C. Plant-based remediation of air pollution: A review. J. Environ. Manag. 2022, 301, 113860. [Google Scholar] [CrossRef] [PubMed]
- Wani, R.A.; Ganai, B.A.; Shah, M.A.; Uqab, B. Heavy metal uptake potential of aquatic plants through phytoremediation technique—A review. J. Bioremediat. Biodegrad. 2017, 8, 404. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Khellaf, N.; Djelal, H.; Amrane, A. An Overview of the valorization of aquatic plants in effluent depuration through phytoremediation processes. Appl. Microbiol. 2022, 2, 309–318. [Google Scholar] [CrossRef]
- Emiliani, J.; Oyarce, W.G.L.; Salvatierra, L.; Novo, L.A.B.; Pérez, L.M. Evaluation of Cadmium Evaluation of cadmium bioaccumulation-related physiological effects in Salvinia biloba an insight towards its use as pollutant bioindicator in water reservoirs. Plants 2021, 10, 2679. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Pardha Saradhi, P.; Nasim, S.A. Physiological and antioxidant responses of Salvinia natans exposed to chromium-rich wastewater. Ecotoxicol. Environ. Saf. 2009, 72, 1790–1797. [Google Scholar] [CrossRef]
- Horvat, T.; Vidakovic-Cifrek, Z.; Orescanin, V.; Tkalec, M.; Pevalek-Kozlina, B. Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci. Total Environ. 2007, 384, 229–238. [Google Scholar] [CrossRef]
- Torok, A.; Gulyas, Z.; Szalai, G.; Kocsy, G.; Majdik, C. Phytoremediation capacity of aquatic plants is associated with the degree of phytochelatin polymerization. J. Hazard. Mater. 2015, 299, 371–378. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Saradhi, P.P. Potential of Aquatic Macrophytes for Removing Contaminants from the Environment. Crit. Rev. Environ. Sci. Technol. 2009, 39, 754–781. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-DAIM, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of floating aquatic plants in phytoremediation of heavy metals polluted water A. Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Xing, W.; Wu, H.; Hao, B.; Liu, G. Metal accumulation by submerged macrophytes in eutrophic lakes at the watershed scale. Environ. Sci. Pollut. Res. Int. 2013, 20, 6999–7008. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Quiñones, F.R.; Módenes, A.N.; Thomé, L.P.; Palácio, S.M.; Trigueros, D.E.G.; Oliveira, A.P.; Szymanski, N. Study of the bioaccumulation kinetic of lead by living aquatic macrophyte Salvinia auriculata. Chem. Eng. J. 2009, 150, 316–322. [Google Scholar] [CrossRef]
- Prado, C.; Rosa, M.; Pagano, E.; Prado, F. Metabolic interconnectivity among alternative respiration, residual respiration, carbohydrates and phenolics in leaves of Salvinia minima exposed to Cr(VI). Environ. Exp. Bot. 2013, 87, 32–38. [Google Scholar] [CrossRef]
- Polechońska, L.; Klink, A.; Dambiec, M. Trace element accumulation in Salvinia natans from areas of various land use types. Environ. Sci. Pollut. Res. 2019, 26, 30242–30251. [Google Scholar] [CrossRef]
- Dhir, B.; Srivastava, S. Heavy metal tolerance in metal hyperaccumulator plant, Salvinia natans. Bull. Environ. Contam. Toxicol. 2013, 90, 720–724. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Ueda, K.; Maki, T.; Rahman, M.M. Influence of phosphate and iron ions in selective uptake of arsenic species by water fern (Salvinia natans L.). Chem. Eng. J. 2008, 145, 179–184. [Google Scholar] [CrossRef]
- Jampeetong, A.; Brix, H. Nitrogen nutrition of Salvinia natans: Effects of inorganic nitrogen form on growth, morphology, nitrate reductase activity and uptake kinetics of ammonium and nitrate. Aquat. Bot. 2009, 90, 67–73. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, S. Adsorptive uptake of arsenic (V) from water by aquatic fern Salvinia natans. J. Water Supply Res. Technol. 2005, 54, 47–53. [Google Scholar] [CrossRef]
- Kumari, M.; Tripathi, B.D. Effect of aeration and mixed culture of Eichhornia crassipes and Salvinia natans on removal of wastewater pollutants. Ecol. Eng. 2014, 62, 48–53. [Google Scholar] [CrossRef]
- Rápó, E.; Posta, K.; Csavdári, A.; Vincze, B.É.; Mara, G.; Kovács, G.; Haddidi, I.; Tonk, S. Performance comparison of Eichhornia crassipes and Salvinia natans on azo-dye (Eriochrome Black T) phytoremediation. Crystals 2020, 10, 565. [Google Scholar] [CrossRef]
- Szmeja, J.; Gałka, A. Survival and reproduction of the aquatic fern Salvinia natans (L.) All. during expansion in the Vistula Delta, south Baltic Sea coast. J. Freshw. Ecol. 2013, 28, 113–123. [Google Scholar] [CrossRef]
- Cui, R.; Nam, S.-H.; An, Y.-J. Salvinia natans: A potential test species for ecotoxicity testing. Environ. Pollut. 2020, 267, 115650. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dou, H.; Liu, C.; Yu, D. Decoupling between plant growth and functional traits of the free-floating fern Salvinia natans under shifted water nutrient stoichiometric regimes. Flora 2021, 281, 151876. [Google Scholar] [CrossRef]
- Dhir, B.; Srivastava, S. Heavy metal removal from a multi-metal solution and wastewater by Salvinia natans. J. Ecol. Eng. 2011, 37, 893–896. [Google Scholar] [CrossRef]
- Mandal, C.; Ghosh, N.; Maiti, S.; Das, K.; Gupta, S.; Dey, N.; Adak, M.K. Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it’s modulation by polyamine. Physiol. Mol. Biol. Plants 2013, 19, 91–103. [Google Scholar] [CrossRef]
- Liu, N.; Zhong, G.; Zhou, J.; Liu, Y.; Pang, Y.; Cai, H.; Wu, Z. Separate and combined effects of glyphosate and copper on growth and antioxidative enzymes in Salvinia natans (L.) All. Sci. Total Environ. 2019, 655, 1448–1456. [Google Scholar] [CrossRef]
- Parola, V.L.; Liveri, V.T.; Todaro, L.; Lombardo, D.; Bauer, E.M.; Dell’Era, A.; Longo, A.; Caschera, D.; de Caro, T.; Toro, R.G.; et al. Iron and lithium-iron alkyl phosphates as nanostructured material for rechargeable batteries. Mater. Lett. 2018, 220, 58–61. [Google Scholar] [CrossRef]
- Martins, A.; da Silva, D.D.; Silva, R.; Carvalho, F.; Guilhermino, L. Long-term effects of lithium and lithium-microplastic mixtures on the model species Daphnia magna: Toxicological interactions and implications to ‘One Health’. Sci. Total Environ. 2022, 838, 155934. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Tanveer, M.; Wang, L.; Bolan, S.; Sooriyakumar, P.; Robinson, B.; Wijesekara, H.; Wijesooriya, M.; Keerthanan, S.; et al. From mine to mind and mobiles—Lithium contamination and its risk management. Environ. Pollut. 2021, 290, 118067. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities—A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, O.I.; Gutyj, B.V.; Darmohray, L.M.; Sobolieva, S.V.; Ivanina, V.V.; Kuzmenko, O.A.; Karkach, P.M.; Fesenko, V.F.; Bilkevych, V.V.; Mashkin, Y.O.; et al. Lithium in the natural environment and its migration in the trophic chain. Ukr. J. Ecol. 2019, 9, 195–203. [Google Scholar]
- Kszos, L.A.; Stewart, A.J. Review of lithium in the aquatic environment: Distribution in the United States, toxicity and case example of groundwater contamination. Ecotoxicology 2003, 12, 439–447. [Google Scholar] [CrossRef]
- Bauer, B.; Kavrakovski, Z.; Kostik, V. Lithium content in potable water, surface water, ground water, and mineral water on the territory of Republic of Macedonia. Int. J. Environ. Res. Public Health 2014, 4, 189–193. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Cleary, J.; Garcia Cabellos, G.; Lloyd, A. Lithium in the natural waters of the south east of Ireland. Int. J. Environ. Res. Public Health 2017, 14, 561. [Google Scholar] [CrossRef]
- Liaugaudaite, V.; Mickuviene, N.; Raskauskiene, N.; Naginiene, R.; Sher, L. Lithium levels in the public drinking water supply and risk of suicide: A pilot study. J. Trace Elem. Med. Biol. 2017, 43, 197–201. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment-a literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Dippong, T.; Hoaghia, M.A.; Mihali, C.; Cical, E.; Calugaru, M. Human health risk assessment of some bottled waters from Romania. Environ. Pollut. 2020, 267, 115409. [Google Scholar] [CrossRef]
- Zsigmond, A.R.; Kékedy-Nagy, L.; Cordoş, E.A.; Măruţoiu, C. Assessment of lithium in transylvanian mineral waters using the platinum-wire loop faes technique. Environ. Eng. Manag. J. 2012, 11, 1561–1566. [Google Scholar] [CrossRef]
- Török, A.I.; Moldovan, A.; Levei, E.A.; Cadar, O.; Tănăselia, C.; Moldovan, O.T. Assessment of lithium, macro- and microelements in water, soil and plant samples from karst areas in Romania. Materials 2021, 14, 4002. [Google Scholar] [CrossRef]
- Naeem, A.; Aslam, M.; Saifullah; Mühling, K.H. Lithium: Perspectives of nutritional beneficence, dietary intake, biogeochemistry, and biofortification of vegetables and mushrooms. Sci. Total Environ. 2021, 798, 149249. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, N.; Adeel, M.; Azeem, I.; Ahmad, M.A.; Zain, M.; Abbas, A.; Zhou, P.; Li, Y.; Ming, X.; Rui, Y. Responses of Agricultural plants to Lithium pollution: Trends, Meta-Analysis, and Perspectives. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture method for growing plants without soil. In California Agricultural Experiment Station; University of California, Agricultural Experiment Station: Berkeley, CA, USA, 1938; pp. 29–32. [Google Scholar]
- Babenko, L.; Vasheka, O.; Shcherbatiuk, M.; Romanenko, P.; Voytenko, L.; Kosakivska, I. Biometric characteristics and surface microstructure of vegetative and reproductive organs of heterosporous water fern Salvinia natans. Flora 2019, 252, 44–50. [Google Scholar] [CrossRef]
- Gałka, A.; Szmeja, J. Phenology of the aquatic fern Salvinia natans (L.) All. in the Vistula Delta in the context of climate warming. Limnologica 2013, 43, 100–105. [Google Scholar] [CrossRef]
- Buta, E.; Török, A.; Zongo, B.; Cantor, M.; Buta, M.; Majdik, C. Comparative studies of the phytoextraction capacity of five aquatic plants in heavy metal contaminated water. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 173–179. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Pachura, P.; Ociepa-Kubicka, A.; Skowron-Grabowska, B. Assessment of the availability of heavy metals to plants based on the translocation index and the bioaccumulation factor. Desalination Water Treat. 2015, 57, 1469–1477. [Google Scholar] [CrossRef]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of Trace Elements by Wetland Plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Kalinowska, M.; Szymańska, M. A Study on selected physiological parameters of plants grown under lithium supplementation. Biol. Trace Elem. Res. 2012, 149, 425–430. [Google Scholar] [CrossRef]
- Kalinowska, M.; Hawrylak-Nowak, B.; Szymańska, M. The influence of two lithium forms on the growth, l-ascorbic acid content and lithium accumulation in lettuce plants. Biol. Trace Elem. Res. 2013, 152, 251–257. [Google Scholar] [CrossRef] [PubMed]
- McStay, N.G.; Rogers, H.H.; Anderson, C.E. Effects of lithium on Phaseolus vulgaris L. Sci. Total Environ. 1980, 16, 185–191. [Google Scholar] [CrossRef]
- Das, S.; Mazumdar, K. Phytoremediation potential of a novel fern, Salvinia cucullata, Roxb. Ex Bory, to pulp and paper mill effluent: Physiological and anatomical response. Chemosphere 2016, 163, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Favas, P.J.C.; Pratas, J.; Rodrigues, N.; D’Souza, R.; Varun, M.; Paul, M.S. Metal(loid) accumulation in aquatic plants of a mining area: Potential for water quality biomonitoring and biogeochemical prospecting. Chemosphere 2018, 194, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements of Group 1 (Previously Group Ia). In Trace Elements from Soil to Human; Kabata-Pendias, A., Mukherjee, A.B., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2007; pp. 87–104. [Google Scholar] [CrossRef]
- Suñe, N.; Sánchez, G.; Caffaratti, S.; Maine, M.A. Cadmium and chromium removal kinetics from solution by two aquatic macrophytes. Environ. Pollut. 2007, 145, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ranade-Malvi, U. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J. Agric. Sci. 2011, 24, 106–109. [Google Scholar]
- Hemantaranjan, A. Advances in Plant Physiology (Vol. 18); Scientific Publishers: Jodhpur, India, 2019; p. 365. [Google Scholar]
- Robinson, B.H.; Yalamanchali, R.; Reiser, R.; Dickinson, N.M. Lithium as an emerging environmental contaminant: Mobility in the soil-plant system. Chemosphere 2018, 197, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dhir, B.; Sharmila, P.; Pardha Saradhi, P.; Sharma, S.; Kumar, R.; Mehta, D. Heavy metal induced physiological alterations in Salvinia natans. Ecotoxicol. Environ. Saf. 2011, 74, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; Baker, A.J.M. Metal-Accumulating Plants. In Phytoremediation of Toxic Metals-Using Plants to Clean Up the Environment; Raskin, I., Ensley, B.D., Eds.; John Wiley and Sons: New York, NY, USA, 2000; pp. 193–229. [Google Scholar]
- Adamczyk-Szabela, D.; Lisowska, K.; Romanowska-Duda, Z.; Wolf, W.M. Combined cadmium-zinc interactions alter manganese, lead, copper uptake by Melissa officinalis. Sci. Rep. 2020, 10, 1675. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Luan, Y. Meta-analysis of the copper, zinc, and cadmium absorption capacities of aquatic plants in heavy metal-polluted water. Int. J. Environ. Res. Public Health 2015, 12, 14958–14973. [Google Scholar] [CrossRef] [PubMed]
- Samecka-Cymerman, A.; Kempers, A.J. Concentrations of heavy metals and plant nutrients in water, sediments and aquatic macrophytes of anthropogenic lakes (former open cut brown coal mines) differing in stage of acidification. Sci. Total Environ. 2001, 281, 87–98. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, N.; Geng, Y.; Zhou, J.; Lei, J. Effects of the combined pollution of cadmium, lead and zinc on the phytoextraction efficiency of ryegrass (Lolium perenne L.). RSC Adv. 2019, 9, 20603–20611. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Hu, S.; Fei, L.; Liu, L.; Wang, Z. Interaction between Cd and Zn on Metal Accumulation, Translocation and Mineral Nutrition in Tall Fescue (Festuca arundinacea). Int. J. Mol. Sci. 2019, 20, 3332. [Google Scholar] [CrossRef] [PubMed]
- Krayem, M.; Khatib, S.E.; Hassan, Y.; Deluchat, V.; Labrousse, P. In search for potential biomarkers of copper stress in aquatic plants. Aquat. Toxicol. 2021, 239, 105952. [Google Scholar] [CrossRef] [PubMed]
- Liliana, C.; Inga, Z.; Ludmila, R.; Tatiana, C.; Ana, P.; Andrei, A.; Svetlana, D.; Larisa, G.; Decebal, I. Biomass of Arthrospira platensis enriched with lithium by bioaccumulation and biosorption process. Food Biosci. 2021, 41, 100950. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Mu, S.-Y.; Tian, C.-Y. Apocynum venetum: A newly found lithium accumulator. Flora 2014, 209, 285–289. [Google Scholar] [CrossRef]
- Li, X.; Gao, P.; Gjetvaj, B.; Westcott, N.; Gruber, M.Y. Analysis of the metabolome and transcriptome of Brassica carinata seedlings after lithium chloride exposure. Plant Sci. 2009, 177, 68–80. [Google Scholar] [CrossRef]
- Emiliani, J.; Llatance Oyarce, W.G.; Bergara, C.D.; Salvatierra, L.M.; Novo, L.A.B.; Pérez, L.M. Variations in the phytoremediation efficiency of metal-polluted water with salvinia biloba: Prospects and toxicological impacts. Water 2020, 12, 1737. [Google Scholar] [CrossRef]
- Dridi, N.; Bouslimi, H.; Duarte, B.; Caçador, I.; Sleimi, N. Evaluation of physiological and biochemical parameters and some bioindicators of barium tolerance in Limbarda crithmoides and Helianthus annuus. Int. J. Plant Biol. 2022, 13, 115–131. [Google Scholar] [CrossRef]
- Suwa, R.; Jayachandran, K.; Nguyen, N.T.; Boulenouar, A.; Fujita, K.; Saneoka, H. Barium toxicity effects in soybean plants. Arch. Environ. Contam. Toxicol. 2008, 55, 397–403. [Google Scholar] [CrossRef]
- Marisamy, K.; Duraipandian, M.; Sevugaperumal, R.; Ramasubramanian, V. Barium toxicity induced changes on growth, photosynthetic, biochemical and antioxidative enzymes level in helianthus annuus (L.). Int. J. Recent Sci. Res. 2016, 7, 9781–9787. [Google Scholar]
- Upadhyay, A.K.; Singh, N.K.; Rai, U.N. Comparative metal accumulation potential of Potamogeton pectinatus L. and Potamogeton crispus L.: Role of enzymatic and non-enzymatic antioxidants in tolerance and detoxification of metals. Aquat. Bot. 2014, 117, 27–32. [Google Scholar] [CrossRef]
- Dhir, B.; Srivastava, S. Disposal of metal treated Salvinia biomass in soil and its effect on growth and photosynthetic Efficiency of wheat. Int. J. Phytoremediation 2012, 14, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Francioso, O.; Ferrari, E.; Schiavon, M.; Nardi, S. Spectroscopic-chemical fingerprint and biostimulant activity of a protein-based product in solid form. Molecules 2018, 23, 1031. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, Y.; Zhang, L.; Yang, R.; Zhou, Y. Extraction, preliminary structural characterization, and antioxidant activities of polysaccharides from Salvia miltiorrhiza Bunge. Carbohydr. Polym. 2012, 87, 1348–1353. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatt, V.; Kumar, N. Chapter 9—Amides From Plants: Structures and Biological Importance. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, Netherlands, 2018; Volume 56, pp. 287–333. [Google Scholar]
- Cajamarca, F.A.B.; Corazza, M.Z.; Prete, M.C.; Dragunski, D.C.; Rocker, C.; Caetano, J.; Gonçalves, J.A.C.; Tarley, C.R.T. Investigation on the performance of chemically modified aquatic macrophytes—Salvinia molesta for the micro-solid phase preconcentration of Cd(II) on-line coupled to FAAS. Bull. Environ. Contam. Toxicol. 2016, 97, 863–869. [Google Scholar] [CrossRef]
- Lima, L.K.S.; Silva, M.G.C.; Vieira, M.G. Study of binary and single biosorption by the floating aquatic macrophyte Salvinia natans. Braz. J. Chem. Eng. 2016, 33, 649–660. [Google Scholar] [CrossRef]
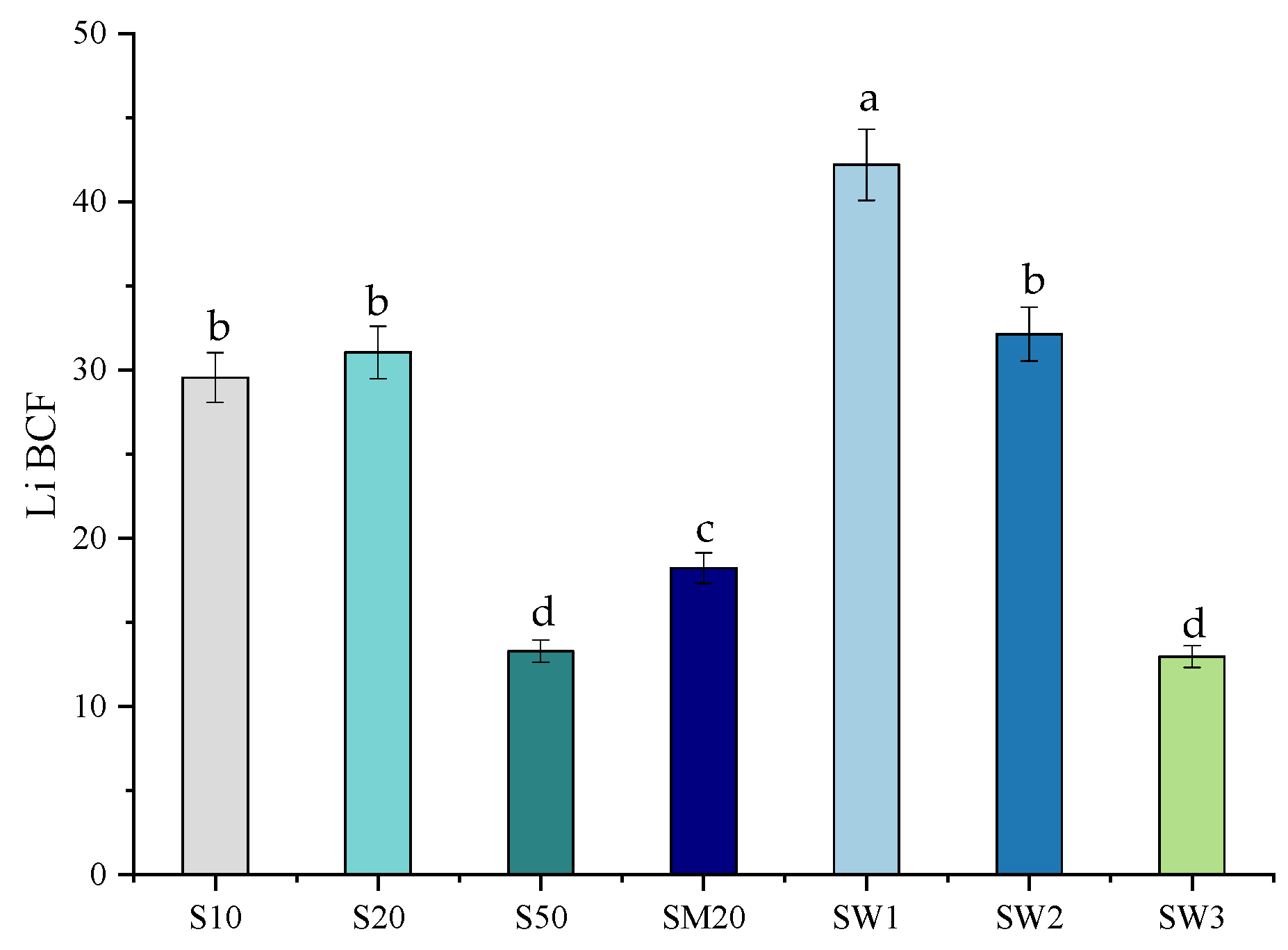
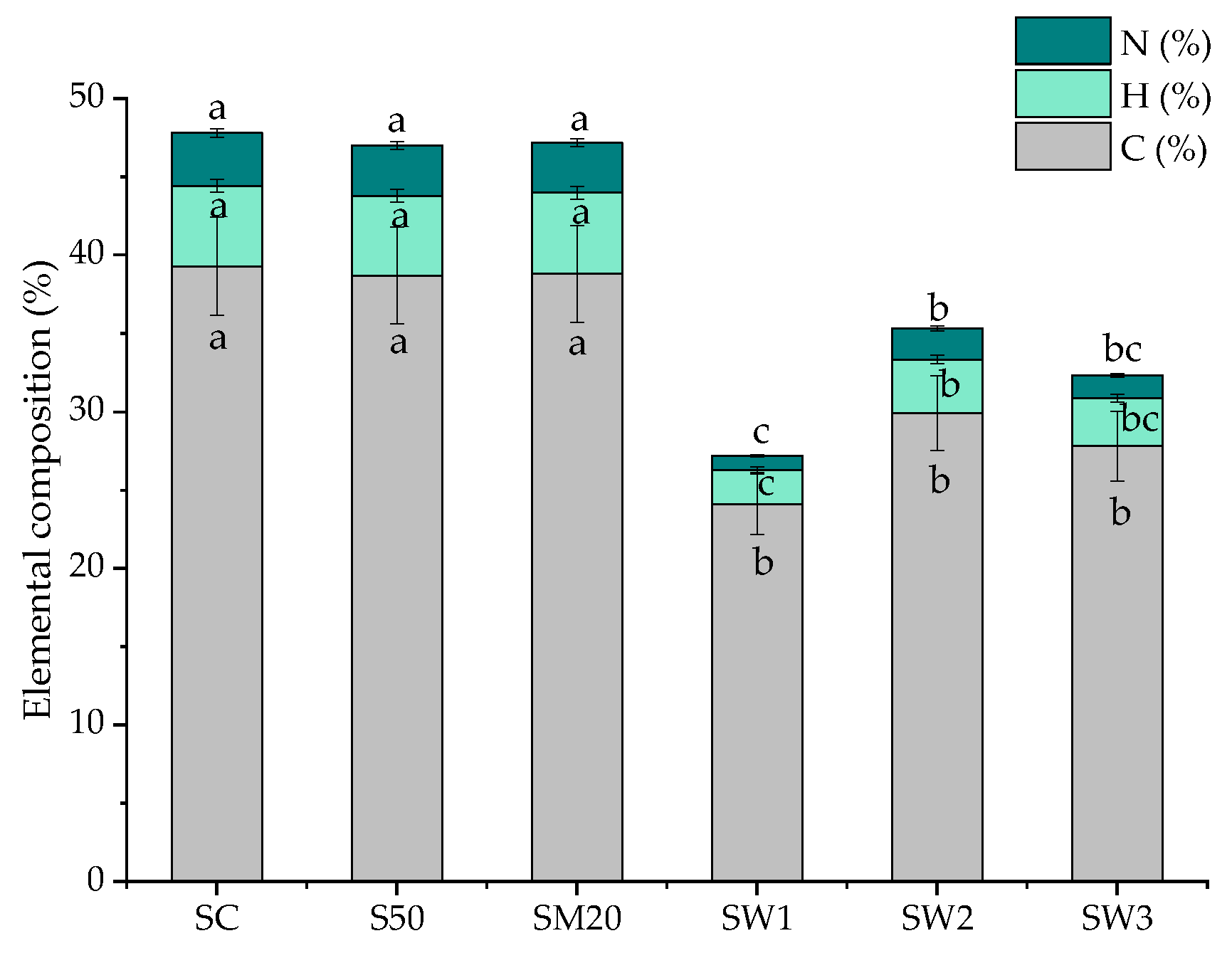

| W1 | W2 | W3 | ||
|---|---|---|---|---|
| Major elements (mg/L) | Na | 20.2 ± 1.6 | 484 ± 24 | 599 ± 30 |
| Mg | 44.8 ± 3.6 | 52.8 ± 4.2 | 44.5 ± 3.6 | |
| K | 2.54 ± 0.20 | 7.18 ± 0.36 | 24.5 ±1.2 | |
| Ca | 386 ± 19 | 126 ± 10 | 154 ± 12 | |
| Trace elements (µg/L) | Li | 68.6 ± 3.4 | 233 ± 12 | 2090 ± 104 |
| Mn | 104 ± 5 | 351 ± 18 | 700 ± 35 | |
| Fe | 176 ± 9 | 35.3 ± 1.8 | 42.3 ± 2.1 | |
| Ni | 15.0 ± 0.7 | 11.0 ± 0.6 | 14.3 ± 0.7 | |
| Cu | <0.21 | 3.49 ± 0.28 | 3.81 ± 0.72 | |
| Zn | 5.04 ± 0.46 | 11.7 ± 1.1 | 2.56 ± 0.23 | |
| Sr | 658 ± 33 | 1110 ± 56 | 1350 ± 67 | |
| Ba | 1010 ±51 | 46.6 ± 22.3 | 301 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Török, A.I.; Moldovan, A.; Kovacs, E.; Cadar, O.; Becze, A.; Levei, E.A.; Neag, E. Lithium Accumulation in Salvinia natans Free-Floating Aquatic Plant. Materials 2022, 15, 7243. https://doi.org/10.3390/ma15207243
Török AI, Moldovan A, Kovacs E, Cadar O, Becze A, Levei EA, Neag E. Lithium Accumulation in Salvinia natans Free-Floating Aquatic Plant. Materials. 2022; 15(20):7243. https://doi.org/10.3390/ma15207243
Chicago/Turabian StyleTörök, Anamaria Iulia, Ana Moldovan, Eniko Kovacs, Oana Cadar, Anca Becze, Erika Andrea Levei, and Emilia Neag. 2022. "Lithium Accumulation in Salvinia natans Free-Floating Aquatic Plant" Materials 15, no. 20: 7243. https://doi.org/10.3390/ma15207243
APA StyleTörök, A. I., Moldovan, A., Kovacs, E., Cadar, O., Becze, A., Levei, E. A., & Neag, E. (2022). Lithium Accumulation in Salvinia natans Free-Floating Aquatic Plant. Materials, 15(20), 7243. https://doi.org/10.3390/ma15207243










