Green Synthesis of Silver Nanoparticles (AgNPs) of Angelica Gigas Fabricated by Hot-Melt Extrusion Technology for Enhanced Antifungal Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Plant Extract
2.3. Synthesis of AgNPs
2.4. Visual Inspection
2.5. Characterization of AgNPs
2.6. Antifungal Effect
2.7. Statistical Analysis
3. Results and Discussion
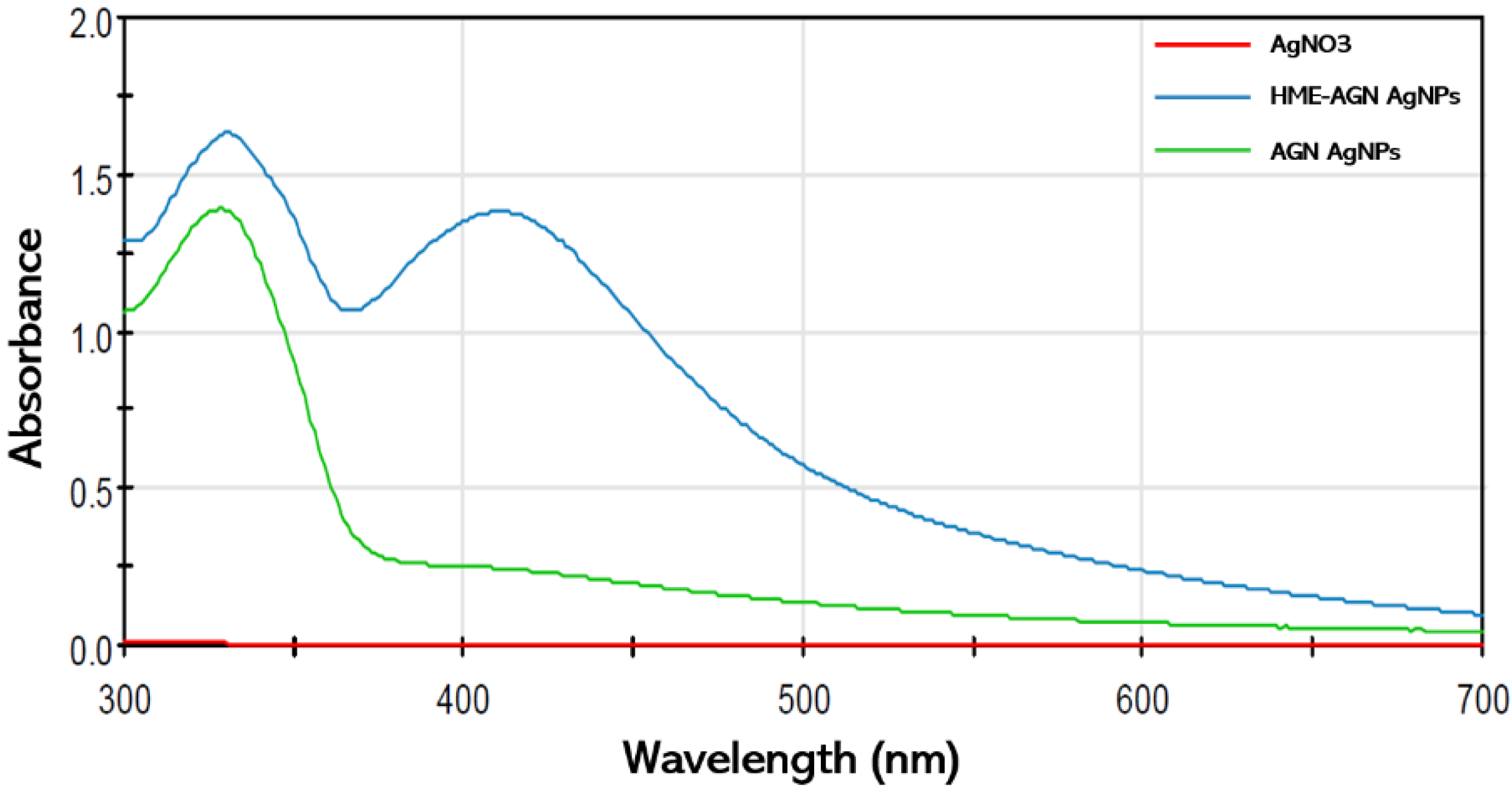
3.1. Synthesis of AgNPs
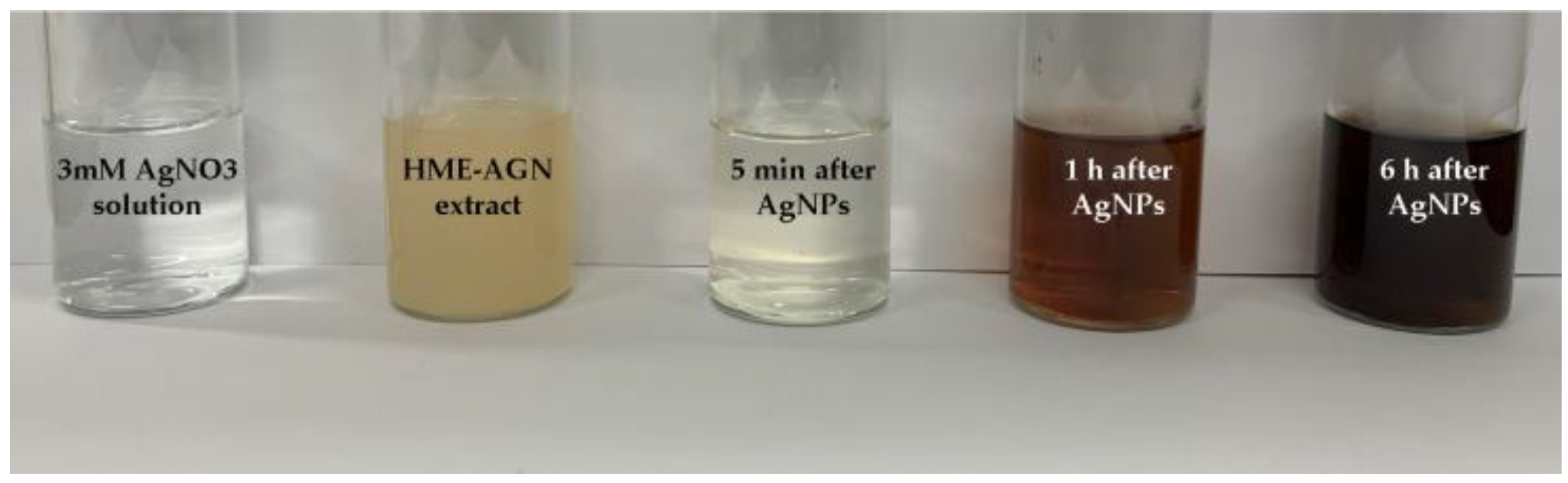
3.2. Visual Inspection
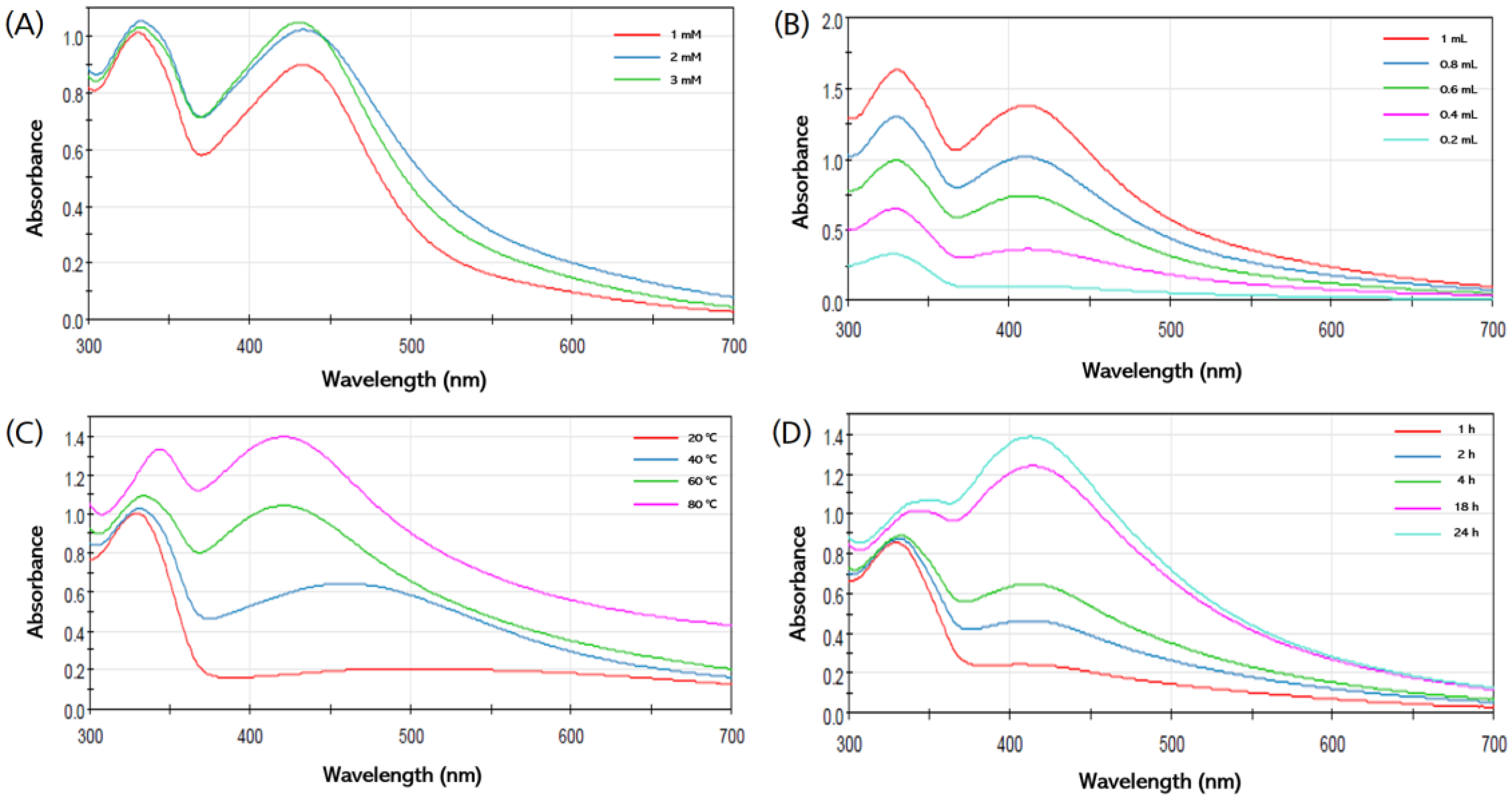
3.3. Optimization of Synthesis of AgNPs
3.3.1. Effect of the AgNO3 Concentration
3.3.2. Effect of Ratio of HME-AGN Extract
3.3.3. Effect of Incubation Temperature
3.3.4. Effect of Incubation Time
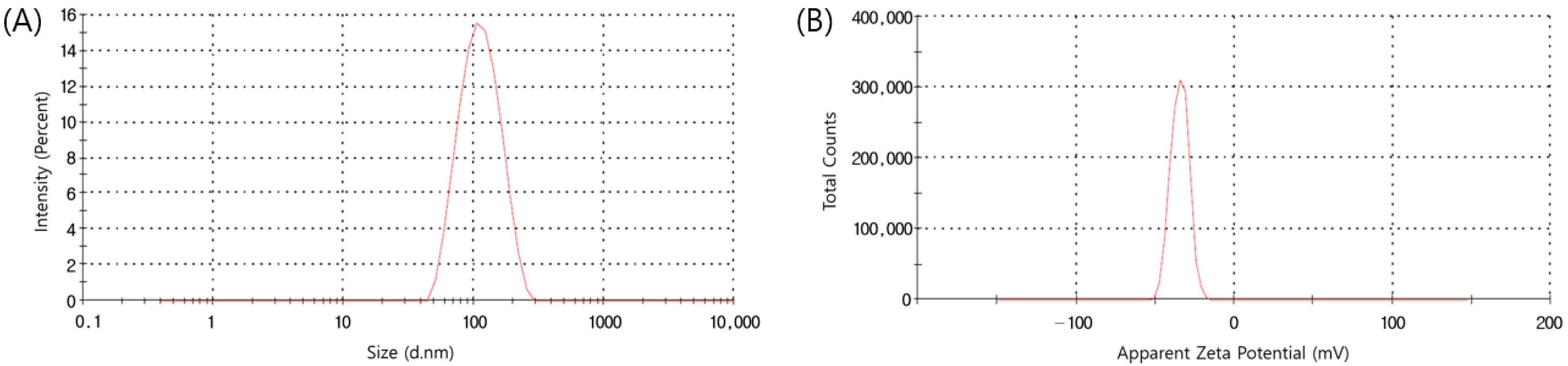
3.4. Dynamic Light Scattering (DLS) and Zeta Potential Analysis
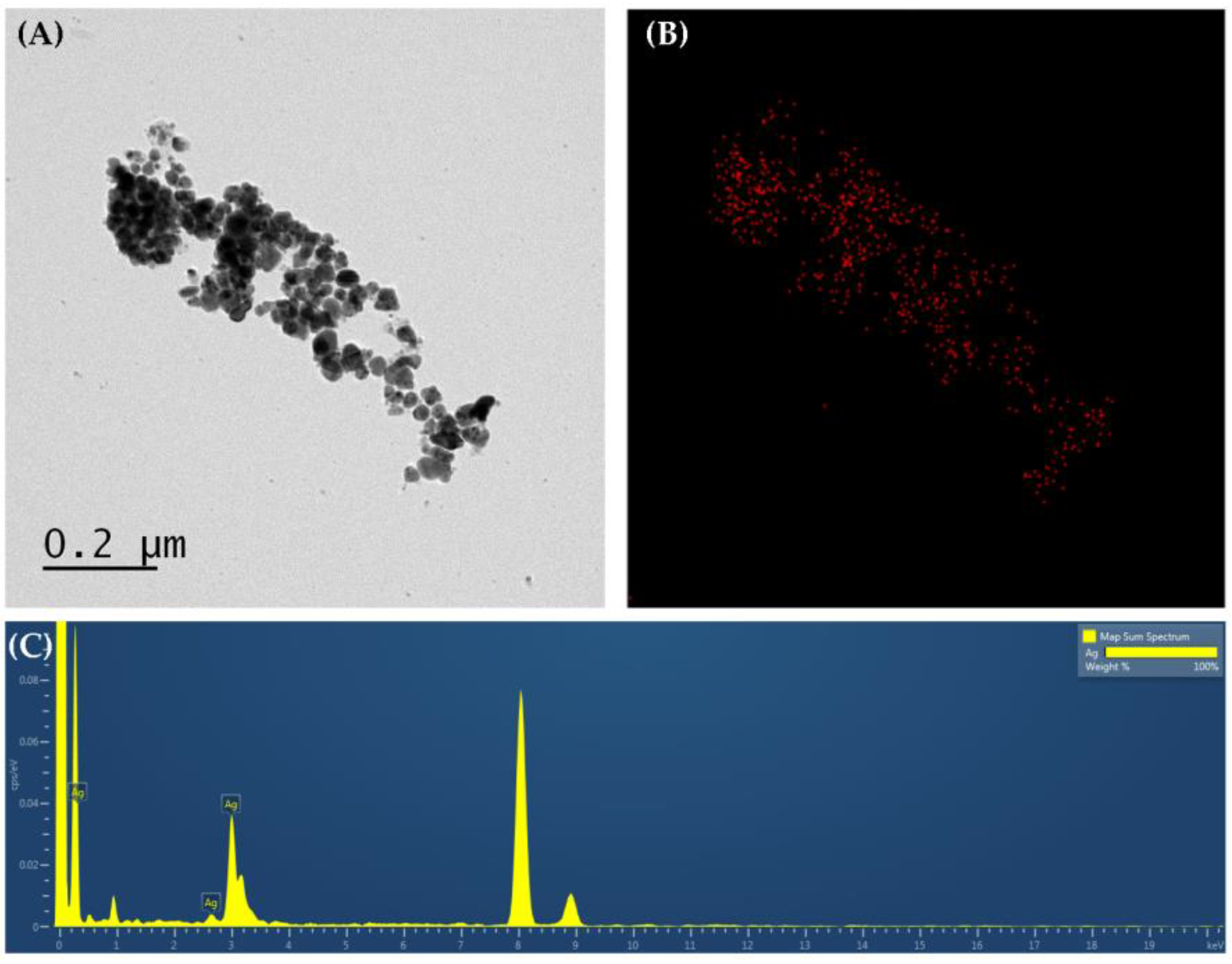
3.5. TEM Analysis
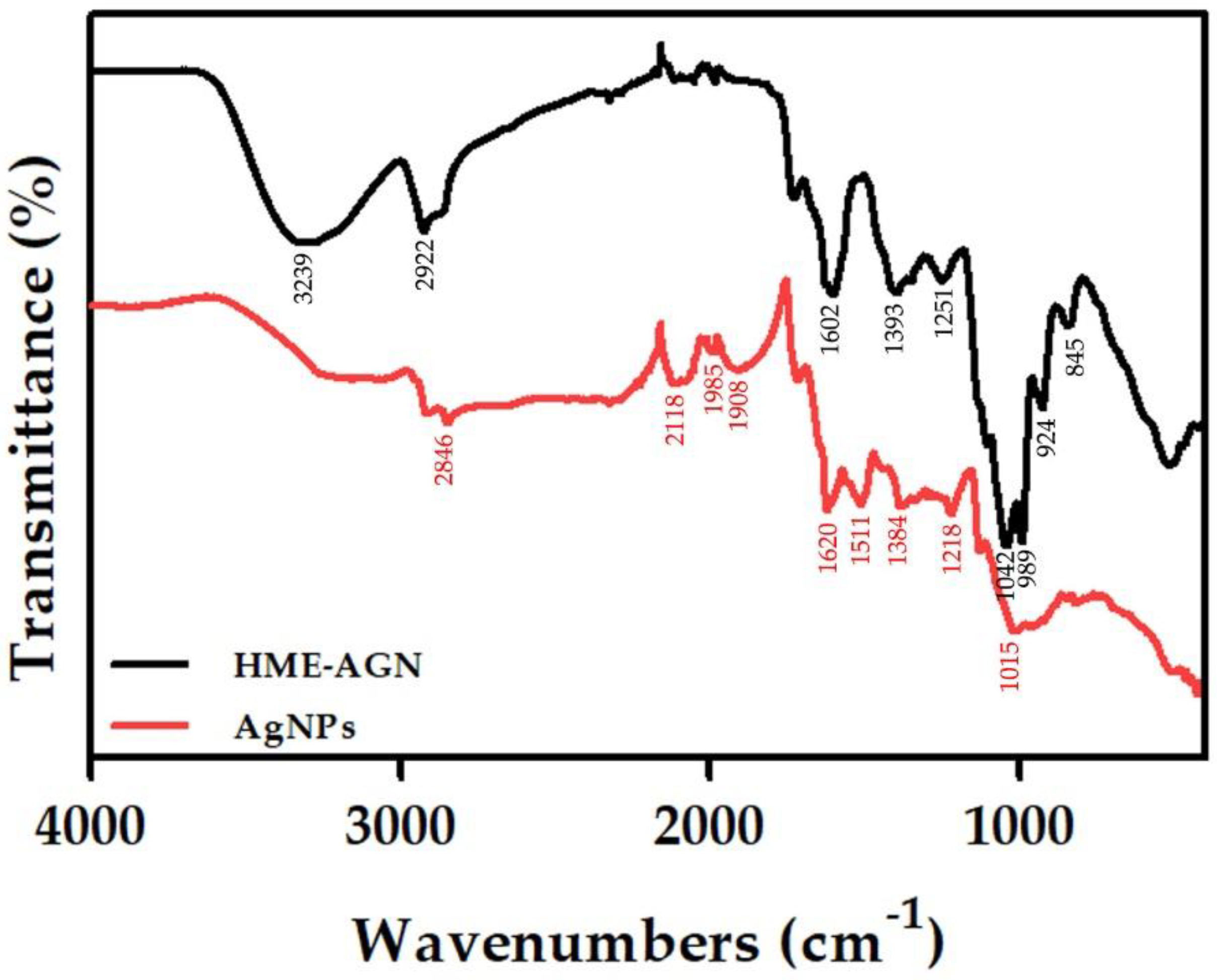
3.6. FT-IR Analysis
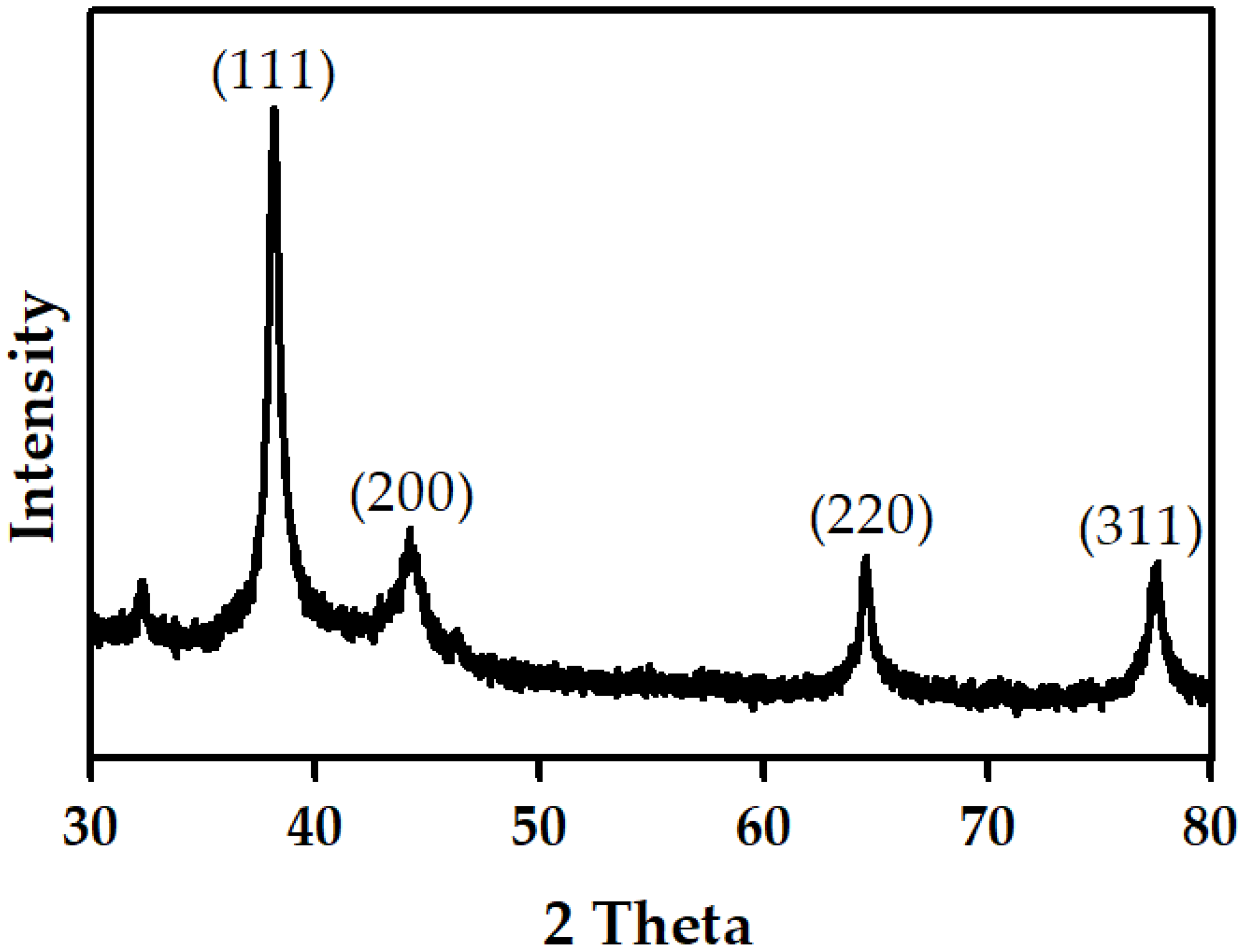
3.7. XRD Analysis
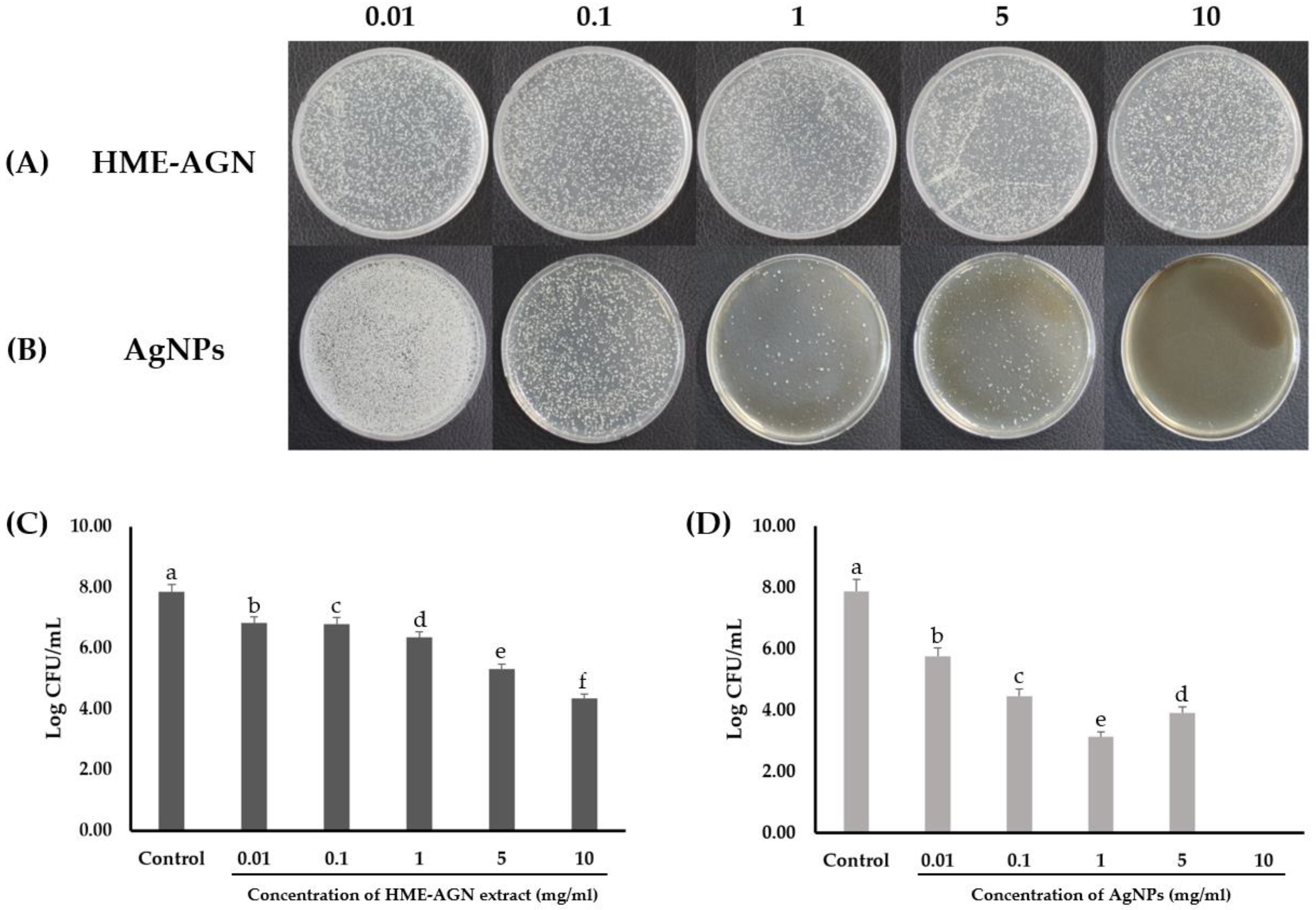
3.8. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryu, S.; Jin, M.; Lee, H.-K.; Wang, M.-H.; Cho, C.-W. Effects of lipid nanoparticles on physicochemical properties, cellular uptake, and lymphatic uptake of 6-methoxflavone. J. Pharm. Investig. 2022, 52, 233–241. [Google Scholar] [CrossRef]
- Kim, M.-K.; Ki, D.-H.; Na, Y.-G.; Lee, H.-S.; Baek, J.-S.; Lee, J.-Y.; Lee, H.-K.; Cho, C.-W. Optimization of Mesoporous Silica Nanoparticles through Statistical Design of Experiment and the Application for the Anticancer Drug. Pharmaceutics 2021, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Baek, J.-S.; Park, J.-K.; Lee, B.-J.; Kim, M.-S.; Hwang, S.-J.; Lee, J.-Y.; Cho, C.-W. Development of Houttuynia cordata Extract-Loaded Solid Lipid Nanoparticles for Oral Delivery: High Drug Loading Efficiency and Controlled Release. Molecules 2017, 22, 2215. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; EL-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef]
- Salem, S.S.; Ali, O.M.; Reyad, A.M.; Abd-Elsalam, K.A.; Hashem, A.H. Pseudomonas indica-Mediated Silver Nanoparticles: Antifungal and Antioxidant Biogenic Tool for Suppressing Mucormycosis Fungi. J. Fungi 2022, 8, 126. [Google Scholar] [CrossRef]
- Mare, A.D.; Man, A.; Ciurea, C.N.; Toma, F.; Cighir, A.; Mareș, M.; Berța, L.; Tanase, C. Silver Nanoparticles Biosynthesized with Spruce Bark Extract—A Molecular Aggregate with Antifungal Activity against Candida Species. Antibiotics 2021, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Mare, A.D.; Ciurea, C.N.; Man, A.; Mareș, M.; Toma, F.; Berța, L.; Tanase, C. In Vitro Antifungal Activity of Silver Nanoparticles Biosynthesized with Beech Bark Extract. Plants 2021, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.; Abdallah, B.M. Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles. Nanomaterials 2020, 10, 422. [Google Scholar] [CrossRef]
- Musa, S.F.; Yeat, T.S.; Kamal, L.; Tabana, Y.M.; Ahmed, M.A.; El Ouweini, A.; Lim, V.; Keong, L.C.; Sandai, D. Pleurotus sajor-caju can be used to synthesize silver nanoparticles with antifungal activity against Candida albicans. J. Sci. Food Agric. 2018, 98, 1197–1207. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Bajwa, A.A.; Parveen, A. Green Synthesis of Silver Nanoparticles Using Parthenium Hysterophorus: Optimization, Characterization and In Vitro Therapeutic Evaluation. Molecules 2020, 25, 3224. [Google Scholar] [CrossRef]
- Handoko, C.T.; Huda, A.; Bustan, M.D.; Bambang, Y. Green synthesis of silver nanoparticle and its antibacterial activity. Rasayan J. Chem. 2017, 10, 1137–1144. [Google Scholar]
- Kalainila, P.; Subha, V.; Ernest Ravindran, R.S.; Renganathan, S. Synthesis and characterization of silver nanoparticle from erythrina indica. Asian J. Pharm. Clin. Res. 2014, 7, 39–43. [Google Scholar]
- Rajaram, K.; Aiswarya, D.C.; Sureshkumar, P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater. Lett. 2015, 138, 251–254. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Individual and Combined Effects of Extracellular Polymeric Substances and Whole Cell Components of Chlamydomonas reinhardtii on Siver Nanoparticle Synthesis and Stability. Molecules 2019, 24, 956. [Google Scholar] [CrossRef] [PubMed]
- Tan Sian Hui Abdullah, H.S.; Aqlili Riana Mohd Asseri, S.N.; Khursyiah Wan Mohamad, W.N.; Kan, S.-Y.; Azmi, A.A.; Yong Julius, F.S.; Chia, P.W. Green synthesis, characterization and applications of silver nanoparticle mediated by the aqueous extract of red onion peel. Environ. Pollut. 2021, 271, 116295. [Google Scholar] [CrossRef]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef]
- Iqtedar, M.; Aslam, M.; Akhyar, M.; Shehzaad, A.; Abdullah, R.; Kaleem, A. Extracellular biosynthesis, characterization, optimization of silver nanoparticles (AgNPs) using Bacillus mojavensis BTCB15 and its antimicrobial activity against multidrug resistant pathogens. Prep. Biochem. Biotechnol. 2019, 49, 136–142. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Taghdiri, M.; Makari, V.; Rahimi-Nasrabadi, M. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1249–1254. [Google Scholar] [CrossRef]
- Allafchian, A.R.; Mirahmadi-Zare, S.Z.; Jalali, S.A.H.; Hashemi, S.S.; Vahabi, M.R. Green synthesis of silver nanoparticles using phlomis leaf extract and investigation of their antibacterial activity. J. Nanostruct. Chem. 2016, 6, 129–135. [Google Scholar] [CrossRef]
- Logaranjan, K.; Raiza, A.J.; Gopinath, A.C.B.; Chen, Y.; Pandian, K. Shape-and Size-Controlled Synthesis of Silver Nanoparticles Using Aloe vera Plant Extract and Their Antimicrobial Activity. Nanoscale Res. Lett. 2016, 11, 520. [Google Scholar] [CrossRef]
- Piao, J.; Lee, J.-Y.; Weon, J.B.; Ma, C.J.; Ko, H.-J.; Kim, D.-D.; Kang, W.-S.; Cho, H.-J. Angelica gigas Nakai and Soluplus-Based Solid Formulations Prepared by Hot-Melting Extrusion: Oral Absorption Enhancing and Memory Ameliorating Effects. PLoS ONE 2015, 10, e0124447. [Google Scholar]
- Jiang, Y.; Piao, J.; Cho, H.-J.; Kang, W.-S.; Kim, H.-Y. Improvement in antiproliferative activity of angelica gigas Nakai by solid dispersion formation via hot-melt extrusion and induction of cell cycle arrest and apoptosis in HeLa cells. Biosci. Biotechnol. Biochem. 2015, 79, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Heo, S.H.; Lee, J.; Shin, M.S. Extraction of Active Compounds from Angelica gigas using Supercritical Carbon Dioxide and its Physiological Activity. J. Converg. Inf. Technol. 2021, 11, 206–212. [Google Scholar]
- Park, S.-J.; Yoon, J.-H.; Kim, Y.-E.; Yoon, W.-B.; Kim, J.-D. In vitro Antioxidant Activity of the Aqueous of Angelicae gigas Nakai Leaves. Korean J. Food Preserv. 2011, 18, 817–823. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Jiang, C.; Xing, C.; Kim, S.-H.; Lu, J. Anti-cancer and Other Bioactivities of Korean Angelica gigas Nakai (AGN) and its Major Pyranocoumarin Compounds. Anticancer Agents Med. Chem. 2012, 12, 1239–1254. [Google Scholar] [CrossRef]
- OK, S.; Oh, S.-R.; Jung, T.-S.; Jeon, S.-O.; Jung, J.-W.; Ryu, D.-S. Effects of Angelica gigas Nakai as an Anti-Inflammatory Agent in In Vitro and In Vivo Atopic Dermatitis Models. eCAM 2018, 2018, 2450712. [Google Scholar]
- Azad, M.O.K.; Adnan, M.; Sung, I.J.; Lim, J.D.; Baek, J.-S.; Lim, Y.S.; Park, C.H. Development of value-added functional food by fusion of colored potato and buckwheat flour through hot-melt extrusion. J. Food Process. Preserv. 2022, 46, e15312. [Google Scholar] [CrossRef]
- Wang, W.; Kang, Q.; Liu, N.; Zhang, Q.; Zhang, Y.; Li, H.; Zhao, B.; Chen, Y.; Lan, Y.; Ma, Q.; et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia 2015, 102, 189–197. [Google Scholar] [CrossRef]
- Giri, B.R.; Kwon, J.; Vo, A.Q.; Bhagurkar, A.M.; Bandari, S.; Kim, D.W. Hot-Melt Extruded Amorphous Solid Dispersion for Solubility, Stability, and Bioavailability Enhancement of Telmisartan. Pharmaceuticals 2021, 14, 73. [Google Scholar] [CrossRef]
- Go, E.-J.; Ryu, B.-R.; Ryu, S.-J.; Kim, H.-B.; Lee, H.-T.; Kwon, J.-W.; Baek, J.-S.; Lim, J.-D. An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion. Int. J. Mol. Sci. 2021, 22, 12377. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, H.Y.; Nam, S.-H.; Baek, J.-S. Antifungal Activity of Angelica gigas with Enhanced Water Solubility of Decursin and Decursinol Angelate by Hot-Melt Extrusion Technology against Candida albicans. Int. J. Transl. Med. 2022, 2, 515–521. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Nasrollahzadeh, M.; Alizadeh, M. Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and Congo red. J. Alloys Compd. 2016, 680, 309–314. [Google Scholar] [CrossRef]
- Krajczewski, J.; Kołątaj, K.; Kudelski, A. Plasmonic nanoparticles in chemical analysis. RSC Adv. 2017, 7, 17559–17576. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, X.; Wei, J. Localized Surface Plasmon Resonance of Silver Nanotriangles Synthesized by a Versatile Solution Reaction. Nanoscale Res. Lett. 2015, 10, 354. [Google Scholar] [CrossRef]
- Ali, Z.A.; Yahya, R.; Sekaran, S.D.; Puteh, R. Green Synthesis of Silver Nanoparticles Using Apple Extract and Its Antibacterial Properties. Adv. Mater. Sci. Eng. 2015, 2016, 4102196. [Google Scholar] [CrossRef]
- Vu, X.H.; Duong, T.T.T.; Pham, T.T.H.; Trinh, D.K.; Nguyen, X.H.; Dang, V.-S. Synthesis and study of silver nanoparticles for antibacterial activity against Escherichia coli and Staphylococcus aureus. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 25019. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arab. J. Chem. 2017, 13, 1011–1019. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Kumari, M.; Nayak, B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 44–52. [Google Scholar] [CrossRef]
- Nahar, K.; Rahaman, M.M.; Khan, G.M.A.; Islam, M.K.; Al-Reza, S.M. Green synthesis of silver nanoparticles from citrus sinensis peel extract and its antibacterial potential. Asian J. Green Chem. 2021, 5, 135–150. [Google Scholar]
- Ameen, F.; Abdullah, M.M.S.; Al-Homaidan, A.A.; Al-Lohedan, H.A.; Al-Ghanayem, A.A.; Almansob, A. Fabrication of silver nanoparticles employing the cyanobacterium Spirulina platensis and its bactericidal effect against opportunistic nosocomial pathogens of the respiratory tract. J. Mol. Struct. 2020, 1217, 128392. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Ramezani, T.; Hosseini, N.; Mohamad, R. Green Synthesis of Silver Nanoparticles using Achillea biebersteinii Flower Extract and Its Anti-Angiogenic Properties in the Rat Aortic Ring Model. Molecules 2014, 19, 4624–4634. [Google Scholar] [CrossRef] [PubMed]
- Saeb, A.T.M.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Salem, S.S.; Alharbi, A.A.; Abdelghany, T.M. Ecofriendly synthesis of silver nanoparticles using Kei-apple (Dovyalis caffra) fruit and their efficacy against cancer cells and clinical pathogenic microorganisms. Arab. J. Chem. 2022, 15, 103927. [Google Scholar] [CrossRef]
- Coseri, S.; Spatareanu, A.; Sacarescu, L.; Rimbu, C.; Suteu, D.; Spirk, S.; Harabagiu, V. Green synthesis of the silver nanoparticles mediated by pullulan and 6-carboxypullulan. Carbohydr. Polym. 2015, 116, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bindhu, M.R.; Umadevi, M.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111836. [Google Scholar]
- Abbasi, Z.; Feizi, S.; Taghipour, E.; Ghadam, P. Green synthesis of silver nanoparticles using aqueous extract of dried Juglans regia green husk and examination of its biological properties. Green Process. Synth. 2017, 6, 477–485. [Google Scholar] [CrossRef]
- Mochochoko, T.; Oluwafemi, O.S.; Jumbam, D.N.; Songca, S.P. Green synthesis of silver nanoparticles using cellulose extracted from an aquatic weed; water hyacinth. Carbohydr. Polym. 2013, 98, 290–294. [Google Scholar] [CrossRef]
- Mehta, B.K.; Chhajlani, M.; Shrivastava, B.D. Green synthesis of silver nanoparticles and their characterization by XRD. J. Phys. Conf. Ser. 2017, 836, 12050. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C. 2015, 49, 373–381. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hussein, G.; Brza, M.A.; Mohammed, J.S.; Abdulwahid, T.R.; Raza Saeed, S.; Hassanzadeh, A. Fabrication of Interconnected Plasmonic Spherical Silver Nanoparticles with Enhanced Localized Surface Plasmon Resonance (LSPR) Peaks Using Quince Leaf Extract Solution. Nanomaterials 2019, 9, 1557. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Kong, Y.; Wang, X.; Pandoli, O.; Gao, G. Synergetic Antibacterial Effects of Silver Nanoparticles@Aloe Vera Prepared via a Green Method. Nano Biomed. Eng. 2010, 2, 252–257. [Google Scholar] [CrossRef]
- Banala, R.R.; Nagati, V.B.; Karnati, P.R. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Biol. Sci. 2015, 22, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 368, 58–63. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ali, E.M. Green Synthesis of Silver Nanoparticles Using the Lotus lalambensis Aqueous Leaf Extract and Their Anti-Candidal Activity against Oral Candidiasis. ACS Omega 2021, 6, 8151–8162. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar] [CrossRef]
- Golabiazar, R.; Othman, K.I.; Khalid, K.M.; Maruf, D.H.; Aulla, S.M.; Yusif, P.A. Green Synthesis, Characterization, and Investigation Antibacterial Activity of Silver Nanoparticles Using Pistacia atlantica Leaf Extract. Bionanoscience 2019, 9, 323–333. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, S.; Nam, S.-H.; Baek, J.-S. Green Synthesis of Silver Nanoparticles (AgNPs) of Angelica Gigas Fabricated by Hot-Melt Extrusion Technology for Enhanced Antifungal Effects. Materials 2022, 15, 7231. https://doi.org/10.3390/ma15207231
Ryu S, Nam S-H, Baek J-S. Green Synthesis of Silver Nanoparticles (AgNPs) of Angelica Gigas Fabricated by Hot-Melt Extrusion Technology for Enhanced Antifungal Effects. Materials. 2022; 15(20):7231. https://doi.org/10.3390/ma15207231
Chicago/Turabian StyleRyu, Suji, Seoul-Hee Nam, and Jong-Suep Baek. 2022. "Green Synthesis of Silver Nanoparticles (AgNPs) of Angelica Gigas Fabricated by Hot-Melt Extrusion Technology for Enhanced Antifungal Effects" Materials 15, no. 20: 7231. https://doi.org/10.3390/ma15207231
APA StyleRyu, S., Nam, S.-H., & Baek, J.-S. (2022). Green Synthesis of Silver Nanoparticles (AgNPs) of Angelica Gigas Fabricated by Hot-Melt Extrusion Technology for Enhanced Antifungal Effects. Materials, 15(20), 7231. https://doi.org/10.3390/ma15207231







